Active Continuous-Flow Micromixer Using an External Braille Pin Actuator Array
Abstract
:1. Introduction
2. Material and Method
2.1. Principle of Braille Pin Controlled Continuous Micromixer

2.2. Chip Fabrication
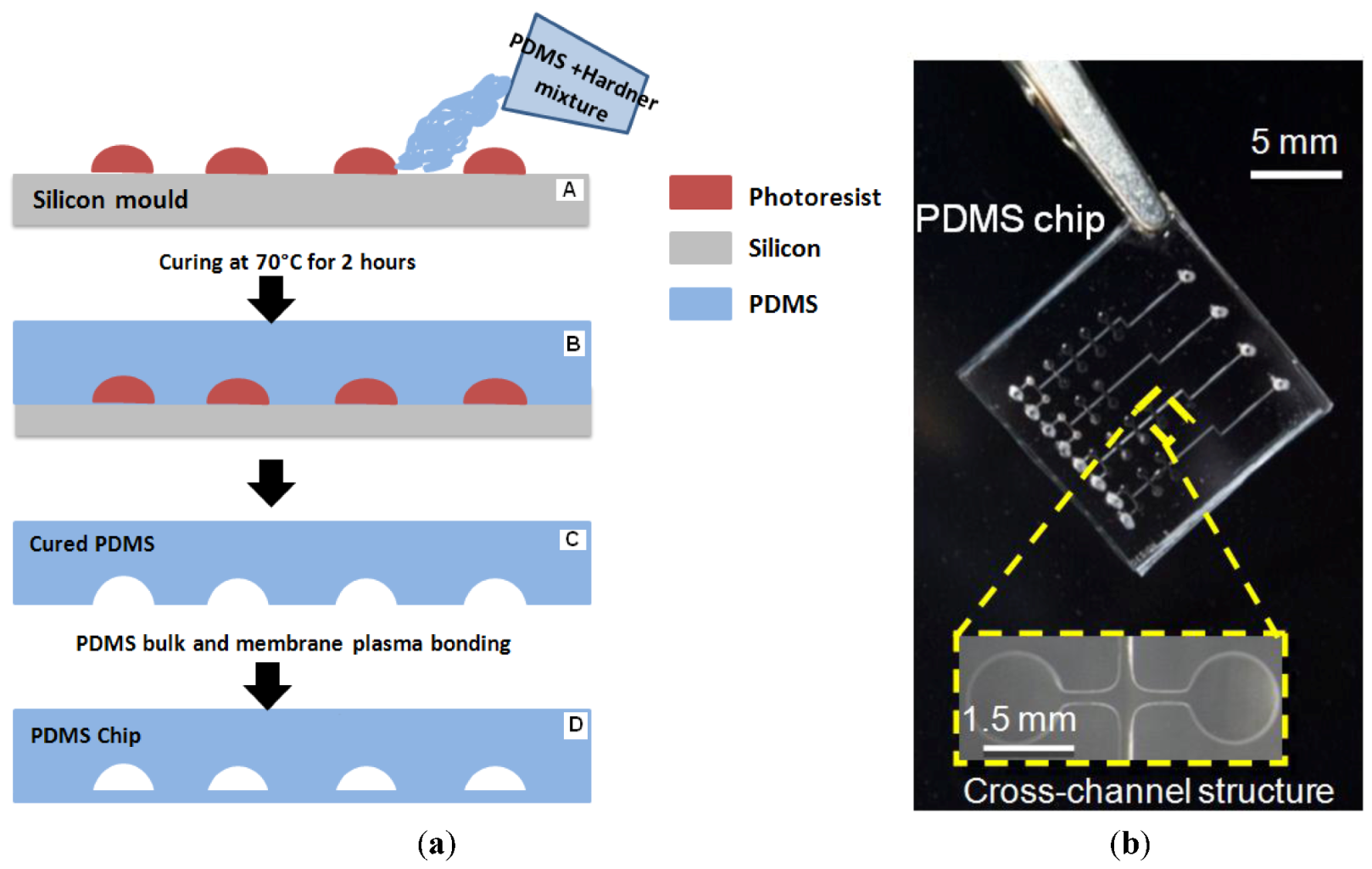
2.3. Braille Pin Actuator
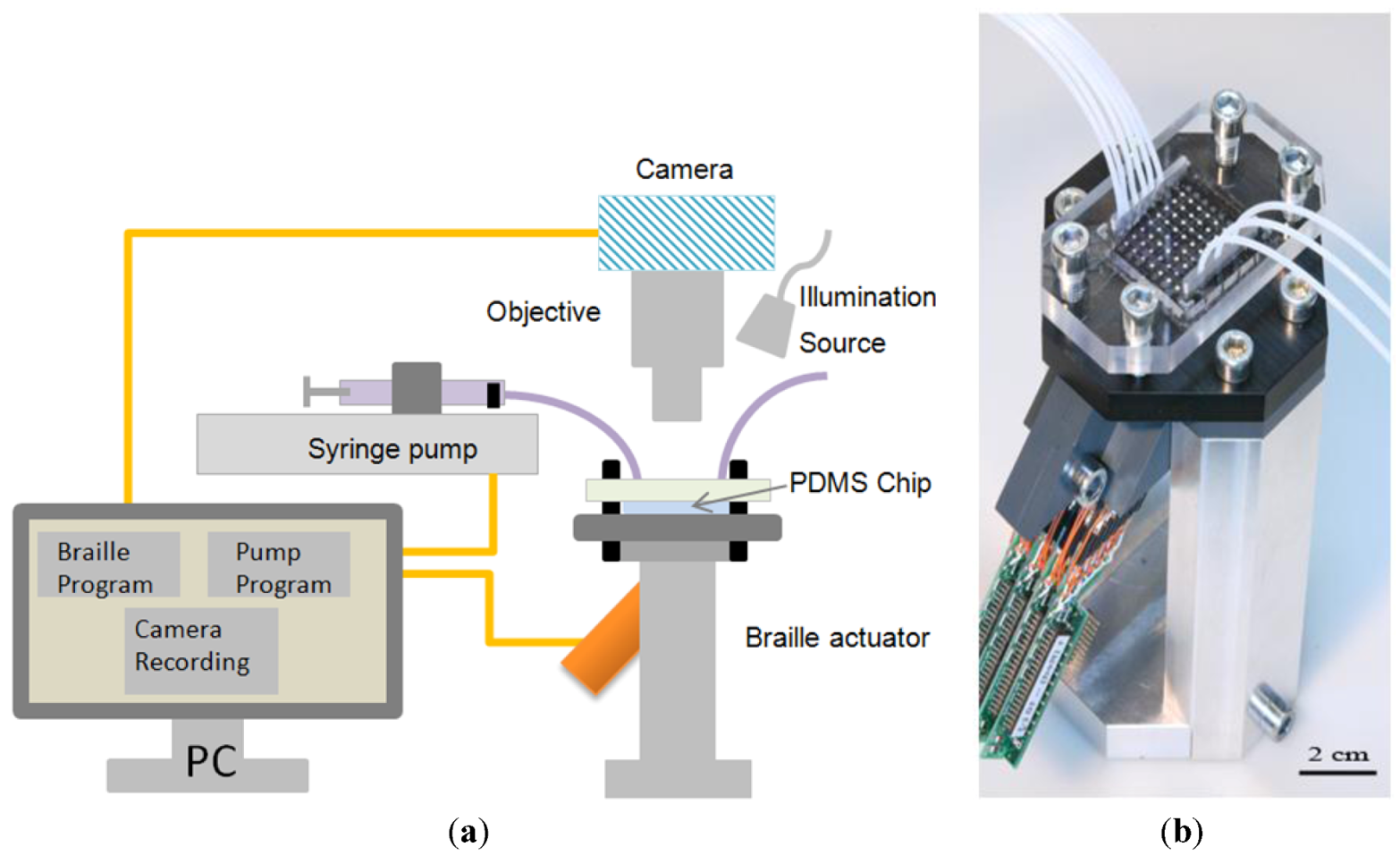
2.4. Experimental Setup
2.5. Mixing Quantification Technique

3. Results and Discussion
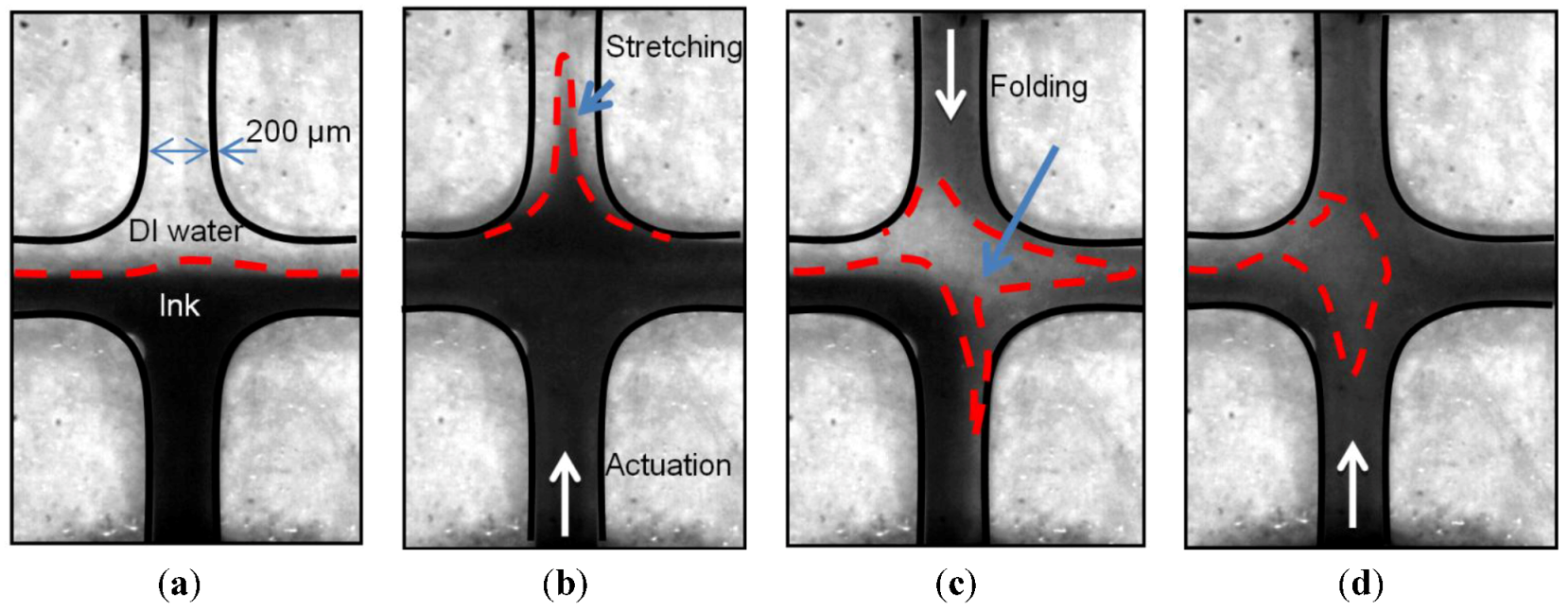
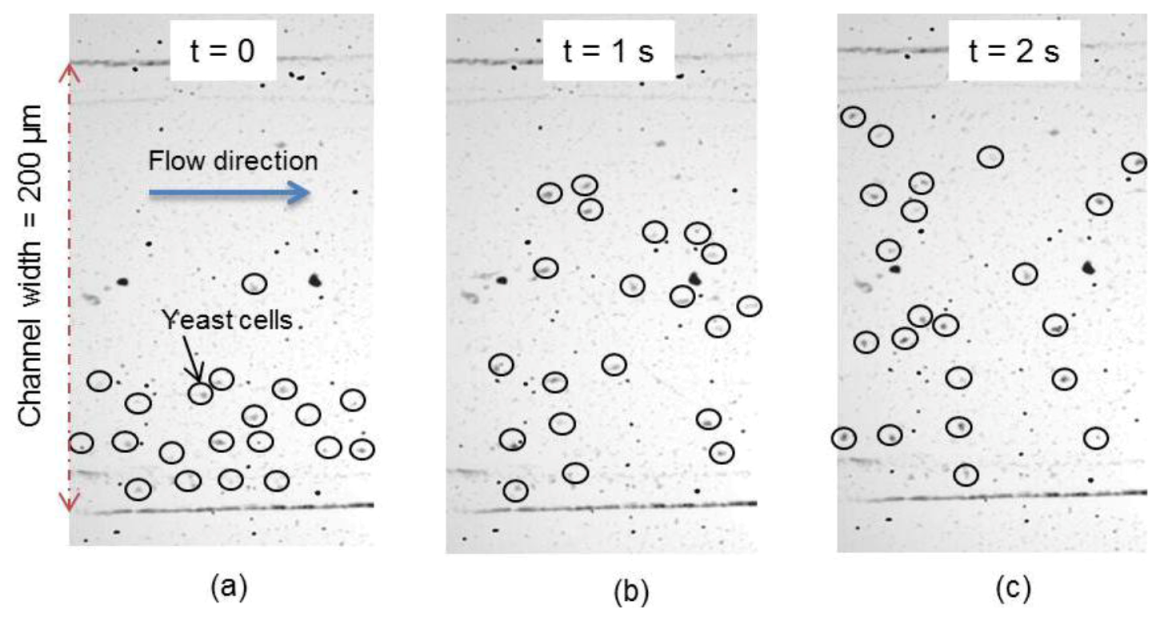
3.1. Qualitative Analysis
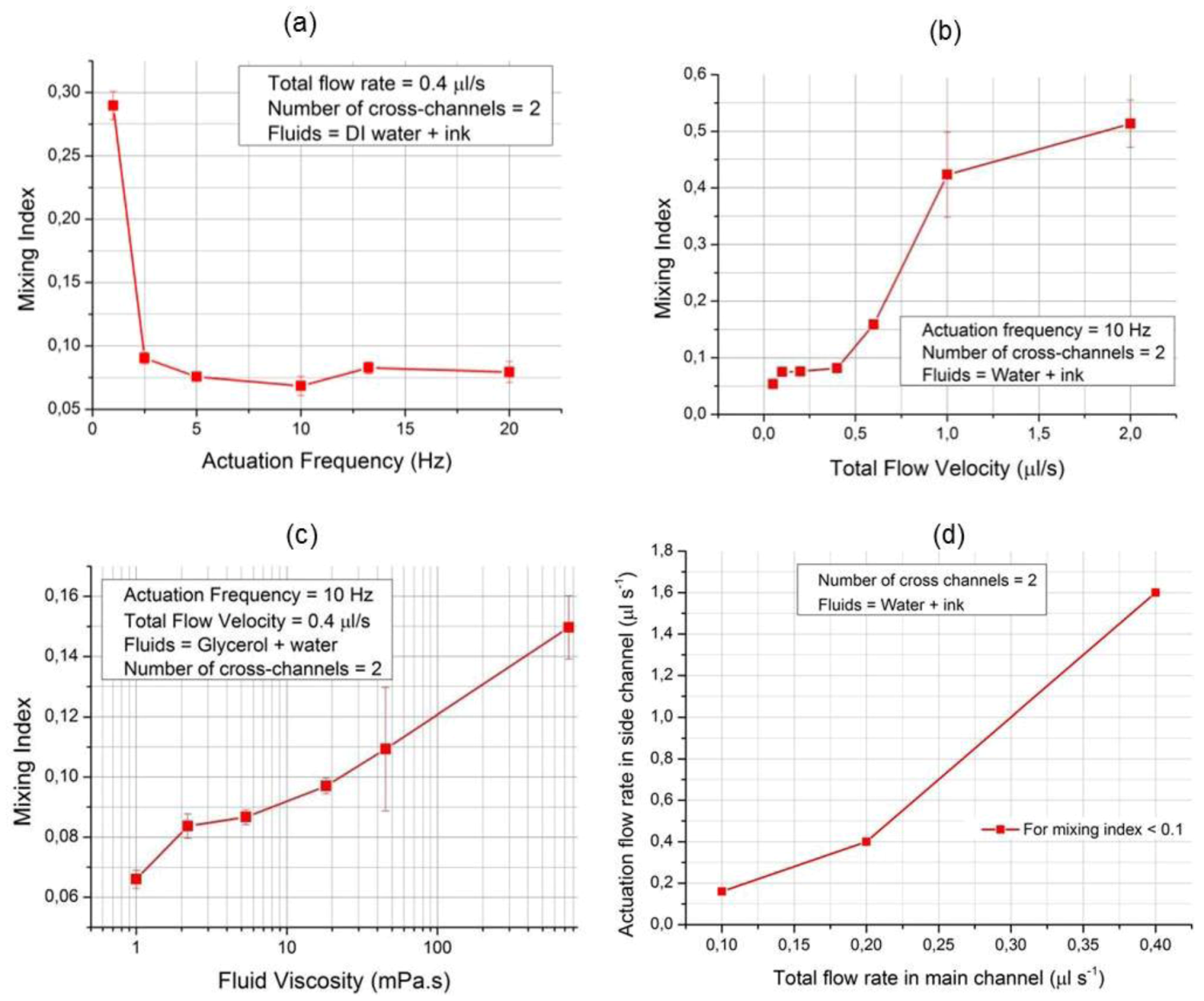
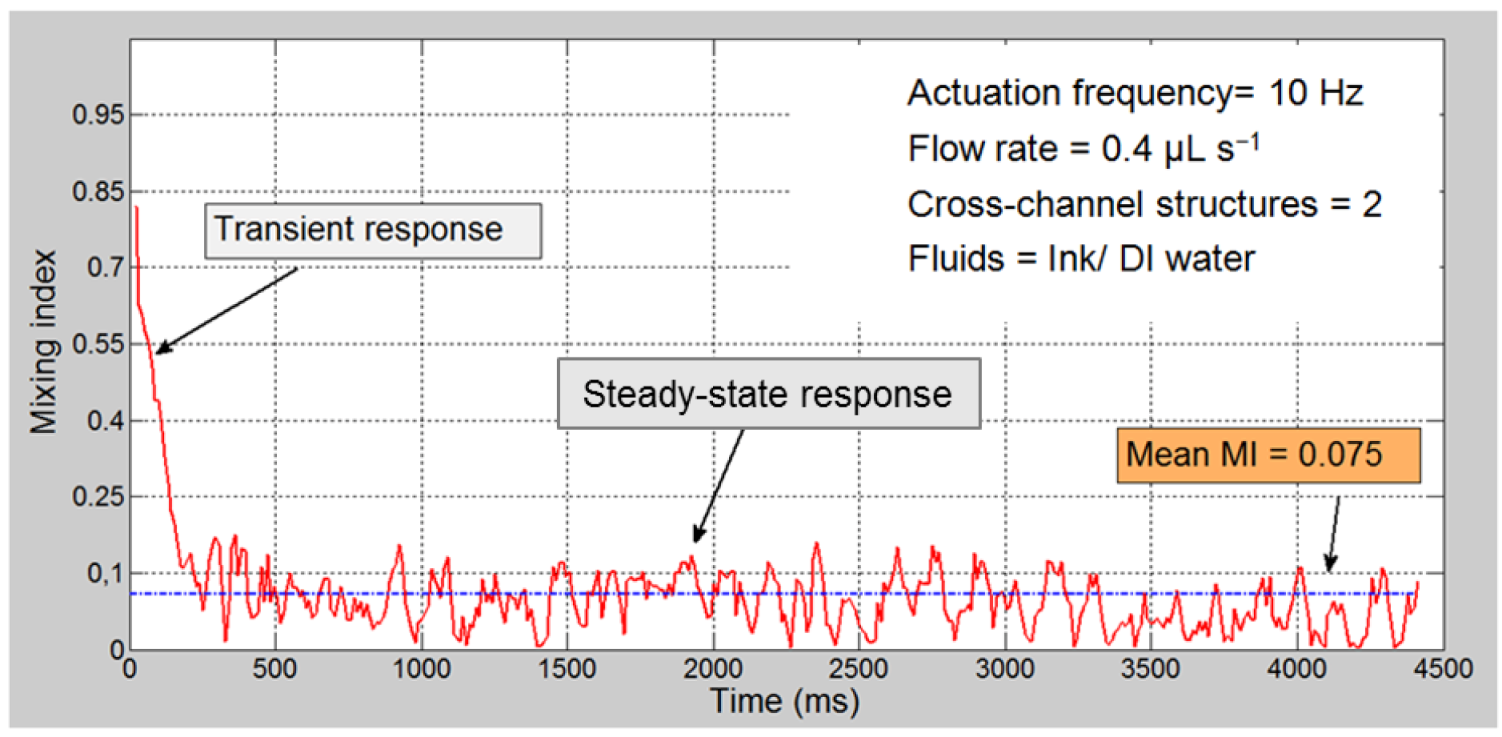
3.2. Quantitative Analysis
4. Conclusions
Acknowledgments
References
- Nguyen, N.-T. Micromixers, Fundamental, Design and Fabriaction, 2nd ed; Elsevier: Waltham, MA, USA, 2012. [Google Scholar]
- Jeong, G.S.; Chung, S.; Kim, C.B.; Lee, S.H. Applications of micromixing technology. Analyst 2010, 135, 460–473. [Google Scholar] [CrossRef]
- Nguyen, N.-T.; Wu, Z. Micromixers—a review. J. Micromech. Microeng. 2005, 15, R1–R16. [Google Scholar] [CrossRef]
- Tabeling, P.; Chabert, M.; Dodge, A.; Jullien, C.; Okkels, F. Chaotic mixing in cross-channel micromixers. Philos. Transact. A Math. Phys. Eng. Sci. 2004, 362, 987–1000. [Google Scholar] [CrossRef]
- Gouillart, E.; Dauchot, O.; Thiffeault, J.L. Measures of mixing quality in open flows with chaotic advection. Phys. Fluids 2011, 23. [Google Scholar] [CrossRef]
- Stroock, A.D.; Dertinger, S.K.W.; Ajdari, A.; Mezić, I.; Stone, H.A.; Whitesides, G.M. Chaotic mixer for micro channels. Science 2002, 295, 647–651. [Google Scholar] [CrossRef]
- Liu, R.H.; Stremler, M.A.; Sharp, K.V.; Olsen, M.G.; Santiago, J.G.; Adrian, R.J.; Aref, H.; Beebe, D.J. Passive mixing in a three-dimensional serpentine microchannel. J. Microelectromech. Syst. 2000, 9, 190–197. [Google Scholar] [CrossRef]
- Jen, C.P.; Wu, C.Y.; Lin, Y.C. Design and simulation of the micromixer with chaotic advection in twisted micro channels. Lab Chip 2003, 3, 77–81. [Google Scholar] [CrossRef]
- Xia, H.; Wan, S.; Shu, C.; Chew, Y. Chaotic micromixers using two-layer crossing channels to exhibit fast mixing at low Reynolds numbers. Lab Chip 2005, 5, 748–755. [Google Scholar] [CrossRef]
- Lu, L.H.; Ryu, K.S.; Liu, C. A magnetic microstirrer and array for microfluidic mixing. J. Microelectromech. Syst. 2002, 11, 462–469. [Google Scholar] [CrossRef]
- Tekin, H.C.; Sivagnanam, V.; Ciftlik, A.T.; Sayah, A.; Vandevyver, C.; Gijs, M.A.M. Chaotic mixing using source–sink microfluidic flows in a PDMS chip. Microfluid. Nanofluid. 2011, 10, 749–759. [Google Scholar] [CrossRef]
- Lee, Y.K.; Deval, J.; Tabeling, P.; Ho, C.M. Chaotic mixing in electrokinetically and pressure driven micro flows. In Proceedings of the 14th IEEE International Conference on Micro Electro Mechanical Systems, Interlaken, Switzerland, 21–25 January 2001; pp. 483–486.
- Gu, W.; Zhu, X.; Futai, N.; Cho, B.S.; Takayama, S. Computerized microfluidic cell culture using elastomeric channels and Braille displays. Proc. Natl. Acad. Sci. USA 2004, 101, 15861–15866. [Google Scholar]
- Erickson, B.E. Braille pins control microfluidic flow. Anal. Chem. 2005, 77, 93–93. [Google Scholar] [CrossRef]
- Duffy, D.C.; McDonald, J.C.; Schueller, O.J.A.; Whitesides, G.M. Rapid prototyping of microfluidic systems in poly (dimethylsiloxane). Anal. Chem. 1998, 70, 4974–4984. [Google Scholar] [CrossRef]
- Futai, N.; Gu, W.; Song, J.W.; Takayama, S. Handheld recirculation system and customized media for microfluidic cell culture. Lab Chip 2005, 6, 149–154. [Google Scholar]
- BioFluidix GmbH Web site. Available online: http://www.biofluidix.com (accessed on 3 November 2012).
- Mao, X.; Juluri, B.K.; Lapsley, M.I.; Stratton, Z.S.; Huang, T.J. Milliseconds microfluidic chaotic bubble mixer. Microfluid. Nanofluid. 2010, 8, 139–144. [Google Scholar] [CrossRef]
- Lange, H.; Taillandier, P.; Riba, J.P. Effect of high shear stress on microbial viability. J. Chem. Technol. Biotechnol. 2001, 76, 501–505. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Abbas, Y.; Miwa, J.; Zengerle, R.; Von Stetten, F. Active Continuous-Flow Micromixer Using an External Braille Pin Actuator Array. Micromachines 2013, 4, 80-89. https://doi.org/10.3390/mi4010080
Abbas Y, Miwa J, Zengerle R, Von Stetten F. Active Continuous-Flow Micromixer Using an External Braille Pin Actuator Array. Micromachines. 2013; 4(1):80-89. https://doi.org/10.3390/mi4010080
Chicago/Turabian StyleAbbas, Yawar, Junichi Miwa, Roland Zengerle, and Felix Von Stetten. 2013. "Active Continuous-Flow Micromixer Using an External Braille Pin Actuator Array" Micromachines 4, no. 1: 80-89. https://doi.org/10.3390/mi4010080
APA StyleAbbas, Y., Miwa, J., Zengerle, R., & Von Stetten, F. (2013). Active Continuous-Flow Micromixer Using an External Braille Pin Actuator Array. Micromachines, 4(1), 80-89. https://doi.org/10.3390/mi4010080




