Generation of Nanoliter Droplets on Demand at Hundred-Hz Frequencies
Abstract
:1. Introduction
2. Experimental Section
2.1. Microfluidic Chip
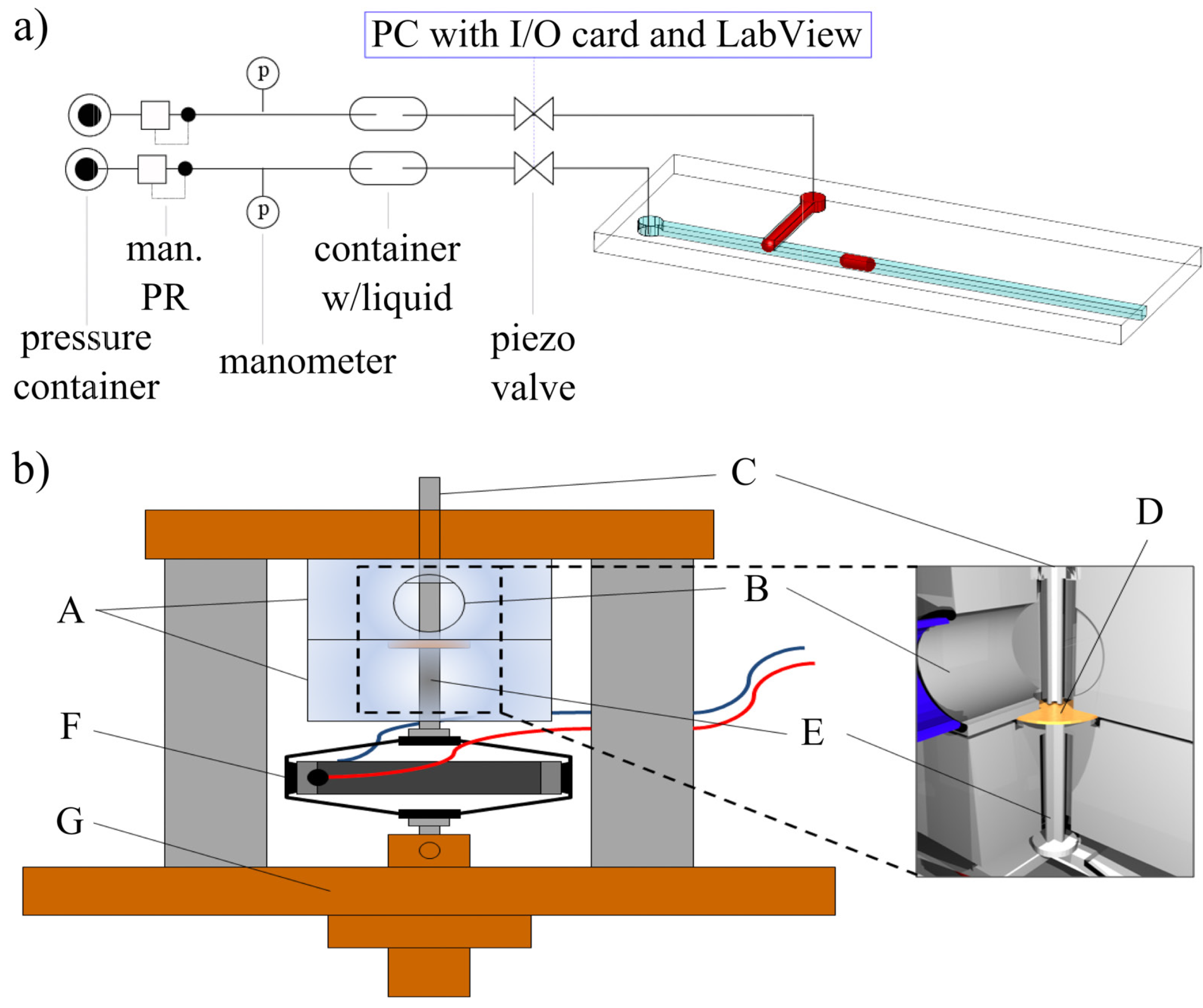
2.2. The Valve
2.3. Amplifier
2.4. Protocol of Generation of Droplets on Demand
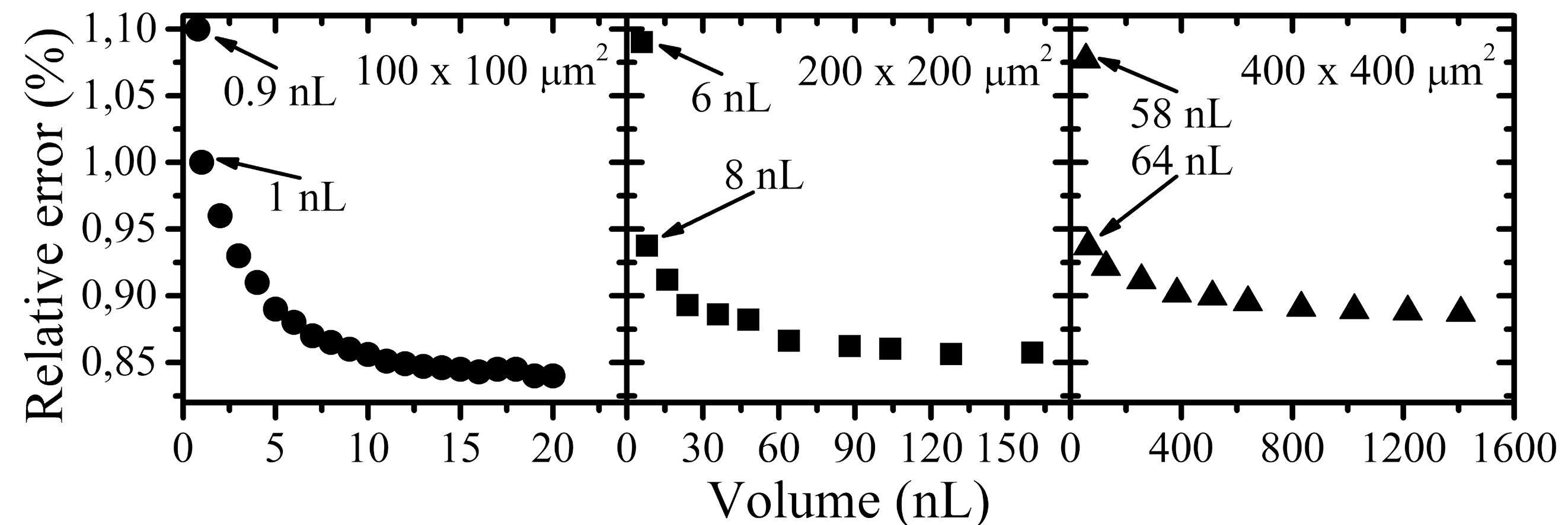
2.5. Measurement of the Volume of Droplets
3. Results and Discussion
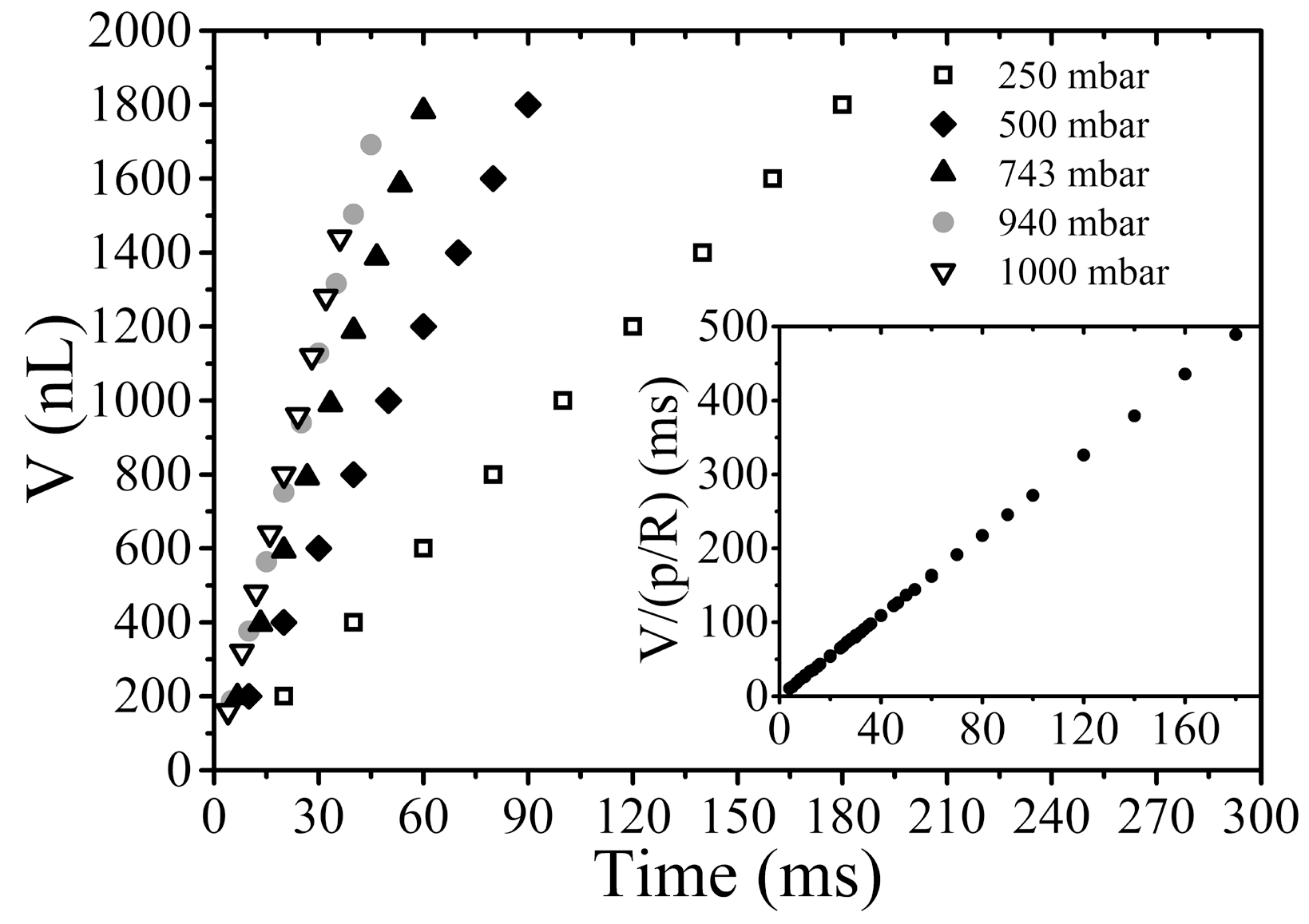
3.1. Tuning the Volume of Droplets via Timing the Valves
3.2. Tuning the Volume of Droplets Generated at a Constant Frequency

3.3. Analogue Control
3.4. Maximum Frequency of Operation
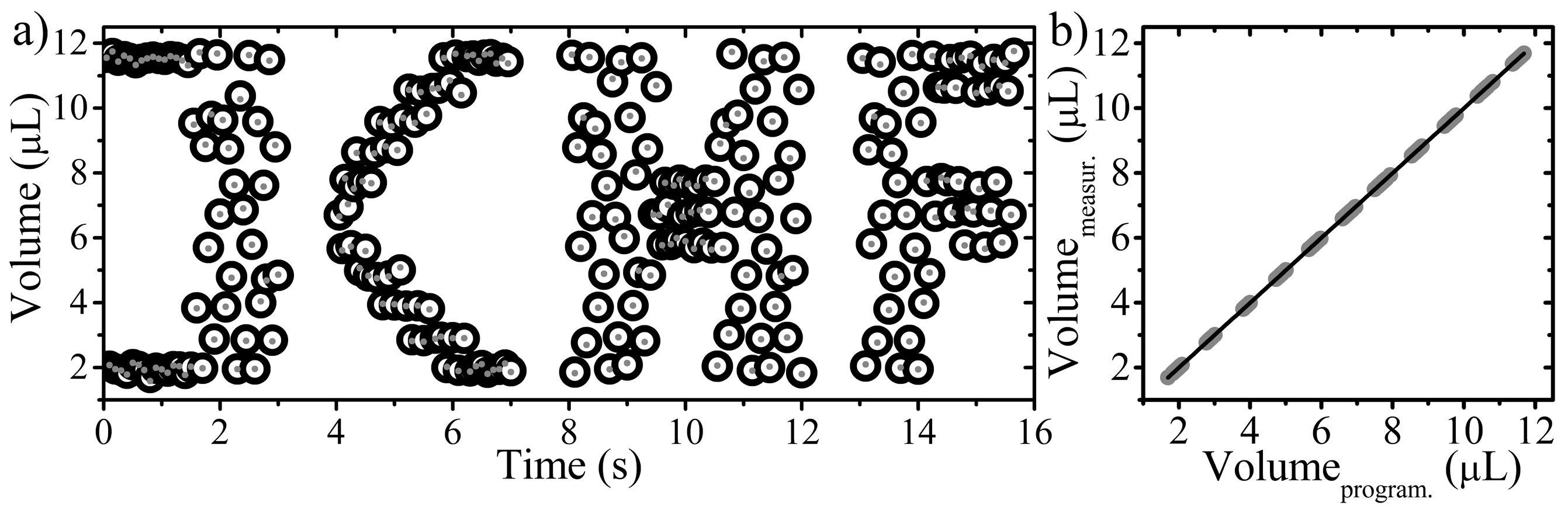
3.5. Generation of Arbitrary Sequences of Volumes of Droplets
3.6. Durability of the Valve
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Theberge, A.B.; Courtois, F.; Schaerli, Y.; Fischlechner, M.; Abell, C.; Hollfelder, F.; Huck, W.T.S. Microdroplets in microfluidics: An evolving platform for discoveries in chemistry and biology. Angew. Chem. Int. Ed. Engl. 2010, 49, 5846–5868. [Google Scholar] [CrossRef]
- Pompano, R.R.; Liu, W.; Du, W.; Ismagilov, R.F. Microfluidics using spatially defined arrays of droplets in one, two, and three dimensions. Annu. Rev. Anal. Chem. 2011, 4, 59–81. [Google Scholar] [CrossRef]
- Squires, T.M.; Quake, S.R. Microfluidics: Fluid physics at the nanoliter scale. Rev. Mod. Phys. 2005, 77, 977–1026. [Google Scholar] [CrossRef]
- Leng, X.; Zhang, W.; Wang, C.; Cui, L.; Yang, C.J. Agarose droplet microfluidics for highly parallel and efficient single molecule emulsion PCR. Lab Chip 2010, 10, 2841–2843. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.S.; Nitin, N. Rapid detection of bacteriophages in starter culture using water-in-oil-in-water emulsion microdroplets. Appl. Microbiol. Biotechnol. 2014, 98, 8347–8355. [Google Scholar] [CrossRef] [PubMed]
- Witters, D.; Sun, B.; Begolo, S.; Rodriquez-Manzano, J.; Robles, W.; Ismagilov, R.F. Digital biology and chemistry. Lab Chip 2014, 14, 3225–3232. [Google Scholar] [CrossRef] [PubMed]
- Teste, B.; Ali-Cherif, A.; Viovy, J.L.; Malaquin, L. A low cost and high throughput magnetic bead-based immuno-agglutination assay in confined droplets. Lab Chip 2013, 13, 2344–2349. [Google Scholar] [PubMed]
- Churski, K.; Korczyk, P.; Garstecki, P. High-throughput automated droplet microfluidic system for screening of reaction conditions. Lab Chip 2010, 10, 816–818. [Google Scholar] [CrossRef] [PubMed]
- Churski, K.; Kaminski, T.S.; Jakiela, S.; Kamysz, W.; Baranska-Rybak, W.; Weibel, D.B.; Garstecki, P. Rapid screening of antibiotic toxicity in an automated microdroplet system. Lab Chip 2012, 12, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Perccin, G.; Levin, L.; Khuri-Yakub, B.T. Piezoelectrically actuated droplet ejector. Rev. Sci. Instrum. 1997, 68, 4561–4563. [Google Scholar] [CrossRef]
- Chen, C.H.; Cho, S.H.; Tsai, F.; Erten, A.; Lo, Y.-H. Microfluidic cell sorter with integrated piezoelectric actuator. Biomed. Microdevices 2009, 11, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Bransky, A.; Korin, N.; Khoury, M.; Levenberg, S. A microfluidic droplet generator based on a piezoelectric actuator. Lab Chip 2009, 9, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, J.; Bransky, A.; Khoury, M.; Levenberg, S. Advanced microfluidic droplet manipulation based on piezoelectric actuation. Biomed. Microdevices 2010, 12, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, J.; Nir, A.; Bransky, A.; Levenberg, S. Coalescence-assisted generation of single nanoliter droplets with predefined composition. Lab Chip 2011, 11, 3225–3230. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Attinger, D. Drop on demand in a microfluidic chip. J. Micromech. Microeng. 2008, 18, 065020. [Google Scholar] [CrossRef]
- Abate, A.R.; Hung, T.; Mary, P.; Agresti, J.J.; Weitz, D.A. High-throughput injection with microfluidics using picoinjectors. Proc. Natl. Acad. Sci. 2010, 107, 19163–19166. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Jofre, A.M.; Kishore, R.B.; Reiner, J.E.; Greene, M.E.; Lowman, G.M.; Denker, J.S.; Willis, C.C.C.; Helmerson, K.; Goldner, L.S. Generation and mixing of subfemtoliter aqueous droplets on demand. Anal. Chem. 2009, 81, 8041–8047. [Google Scholar] [CrossRef] [PubMed]
- Thorsen, T.; Roberts, R.W.; Arnold, F.H.; Quake, S.R. Dynamic pattern formation in a vesicle-generating microfluidic device. Phys. Rev. Lett. 2001, 86, 4163–4166. [Google Scholar] [CrossRef] [PubMed]
- Garstecki, P.; Fuerstman, M.J.; Stone, H.A.; Whitesides, G.M. Formation of droplets and bubbles in a microfluidic T-junction—Scaling and mechanism of break-up. Lab Chip 2006, 6, 437–446. [Google Scholar] [CrossRef]
- Jakiela, S.; Zaslona, J.; Michalski, J.A. Square wave driver for piezoceramic actuators. Actuators 2012, 1, 12–20. [Google Scholar] [CrossRef]
- Churski, K.; Nowacki, M.; Korczyk, P.M.; Garstecki, P. Simple modular systems for generation of droplets on demand. Lab Chip 2013, 13, 3689–3697. [Google Scholar] [CrossRef] [PubMed]
- Jakiela, S.; Kaminski, T.S.; Cybulski, O.; Weibel, D.B.; Garstecki, P. Bacterial growth and adaptation in microdroplet chemostats. Angew. Chem. Int. Ed. Engl. 2013, 52, 8908–8911. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Kürsten, D.; Schneider, S.; Knauer, A.; Günther, P.M.; Köhler, J.M. Uncovering toxicological complexity by multi-dimensional screenings in microsegmented flow: Modulation of antibiotic interference by nanoparticles. Lab Chip 2012, 12, 474–484. [Google Scholar] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakiela, S.; Debski, P.R.; Dabrowski, B.; Garstecki, P. Generation of Nanoliter Droplets on Demand at Hundred-Hz Frequencies. Micromachines 2014, 5, 1002-1011. https://doi.org/10.3390/mi5041002
Jakiela S, Debski PR, Dabrowski B, Garstecki P. Generation of Nanoliter Droplets on Demand at Hundred-Hz Frequencies. Micromachines. 2014; 5(4):1002-1011. https://doi.org/10.3390/mi5041002
Chicago/Turabian StyleJakiela, Slawomir, Pawel R. Debski, Bogdan Dabrowski, and Piotr Garstecki. 2014. "Generation of Nanoliter Droplets on Demand at Hundred-Hz Frequencies" Micromachines 5, no. 4: 1002-1011. https://doi.org/10.3390/mi5041002
APA StyleJakiela, S., Debski, P. R., Dabrowski, B., & Garstecki, P. (2014). Generation of Nanoliter Droplets on Demand at Hundred-Hz Frequencies. Micromachines, 5(4), 1002-1011. https://doi.org/10.3390/mi5041002





