Au@Cu Nanoarrays with Uniform Long-Range Ordered Structure: Synthesis and SERS Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Au Decorated Cu Ordered Nanoarrays (Au@Cu NAs)
2.3. Surface-Enhanced Raman Scattering (SERS) Measurements
2.4. Characterization
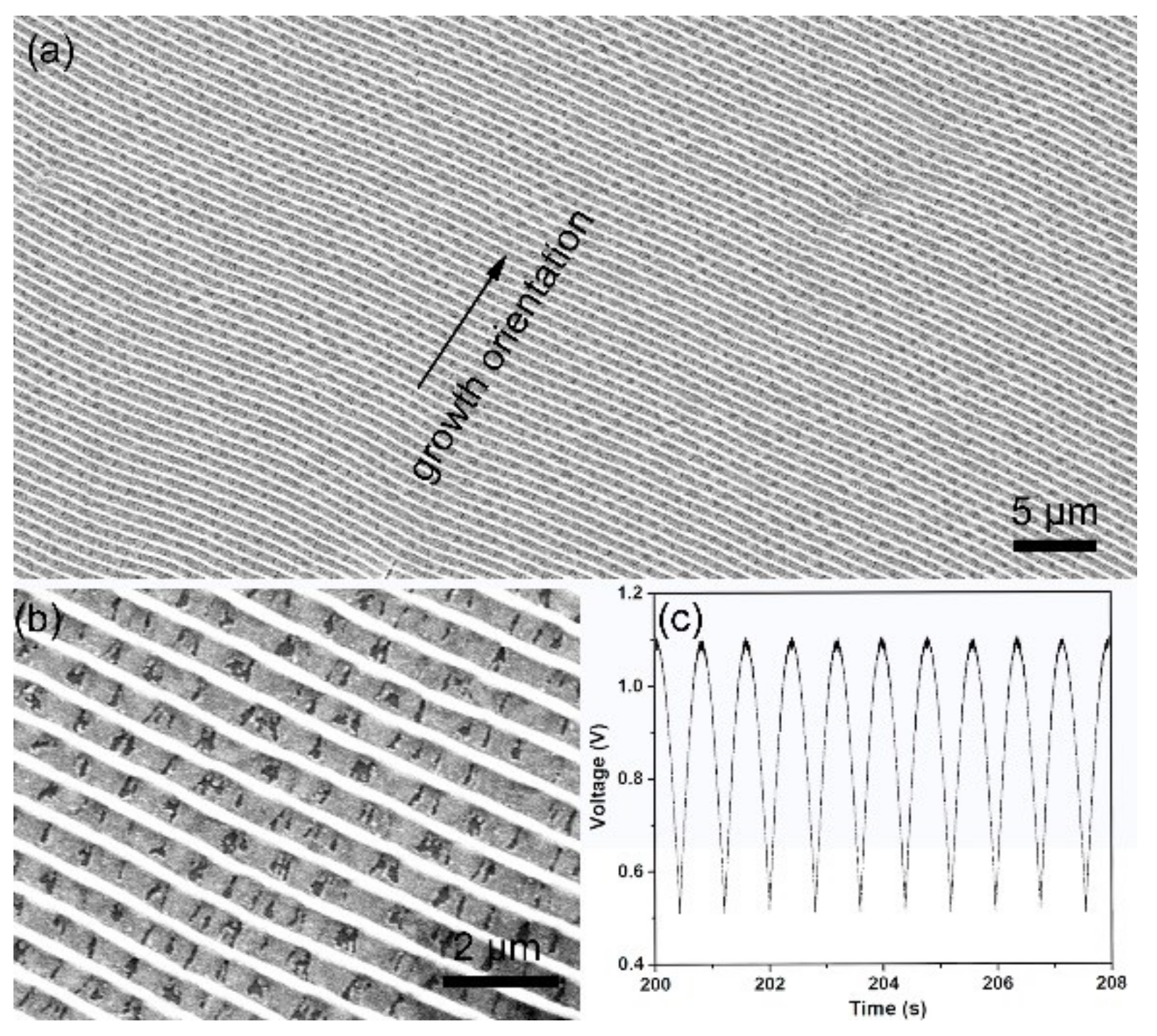
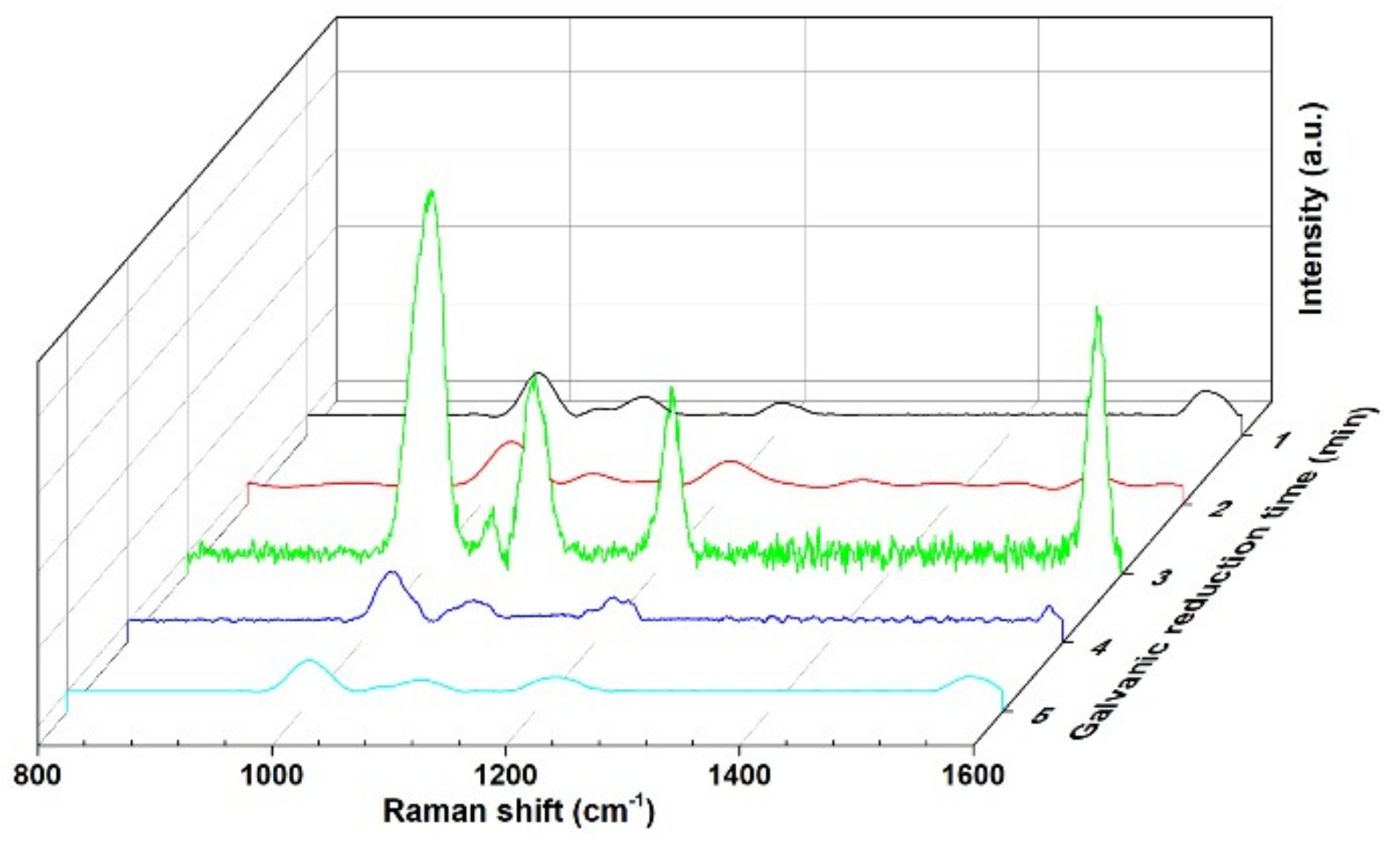
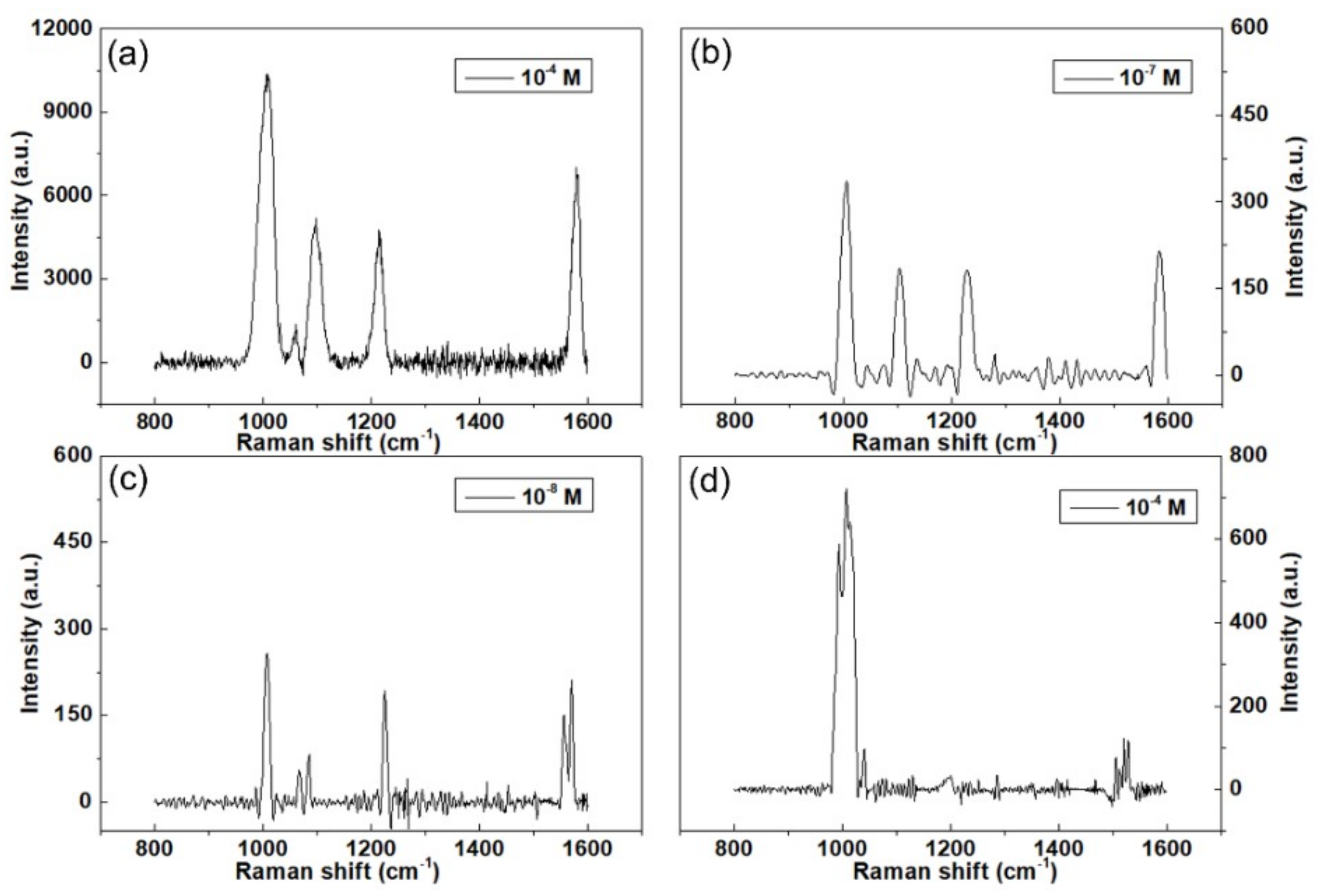
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abell, J.L.; Driskell, J.D.; Zhao, Y. Controllable and reversible hot spot formation on silver nanorod arrays. Chem. Commun. 2014, 50, 106–108. [Google Scholar] [CrossRef]
- Nie, S.; Emory, S.R. Probing Single Molecules and Single Nanoparticles by Surface-Enhanced Raman Scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef]
- Chen, A.; DePrince, A.E.; Demortière, A.; Joshi-Imre, A.; Shevchenko, E.V.; Gray, S.K.; Welp, U.; Vlasko-Vlasov, V.K. Self-Assembled Large Au Nanoparticle Arrays with Regular Hot Spots for SERS. Small 2011, 7, 2365–2371. [Google Scholar] [CrossRef]
- Chen, B.; Meng, G.; Huang, Q.; Huang, Z.; Xu, Q.; Zhu, C.; Qian, Y.; Ding, Y. Green Synthesis of Large-Scale Highly Ordered Core@Shell Nanoporous Au@Ag Nanorod Arrays as Sensitive and Reproducible 3D SERS Substrates. ACS Appl. Mater. Interfaces 2014, 6, 15667–15675. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, X.; MacFarlane, D.R. Ultrasensitive surface-enhanced Raman scattering detection of urea by highly ordered Au/Cu hybrid nanostructure arrays. Chem. Commun. 2017, 53, 7949–7952. [Google Scholar] [CrossRef]
- Candioti, L.V.; De Zan, M.M.; Cámara, M.S.; Goicoechea, H.C. Experimental design and multiple response optimization. Using the desirability function in analytical methods development. Talanta 2014, 124, 123–138. [Google Scholar] [CrossRef]
- Haynes, C.L.; Yonzon, C.R.; Zhang, X.Y.; Van Duyne, R.P. Surface-enhanced Raman sensors: Early history and the development of sensors for quantitative biowarfare agent and glucose detection. J. Raman Spectrosc. 2005, 36, 471–484. [Google Scholar] [CrossRef]
- Zheng, J.K.; Pang, S.; Labuza, T.P.; Labuzac, T.P.; He, L.L. Semi-quantification of surface-enhanced Raman scattering using a handheld Raman spectrometer: A feasibility study. Analyst 2013, 138, 7075–7078. [Google Scholar] [CrossRef]
- Shan, Y.; Yang, Y.; Cao, Y.; Fu, C.L.; Huang, Z.R. Synthesis of wheatear-like ZnO nanoarrays decorated with Ag nanoparticles and its improved SERS performance through hydrogenation. Nanotechnology 2016, 27, 145502. [Google Scholar] [CrossRef]
- Chen, L.; Luo, L.; Chen, Z.; Zhang, M.L.; Zapien, J.A.; Lee, C.X.; Lee, S.Y. ZnO/Au composite nanoarrays as substrates for surface-enhanced Raman scattering detection. J. Phys. Chem. C 2009, 114, 93–100. [Google Scholar] [CrossRef]
- Li, X.H.; Chen, G.Y.; Yang, L.B.; Jin, Z.; Liu, J.H. Multifunctional Au-Coated TiO2 Nanotube Arrays as Recyclable SERS Substrates for Multifold Organic Pollutants Detection. Adv. Funct. Mater. 2010, 20, 2815–2824. [Google Scholar] [CrossRef]
- Rider, A.E.; Kumar, S.; Furman, S.A.; Ostrikov, K. Self-organized Au nanoarrays on vertical graphenes: An advanced three-dimensional sensing platform. Chem. Commun. 2012, 48, 2659–2661. [Google Scholar] [CrossRef]
- Xie, W.; Qiu, P.H.; Mao, C.B. Bio-imaging, detection and analysis by using nanostructures as SERS substrates. J. Mater. Chem. 2011, 21, 106–108. [Google Scholar] [CrossRef]
- Liu, H.Y.; Yang, W.; Wang, M.X.; Xiao, L.; Liu, S.T. Fabrication of lotus-like Au@TiO2 nanocomposites with enhanced gas-sensing properties. Sens. Actuators B Chem. 2016, 236, 490–498. [Google Scholar] [CrossRef]
- Sun, S.D. Recent advances in hybrid Cu2O-based heterogeneous nanostructures. Nanoscale 2015, 7, 10850–10882. [Google Scholar] [CrossRef]
- Zhang, P.H.; Cui, G.L.; Xiao, C.H.; Zhang, M.Z.; Chen, L.; Shi, C.M. Synthesis and characterization of Ag@Cu nano/microstructure ordered arrays as SERS-active substrates. Mater. Res. Express 2016, 3, 065018. [Google Scholar] [CrossRef]
- Zalduendo, M.M.; Langer, J.; Giner-Casares, J.J.; Halac, E.B.; Soler-Illia, G.J.A.A.; Liz-Marzán, L.M.; Angelomé, P.C. Au Nanoparticles–Mesoporous TiO2 Thin Films Composites as SERS Sensors: A Systematic Performance Analysis. J. Phys. Chem. C 2018, 122, 13095–13105. [Google Scholar] [CrossRef]
- Zhong, L.B.; Liu, Q.; Wu, P.; Niu, Q.F.; Zhang, H.; Zheng, Y.M. Facile On-Site Aqueous Pollutant Monitoring Using a Flexible, Ultralight, and Robust Surface-Enhanced Raman Spectroscopy Substrate: Interface Self-Assembly of Au@Ag Nanocubes on a Polyvinyl Chloride Template. Environ. Sci. Technol. 2018, 52, 5812–5820. [Google Scholar] [CrossRef]
- Wu, X.L.; Hao, C.L.; Kumar, J.; Kuang, H.; Kotov, N.A.; Liz-Marzán, L.M.; Xu, C.L. Environmentally responsive plasmonic nanoassemblies for biosensing. Chem. Soc. Rev. 2018, 47, 4677–4696. [Google Scholar] [CrossRef]
- Cui, G.L.; Zhang, P.H.; Chen, L.; Wang, X.L.; Li, J.F.; Shi, C.M.; Wang, D.C. Highly sensitive H2S sensors based on Cu2O/Co3O4 nano/microstructure heteroarrays at and below room temperature. Sci. Rep. 2017, 7, 43887. [Google Scholar] [CrossRef]
- Cui, G.L.; Xiao, C.H.; Zhang, P.H.; Zhang, M.Z. In situ electrodeposition of a Cu2O/SnO2 periodical heterostructure film for photosensor applications. Phys. Chem. Chem. Phys. 2016, 18, 10918–10923. [Google Scholar] [CrossRef]
- Wang, S.M.; Liu, J.Y.; Cui, G.L.; Yao, B.B.; Zhang, M.Z. Novel ZnO parallel ordered arrays with lamellar structure: Fabrication and characterization. Mater. Lett. 2013, 94, 186–188. [Google Scholar] [CrossRef]
- Zong, Z.C.; Wang, H.X.; Kong, L.M. Assembly of PbTe/Pb-based nanocomposite and photoelectric property. Nanoscale Res. Lett. 2013, 8, 191. [Google Scholar] [CrossRef]
- Meng, S.G.; Li, D.Z.; Fu, X.L.; Fu, X.Z. Integrating photonic bandgaps with surface plasmon resonance for the enhancement of visible-light photocatalytic performance. J. Mater. Chem. A 2015, 3, 23501–23511. [Google Scholar] [CrossRef]
- Zheng, X.S.; Hu, P.; Zhong, J.H.; Zong, C.; Wang, X.; Liu, B.J.; Ren, B. Laser power dependent surface-enhanced Raman spectroscopic study of 4-mercaptopyridine on uniform gold nanoparticle-assembled substrates. J. Phys. Chem. C 2014, 118, 3750–3757. [Google Scholar] [CrossRef]
- Yamamoto, Y.S.; Itoh, T. Why and how do the shapes of surface-enhanced Raman scattering spectra change? Recent progress from mechanistic studies. J. Raman Spectrosc. 2016, 47, 78–88. [Google Scholar] [CrossRef]
- Huang, Z.L.; Meng, G.W.; Huang, Q.; Yang, Y.J.; Zhu, C.H.; Tang, C.T. Improved SERS Performance from Au Nanopillar Arrays by Abridging the Pillar Tip Spacing by Ag Sputtering. Adv. Mater. 2010, 22, 4136–4139. [Google Scholar] [CrossRef]
- Hou, X.M.; Zhang, X.L.; Chen, S.T.; Fang, Y.; Li, N.; Zhai, X.M.; Liu, Y. Size-controlled synthesis of Au nanoparticles and nanowires and their application as SERS substrates. Colloids Surf. A 2011, 384, 345–351. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Sun, H.; Guan, W.; Liang, J.; Zhu, X.; Zhang, J.; Chen, M.; Cao, M.; Qian, W.; Gao, K.; et al. Au@Cu Nanoarrays with Uniform Long-Range Ordered Structure: Synthesis and SERS Applications. Micromachines 2018, 9, 678. https://doi.org/10.3390/mi9120678
Zhang P, Sun H, Guan W, Liang J, Zhu X, Zhang J, Chen M, Cao M, Qian W, Gao K, et al. Au@Cu Nanoarrays with Uniform Long-Range Ordered Structure: Synthesis and SERS Applications. Micromachines. 2018; 9(12):678. https://doi.org/10.3390/mi9120678
Chicago/Turabian StyleZhang, Pinhua, Haoming Sun, Wenhui Guan, Jinjin Liang, Xiaomeng Zhu, Junkai Zhang, Min Chen, Meng Cao, Wenbing Qian, Kefu Gao, and et al. 2018. "Au@Cu Nanoarrays with Uniform Long-Range Ordered Structure: Synthesis and SERS Applications" Micromachines 9, no. 12: 678. https://doi.org/10.3390/mi9120678
APA StyleZhang, P., Sun, H., Guan, W., Liang, J., Zhu, X., Zhang, J., Chen, M., Cao, M., Qian, W., Gao, K., & Cui, G. (2018). Au@Cu Nanoarrays with Uniform Long-Range Ordered Structure: Synthesis and SERS Applications. Micromachines, 9(12), 678. https://doi.org/10.3390/mi9120678



