A Trace C2H2 Sensor Based on an Absorption Spectrum Technique Using a Mid-Infrared Interband Cascade Laser
Abstract
1. Introduction
2. Detection Principle of C2H2 Using Absorption Spectroscopy
2.1. Selection of C2H2 Absorption Line
2.2. Derivation of TDLAS-WMS
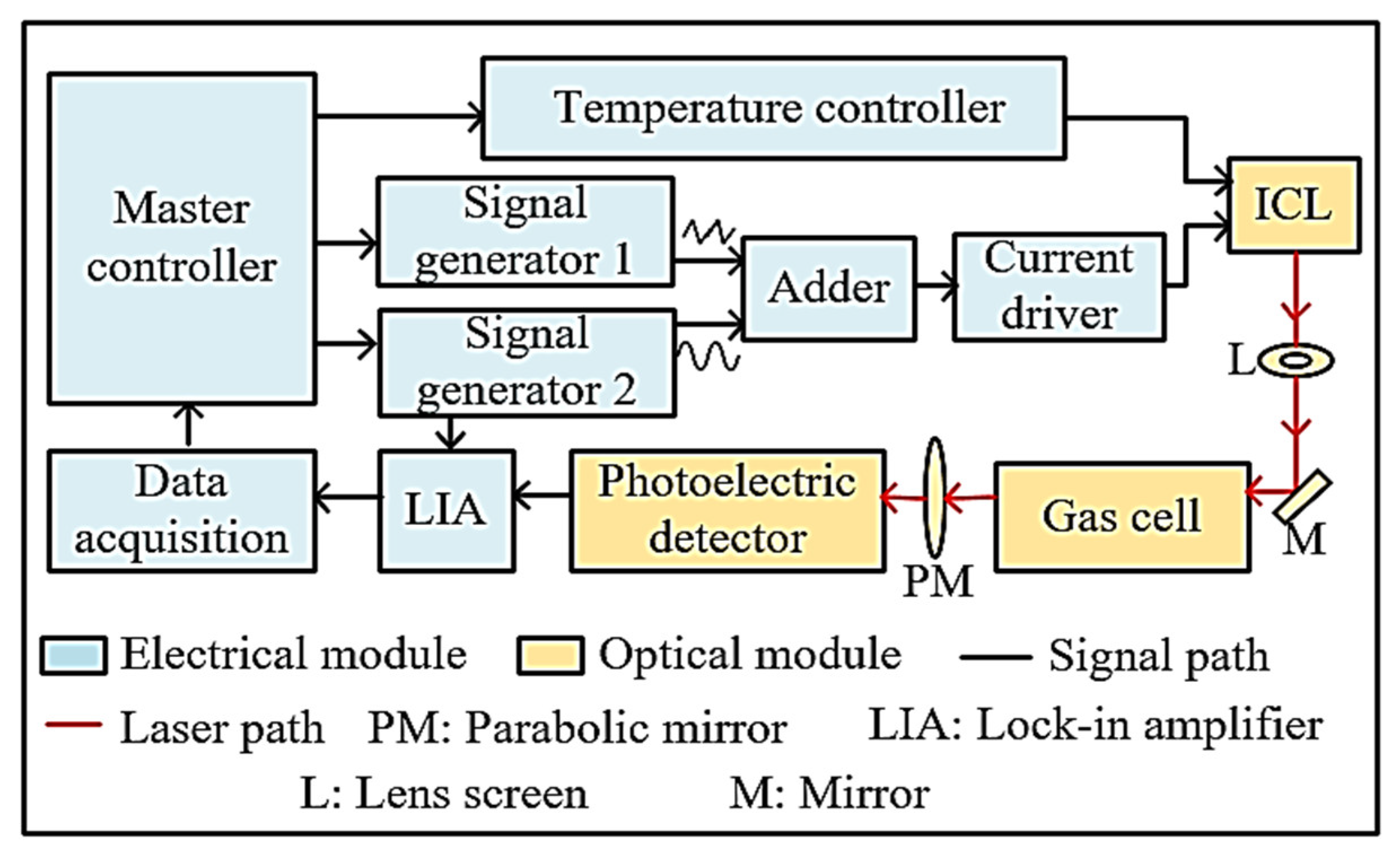
3. System Configuration
4. Experiment
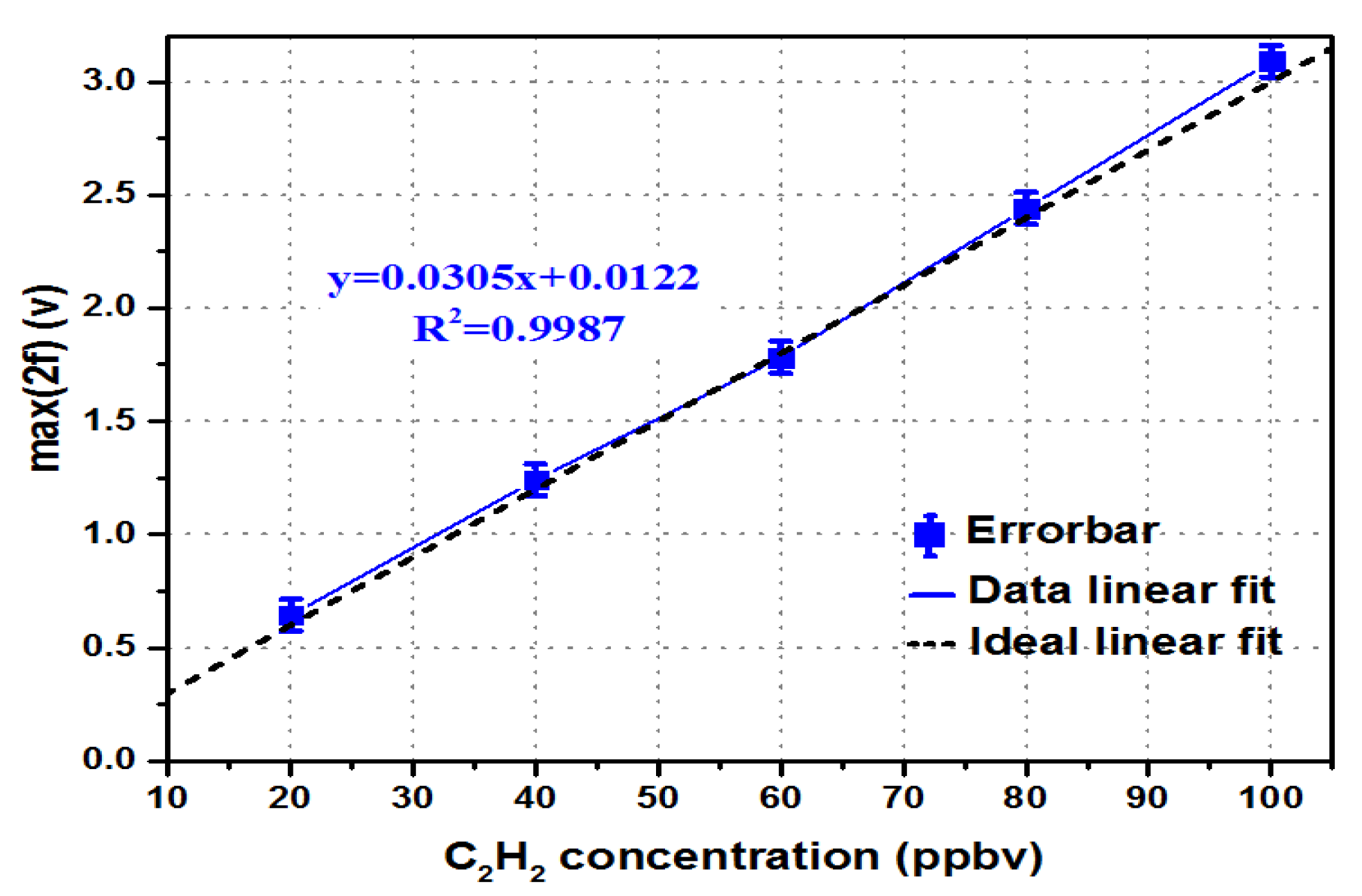
4.1. Response
4.2. Precision
4.3. Stability
4.4. MDL
4.5. Recovery Time and Reproducibility
4.6. Performance Comparison
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Chong, X.; Li, E.; Squire, K.; Wang, A.X. On-chip near-infrared spectroscopy of CO2 using high resolution plasmonic filter array. Appl. Phys. Lett. 2016, 108, 221106. [Google Scholar] [CrossRef]
- Chen, B.; Han, C.; Liu, G. Detection on Particulate Pollutantin Transformer Oil Based on the Mid-Infrared Spectrum. Acta Photonica Sin. 2016, 45, 530002. [Google Scholar] [CrossRef]
- Zhang, L.; Cui, X. The Design of Carbon Monoxide Detector Based on Tunable Diode Lasers Absorption Spectroscope. Laser J. 2014, 35, 54–56. [Google Scholar]
- Xie, Y.; Li, F.; Fan, X.; Hu, S.; Xiao, X.; Wang, J. Components Analysis of Biochar Based on Near Infrared Spectroscopy Technology. Chin. J. Anal. Chem. 2018, 46, 609–615. [Google Scholar] [CrossRef]
- Li, M.; Bai, F. Design of High Sensitivity Infrared Methane Detector Based on TDLAS-WMS. Laser J. 2018, 39, 75–79. [Google Scholar]
- Northern, H.; O’Hagan, S.; Hamilton, M.L.; Ewart, P. Mid-infrared multi-mode absorption spectroscopy, MUMAS, using difference frequency generation. Appl. Phys. B Lasers Opt. 2015, 118, 343–351. [Google Scholar] [CrossRef]
- Chen, C.; Wang, B.; Li, C.; Li, J.; Wang, Y. A Trace Gas Sensor Using Mid-infrared Quantum Cascaded Laser at 4.8 μm to Detect Carbon Monoxide. Spectrosc. Spectr. Anal. 2014, 34, 838–842. [Google Scholar]
- Yan, M.; Luo, P.; Iwakuni, K.; Millot, G.; Hänsch, T.; Picqué, N. Mid-infrared dual-comb spectroscopy with electro-optic modulators. Light Sci. Appl. 2017, 6, e17076. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Li, Z.; Li, B.; Alwahabi, Z.; Aldén, M. Quantitative C2H2 Measurements in Sooty Flames Using Mid-Infrared Polarization Spectroscopy. Appl. Phys. B Lasers Opt. 2010, 101, 423–432. [Google Scholar] [CrossRef]
- Chen, K.; Mei, M. Detection of Gas Concentrations Based on Wireless Sensor and Laser Technology. Laser J. 2014, 35, 50–54. [Google Scholar]
- KC, U.; Nasir, E.; Farooq, A. A mid-infrared absorption diagnostic for acetylene detection. Appl. Phys. B Lasers Opt. 2015, 120, 223–232. [Google Scholar] [CrossRef]
- Van Helden, J.H.; Lang, N.; Macherius, U.; Zimmermann, H.; Ropcke, J. Sensitive trace gas detection with cavity enhanced absorption spectroscopy using a continuous wave external-cavity quantum cascade laser. Appl. Phys. Lett. 2013, 103, 553. [Google Scholar] [CrossRef]
- Jin, M.; Lu, F.; Belkin, M. High-sensitivity infrared vibrational nanospectroscopy in water. Light Sci. Appl. 2017, 6, e17096. [Google Scholar] [CrossRef] [PubMed]
- Rothman, L.S.; Gamache, R.R.; Goldman, A.; Brown, L.R.; Toth, R.A.; Pickett, H.M.; Poynter, R.; Flaud, J.M.; Camy-Peyret, C.; Barbe, A.; et al. The HITRAN database: 1986 edition. Appl. Opt. 1987, 26, 4058–4097. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, B.; Zhao, W.; Chen, W. A Review of Signal Enhancement and Noise Reduction Techniques for Tunable Diode Laser Absorption Spectroscopy. Appl. Spectrosc. Rev. 2014, 49, 666–691. [Google Scholar] [CrossRef]
- Zhu, X.; Lu, W.; Rao, Y.; Li, Y.; Lu, Z.; Yao, S. Selection of baseline method in TDLAS direct absorption CO2 measurement. Chin. Opt. 2017, 10, 455–461. [Google Scholar]
- Klein, A.; Witzel, O.; Ebert, V. Rapid, Time-Division Multiplexed, Direct Absorption- and Wavelength Modulation-Spectroscopy. Sensors 2014, 14, 21497–21513. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Chen, B.; Hu, B. A system for gas sensing employing correlation spectroscopy and wavelength modulation techniques with a multimode diode laser. Measurement 2013, 46, 1657–1662. [Google Scholar] [CrossRef]
- Qu, S.; Wang, M.; Li, N. Mid-Infrared Trace CH4 Detector Based on TDLAS-WMS. Spectrosc. Spectr. Anal. 2016, 36, 3174–3178. [Google Scholar]
- Wang, M.; Zhang, Y.; Liu, W.; Liu, J.; Wang, T.; Tu, X.; Gao, S.; Kan, R. Second-harmonic Detection Research with Tunable Diode Laser Absorption Spectroscope. Opt. Tech. 2005, 31, 279–285. [Google Scholar]
- Li, C.; Dong, L.; Zheng, C.; Tittel, F.K. Compact TDLAS based optical sensor for ppb-level ethane detection by use of a 3.34 μm room-temperature CW interband cascade laser. Sens. Actuators B Chem. 2016, 232, 188–194. [Google Scholar] [CrossRef]
- Jacob, F. Handbook of Modern Sensors, 4th ed.; Springer: New York, NY, USA, 2010; pp. 13–52. ISBN 978-1-4419-6465-6. [Google Scholar]
- Allan, D.W.; Barnes, J.A. A modified “Allan Variance” with increased oscillator characterization ability. In Proceedings of the 35th Annual Symposium on Frequency Control, Philadelphia, PA, USA, 27–29 May 1981; pp. 470–475. [Google Scholar]
- Deng, H. Research on Near Infrared Absorption Spectrum Theory and Detection Technology for Acetylene. Master’s Thesis, Anhui University, Hefei, China, 2017. [Google Scholar]
- He, Q.; Liu, H.; Li, B.; Pan, J.; Wang, L.; Zheng, C.; Wang, Y. Online Detection System on Acetylene with Tunable Diode Laser Absorption Spectroscopy Method. Spectrosc. Spectr. Anal. 2016, 36, 3501–3505. [Google Scholar]
- Schmidt, F.M.; Vaittinen, O.; Metsälä, M.; Kraus, P.; Halonen, L. Direct detection of acetylene in air by continuous wave cavityring-down spectroscopy. Appl. Phys. B Lasers Opt. 2010, 101, 671–682. [Google Scholar] [CrossRef]
- Amiot, C.; Aalto, A.; Ryczkowski, P.; Toivonen, J.; Genty, G. Cavity enhanced absorption spectroscopy in the mid-infrared using a supercontinuum source. Appl. Phys. Lett. 2017, 111, 061103. [Google Scholar] [CrossRef]





| Ref/Type | Wavelength/Maximum Intensity | Technique | MDL (ppmv) | Error (%) |
|---|---|---|---|---|
| [24] | 1.533 µm/1.211 × 10−20 | DAS | 1.8 | 4 |
| [25] | 1.534 nm/8.572 × 10−21 | TDLAS-WMS | 2 | 1 |
| LGA-4500 | 1.533 µm/1.211 × 10−20 | TDLAS-WMS | 0.1 | 1 |
| [26] | 1.523 µm/3.145 × 10−20 | CRDS | 0.00034 | / |
| [27] | Broad mid-infrared range | CEAS | 0.5 | / |
| This study | 1.533 µm/1.211 × 10−20 | TDLAS-WMS | 0.001 | 0.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mu, Y.; Hu, T.; Gong, H.; Ni, R.; Li, S. A Trace C2H2 Sensor Based on an Absorption Spectrum Technique Using a Mid-Infrared Interband Cascade Laser. Micromachines 2018, 9, 530. https://doi.org/10.3390/mi9100530
Mu Y, Hu T, Gong H, Ni R, Li S. A Trace C2H2 Sensor Based on an Absorption Spectrum Technique Using a Mid-Infrared Interband Cascade Laser. Micromachines. 2018; 9(10):530. https://doi.org/10.3390/mi9100530
Chicago/Turabian StyleMu, Ye, Tianli Hu, He Gong, Ruiwen Ni, and Shijun Li. 2018. "A Trace C2H2 Sensor Based on an Absorption Spectrum Technique Using a Mid-Infrared Interband Cascade Laser" Micromachines 9, no. 10: 530. https://doi.org/10.3390/mi9100530
APA StyleMu, Y., Hu, T., Gong, H., Ni, R., & Li, S. (2018). A Trace C2H2 Sensor Based on an Absorption Spectrum Technique Using a Mid-Infrared Interband Cascade Laser. Micromachines, 9(10), 530. https://doi.org/10.3390/mi9100530





