Rapid Fabrication of Hydrogel Microstructures Using UV-Induced Projection Printing
Abstract
:1. Introduction
2. Experimental Section
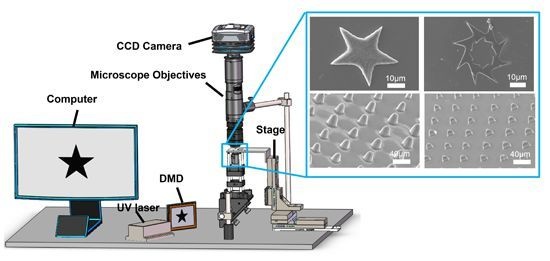
2.1. DMD-Based Hydrogel Microstructures Fabricating System
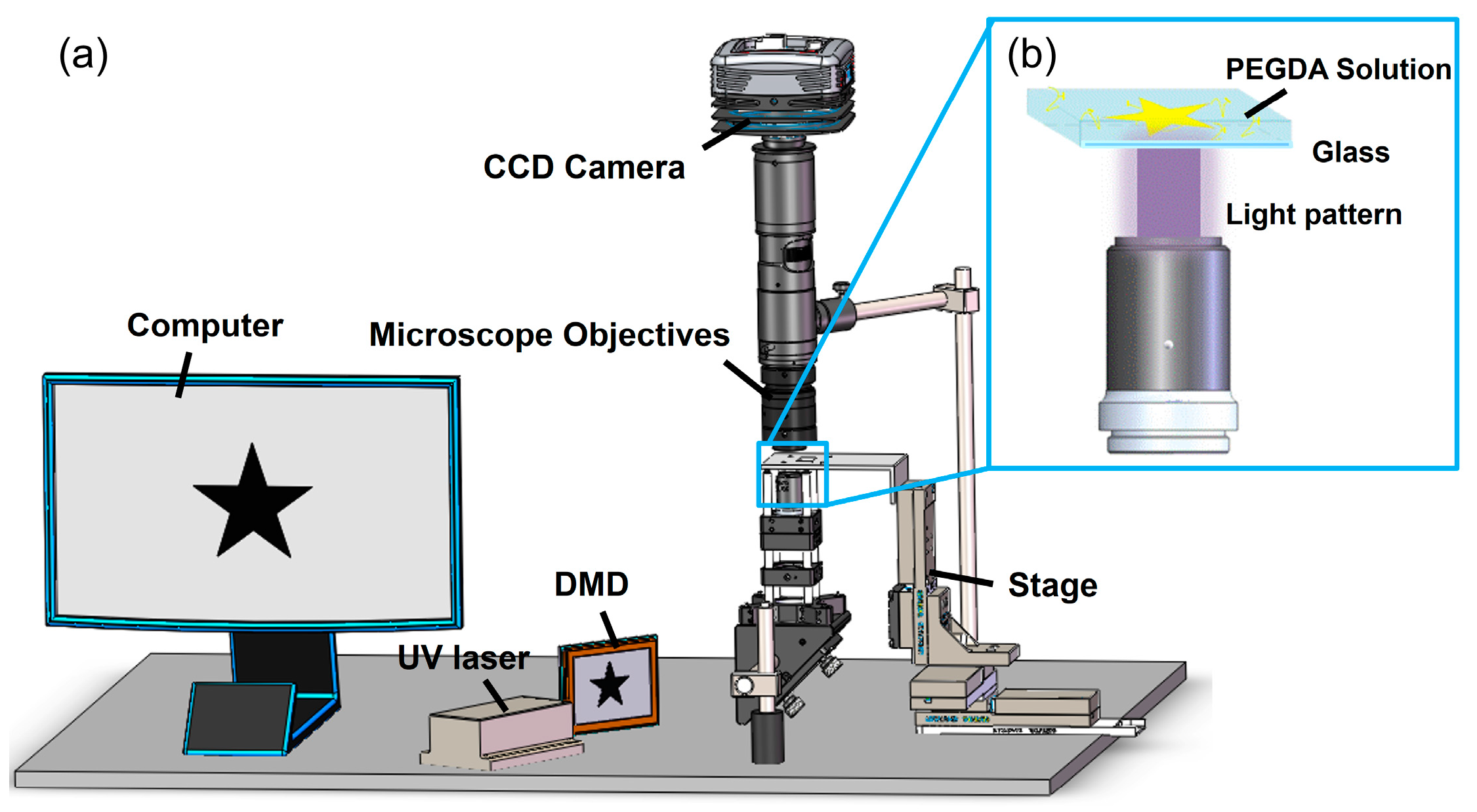
2.2. Prparing Pre-Polymer Solution

2.3. Cell Culture and Fluorescent Staining
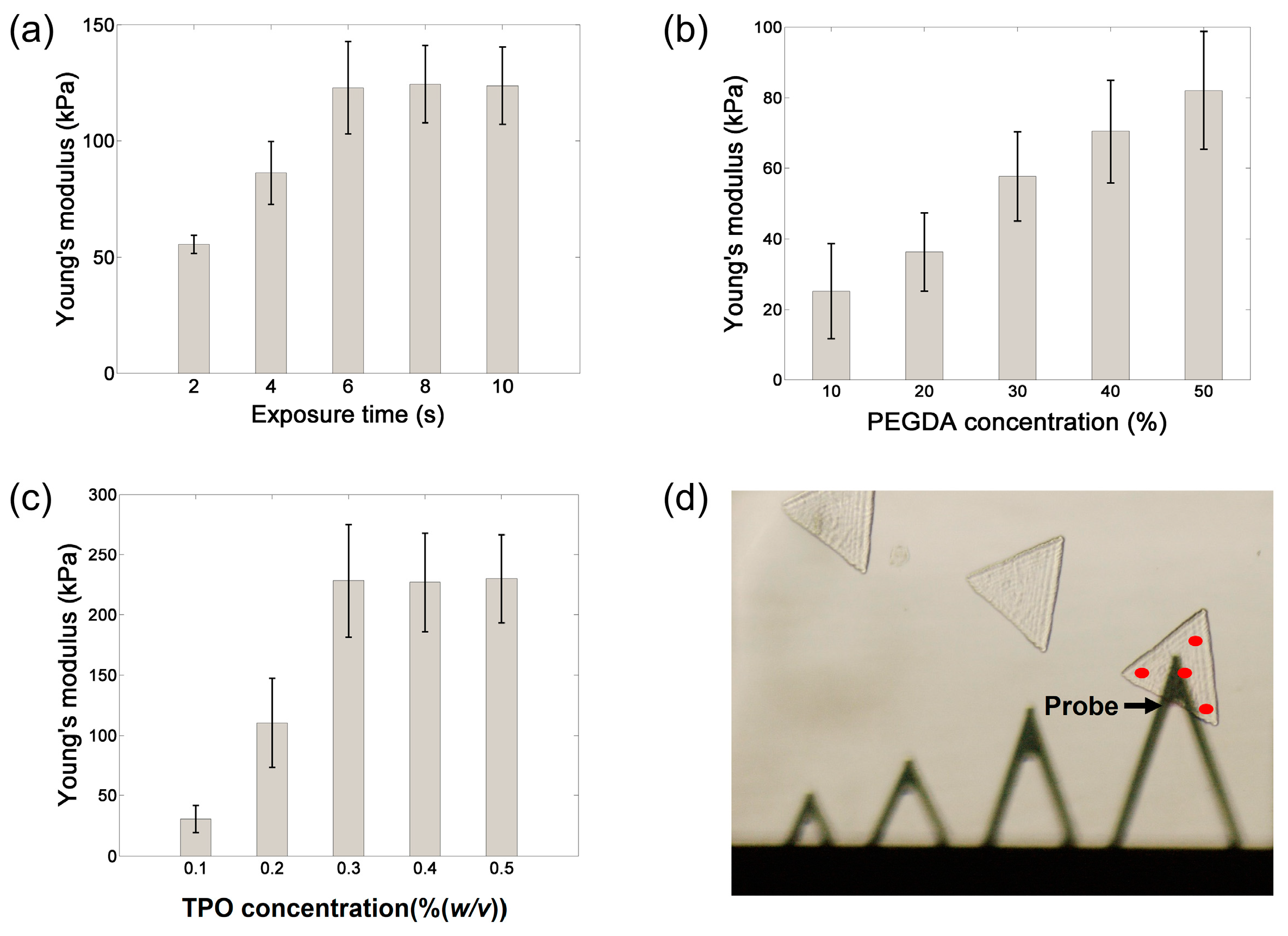
2.4. Measurement of Mechanical Properties
3. Results and Discussion
3.1. 2D Microstructure Arrays Fabricated by Printing System
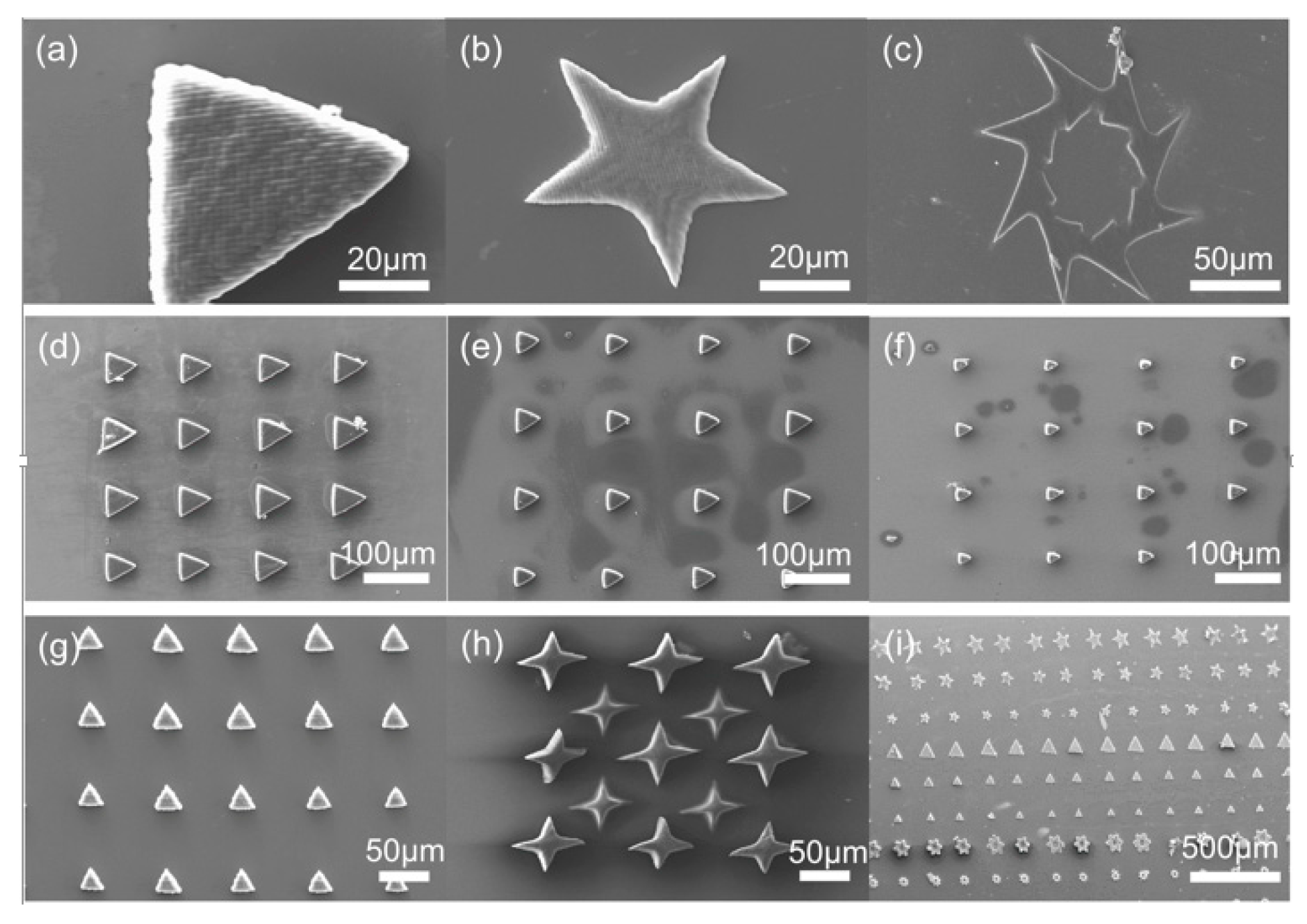
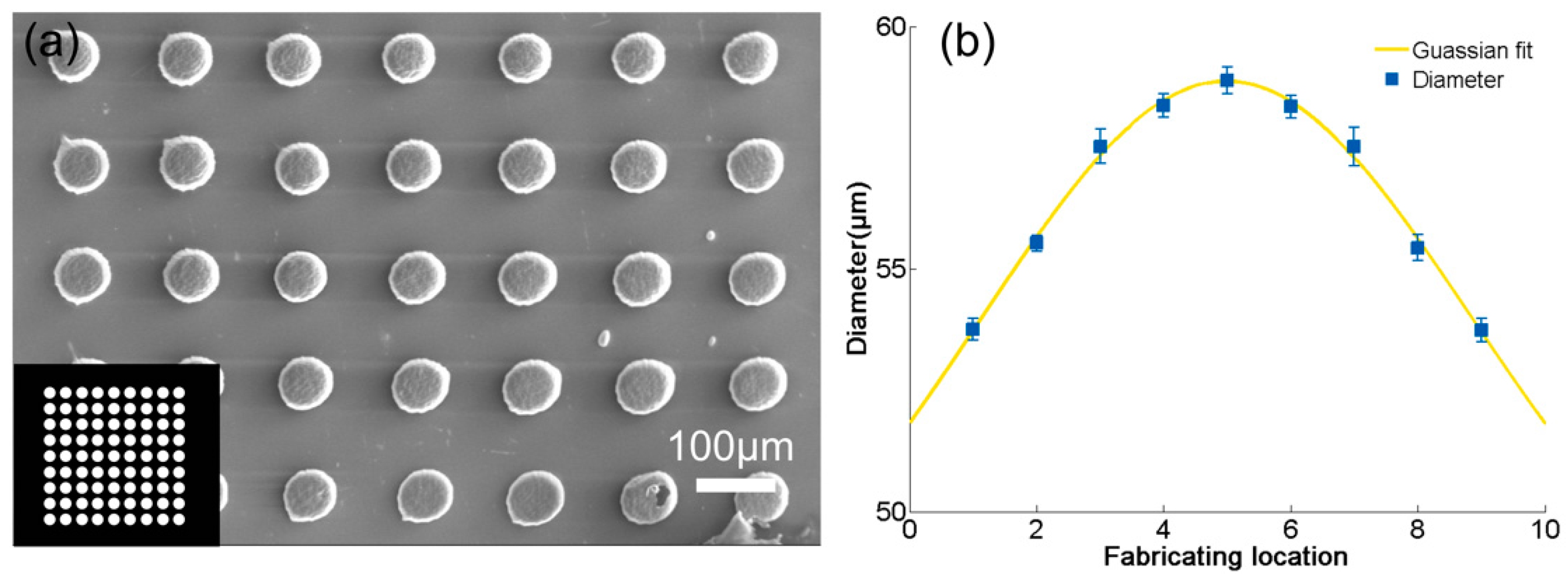
3.2. Micropillar Array Fabrication
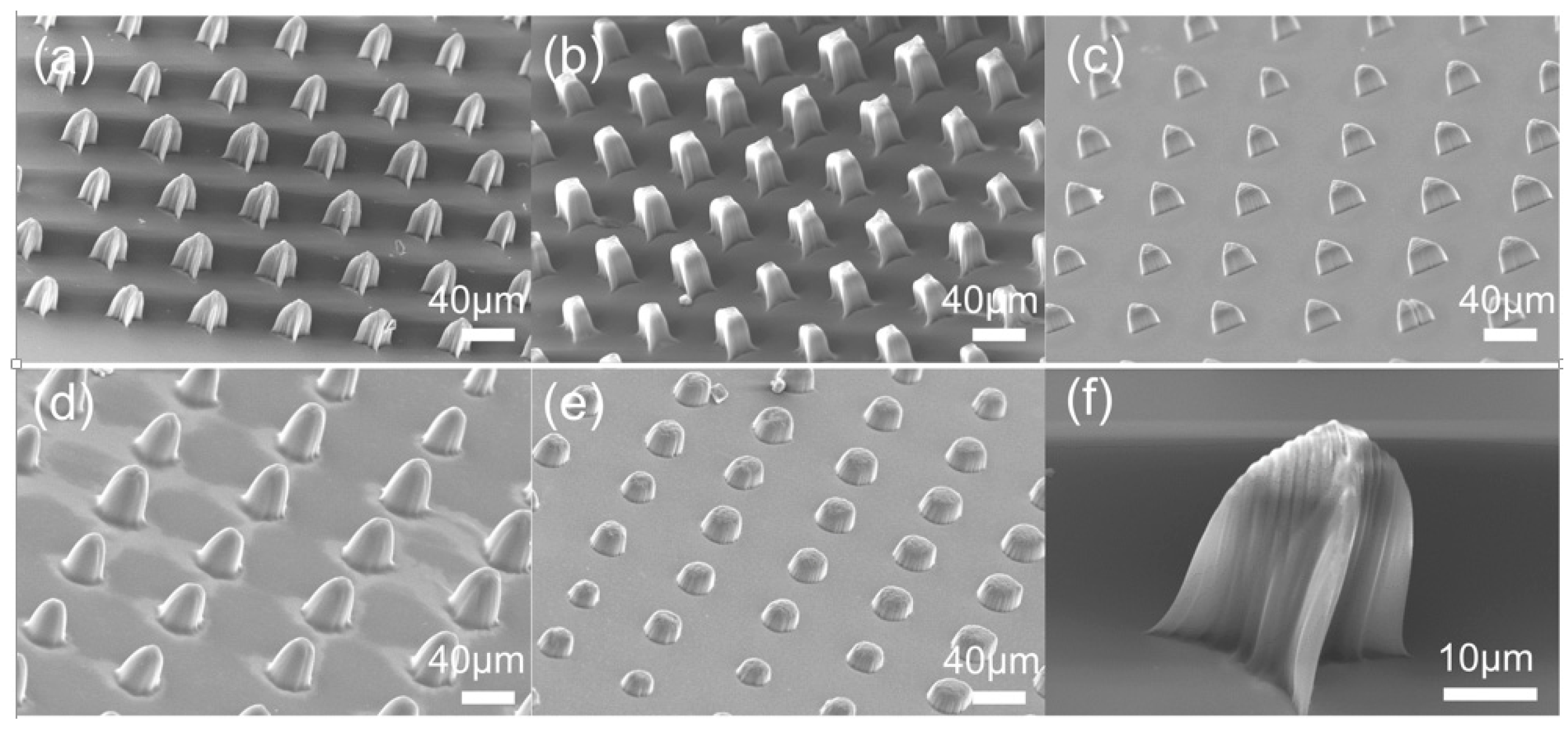
3.3. Mechanical Properties of Hydrogel Microstructures
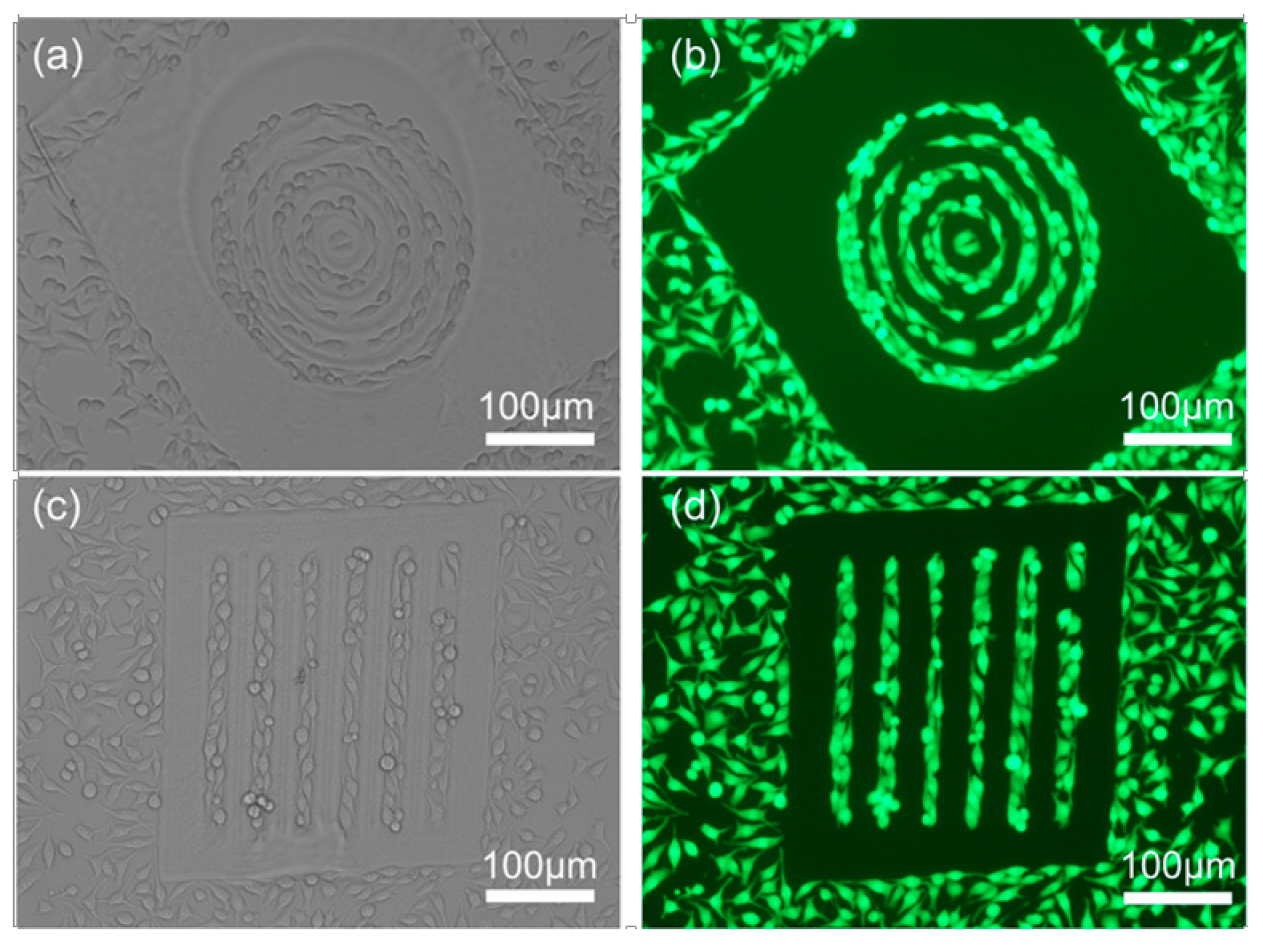
3.4. Formation of Cell Chains
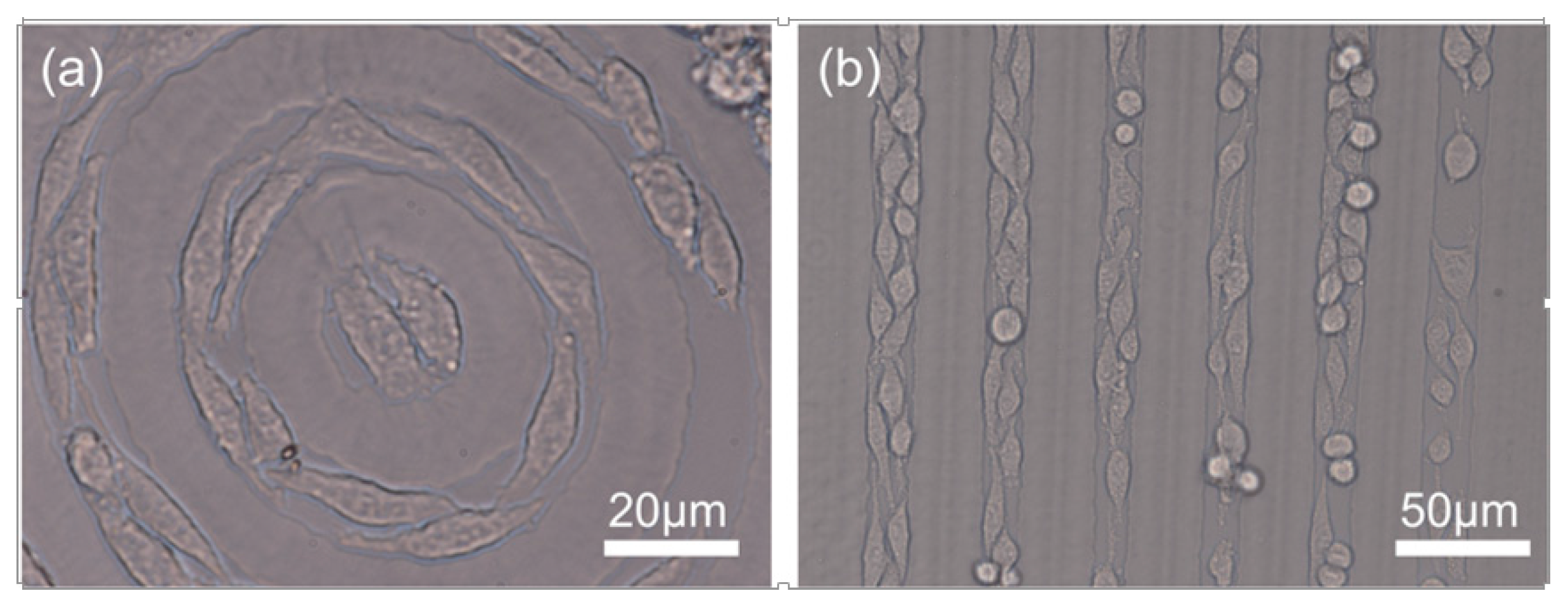
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Khademhosseini, A.; Langer, R. Microengineered hydrogels for tissue engineering. Biomaterials 2007, 28, 5087–5092. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.M.; Sant, S.; Masaeli, M.; Kachouie, N.N.; Zamanian, B.; Lee, S.H.; Khademhosseini, A. Fabrication of three-dimensional porous cell-laden hydrogel for tissue engineering. Biofabrication 2010, 2, 035003. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yu, H.; Wei, F.; Li, G.; Wang, Y.; Liu, L. Selective pattern of cancer cell accumulation and growth using UV modulating printing of hydrogels. Biomed. Microdevices 2015, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2012, 64, 49–60. [Google Scholar] [CrossRef]
- Li, J.J.; Guo, S.Z.; Wang, M.; Ye, L.; Yao, F.L. Poly(lactic acid)/poly(ethylene glycol) block copolymer based shell or core cross-linked micelles for controlled release of hydrophobic drug. RSC Adv. 2015, 5, 19484–19492. [Google Scholar] [CrossRef]
- Dang, S.M.; Kyba, M.; Perlingeiro, R.; Daley, G.Q.; Zandstra, P.W. Efficiency of embryoid body formation and hematopoietic development from embryonic stem cells in different culture systems. Biotechnol. Bioeng. 2002, 78, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Buenger, D.; Topuz, F.; Groll, J. Hydrogels in sensing applications. Prog. Polym. Sci. 2012, 37, 1678–1719. [Google Scholar] [CrossRef]
- Roullier, V.; Grasset, F.; Boulmedais, F.; Artzner, F.; Cador, O.; Marchi-Artzner, V. Small bioactivated magnetic quantum dot micelles. Chem. Mater. 2008, 20, 6657–6665. [Google Scholar] [CrossRef]
- Hu, Y.L.; Lao, Z.X.; Cumming, B.P.; Wu, D.; Li, J.W.; Liang, H.Y.; Chu, J.R.; Huang, W.H.; Gu, M. Laser printing hierarchical structures with the aid of controlled capillary-driven self-assembly. Proc. Natl. Acad. Sci. USA 2015, 112, 6876–6881. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Gautrot, J.E.; Connelly, J.T. Directing cell migration using micropatterned and dynamically adhesive polymer brushes. Acta Biomater. 2014, 10, 2415–2422. [Google Scholar] [CrossRef] [PubMed]
- Park, K.J.; Lee, K.G.; Seok, S.; Choi, B.G.; Lee, M.K.; Park, T.J.; Park, J.Y.; Kim, D.H.; Lee, S.J. Micropillar arrays enabling single microbial cell encapsulation in hydrogels. Lab Chip 2014, 14, 1873–1879. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Liang, W.F.; Liu, L.Q.; Wang, Y.C.; Mai, J.D.; Lee, G.B.; Li, W.J. Extracellular-controlled breast cancer cell formation and growth using non-UV patterned hydrogels via optically-induced electrokinetics. Lab Chip 2014, 14, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Xia, Y.; Whitesides, G.M. Soft lithography for micro- and nanoscale patterning. Nat. Protoc. 2010, 5, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Suh, K.Y.; Seong, J.; Khademhosseini, A.; Laibinis, P.E.; Langer, R. A simple soft lithographic route to fabrication of poly(ethylene glycol) microstructures for protein and cell patterning. Biomaterials 2004, 25, 557–563. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Ostuni, E.; Takayama, S.; Jiang, X.Y.; Ingber, D.E. Soft lithography in biology and biochemistry. Annu. Rev. Biomed. Eng. 2001, 3, 335–373. [Google Scholar] [CrossRef] [PubMed]
- Occhetta, P.; Sadr, N.; Piraino, F.; Redaelli, A.; Moretti, M.; Rasponi, M. Fabrication of 3D cell-laden hydrogel microstructures through photo-mold patterning. Biofabrication 2013, 5, 035002. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Khademhosseini, A.; Langer, R. Fabrication of gradient hydrogels using a microfluidics/photopolymerization process. Langmuir 2004, 20, 5153–5156. [Google Scholar] [CrossRef] [PubMed]
- Revzin, A.; Russell, R.J.; Yadavalli, V.K.; Koh, W.G.; Deister, C.; Hile, D.D.; Mellott, M.B.; Pishko, M.V. Fabrication of poly(ethylene glycol) hydrogel microstructures using photolithography. Langmuir 2001, 17, 5440–5447. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.N.; Lee, W.; Koh, W.G. Micropatterning of proteins on the surface of three-dimensional poly(ethylene glycol) hydrogel microstructures. Anal. Chim. Acta 2008, 609, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, J.; Khademhosseini, A.; Yeo, Y.; Yang, X.; Yeh, J.; Eng, G.; Blumling, J.; Wang, C.F.; Kohane, D.S.; Langer, R. Micromolding of photocrosslinkable chitosan hydrogel for spheroid microarray and co-cultures. Biomaterials 2006, 27, 5259–5267. [Google Scholar] [CrossRef] [PubMed]
- Hirai, Y.; Inamoto, Y.; Sugano, K.; Tsuchiya, T.; Tabata, O. Moving mask UV lithography for three-dimensional structuring. J. Micromech. Microeng. 2007, 17, 199–206. [Google Scholar] [CrossRef]
- Liu, R.H.; Yu, Q.; Beebe, D.J. Fabrication and characterization of hydrogel-based microvalves. J. Microelectromech. Syst. 2002, 11, 45–53. [Google Scholar] [CrossRef]
- Chan, V.; Jeong, J.H.; Bajaj, P.; Collens, M.; Saif, T.; Kong, H.; Bashir, R. Multi-material bio-fabrication of hydrogel cantilevers and actuators with stereolithography. Lab Chip 2012, 12, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Chan, V.; Zorlutuna, P.; Jeong, J.H.; Kong, H.; Bashir, R. Three-dimensional photopatterning of hydrogels using stereolithography for long-term cell encapsulation. Lab Chip 2010, 10, 2062–2070. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Akiyama, M.; Totani, K.; Kuebler, S.M.; Stellacci, F.; Wenseleers, W.; Braun, K.; Marder, S.R.; Perry, J.W. Photoresponsive hydrogel microstructure fabricated by two-photon initiated polymerization. Adv. Funct. Mater. 2002, 12, 611–614. [Google Scholar] [CrossRef]
- Jhaveri, S.J.; McMullen, J.D.; Sijbesma, R.; Tan, L.S.; Zipfel, W.; Ober, C.K. Direct three-dimensional microfabrication of hydrogels via two-photon lithography in aqueous solution. Chem. Mater. 2009, 21, 2003–2006. [Google Scholar] [CrossRef] [PubMed]
- Ciuciu, A.I.; Cywinski, P.J. Two-photon polymerization of hydrogels—Versatile solutions to fabricate well-defined 3D structures. RSC Adv. 2014, 4, 45504–45516. [Google Scholar] [CrossRef]
- Bohandy, J.; Kim, B.F.; Adrian, F.J. Metal-deposition from a supported metal-film using an excimer laser. J. Appl. Phys. 1986, 60, 1538–1539. [Google Scholar] [CrossRef]
- Heath, D.J.; Feinaeugle, M.; Grant-Jacob, J.A.; Mills, B.; Eason, R.W. Dynamic spatial pulse shaping via a digital micromirror device for patterned laser-induced forward transfer of solid polymer films. Opt. Mater. Express 2015, 5, 1129–1136. [Google Scholar] [CrossRef]
- Delaney, J.T.; Liberski, A.R.; Perelaer, J.; Schubert, U.S. Reactive inkjet printing of calcium alginate hydrogel porogens—A new strategy to open-pore structured matrices with controlled geometry. Soft Matter 2010, 6, 866–869. [Google Scholar] [CrossRef]
- Bertassoni, L.E.; Cecconi, M.; Manoharan, V.; Nikkhah, M.; Hjortnaes, J.; Cristino, A.L.; Barabaschi, G.; Demarchi, D.; Dokmeci, M.R.; Yang, Y.Z.; et al. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip 2014, 14, 2202–2211. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Brauer, N.B.; Fakhfouri, V.; Boiko, D.L.; Charbon, E.; Grutzner, G.; Brugger, J. Hybrid polymer microlens arrays with high numerical apertures fabricated using simple ink-jet printing technique. Opt. Mater. Express 2011, 1, 259–269. [Google Scholar] [CrossRef]
- Kim, H.C.; Yoon, H.R.; Lee, I.H.; Ko, T.J. Exposure time variation method using DMD for microstereolithography. J. Adv. Mech. Des. Syst. 2012, 6, 44–51. [Google Scholar] [CrossRef]
- Grogan, S.P.; Chung, P.H.; Soman, P.; Chen, P.; Lotz, M.K.; Chen, S.C.; D’Lima, D.D. Digital micromirror device projection printing system for meniscus tissue engineering. Acta Biomater. 2013, 9, 7218–7226. [Google Scholar] [CrossRef] [PubMed]
- Cuchiara, M.P.; Allen, A.C.B.; Chen, T.M.; Miller, J.S.; West, J.L. Multilayer microfluidic pegda hydrogels. Biomaterials 2010, 31, 5491–5497. [Google Scholar] [CrossRef] [PubMed]
- Durst, C.A.; Cuchiara, M.P.; Mansfield, E.G.; West, J.L.; Grande-Allen, K.J. Flexural characterization of cell encapsulated PEGDA hydrogels with applications for tissue engineered heart valves. Acta Biomater. 2011, 7, 2467–2476. [Google Scholar] [CrossRef] [PubMed]
- Harmon, M.E.; Kuckling, D.; Frank, C.W. Photo-cross-linkable pnipaam copolymers. 2. Effects of constraint on temperature and pH-responsive hydrogel layers. Macromolecules 2003, 36, 162–172. [Google Scholar] [CrossRef]
- Touhami, A.; Nysten, B.; Dufrene, Y.F. Nanoscale mapping of the elasticity of microbial cells by atomic force microscopy. Langmuir 2003, 19, 4539–4543. [Google Scholar] [CrossRef]
- Chiu, Y.C.; Cheng, M.H.; Engel, H.; Kao, S.W.; Larson, J.C.; Gupta, S.; Brey, E.M. The role of pore size on vascularization and tissue remodeling in peg hydrogels. Biomaterials 2011, 32, 6045–6051. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, P.; Marchwiany, D.; Duarte, C.; Bashir, R. Patterned three-dimensional encapsulation of embryonic stem cells using dielectrophoresis and stereolithography. Adv. Healthc. Mater. 2013, 2, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Neiman, J.A.S.; Raman, R.; Chan, V.; Rhoads, M.G.; Raredon, M.S.B.; Velazquez, J.J.; Dyer, R.L.; Bashir, R.; Hammond, P.T.; Griffith, L.G. Photopatterning of hydrogel scaffolds coupled to filter materials using stereolithography for perfused 3D culture of hepatocytes. Biotechnol. Bioeng. 2015, 112, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Chan, V.; Park, K.; Collens, M.B.; Kong, H.; Saif, T.A.; Bashir, R. Development of miniaturized walking biological machines. Sci. Rep. 2012, 2, 857. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.P.; Qu, X.; Soman, P.; Hribar, K.C.; Lee, J.W.; Chen, S.C.; He, S.L. Rapid fabrication of complex 3D extracellular microenvironments by dynamic optical projection stereolithography. Adv. Mater. 2012, 24, 4266–4270. [Google Scholar] [CrossRef] [PubMed]
- Cha, C.Y.; Soman, P.; Zhu, W.; Nikkhah, M.; Camci-Unal, G.; Chen, S.C.; Khademhosseini, A. Structural reinforcement of cell-laden hydrogels with microfabricated three dimensional scaffolds. Biomater. Sci. 2014, 2, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.Y.; Zhou, C.; Chen, Y. A fast mask projection stereolithography process for fabricating digital models in minutes. J. Manuf. Sci. Eng. 2012, 134, 051011. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Yu, H.; Liang, W.; Wang, Y.; Liu, L. Rapid Fabrication of Hydrogel Microstructures Using UV-Induced Projection Printing. Micromachines 2015, 6, 1903-1913. https://doi.org/10.3390/mi6121464
Yang W, Yu H, Liang W, Wang Y, Liu L. Rapid Fabrication of Hydrogel Microstructures Using UV-Induced Projection Printing. Micromachines. 2015; 6(12):1903-1913. https://doi.org/10.3390/mi6121464
Chicago/Turabian StyleYang, Wenguang, Haibo Yu, Wenfeng Liang, Yuechao Wang, and Lianqing Liu. 2015. "Rapid Fabrication of Hydrogel Microstructures Using UV-Induced Projection Printing" Micromachines 6, no. 12: 1903-1913. https://doi.org/10.3390/mi6121464
APA StyleYang, W., Yu, H., Liang, W., Wang, Y., & Liu, L. (2015). Rapid Fabrication of Hydrogel Microstructures Using UV-Induced Projection Printing. Micromachines, 6(12), 1903-1913. https://doi.org/10.3390/mi6121464