Fabrication of Magnetically Actuated Fluidic Drug Delivery Device Using Polyvinyl Chloride Adhesive Stencils
Abstract
1. Introduction
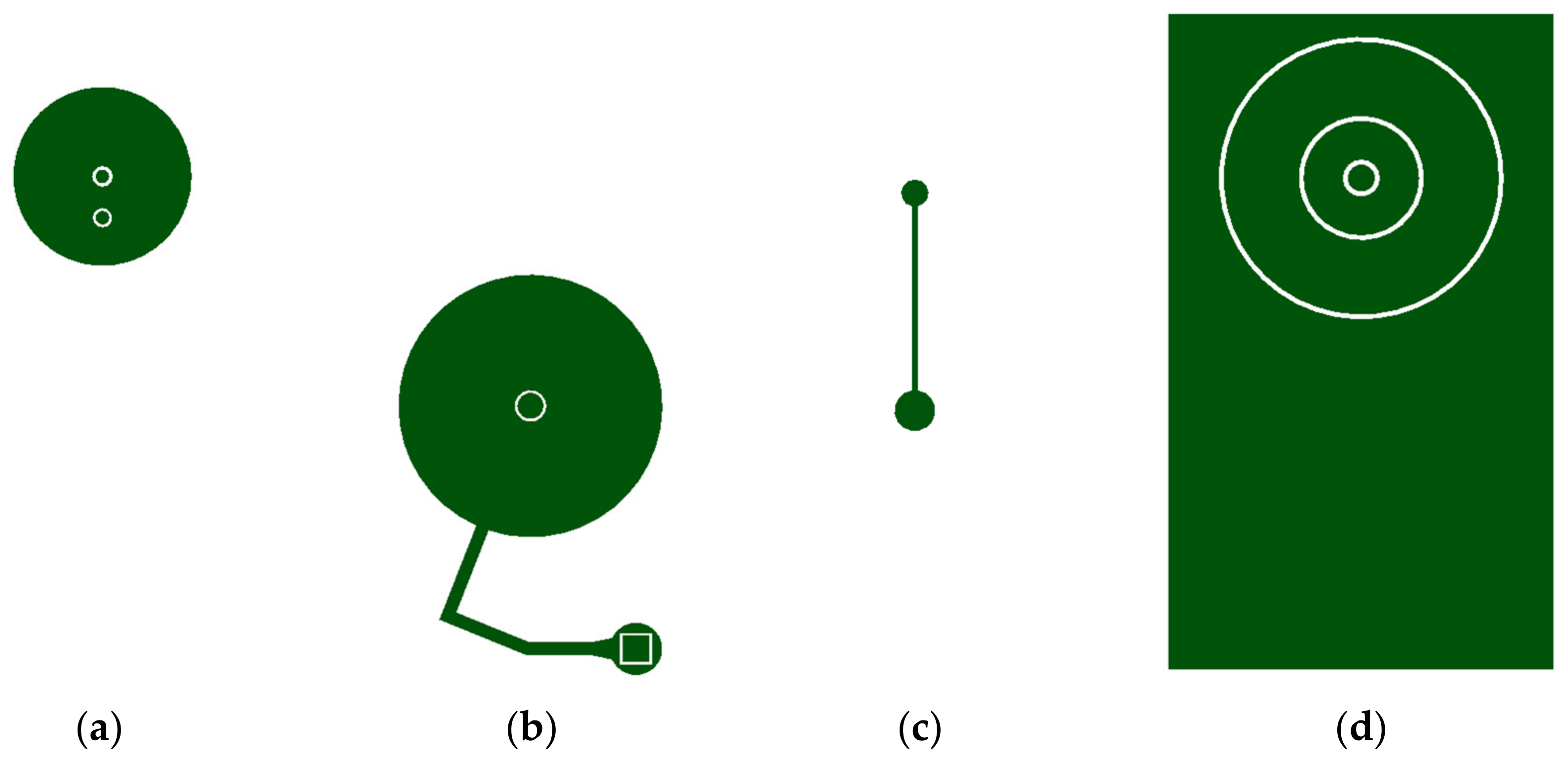
2. Design and Fabrication
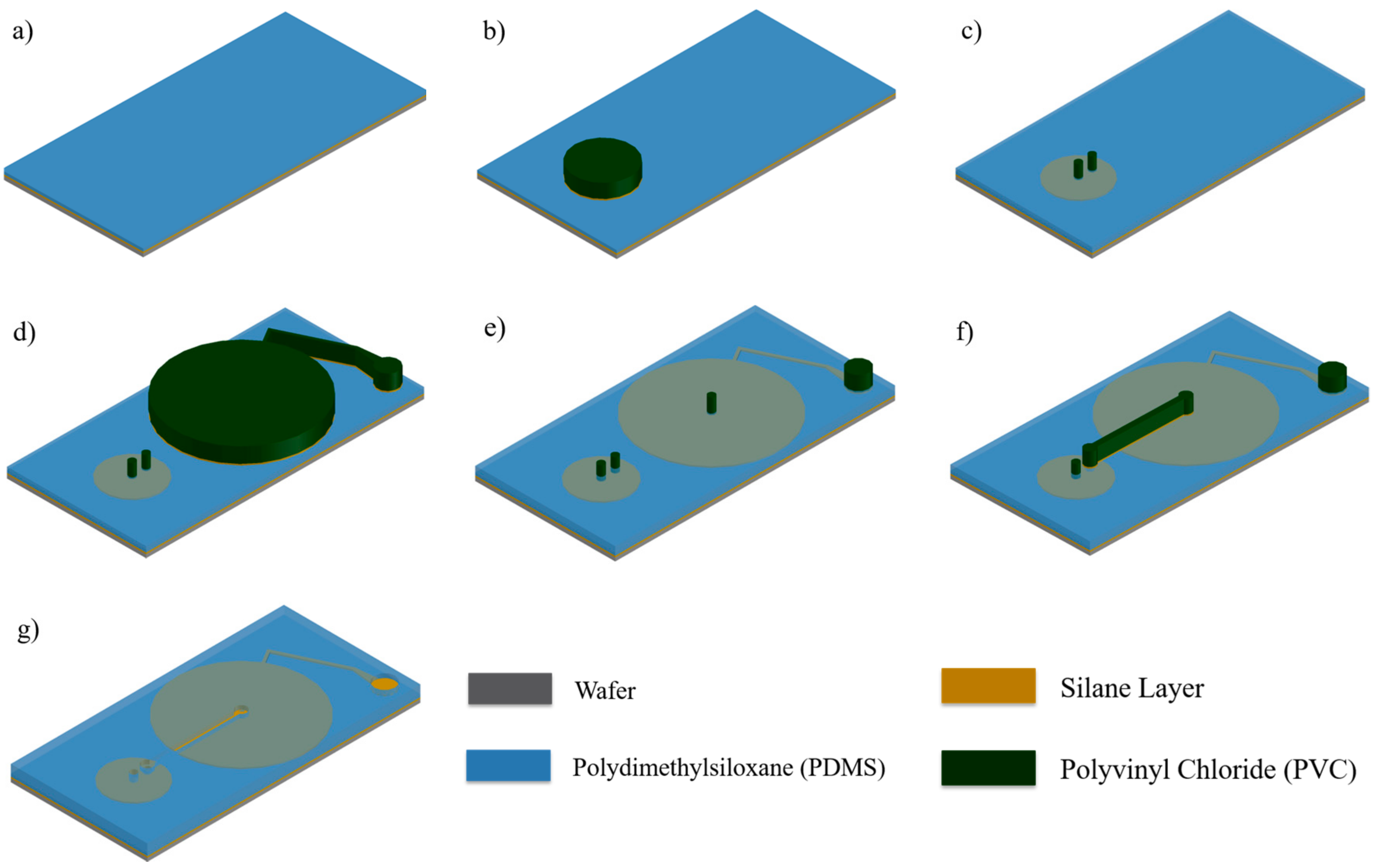
2.1. Selective Bonding and Inflatable Chamber Fabrication
2.2. Flow Rate Regulating Microchannel
2.3. Magnetically Actuated Microvalve
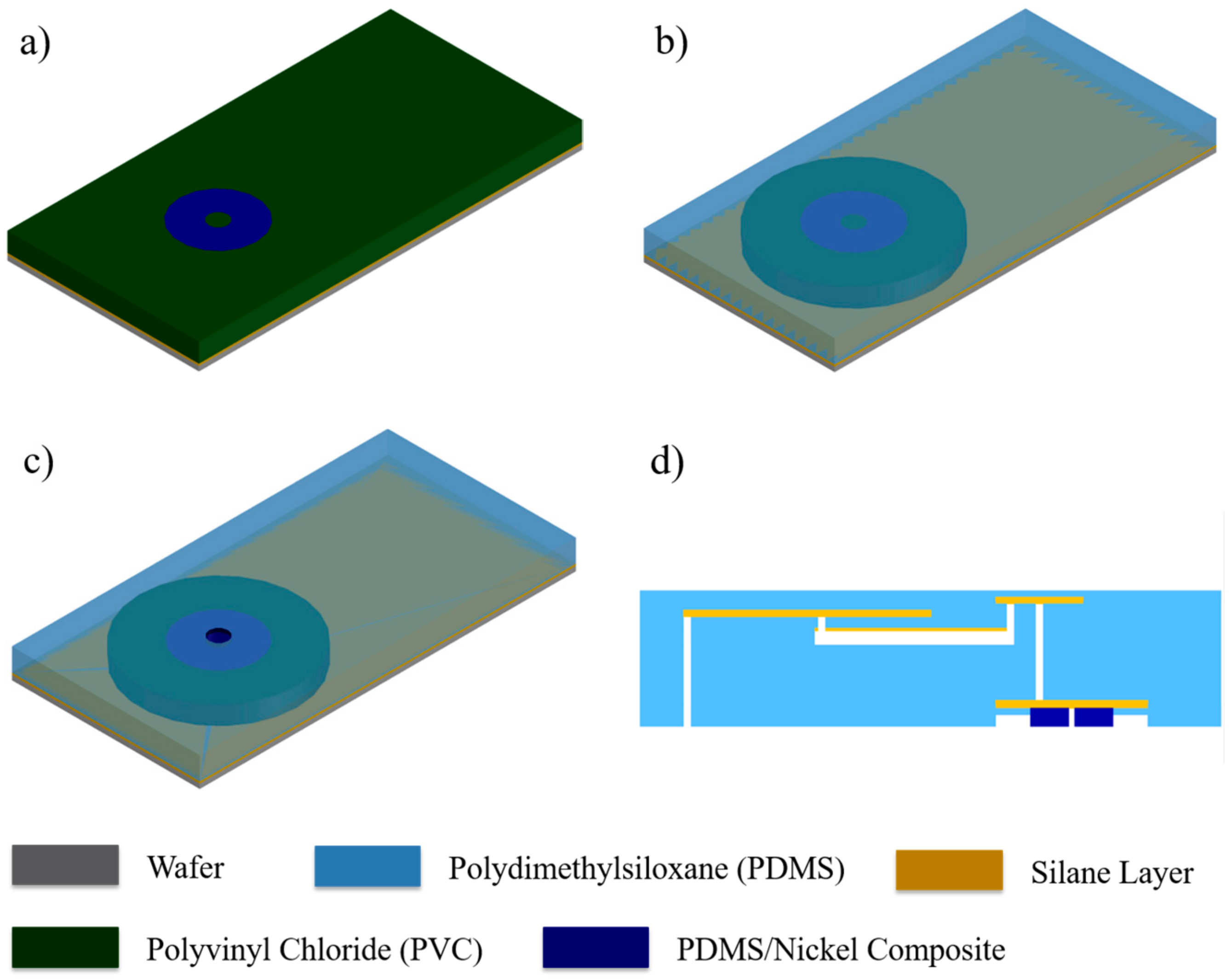
2.4. Integration via Plasma Bonding
2.5. Experimental Setup
3. Results
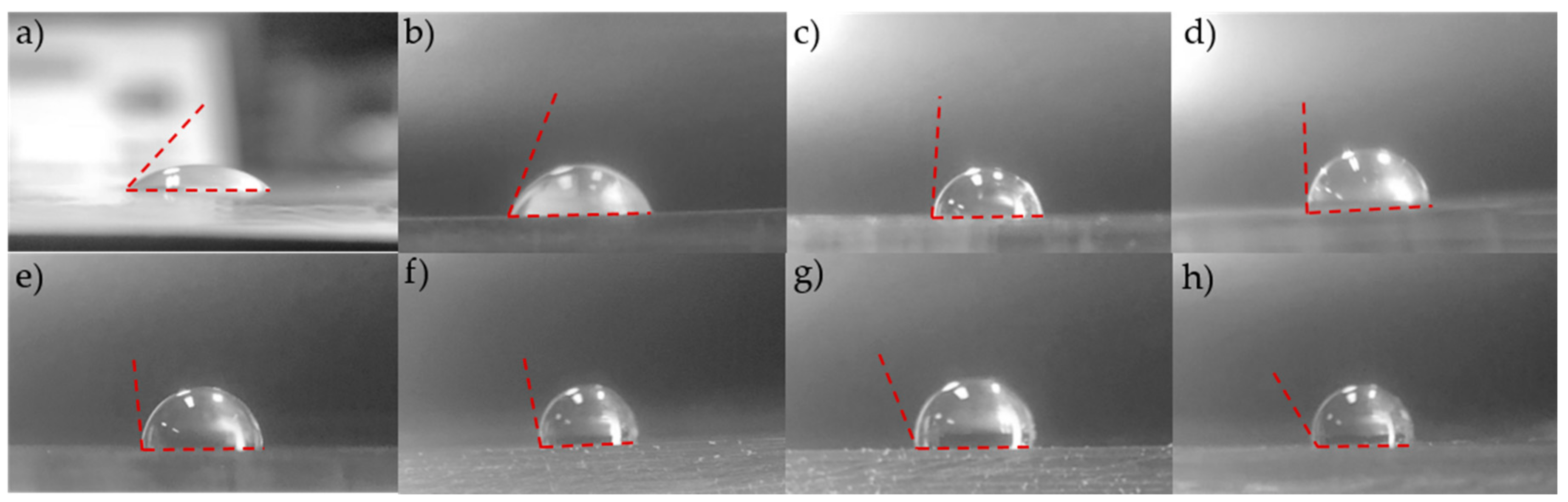
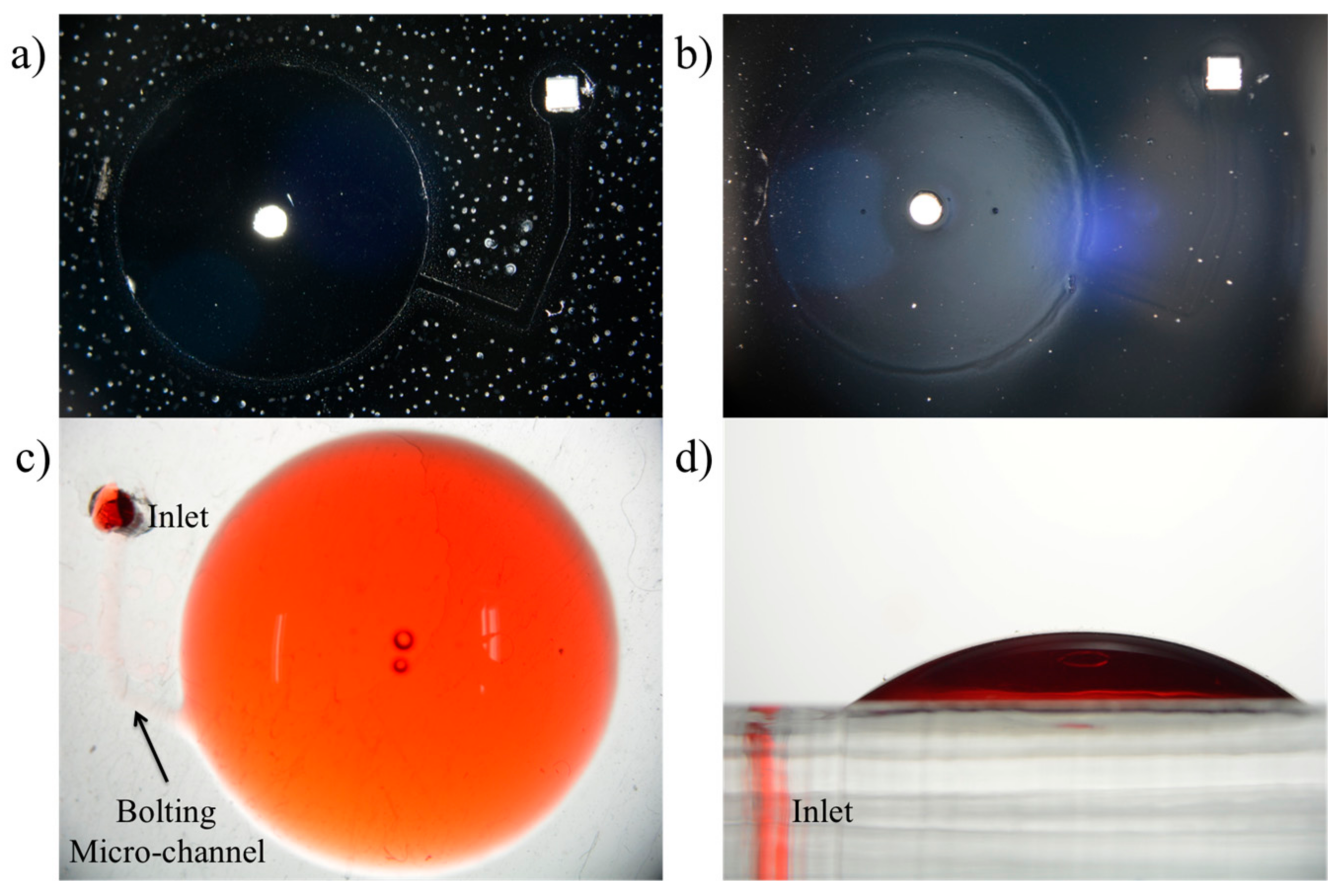
3.1. Surface Modification and Selective Bonding
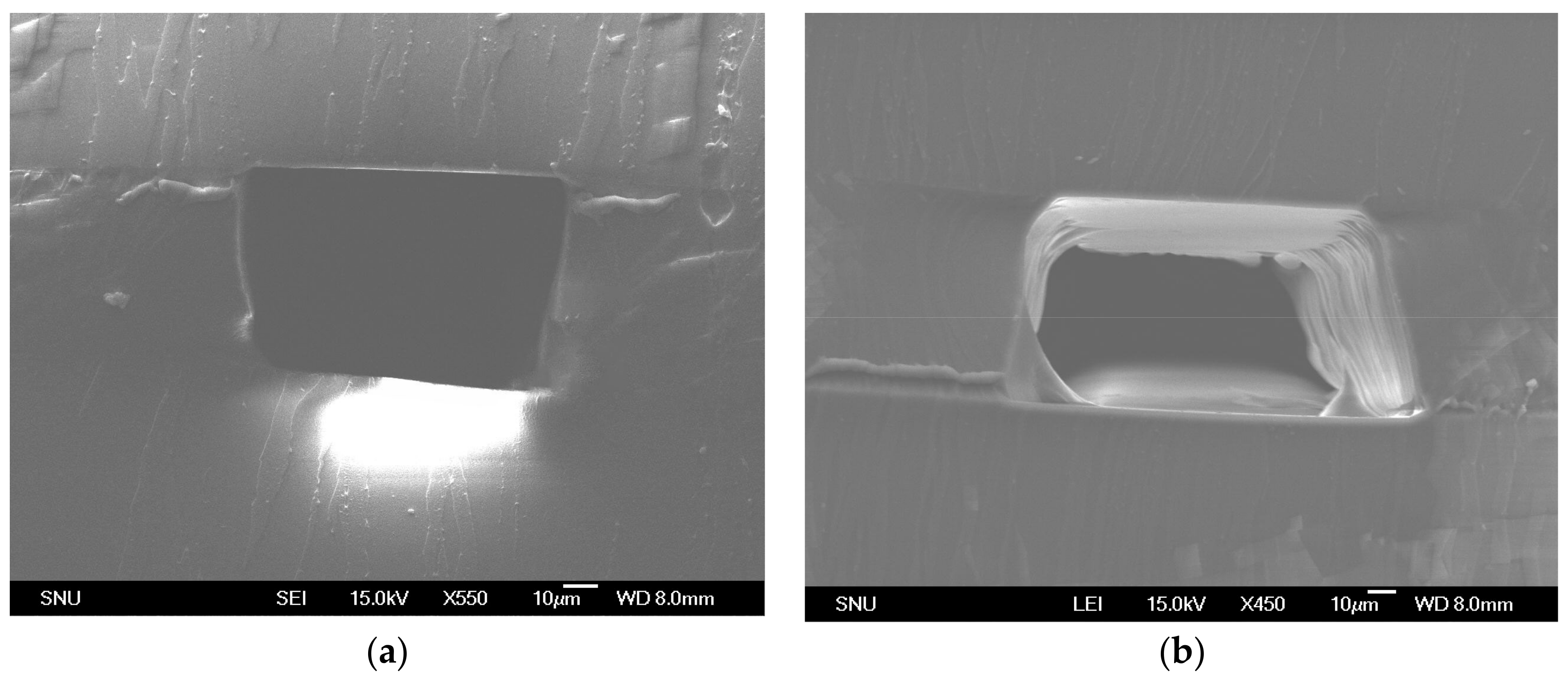
3.2. Microchannel Fabrication
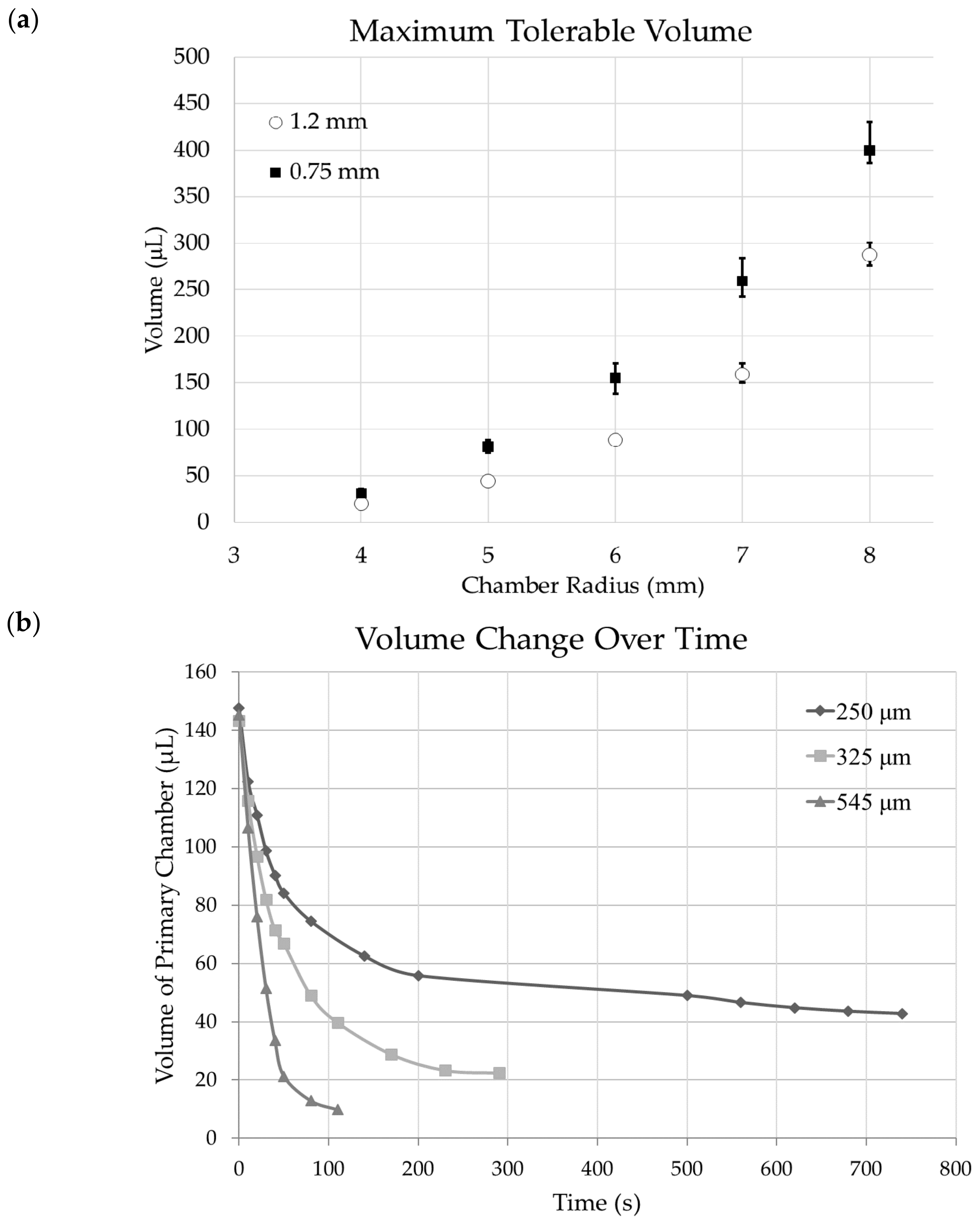
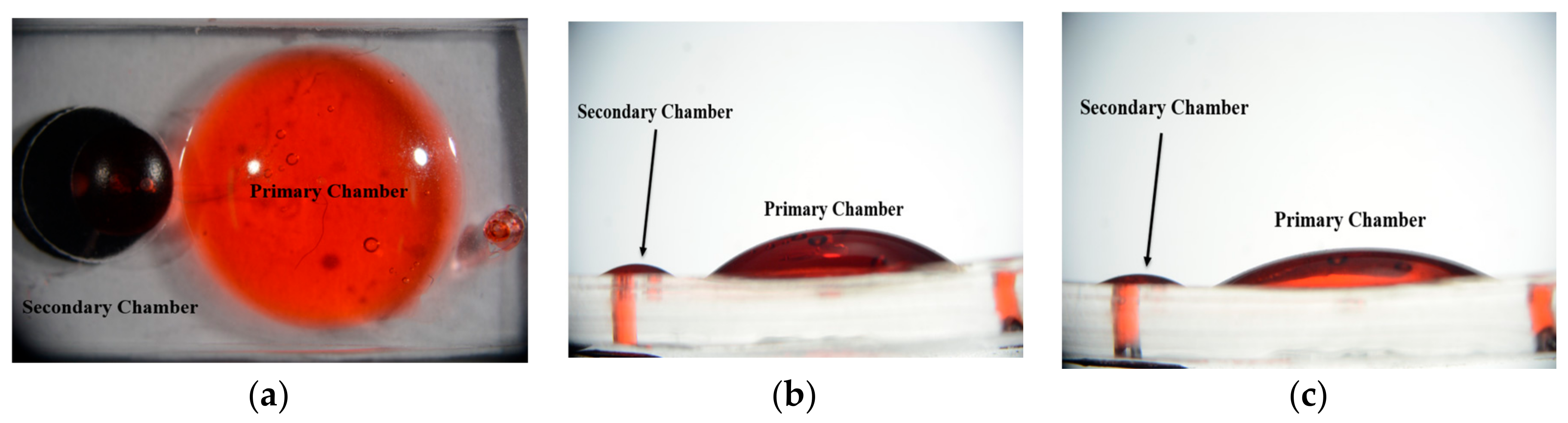
3.3. Chamber Maximum Volume
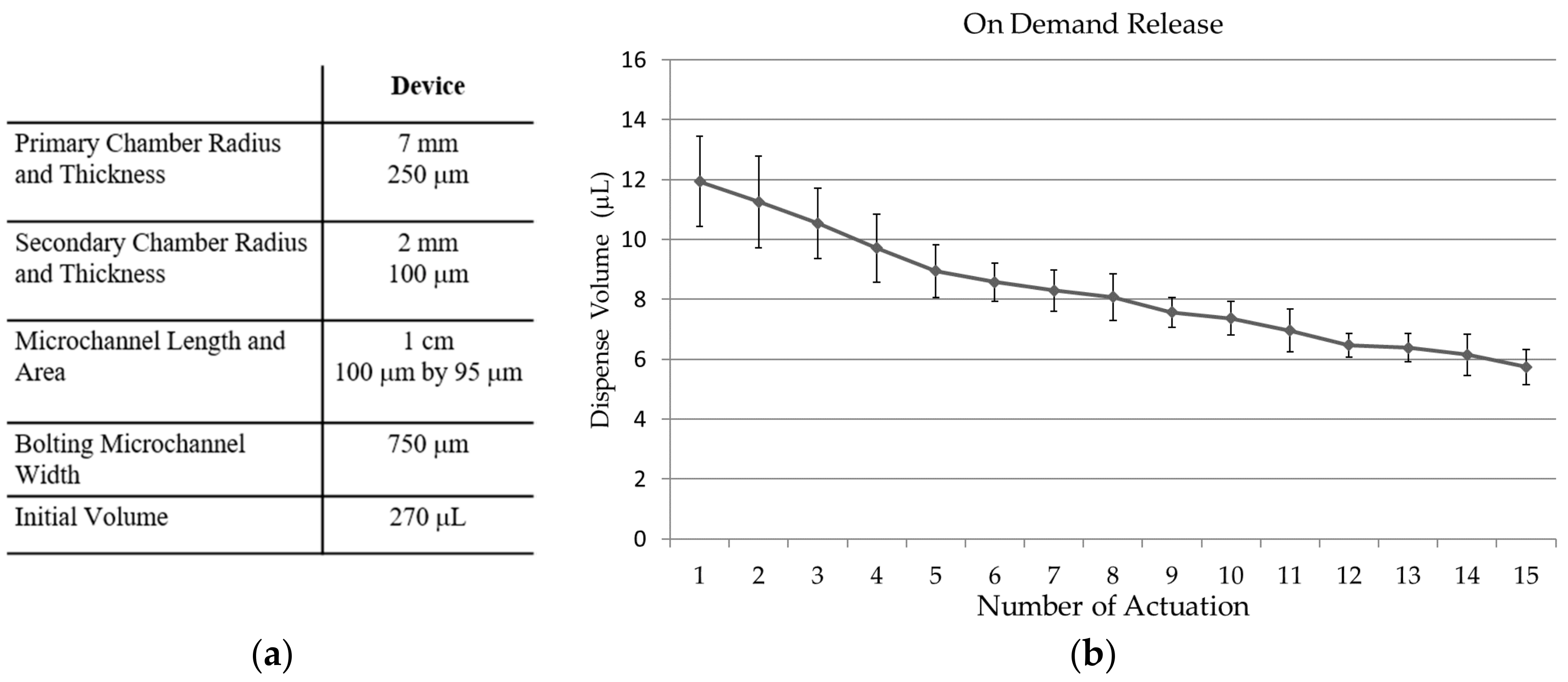
3.4. Fluid Release
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lavan, D.A.; McGuire, T.; Langer, R. Small-scale systems for in vivo drug delivery. Nat. Biotechnol. 2003, 21, 1184. [Google Scholar] [CrossRef] [PubMed]
- Santini, J.T., Jr.; Richards, A.C.; Scheidt, R.; Cima, M.J.; Langer, R. Microchips as controlled drug-delivery devices. Angew. Chem. Int. Ed. 2000, 39, 2396–2407. [Google Scholar] [CrossRef]
- Santini, J.T., Jr.; Cima, M.J.; Langer, R. A controlled-release microchip. Nature 1999, 397, 335. [Google Scholar] [CrossRef] [PubMed]
- Göpferich, A. Mechanisms of polymer degradation and erosion. In The Biomaterials: Silver Jubilee Compendium; Elsevier: New York, NY, USA, 2006; pp. 117–128. [Google Scholar]
- Zhang, Y.; Ji, X.; Mohapatra, R.N. A naturally light sterile neutrino in an asymmetric dark matter model. J. High Energy Phys. 2013, 2013, 104. [Google Scholar] [CrossRef]
- Singh, A.; Agarwal, R.; Diaz-Ruiz, C.A.; Willett, N.J.; Wang, P.; Lee, L.A.; Wang, Q.; Guldberg, R.E.; García, A.J. Nanoengineered Particles for Enhanced Intra-Articular Retention and Delivery of Proteins. Adv. Healthc. Mater. 2014, 3, 1562–1567. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Siepmann, F. Modeling of diffusion controlled drug delivery. J. Control. Release 2012, 161, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Santus, G.; Baker, R.W. Osmotic drug delivery: A review of the patent literature. J. Control. Release 1995, 35, 1–21. [Google Scholar] [CrossRef]
- Ma, Y.; Pidaparti, R.M. Simulation of Drug-Loaded Nanoparticles Transport through Drug Delivery Microchannels. J. Nanotechnol. Eng. Med. 2014, 5, 031002. [Google Scholar] [CrossRef]
- Liu, T.Y.; Hu, S.H.; Liu, K.H.; Liu, D.M.; Chen, S.Y. Study on controlled drug permeation of magnetic-sensitive ferrogels: Effect of Fe3O4 and PVA. J. Control. Release 2008, 126, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Riahi, R.; Tamayol, A.; Shaegh, S.A.M.; Ghaemmaghami, A.M.; Dokmeci, M.R.; Khademhosseini, A. Microfluidics for advanced drug delivery systems. Curr. Opin. Chem. Eng. 2015, 7, 101–112. [Google Scholar] [CrossRef]
- Shikida, M.; Sato, K.; Tanaka, S.; Kawamura, Y.; Fujisaki, Y. Electrostatically driven gas valve with high conductance. J. Microelectromech. Syst. 1994, 3, 76–80. [Google Scholar] [CrossRef]
- Bae, B.; Kee, H.; Kim, S.; Lee, Y.; Sim, T.; Kim, Y.; Park, K. In vitro experiment of the pressure regulating valve for a glaucoma implant. J. Micromech. Microeng. 2003, 13, 613. [Google Scholar] [CrossRef]
- Zachkani, P.; Jackson, J.K.; Pirmoradi, F.N.; Chiao, M. A cylindrical magnetically-actuated drug delivery device proposed for minimally invasive treatment of prostate cancer. RSC Adv. 2015, 5, 98087–98096. [Google Scholar] [CrossRef]
- Lo, R.; Li, P.Y.; Saati, S.; Agrawal, R.N.; Humayun, M.S.; Meng, E. A passive MEMS drug delivery pump for treatment of ocular diseases. Biomed. Microdevices 2009, 11, 959. [Google Scholar] [CrossRef] [PubMed]
- Barth, P.W. Silicon microvalves for gas flow control. In Proceedings of the International Solid-State Sensors and Actuators Conference, Stockholm, Sweden, 25–29 June 1995; Volume 2, pp. 276–279. [Google Scholar]
- Roberts, D.C.; Li, H.; Steyn, J.L.; Yaglioglu, O.; Spearing, S.M.; Schmidt, M.A.; Hagood, N.W. A piezoelectric microvalve for compact high-frequency, high-differential pressure hydraulic micropumping systems. J. Microelectromech. Syst. 2003, 12, 81–92. [Google Scholar] [CrossRef]
- Kim, J.H.; Na, K.H.; Kang, C.J.; Jeon, D.; Kim, Y.S. A disposable thermopneumatic-actuated microvalve stacked with PDMS layers and ITO-coated glass. Microelectron. Eng. 2004, 73, 864–869. [Google Scholar] [CrossRef]
- Yang, X.; Grosjean, C.; Tai, Y.C.; Ho, C.M. A MEMS thermopneumatic silicone rubber membrane valve. Sens. Actuators A Phys. 1998, 64, 101–108. [Google Scholar] [CrossRef]
- Baechi, D.; Buser, R.; Dual, J. A high density microchannel network with integrated valves and photodiodes. Sens. Actuators A Phys. 2002, 95, 77–83. [Google Scholar] [CrossRef]
- Kohl, M.; Dittmann, D.; Quandt, E.; Winzek, B. Thin film shape memory microvalves with adjustable operation temperature. Sens. Actuators A Phys. 2000, 83, 214–219. [Google Scholar] [CrossRef]
- Reynaerts, D.; Peirs, J.; Van Brussel, H. An implantable drug-delivery system based on shape memory alloy micro-actuation. Sens. Actuators A Phys. 1997, 61, 455–462. [Google Scholar] [CrossRef]
- Pemble, C.M.; Towe, B.C. A miniature shape memory alloy pinch valve. Sens. Actuators A Phys. 1999, 77, 145–148. [Google Scholar] [CrossRef]
- Tamanaha, C.R.; Whitman, L.J.; Colton, R.J. Hybrid macro-micro fluidics system for a chip-based biosensor. J. Micromech. Microeng. 2002, 12, N7. [Google Scholar] [CrossRef]
- Kim, H.; Yoo, S.Y.; Kim, J.S.; Wang, Z.; Lee, W.H.; Koo, K.I.; Seo, J.M.; Cho, D.I. Simple and fast polydimethylsiloxane (PDMS) patterning using a cutting plotter and vinyl adhesives to achieve etching results. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju Island, Korea, 11–15 July 2017; pp. 1885–1888. [Google Scholar]
- Choi, K.M.; Rogers, J.A. A photocurable poly (dimethylsiloxane) chemistry designed for soft lithographic molding and printing in the nanometer regime. J. Am. Chem. Soc. 2003, 125, 4060–4061. [Google Scholar] [CrossRef] [PubMed]
- Garra, J.; Long, T.; Currie, J.; Schneider, T.; White, R.; Paranjape, M. Dry etching of polydimethylsiloxane for microfluidic systems. J. Vac. Sci. Technol. A Vac. Surf. Films 2002, 20, 975–982. [Google Scholar] [CrossRef]
- Balakrisnan, B.; Patil, S.; Smela, E. Patterning PDMS using a combination of wet and dry etching. J. Micromech. Microeng. 2009, 19, 047002. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Seo, J.-m. Fabrication of Magnetically Actuated Fluidic Drug Delivery Device Using Polyvinyl Chloride Adhesive Stencils. Micromachines 2018, 9, 358. https://doi.org/10.3390/mi9070358
Kim H, Seo J-m. Fabrication of Magnetically Actuated Fluidic Drug Delivery Device Using Polyvinyl Chloride Adhesive Stencils. Micromachines. 2018; 9(7):358. https://doi.org/10.3390/mi9070358
Chicago/Turabian StyleKim, Hyun, and Jong-mo Seo. 2018. "Fabrication of Magnetically Actuated Fluidic Drug Delivery Device Using Polyvinyl Chloride Adhesive Stencils" Micromachines 9, no. 7: 358. https://doi.org/10.3390/mi9070358
APA StyleKim, H., & Seo, J.-m. (2018). Fabrication of Magnetically Actuated Fluidic Drug Delivery Device Using Polyvinyl Chloride Adhesive Stencils. Micromachines, 9(7), 358. https://doi.org/10.3390/mi9070358




