Vorticella: A Protozoan for Bio-Inspired Engineering
Abstract
:1. Introduction
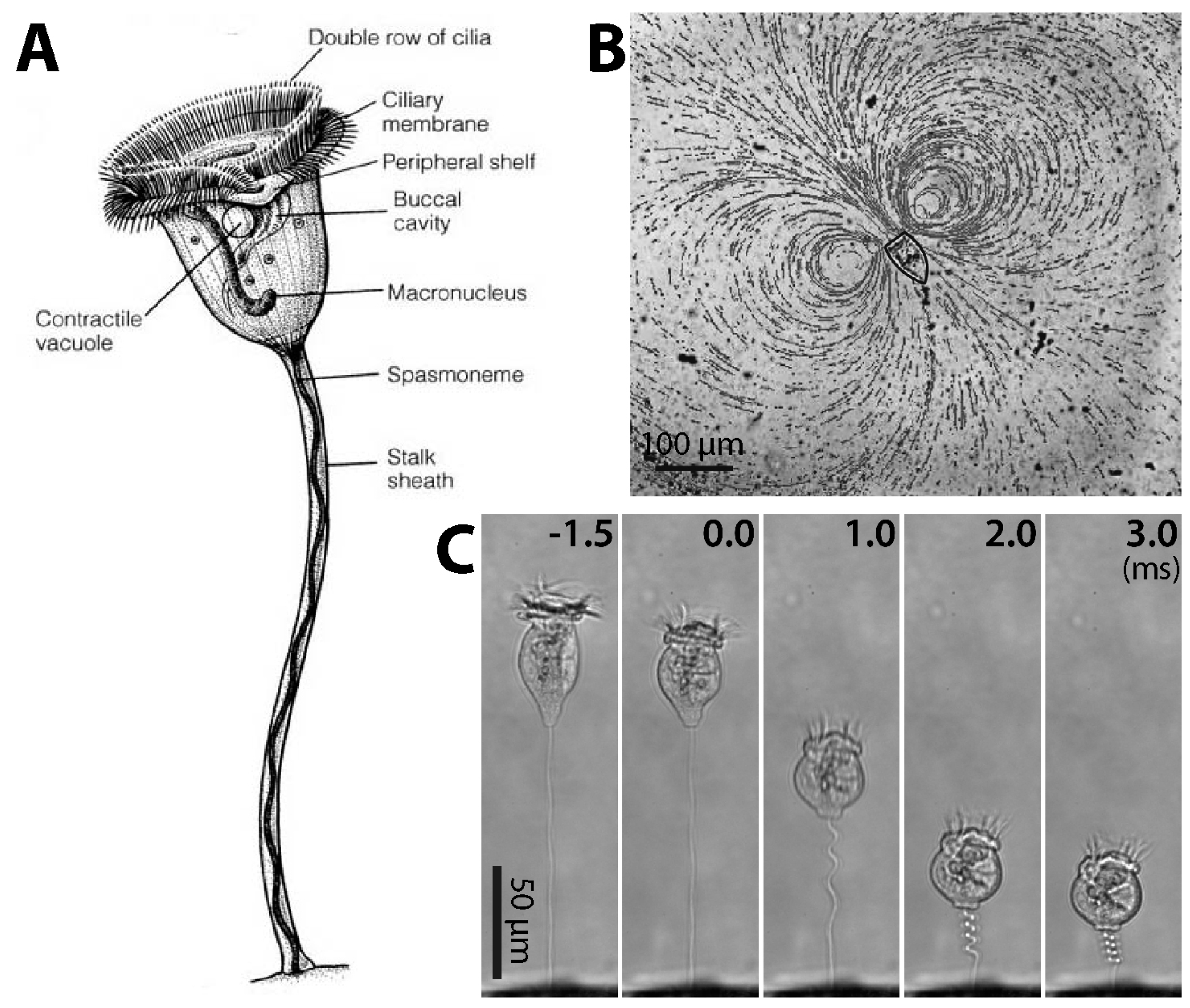
1.1. Overview of Vorticella
1.2. Similar Microorganisms
1.3. Focus of this Review
2. Cilia and Cilia-Generated Flow of Vorticella
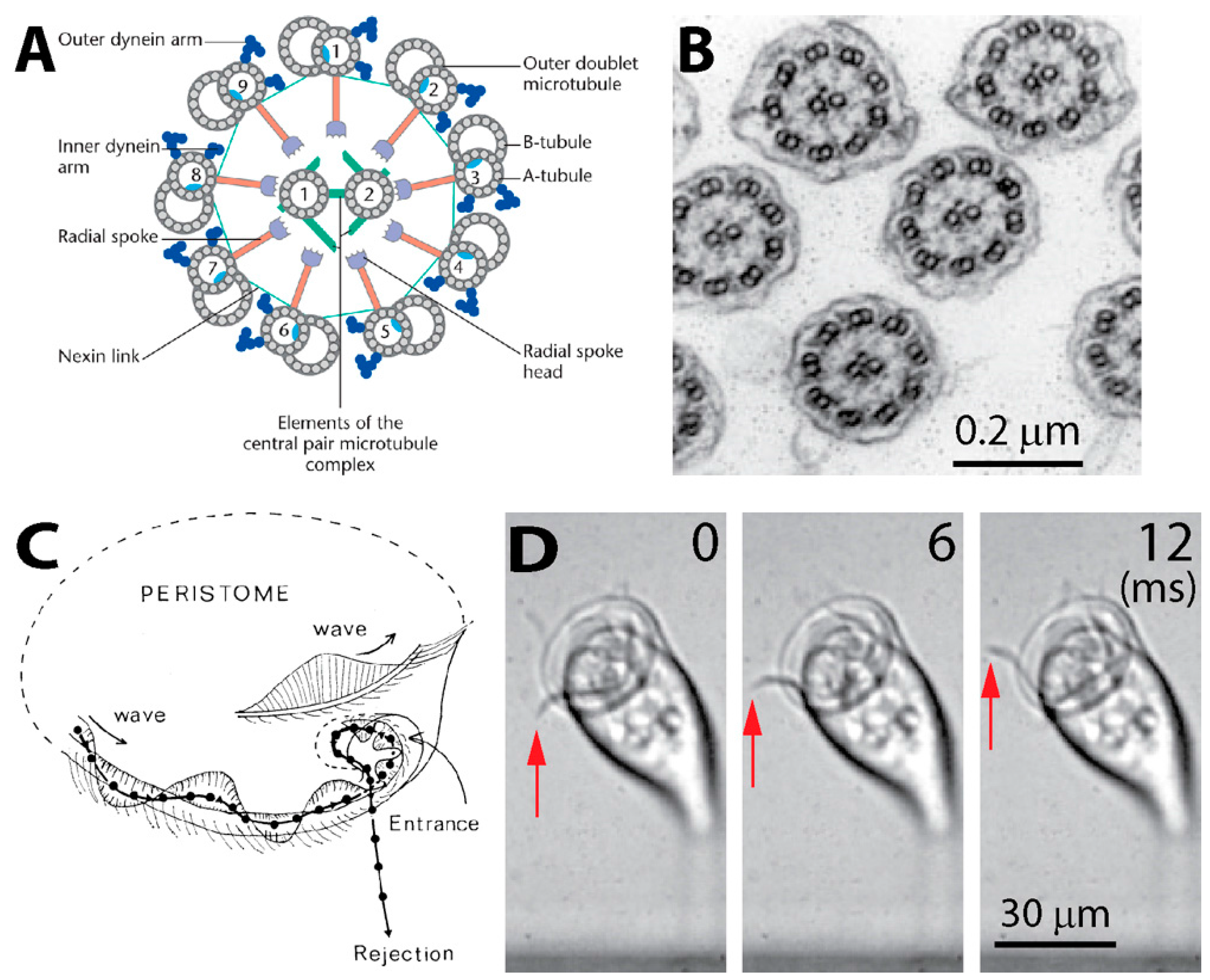
2.1. Structure of Cilia Rows
2.2. Ciliary Performance
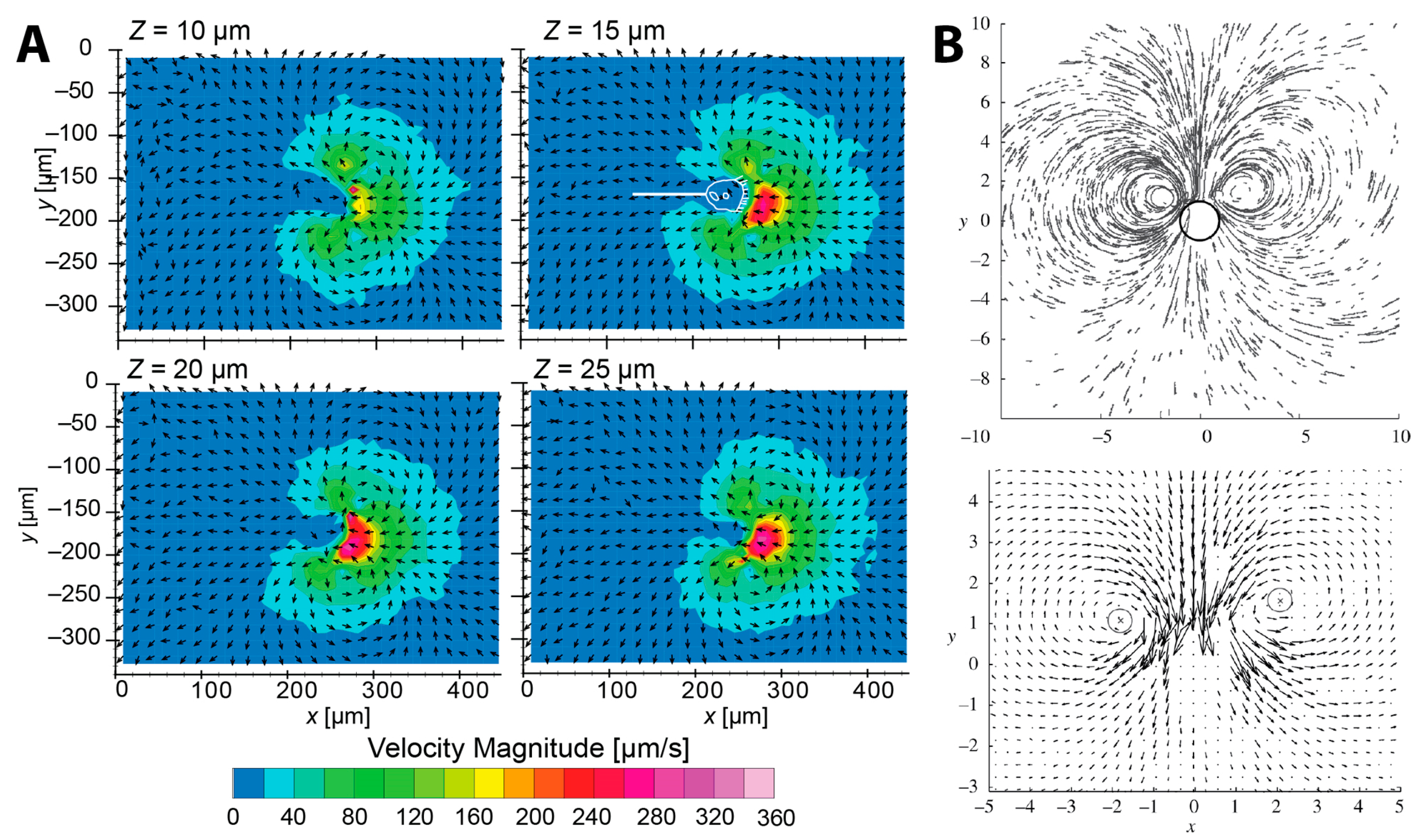
2.2.1. Flow Measurement
2.2.2. Fluid Dynamic Model
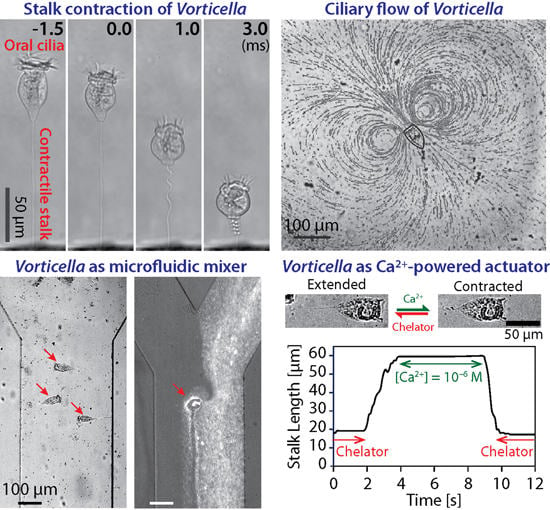
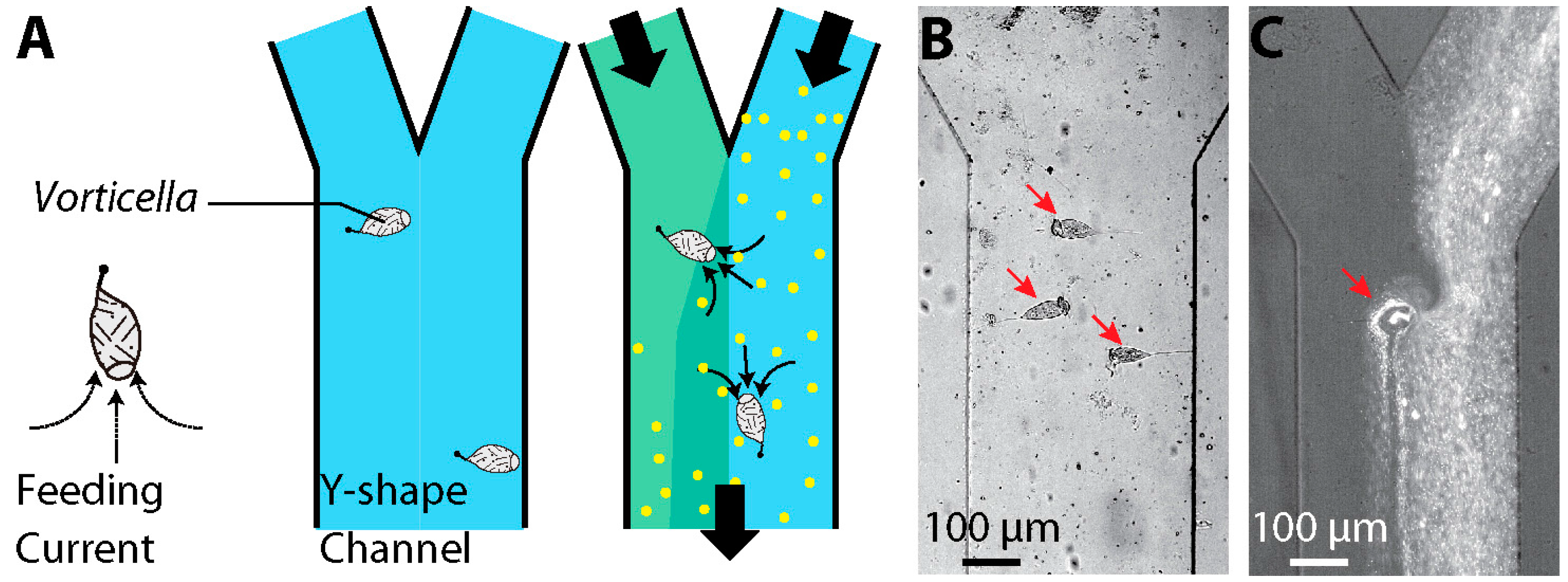
2.3 Engineering Applications
3. Stalk and Ultrafast Contraction of Vorticella
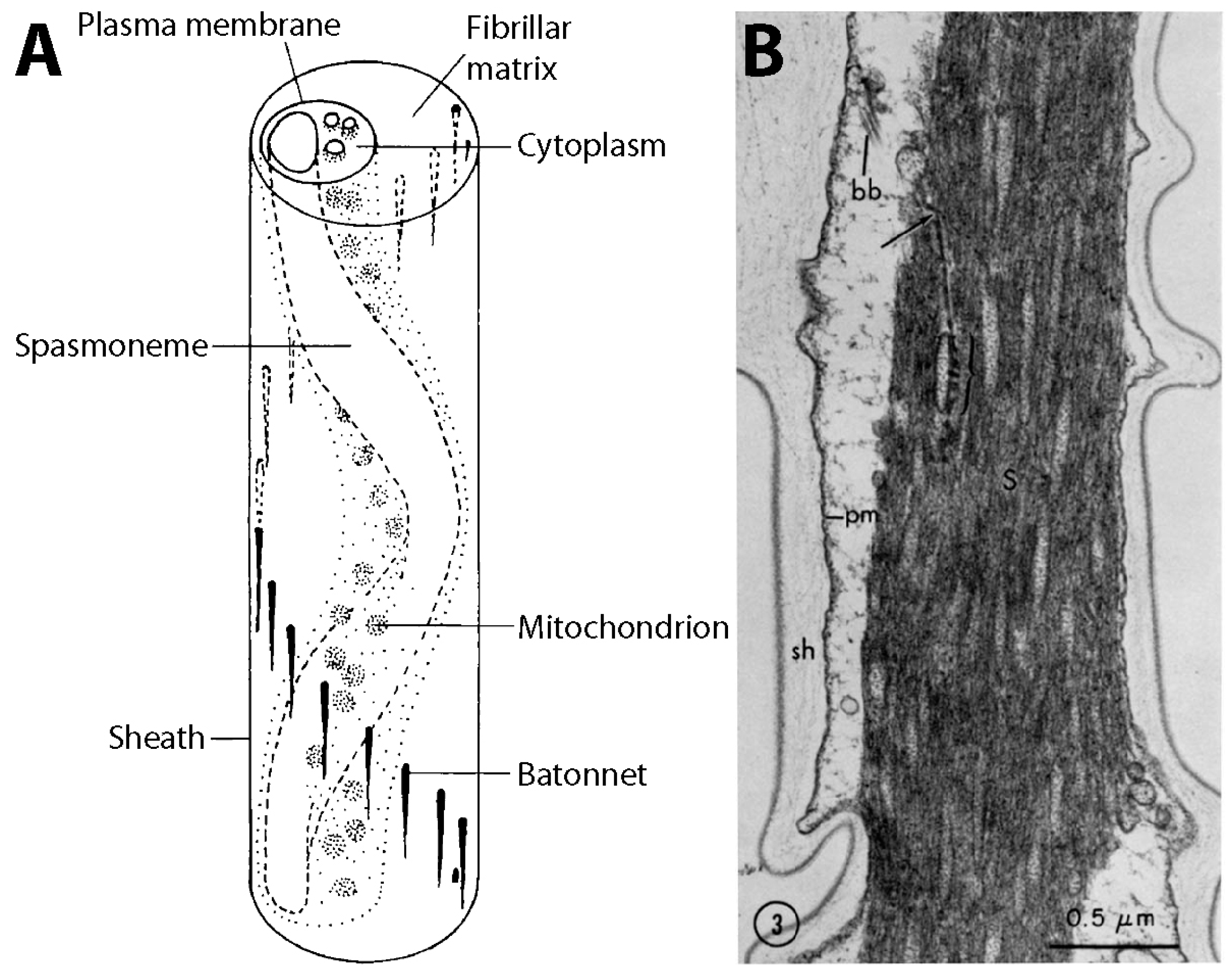
3.1. Structure and Coiling of the Stalk
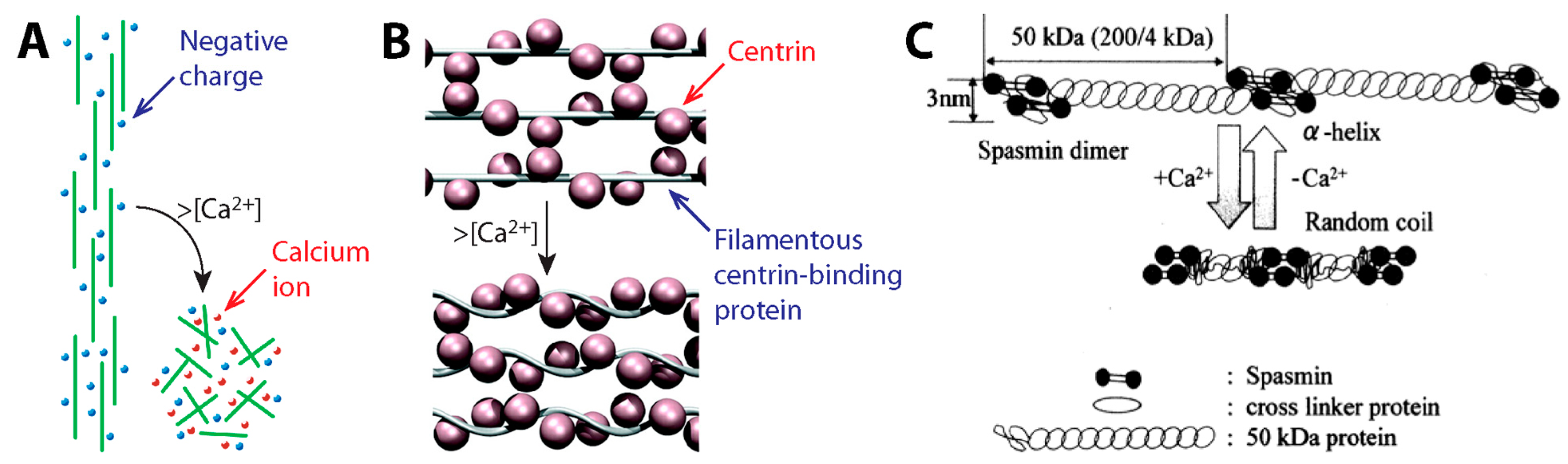
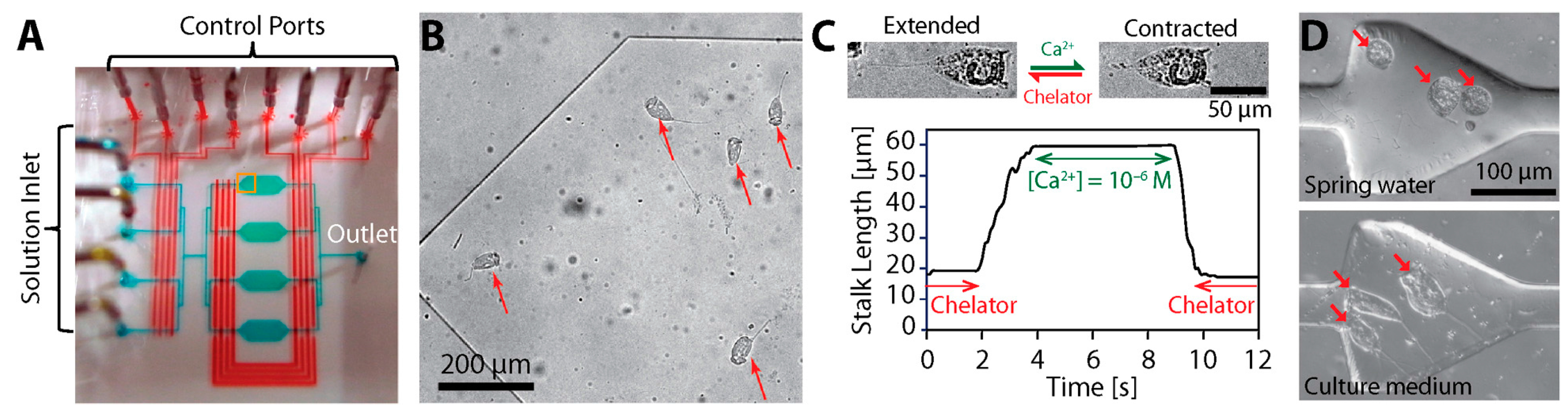
3.2. Contraction Mechanism
3.3. Contractile Performance
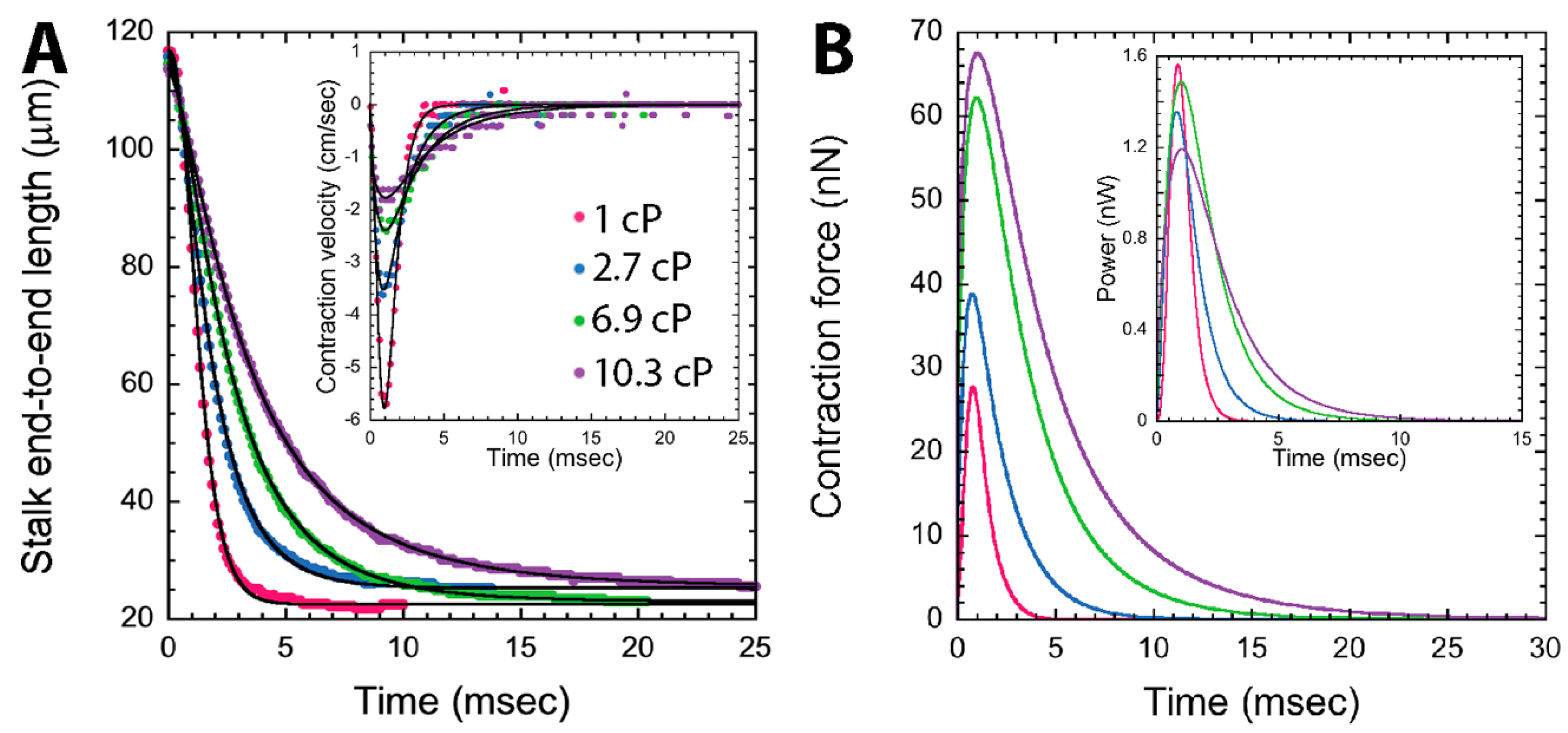
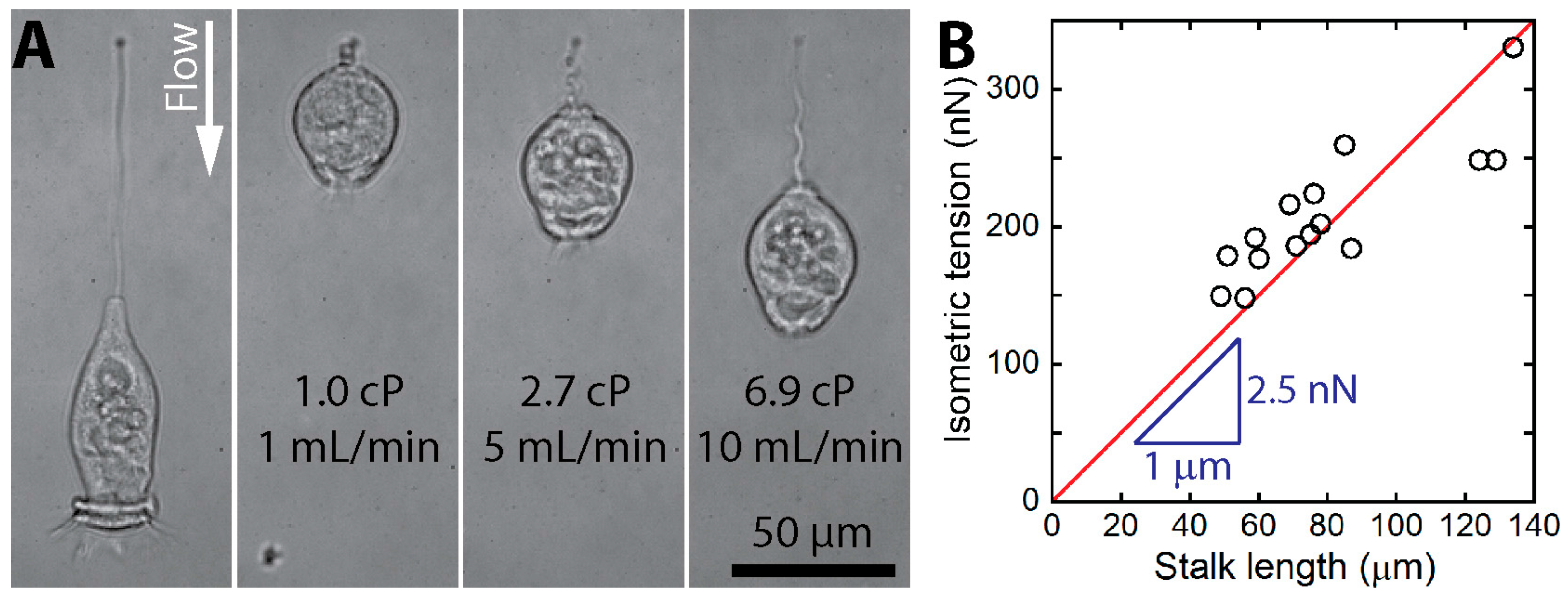
3.3.1. Dynamics of Stalk Contraction
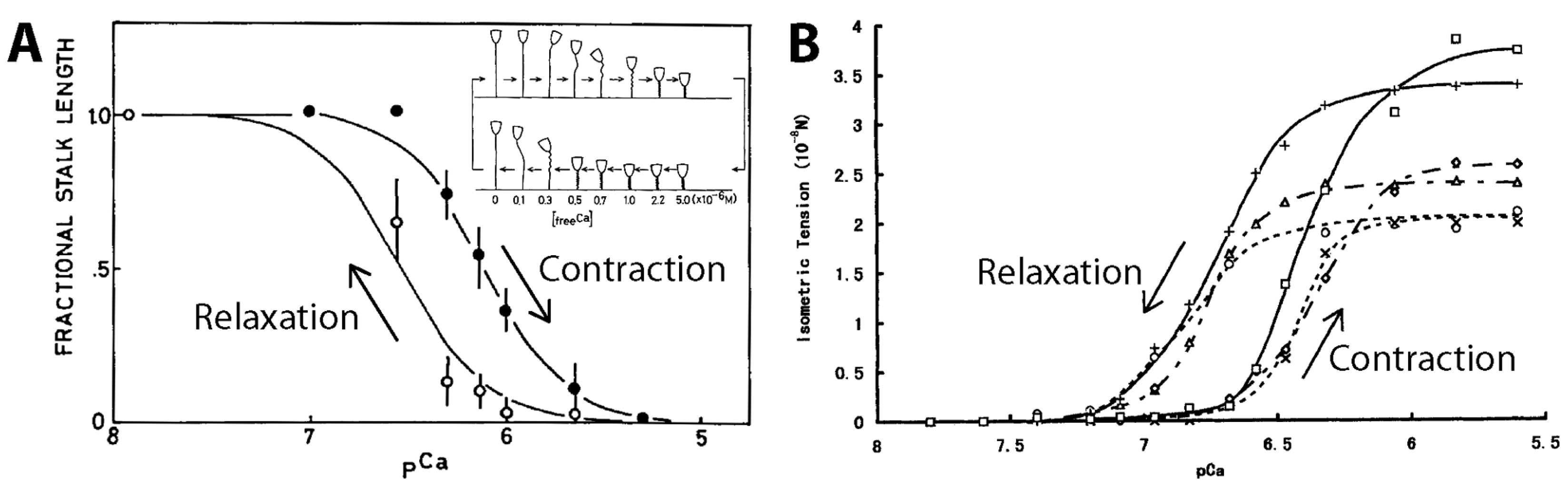
3.3.2. Model-Based Tension Measurements
3.3.3. Experimental Tension Measurements
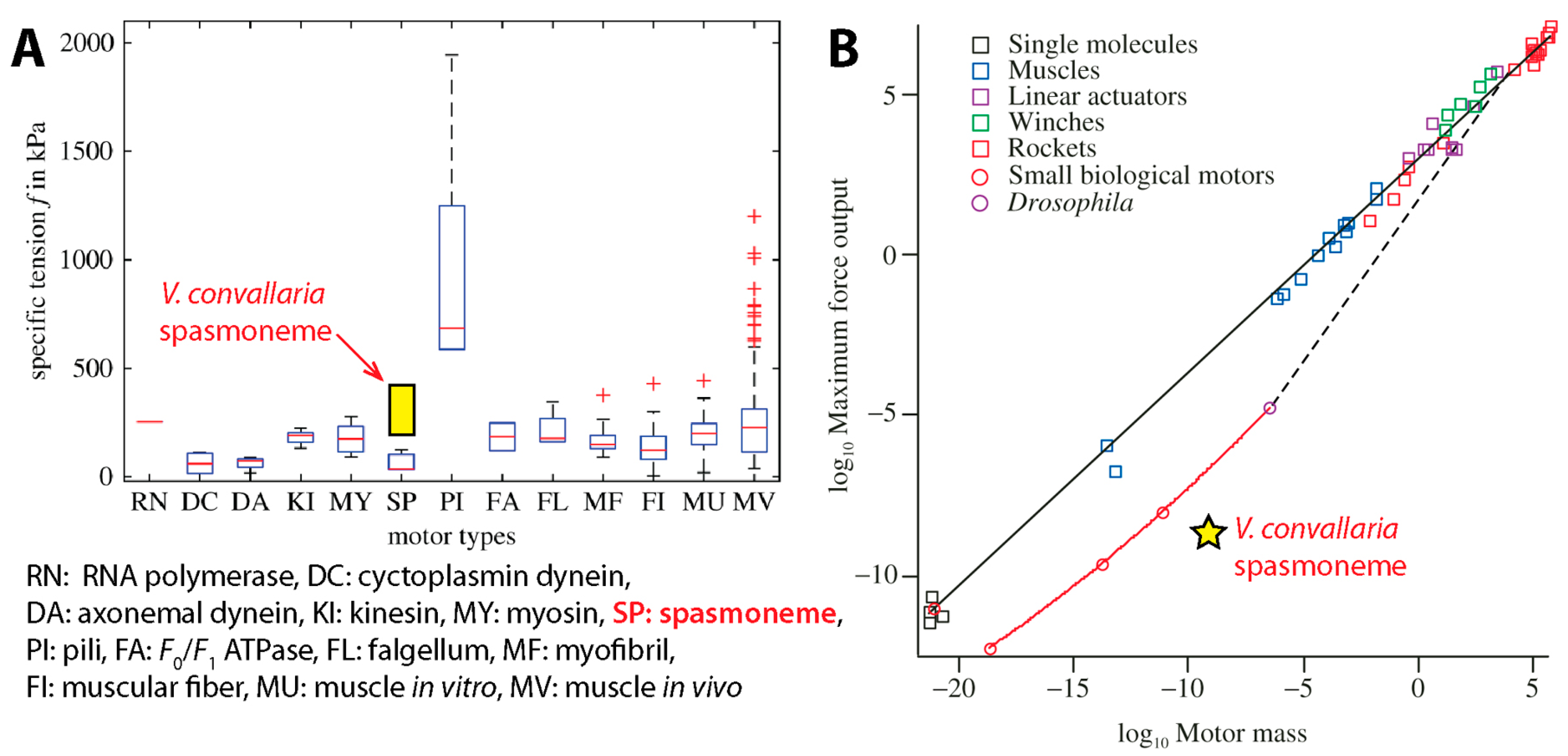
3.4. Comparison with Other Motors
3.5. Possible Engineering Applications
4. Discussion and Future Outlook
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ATP | Adenosine TriPhosphate |
| CFD | Computational Fluid Dynamics |
| ICL | InfraCilliary Lattice |
| PIV | Particle Image Velocimetry |
| PTV | Particle Tracking Velocimetry |
| TEM | Transmission Electron Microscope |
References
- Van Leewenhoeck, A. Observations, communicated to the publisher by Mr. Antony van Leewenhoeck, in a Dutch letter of the 9th of Octob. 1676. Philos. Trans. R. Soc. Lond. 1677, 12, 821–831. [Google Scholar] [CrossRef]
- Dobell, C. Antony van Leeuwenhoek and His ‘Little Animals’; Russell & Russell Inc.: New York, NY, USA, 1958. [Google Scholar]
- Bomfleur, B.; Kerp, H.; Taylor, T.N.; Moestrup, Ø.; Taylor, E.L. Triassic leech cocoon from Antartica contains fossil bell animal. Proc. Nat. Acad. Sci. USA 2012, 109, 20971–20974. [Google Scholar] [CrossRef] [PubMed]
- Buhse, H.E., Jr.; Clamp, J.C. Vorticella. In Encyclopedia of Life Sciences; John Wiley & Sons Ltd.: Chichester, UK, 2001. [Google Scholar]
- Sleigh, M.A.; Barlow, D. Collection of food by Vorticella. Trans. Am. Microsc. Soc. 1976, 95, 482–486. [Google Scholar] [CrossRef]
- Nagai, M.; Oishi, M.; Oshima, M.; Asai, H.; Fujita, H. Three-dimensional two-component velocity measurement of the flow field induced by the Vorticella picta microorganism using a confocal microparticle image velocimetry technique. Biomicrofluidics 2009, 3, 014105. [Google Scholar] [CrossRef] [PubMed]
- Ruppert, E.E.; Fox, R.S.; Barnes, R.D. Invertebrate Zoology: A Functional Evolutionary Approach, 7th ed.; Thomson-Brooks/Cole: Belmont, CA, USA, 2004. [Google Scholar]
- Pepper, R.E.; Roper, M.; Ryu, S.; Matsudaira, P.; Stone, H.A. Nearby boundaries create eddies near microscopic filter feeders. J. R. Soc. Interface 2010, 7, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Matsudaira, P. Unsteady motion, finite Reynolds numbers, and wall effect of Vorticella convallaria contribute contraction force greater than the Stokes drag. Biophys. J. 2010, 98, 2574–2581. [Google Scholar] [CrossRef] [PubMed]
- Sugi, H. Contraction and relaxation in the stalk muscle of Carchesium. Annot. Zool. Jpn. 1959, 32, 163–169. [Google Scholar]
- Katoh, K.; Naitoh, Y. A mechanosensory mechanism for evoking cellular contraction in Vorticella. J. Exp. Biol. 1992, 168, 253–267. [Google Scholar]
- Moriyama, Y.; Hiyama, S.; Asai, H. High-speed video cinematographic demonstration of stalk and zooid contraction of Vorticella convallaria. Biophys. J. 1998, 74, 487–491. [Google Scholar] [CrossRef]
- Amos, W.B. Reversible mechanochemical cycle in the contraction of Vorticella. Nature 1971, 229, 127–128. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, A.; Baraban, M.; Wong, J.; Matsudaira, P.; van Oudenaarden, A.; Mahadevan, L. Power-limited contraction dynamics of Vorticella convallaria: An ultrafast biological spring. Biophys. J. 2008, 94, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, L.; Matsudaira, P. Motility powered by supramolecular springs and ratchets. Science 2000, 288, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Lang, M.J.; Matsudaira, P. Maximal force characterisitics of the Ca2+-powered actuator of Vorticella convallaria. Biophys. J. 2012, 103, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann-Berling, H. The mechanism of a new contraction cycle different from muscle contraction. Biochim. Biophys. Acta 1958, 27, 247–255. [Google Scholar] [CrossRef]
- Townes, M.M.; Brown, D.E.S. The involvement of pH, adenosine triphosphate, calcium, and magnesium in the contraction of the glycerinated stalks of Vorticella. J. Cell. Comp. Physiol. 1965, 65, 261–270. [Google Scholar] [CrossRef]
- Asai, H.; Ochiai, T.; Fukui, K.; Watanabe, M.; Kano, F. Improved preparatioin and cooperative calcium contraction of glycerinated Vorticella. J. Biochem. 1978, 83, 795–798. [Google Scholar] [PubMed]
- Ochiai, T.; Asai, H.; Fukui, K. Hysteresis of contraction-extension cycle of glycerinated Vorticella. J. Protozool. 1979, 26, 420–425. [Google Scholar] [CrossRef]
- Ochiai, T.; Hara, R.; Asai, H. Contraction of the spasmoneme and coiling of the sheath in the glycerinated stalk of Vorticella. Cytobios 1983, 36, 95–105. [Google Scholar]
- Asai, H.; Ochiai, T. Dependence on ionic strength of the calcium-induced contraction of glycerinated stalk of the peritrich ciliate, Vorticella convallaria. Comp. Biochem. Physiol. 1987, 87A, 565–567. [Google Scholar]
- Moriyama, Y.; Yasuda, K.; Ishiwata, S.; Asai, H. Ca2+-induced tension development in the stalks of glycerinated Vorticella convallaria. Cell Motil. Cytoskelet. 1996, 34, 271–278. [Google Scholar] [CrossRef]
- Vincent, J.F.V. Smart by name, smart by nature. Smart Mater. Struct. 2000, 9, 255–259. [Google Scholar] [CrossRef]
- Lee, B.S.; Lee, S.C.; Holliday, L.S. Biochemistry of mechanoenzymes: Biological motors for nanotechnology. Biomed. Microdevices 2003, 5, 269–280. [Google Scholar] [CrossRef]
- Knoblauch, M.; Peters, W.S. Biomimetic actuators: Where technology and cell biology merge. Cell. Mol. Life Sci. 2004, 61, 2497–2509. [Google Scholar] [CrossRef] [PubMed]
- Huck, W.T.S. Responsive polymers for nanoscale actuations. Mater. Today 2008, 11, 24–32. [Google Scholar] [CrossRef]
- Sareh, S.; Rossiter, J. Kirigami artificial muscles with complex biologically inspired morphologies. Smart Mater. Struct. 2013, 22, 014004. [Google Scholar] [CrossRef]
- Chan, V.; Asada, H.H.; Bashir, R. Utilization and control of bioactuators across multiple length scales. Lab Chip 2014, 14, 653–670. [Google Scholar] [CrossRef] [PubMed]
- Itabashi, T.; Mikami, K.; Asai, H. Characterization of the spasmin 1 gene in Zoothamnium arbuscula strain Kawagoe (protozoa, ciliophora) and its relation to other spasmins and centrins. Res. Microbiol. 2003, 154, 361–367. [Google Scholar] [CrossRef]
- Ueda, K. Studies on the stalk muscle of Carchesium (I). Zool. Mag. 1952, 61, 367–371. [Google Scholar]
- Ueda, K. Electri stimulation of the stalk muscle of Carchesium II. Zool. Mag. 1954, 63, 9–14. [Google Scholar]
- Sugi, H. Propagation of contraction in the stalk muscle of Carchesium. J. Fac. Sci. Univ. Tokyo Sect. IV Zool. 1960, 8, 603–615. [Google Scholar]
- Sugi, H. Volume change during contraction in the stalk muscle of Carchesium. J. Fac. Sci. Univ. Tokyo Sect. IV Zool. 1961, 9, 155–170. [Google Scholar]
- Randall, J.T.; Hopkins, J.M. On the stalks of certain peritrichs. Philol. Trans. B 1962, 245, 59–79. [Google Scholar] [CrossRef]
- Rahat, M.; Parnas, I.; Nevo, A.C. Extensibility and tensile strength of the stalk “muscle” of Carchesium sp. Exp. Cell Res. 1969, 54, 69–76. [Google Scholar] [CrossRef]
- Amos, W.B. Structure and coiling of the stalk in the peritrich ciliates Vorticella and Carchesium. J. Cell Sci. 1972, 10, 95–122. [Google Scholar] [PubMed]
- Weis-Fogh, T.; Amos, W.B. Evidence for a new mechanism of cell motility. Nature 1972, 236, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Rahat, M.; Pri-Paz, Y.; Parnas, I. Properties of stalk-’muscle’ contractions of Carchesium sp. J. Exp. Biol. 1973, 58, 463–471. [Google Scholar]
- Routledge, L.M.; Amos, W.B.; Gupta, B.L.; Hall, T.A.; Weis-Fogh, T. Microprobe measurements of calcium binding in the contractile spasmoneme of a Vorticellid. J. Cell Sci. 1975, 19, 195–201. [Google Scholar] [PubMed]
- Amos, W.B.; Routledge, L.M.; Yew, F.F. Calcium-binding proteins in a Vorticellid contractile organelle. J. Cell Sci. 1975, 19, 203–213. [Google Scholar] [PubMed]
- Weis-Fogh, T. Principles of contraction in the spasmoneme of verticellids. A new contractile system. In Comparative Physiology—Functional Aspects of Structural Materials; Bolis, L., Maddrell, H.P., Schmidt-Nielsen, K., Eds.; North-Holland Publishing Company: Amsterdam, The Netherlands, 1975; pp. 83–98. [Google Scholar]
- Amos, W.B. Structure and protein composition of the spasmoneme. In Comparative Physiology—Functional Aspects of Structural Materials; Bolis, L., Maddrell, H.P., Schmidt-Nielsen, K., Eds.; North-Holland Publishing Company: Amsterdam, The Netherlands, 1975; pp. 99–104. [Google Scholar]
- Amos, W.B. Contraction and calcium binding in the Vorticellid ciliates. In Molecules and Cell Movement; Inoue, S., Stephens, R.E., Eds.; Raven Press: New York, NY, USA, 1975; pp. 411–435. [Google Scholar]
- Amos, W.B.; Routledge, L.M.; Weis-Fogh, T.; Yew, F.F. The spasmoneme and calcium-dependent contraction in connection with specific calcium binding proteins. In Proceedings of the Symposia of the Society for Experimental Biology, Englefield Green, Surrey, UK, 9–12 September 1975; Cambridge University Press: Englefield Green, UK, 1976. [Google Scholar]
- Hawkes, R.B.; Rahat, M. Contraction and volume reduction of the glycerolated Carchesium spasmoneme: Effects of alkali earth cations. Experientia 1976, 32, 160–162. [Google Scholar] [CrossRef]
- Hawkes, R.B. Carchesium stalk fibrillar matrix as a highly filled polymer network. J. Cell. Physiol. 1977, 90, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Routledge, L.M.; Amos, W.B.; Yew, F.F.; Weis-Fogh, T. New calcium-binding contractile proteins. In Cell Motility Book A: Motility, Muscle and Non-Muscle Cells; Goldman, R., Pollard, T., Rosenbaum, J., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1976; pp. 93–113. [Google Scholar]
- Routledge, L.M. Calcium-binding proteins in the Vorticellid spasmoneme: Extraction and characterization by gel electrophoresis. J. Cell Biol. 1978, 77, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Moreton, R.B.; Amos, W.B. Electrical recording from the contractile ciliate Zoothamnium geniulatum ayrton. J. Exp. Biol. 1979, 83, 159–167. [Google Scholar]
- Yamada, K.; Asai, H. Extraction and some properties of the proteins, Spastin B, from the spasmoneme of Carchesium polypinum. J. Biochem. 1982, 91, 1187–1195. [Google Scholar] [PubMed]
- Yamada-Horiuchi, K.; Asai, H. Partial purification of the Ca2+-binding proteins from the spasmoneme of Carchesium. Comp. Biochem. Physiol. 1985, 81, 409–413. [Google Scholar] [CrossRef]
- Yamada-Horiuchi, K.; Asai, H. Circular dichroism studies of the Ca2+-binding proteins from the spasmoneme of Carchesium. Comp. Biochem. Physiol. 1985, 81B, 927–931. [Google Scholar] [CrossRef]
- Ochiai, T.; Kato, M.; Ogawa, T.; Asai, H. Spasmin-like proteins in various ciliates revealed by antibody to purified spasmins of Carchesium polypinum. Experientia 1988, 44, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Asai, H.; Ninomiya, T.; Kono, R.; Moriyama, Y. Spasmin and a putative spasmin binding protein(s) isolated from solubilized spasmonemes. J. Eukaryot. Microbiol. 1998, 45, 33–39. [Google Scholar] [CrossRef]
- Moriyama, Y.; Okamoto, H.; Asai, H. Rubber-like elasticity and volume change in the isolated spasmoneme of Giant Zoothamnium sp. under Ca2+-induced contraction. Biophys. J. 1999, 76, 993–1000. [Google Scholar] [CrossRef]
- Itabashi, T.; Terasaki, T.; Asai, H. Novel nuclear and cytoplasmic proteins detected by Anti-Zoothamnium arbuscula (protozoa) spasmin 1 antibody in mammalian cells are dependent on the cell cycle. J. Biochem. 2004, 136, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Asai, H. Ca2+-driven contraction of spasmoneme in Vorticellidae. Jpn. J. Protozool. 2005, 38, 133–152. [Google Scholar]
- Rahat, M.; Friedlaender, H.; Pimstein, R. Colchicine inhibition of stalk elongation in Carchesium sp.: Effect of Ca2+ and Mg2+. J. Cell Sci. 1975, 19, 183–193. [Google Scholar] [PubMed]
- Buhse, H.E., Jr. Vorticella: “A cell for all seasons”. J. Eukaryot. Microbiol. 1998, 45, 469–474. [Google Scholar] [CrossRef]
- Bray, D. Cell Movements: From Molecules to Motility, 2nd ed.; Garland Publishing: New York, NY, USA, 2001. [Google Scholar]
- Lodish, H.; Berk, A.; Matsudaira, P.; Kaiser, C.A.; Krieger, M.; Scott, M.P. Molecular Cell Biology, 5th ed.; Freeman W. H. and Company: New York, NY, USA, 2004. [Google Scholar]
- Linck, R.W. Cilia and flagella. In Encyclopedia of Life Sciences; John Wiley & Sons Ltd.: Chichester, UK, 2009. [Google Scholar]
- Brennen, C.; Winet, H. Fluid mechanics of propulsion by cilia and flagella. Ann. Rev. Fluid Mech. 1977, 9, 339–398. [Google Scholar] [CrossRef]
- Subramanian, G.; Nott, P.R. The fluid dynamics of swimming microorganisms and cells. J. Indian Inst. Sci. 2011, 91, 383–413. [Google Scholar]
- Guasto, J.S.; Rusconi, R.; Stocker, R. Fluid mechanics of planktonic microorganisms. Annu. Rev. Fluid Mech. 2012, 44, 373–400. [Google Scholar] [CrossRef]
- Allen, R.D. Structures linking the myonemes, endoplasmic reticulum, and surface membranes in the contractile ciliate Vorticella. J. Cell Biol. 1973, 56, 559–579. [Google Scholar] [CrossRef] [PubMed]
- Paramecium and Other Ciliates: Richard Allen’s Image Collection. Available online: http://www5.pbrc.hawaii.edu/allen/ch19/21-vor720307–15.html. (accessed on 13 October 2016).
- Vopel, K.; Reick, C.H.; Arlt, G.; Pohn, M.; Ott, J. Flow microenvironment of two marine peritrich ciliates with ecobiotic chemoautotrophic bacteria. Aquat. Microb. Ecol. 2002, 29, 19–28. [Google Scholar] [CrossRef]
- Vogel, S. Life in Moving Fluids; Princeton University Press: Princeton, NJ, USA, 1994. [Google Scholar]
- Blake, J.R.; Liron, N.; Aldis, G.K. Flow patterns around ciliated microorganisms and in ciliated ducts. J. Theor. Biol. 1982, 98, 127–141. [Google Scholar] [CrossRef]
- Blake, J.R.; Otto, S.R. Ciliary propulsion, chaotic filtration and a ‘blinking’ stokeslet. J. Eng. Math. 1996, 30, 151–168. [Google Scholar] [CrossRef]
- Blake, J.R.; Otto, S.R.; Blake, D.A. Filter feeding, chaotic filtration and a blinking Stokeslet. Theor. Comput. Fluid Dyn. 1998, 10, 23–36. [Google Scholar] [CrossRef]
- Liron, N.; Blake, J.R. Existence of viscous eddies near boundaries. J. Fluid Mech. 1981, 107, 109–129. [Google Scholar] [CrossRef]
- Liron, N.; Mochon, S. Stokes flow for a stokeslet between two parallel flat plates. J. Eng. Math. 1976, 10, 287–303. [Google Scholar] [CrossRef]
- Pettitt, M.E.; Orme, B.A.A.; Blake, J.R.; Leadbeater, B.S.C. The hydrodynamics of filter feeding in choanoflagellates. Eur. J. Protistol. 2002, 38, 313–332. [Google Scholar] [CrossRef]
- Liron, N. Fluid transport by cilia between parallel plates. J. Fluid Mech. 1978, 86, 705–726. [Google Scholar] [CrossRef]
- Orme, B.A.A.; Blake, J.R.; Otto, S.R. Modelling the motion of particles around choanoflagellates. J. Fluid Mech. 2003, 475, 333–355. [Google Scholar] [CrossRef]
- Fenchel, T. Protozoan filter feeding. Prog. Protistol. 1986, 2, 65–113. [Google Scholar]
- Hartmann, C.; Ozlemzmutlu, O.; Petermeier, H.; Fried, J.; Delgado, A. Analysis of the flow field induced by the sessile peritrichious ciliate Opercularia asymmetrica. J. Biomech. 2007, 40, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Otto, S.R.; Yannacopoulos, A.N.; Blake, J.R. Transport and mixing in Stokes flow: The effect of chaotic dynamics on the blinking stokeslet. J. Fluid Mech. 2001, 430, 1–26. [Google Scholar] [CrossRef]
- Orme, A.A.; Otto, S.R.; Blake, J.R. Enhanced efficiency of feeding and mixing due to chaotic flow patterns around choanoflagellates. Math. Med. Biol. 2001, 18, 293–325. [Google Scholar] [CrossRef]
- Roper, M.; Dayle, M.J.; Pepper, R.E.; Koehl, M.A.R. Cooperatively generated stresslet flows supply fresh fluid to multicellular choanoflagellate colonies. Phys. Rev. Lett. 2013, 110, 228104. [Google Scholar] [CrossRef] [PubMed]
- Pepper, R.E.; Roper, M.; Ryu, S.; Matsumoto, N.; Nagai, M.; Stone, H.A. A new angle on microscopic suspension feeders near boundaries. Biophys. J. 2013, 105, 1796–1804. [Google Scholar] [CrossRef] [PubMed]
- Den Toonder, J.M.J.; Onck, P.R. Microfluidic manipulation with artificial/bioinspired cilia. Trends Biotechnol. 2013, 31, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Den Toonder, J.; Bos, F.; Broer, D.J.; Filippini, L.; Gillies, M.; de Goede, J.; Mol, T.; Reijme, M.; Talen, W.; Wilderbeck, H.; et al. Artificial cilia for active micro-fluidic mixing. Lab Chip 2008, 8, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Baltussen, M.; Anderson, P.; Bos, F.; den Toonder, J. Interial flow effects in a micro-mixer based on artificial cilia. Lab Chip 2009, 9, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Fahrni, F.; Prins, M.W.J.; van IJzendoorn, L.J. Micro-fluidic actuation using magnetic artificial cilia. Lab Chip 2009, 9, 3413–3421. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.; Chung, J.-H.; Devasia, S.; Riley, J.J. Bio-mimetic silicone cilia for microfluidic manipulation. Lab Chip 2009, 9, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Vilfan, M.; Potocnik, A.; Kavcic, B.; Osterman, N.; Poberaj, I.; vilfan, A.; Babic, D. Self-assembled artificial cilia. Proc. Natl. Acad. Sci. USA 2009, 107, 1844–1847. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.; Smith, B.; Devasia, S.; Riley, J.J.; Chung, J.-H. Characterization of mixing performance for bio-mimetic silicone cilia. Microfluid. Nanofluid. 2010, 9, 645–655. [Google Scholar] [CrossRef]
- Shields, A.R.; Fiser, B.L.; Evans, B.A.; Falvo, M.R.; Washburn, S.; Superfine, R. Biomimetic cilia arrays generate simultaneous pumping and mixing regimes. Proc. Natl. Acad. Sci. USA 2010, 107, 15670–15675. [Google Scholar] [CrossRef] [PubMed]
- Coq, N.; Bricard, A.; Delapierre, F.-D.; Malaquin, L.; du Roure, O.; Fermigier, M.; Bartolo, D. Collective beating of artificial microcilia. Phys. Rev. Lett. 2011, 107, 014501. [Google Scholar] [CrossRef] [PubMed]
- Hussong, J.; Schorr, N.; Belardi, J.; Prucker, O.; Ruhe, J.; Westerweel, J. Experimental investigation of the flow induced by artificial cilia. Lab Chip 2011, 11, 2017–2022. [Google Scholar] [CrossRef] [PubMed]
- Khaderi, S.N.; Craus, C.B.; Hussong, J.; Schorr, N.; Belardi, J.; Westerweel, J.; Prucker, O.; Ruhe, J.; den Toonder, J.M.J.; Onck, P.R. Magnetically-actuated artificial cilia for microfluidic propulsion. Lab Chip 2011, 11, 2002–2010. [Google Scholar] [CrossRef] [PubMed]
- Kokot, G.; Vilfan, M.; Osterman, N.; Vilfan, A.; Kavcic, B.; Poberaj, I.; Babic, D. Measurement of fluid flow generated by artificial cilia. Biomicrofluidics 2011, 5, 034103. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, T.; Welch, D.; Nicastro, D.; Dogic, Z. Cilia-like beating of active microtubule bundles. Science 2011, 333, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Darnton, N.; Turner, L.; Breuer, K.; Berg, H.C. Moving fluid with bacterial carpets. Biophys. J. 2004, 86, 1863–1870. [Google Scholar] [CrossRef]
- Kim, M.J.; Breuer, K.S. Use of bacterial carpets to enhance mixing in microfluidic systems. J. Fluid Eng. 2007, 129, 319–324. [Google Scholar] [CrossRef]
- Kim, M.J.; Breuer, K.S. Microfluidic pump powered by self-organizing bacteria. Small 2008, 4, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Nagai, M.; Hasayaka, Y.; Kato, K.; Kawashima, T.; Shibata, T. Mixing of solutions by coordinated ciliary motion in Vorticella convallaria and patterning method for microfluidic applications. Sens. Actuat. B Chem. 2013, 188, 1255–1262. [Google Scholar] [CrossRef]
- Nagai, M.; Hayasaka, Y.; Kawashima, T.; Shibata, T. Active mixing in microchamber using cilia of Vorticella convallaria. IEEJ Trans. Electr. Electron. Eng. 2014, 9, 575–576. [Google Scholar] [CrossRef]
- Nagai, M.; Tanizaki, K.; Hayasaka, Y.; Kawashima, T.; Shibata, T. A microfluidic flow-switching device powered by Vorticella stalk. J. Phys. 2013, 433, 012014. [Google Scholar]
- Allen, R.D. Contractility and its control in peritrich ciliates. J. Eukaryot. Microbiol. 1973, 20, 25–36. [Google Scholar] [CrossRef]
- Katoh, K.; Kikuyama, M. An all-or-nothing rise in cytosolic [Ca2+] in Vorticella sp. J. Exp. Biol. 1997, 200, 35–40. [Google Scholar] [PubMed]
- Yokoyama, Y.; Asai, H. Contractiliy of the spasmoneme in glycerinated Vorticella stalk induced by various divalent metal and lanthanide ions. Cell Motil. Cytoskelet. 1987, 7, 39–45. [Google Scholar] [CrossRef]
- Gogendeau, D.; Beisson, J.; de Loubresse, N.G.; Le Caer, J.-P.; Ruiz, F.; Cohen, J.; Sperling, L.; Koll, F.; Klotz, C. An sfi1p-like centrin-binding protein mediates centrin-based Ca2+-dependent contractility in Paramecium tetraurelia. Eukaryot. Cell 2007, 6, 1992–2000. [Google Scholar] [CrossRef] [PubMed]
- Baek, N.; Park, K. Natural polymer gels with fast responses. In Reflexive Polymers and Hydrogels: Understanding and Designing Fast Responsive Polymeric Systems; Yui, N., Mrsny, R.J., Park, K., Eds.; CRC Press LLC: Boca Raton, FL, USA, 2004; pp. 86–96. [Google Scholar]
- Fang, J.; Zhang, B.; Asai, H. Chemical modification of contractile 3-nm-diameter filaments in Vorticella spasmoneme by diethyl-pyrocarbonate and its reversible renaturation by hydroxylamine. Biochem. Biophys. Res. Commun. 2003, 310, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Asai, H. Structure and function of general motor proteins systems for motility, including the spasmoneme in Vorticellidae stalk. Jpn. J. Protozool. 2006, 39, 104–106. [Google Scholar]
- Schiebel, E.; Bornens, M. In search of a function for centrins. Trends Cell Biol. 1995, 5, 197–201. [Google Scholar] [CrossRef]
- Kono, R.; Ochiai, T.; Asai, H. Chemical modification of amino acid residue in glycerinated Vorticella stalk and Ca2+-induced contractility. Cell Motil. Cytoskelet. 1997, 36, 305–312. [Google Scholar] [CrossRef]
- Dantas, T.J.; Daly, O.M.; Morrison, C.G. Such small hands: The roles of centrins/caltractins in the centriole and in genome maintenance. Cell. Mol. Life Sci. 2012, 69, 2979–2997. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, C.Y. Centrins in unicellular organisms: Functional diversity and specialization. Protoplasma 2012, 249, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Salisbury, J.L. Roots. J. Eukaryot. Microbiol. 1998, 45, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Kilmartin, J.V. Sfi1p has conserved centrin-binding sites and an essential function in budding yeast spindle pole body duplication. J. Cell Biol. 2003, 162, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Salisbury, J.L. Centrosomes: Sfi1p and centrin unravel a structural riddle. Curr. Biol. 2004, 14, R27–R29. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Sandercock, A.M.; Conduit, P.; Robinson, C.V.; Williams, R.L.; Kilmartin, J.V. Structural role of Sfi1p-centrin filaments in budding yeast spindle pole body duplication. J. Cell Biol. 2006, 173, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.R.; Jahn, T.L.; Fonesca, J. Contraction of protoplasm IV. Cinematographic analysis of the contraction of some peritrichs. J. Cell. Physiol. 1970, 75, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Kamiguri, J.; Tsuchiya, N.; Hidema, R.; Tachibana, M.; Yatabe, Z.; Shoji, M.; Hashimoto, C.; Pansu, R.B.; Ushiki, H. Contraction behaviors of Vorticella sp. stalk investigated using high-speed video camera. I: Nucleation and growth model. Biophysics 2012, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kamiguri, J.; Tsuchiya, N.; Hidema, R.; Yatabe, Z.; Shoji, M.; Hashimoto, C.; Pansu, R.B.; Ushiki, H. Contraction behaviors of Vorticella sp. stalk investigated using high-speed video camera. II: Viscosity effect of several types of polymer additives. Biophysics 2012, 8, 11–19. [Google Scholar] [CrossRef] [PubMed]
- France, D.C. Structure and Mechanics of the Spasmoneme, a Biological Spring within the Protozoan Vorticella convallaria; Massachusetts Institute of Technology: Cambridge, MA, USA, 2007. [Google Scholar]
- Apolinar-Iribe, A.; Virgen-Ortiz, A.; Marin, J.L.; Muniz, J. Measurement of Vorticella contraction force using a micropipette technique. Adv. Sci. Lett. 2010, 3, 482–485. [Google Scholar] [CrossRef]
- Knoblauch, M.; Peters, W.S. Forisomes, a novel type of Ca2+-dependent contractile protein motor. Cell Motil. Cytoskelet. 2004, 58, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Tuteja, N.; Umate, P.; van Bel, A.J.E. Forisomes: Calcium-powered protein complexes with potential as ‘smart’ biomaterials. Trends Biotechnol. 2009, 28, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Shen, A.Q.; Hamlington, B.D.; Knoblauch, M.; Peters, W.S.; Pickard, W.F. Forisome based biomimetic smart materials. Smart Struct. Syst. 2006, 2, 225–235. [Google Scholar] [CrossRef]
- Noll, G.A.; Müller, B.; Ernst, A.M.; Rüping, B.; Twyman, R.M.; Prüfer, D. Native and artificial forisomes: Functions and applications. Appl. Microbiol. Biotechnol. 2011, 89, 1675–1682. [Google Scholar] [CrossRef] [PubMed]
- Knoblauch, M.; Peters, W.S.; Ehlers, K.; van Bel, A.J.E. Reversible calcium-regulated stopcoks in legume sieve tubes. Plant Cell 2001, 13, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Knoblauch, M.; Noll, G.A.; Müller, T.; Prüfer, D.; Schneider-Hüther, I.; Scharner, D.; Van Bel, A.J.E.; Peters, W.S. ATP-independent contractile proteins from plants. Nat. Mater. 2003, 2, 600–603. [Google Scholar] [CrossRef] [PubMed]
- Schwan, S.; Fritzsche, M.; Cismak, A.; Heilmann, A.; Spohn, U. In vitro investigation of the geometric contraction behavior of chemo-mechanical P-protein aggregates (forisomes). Biophys. Chem. 2007, 125, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Schwan, S.; Ferrell, N.; Hansford, D.; Spohn, U.; Heilmann, A. Measurement of mechanical forces generated by plant P-protein aggregates (forisomes). Eur. Biophys. J. 2009, 38, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Schwan, S.; Menzel, M.; Fritzsche, M.; Heilmann, A.; Spohn, U. Micromechanical measurements on P-protein aggregates (forisomes) from Vicia faba plants. Biophys. Chem. 2009, 139, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Rospars, J.-P.; Meyer-Vernet, N. Force per cross-sectional area from molecules to muscles: A general property of biological motors. R. Soc. Open Sci. 2016, 3, 160313. [Google Scholar] [CrossRef] [PubMed]
- Marden, J.H.; Allen, L.R. Molecules, muscles, and machines: Universal performance characteristics of motors. Proc. Natl. Acad. Sci. USA 2002, 99, 4161–4166. [Google Scholar] [CrossRef] [PubMed]
- Marden, J.H. Scaling of maximum net force output by motors used for locomotion. J. Exp. Biol. 2005, 208, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Hunter, I.W.; Lafontaine, S. A comparison of muscle with artificial actuators. In Proceedings of the IEEE Solid-State Sensor and Actuator Workshop, Hilton Head Island, SC, USA, 22–25 June 1992; pp. 178–185.
- Madden, J.D.W.; Vandesteeg, N.A.; Anquetil, P.A.; Madden, P.G.A.; Takshi, A.; Pytel, R.Z.; Lafontaine, S.R.; Wieringa, P.A.; Hunter, I.W. Artificial muscle technology: Physical principles and naval prospects. IEEE J. Ocean. Eng. 2004, 29, 706–728. [Google Scholar] [CrossRef]
- Argentiere, S.; Gigli, G.; Mortato, M.; Gerges, I.; Blasi, L. Smart microfluidics: The role of stimuli-responsive polymers in microfluidic devices. In Advances in Microfluidics; Kelly, R., Ed.; InTech: Rijeka, Croatia, 2012; pp. 127–154. [Google Scholar]
- Dong, L.; Jiang, H. Autonomous microfluidics with stimuli-responsive hydrogels. Soft Matter 2007, 3, 1223–1230. [Google Scholar] [CrossRef]
- Beebe, D.J.; Moore, J.S.; Bauer, J.M.; Yu, Q.; Liu, R.H.; Devadoss, C.; Jo, B.-H. Functional hydrogel structures for autonomous flow control inside microfluidic channels. Nature 2000, 404, 588–590. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H.; Yu, Q.; Beebe, D.J. Fabrication and characterization of hydrogel-based microvalves. J. Microelectromech. Syst. 2002, 11, 45–53. [Google Scholar] [CrossRef]
- Dong, L.; Agarwal, A.K.; Beebe, D.J.; Jiang, H. Adaptive liquid microlenses activated by stimuli-responsive hydrogels. Nature 2006, 442, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Agarwal, A.K.; Beebe, D.J.; Jiang, H. Variable-focus liquid microlenses and microlens arrays actuated by thermoresponsive hydrogels. Adv. Mater. 2007, 19, 401–405. [Google Scholar] [CrossRef]
- Nagai, M.; Matsumoto, N.; Kawashima, T.; Shibata, T. Reversible motion control of Vorticella stalk in microchannel. Microelectron. Eng. 2013, 108, 28–32. [Google Scholar] [CrossRef]
- Nagai, M.; Ryu, S.; Thorsen, T.; Matsudaira, P.; Fujita, H. Chemical control of Vorticella bioactuator using microfluidics. Lab Chip 2010, 10, 1574–1578. [Google Scholar] [CrossRef] [PubMed]
- Nagai, M.; Tanizaki, K.; Hayasaka, Y.; Kawashima, T.; Shibata, T. Microfluidic cellular valve powered by linear bioactuator. In Proceedings of the 2013 Transducers & Eurosensors XXVII: The 17th International Conference on Solid-State Sensors, Actuators and Microsystems, Barcelona, Spain, 16–20 June 2013; pp. 1699–1702.
- Nagai, M.; Kumemura, M.; Asai, H.; Fujita, H. Binding of artificial object to Vorticella for a microsystem powered by a microorganism. e-J. Surf. Sci. Nanotechnol. 2009, 7, 673–676. [Google Scholar] [CrossRef]
- Nagai, M.; Asai, H.; Fujita, H. Reciprocation of micro-objects by contraction and extension of Vorticella convallaria using polylysine as adhesive material. Mech. Eng. J. 2014, 1. [Google Scholar] [CrossRef]
- Patterson, D.J. Habituation in a protozoan Vorticella convallaria. Behavior 1973, 45, 304–311. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sato, K.; Shimizu, T.; Yamato, M.; Okano, T.; Kitamori, T. Biological cells on microchips: New technologies and applications. Biosens. Bioelectron. 2007, 23, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Charlsen, R.W.; Sitti, M. Bio-hybrid cell-based actuators for microsystems. Small 2014, 10, 3831–3851. [Google Scholar] [CrossRef] [PubMed]
- Martel, S. Bacterial microsystems and microrobots. Biomed. Microdevices 2012, 14, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Tung, S.; Kim, J.W. Microscale hybrid devices powered by biological flagellar motors. IEEE. Trans. Autom. Sci. Eng. 2006, 3, 260–263. [Google Scholar] [CrossRef]
- Steager, E.B.; Sakar, M.S.; Kim, D.H.; Kumar, V.; Pappas, G.J.; Kim, M.J. Electrokinetic and optical control of bacterial microrobots. J. Micromech. Microeng. 2011, 21, 035001. [Google Scholar] [CrossRef]
- Weibel, D.B.; Garstecki, P.; Ryan, D.; DiLuzio, W.R.; Mayer, M.; Seto, J.E.; Whitesides, G.M. Microoxen: Microorganisms to move microscale loads. Proc. Natl. Acad. Sci. USA 2005, 102, 11963–11967. [Google Scholar] [CrossRef] [PubMed]
- McCord, R.P.; Yukich, J.N.; Bernd, K.K. Analysis of force generation during flagellar assembly through optical trapping of free-swimming Chlamydomonas reinhardtii. Cell Motil. Cytoskelet. 2005, 61, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Itoh, A. Motion control of protozoa for bio-MEMS. IEEE/ASME Trans. Mechatron. 2000, 5, 181–188. [Google Scholar] [CrossRef]
- Vacchiano, E.; Dreisbach, A.; Locascio, D.; Castaneda, L.; Vivian, T.; Bushe, H.E., Jr. Morphogenetic transitions and cytoskeletal elements of the stalked zooid and the telotroch stages in the peritrich ciliate Vorticella convallaria. J. Protozool. 1992, 39, 101–106. [Google Scholar] [CrossRef]
- De Baufer, P.J.; Amin, A.A.; Pak, S.C.; Buhse, H.E., Jr. A method for the synchronous induction of large numbers of telotrochs in vorticella convallaria by monocalcium phosphate at low pH. J. Eukaryot. Microbiol. 1999, 46, 12–16. [Google Scholar] [CrossRef]
- Bramucci, M.G.; Nagarajan, V. Inhibition of Vorticella microstoma stalk formation by wheat germ agglutinin. J. Eukaryot. Microbiol. 2004, 51, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Fearing, R.S. Control of a micro-organism as a prototype micro-robot. In Proceedings of the 2nd International Symposium on Micromachines and Human Sciences, Nagoya, Japan, 9 October 1991.
- Ogawa, N.; Oku, H.; Hashimoto, K. Microrobotic visual control of motile cells using high-speed tracking system. IEEE Trans. Robot. 2005, 21, 704–712. [Google Scholar] [CrossRef]
- Kim, D.H.; Casale, D.; Kohidai, L.; Kim, M.J. Galvanotactic and phototactic control of Tetrahymena pyriformis as a microfluidic workhorse. Appl. Phys. Lett. 2009, 94, 163901. [Google Scholar] [CrossRef]
- Kim, S.; Laschi, C.; Trimmer, B. Soft robotics: A bioinspired evolution in robotics. Trends Biotechnol. 2013, 31, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, A.W. Biological soft robotics. Annu. Rev. Biomed. Eng. 2015, 17, 243–265. [Google Scholar] [CrossRef] [PubMed]
- Rus, D.; Tolley, M.T. Design, fabrication and control of soft robots. Nature 2015, 521, 467–475. [Google Scholar] [CrossRef] [PubMed]


| Reference | Cell | Stalk Length | Peak Contraction Speed | Time for Peak Speed | Contraction Time | Latent Period | Contraction Propagation Speed |
|---|---|---|---|---|---|---|---|
| Ueda (1954) [32] | C. polypinum | 1.05–1.40 mm | 17.8–24.7 cm/s | - | 7–10 ms | 2–3 ms | - |
| Sugi (1960) [33] | C. polypinum | 0.8–2.2 mm | - | - | - | 1–4 ms | 20–50 cm/s |
| Jones et al. (1970) [119] | V. difficilis | 140–200 μm | 1.4–5.6 cm/s | - | ~10 ms | - | 3.5–10.5 cm/s |
| Weis-Fogh and Amos (1972) [38] | Z. geniculatum | 1 mm | 110 length/s (11 cm/s) | - | 5 ms | - | - |
| Rahat et al. (1973) [39] | Carchesium | - | - | - | 40–60 ms | - | - |
| Katoh and Naitoh (1992) [11] | Vorticella | - | 6.7 cm/s | - | - | 1.7 ms | - |
| Moriyama et al. (1998) [12] | V. convallaria | ~250 μm | 8.8 cm/s | 2 ms | ~9 ms | - | - |
| Upadhyaya et al. (2008) [14] | V. convallaria | 150 μm | ~6 cm/s | ~2.5 ms | ~6 ms | - | ~10 cm/s |
| Ryu and Matsudaira (2010) [9] | V. convallaria | 117 μm | 5.8 cm/s | 1 ms | 3.9 ms | - | - |
| Kamiguri et al. (2012) [120] | Vorticella | - | 4.3–7.5 cm/s | - | - | - | - |
| Reference | Cell | Stalk (st) or Spasmoneme (sp) Dimension | Contractile Force or Tension | Isometric Force or Tension | Work | Instantaneous or Maximum Power Output | Energy Efficiency |
|---|---|---|---|---|---|---|---|
| Ueda (1952) [31] | C. polypinum (Live) | 1–2.7 mm long 12–15 μm diameter (st) | 0.092–0.132 mg (0.9–1.3 μN) 65–88 g/cm2 (6.3–8.6 kPa) | - | - | - | - |
| Hoffman-Berling (1958) [17] | Vorticella (Live) | - | ~10 kPa [42] | - | - | ~2.8 W/g [42] | - |
| Amos (1971) [13] | Vorticella (Live) | 80 μm long 1 μm diameter (sp) [44] | 8.7 Nn (11 kPa [44]) 20–30 g/cm2 [39] (1.96–2.94 kPa) | - | 0.69 pJ | 2300 cal/g/h [39] (2.67 W/g) | - |
| Weis-Fogh and Amos (1972) [38] | Z. geniculatum (Glycerinated) | 1 mm long 30 μm diameter (sp) | 30 kPa [42] | - | - | ~4 W/g [42] | - |
| Rahat et al. (1973) [39] | Carchesium (Live) | 5 μm diameter (sp) | 400–800 g/cm2 (39.2–78.4 kPa) | - | - | - | - |
| Moriyama et al. (1996) [23] | V. convallaria (Glycerinated) | 1.0–1.2 μm diameter (sp) | - | 40 nN (avg) 120 nN (max) (35–51 kPa) | - | - | ~7% |
| Moriyama et al. (1998) [12] | V. convallaria (Live) | ~250 μm long | 55.8 nN | - | - | - | - |
| France (2007) [122] | V. convallaria (Live) | - | 109–142 nN | - | 1.93–2.59 pJ | 0.70–0.93 nW | - |
| Upadhyaya et al. (2008) [14] | V. convallaria (Live) | 150 μm long | ~30 nN | - | - | 1–3 nW | - |
| Ryu and Matsudaira (2010) [9] | V. convallaria (Live) | 117 μm long | 28 nN | - | 1.64 pJ | 1.6 nW | 8% |
| Apolinar-Iribe et al. (2010) [123] | Vorticella (Live) | - | 117.7 nN | - | - | - | - |
| Ryu et al. (2012) [16] | V. convallaria (Live) | 49–134 μm long | - | 150–330 nN | - | - | - |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, S.; Pepper, R.E.; Nagai, M.; France, D.C. Vorticella: A Protozoan for Bio-Inspired Engineering. Micromachines 2017, 8, 4. https://doi.org/10.3390/mi8010004
Ryu S, Pepper RE, Nagai M, France DC. Vorticella: A Protozoan for Bio-Inspired Engineering. Micromachines. 2017; 8(1):4. https://doi.org/10.3390/mi8010004
Chicago/Turabian StyleRyu, Sangjin, Rachel E. Pepper, Moeto Nagai, and Danielle C. France. 2017. "Vorticella: A Protozoan for Bio-Inspired Engineering" Micromachines 8, no. 1: 4. https://doi.org/10.3390/mi8010004
APA StyleRyu, S., Pepper, R. E., Nagai, M., & France, D. C. (2017). Vorticella: A Protozoan for Bio-Inspired Engineering. Micromachines, 8(1), 4. https://doi.org/10.3390/mi8010004