Research Progress of Hyperfluorescent Organic Electroluminescent Devices
Abstract
1. Introduction
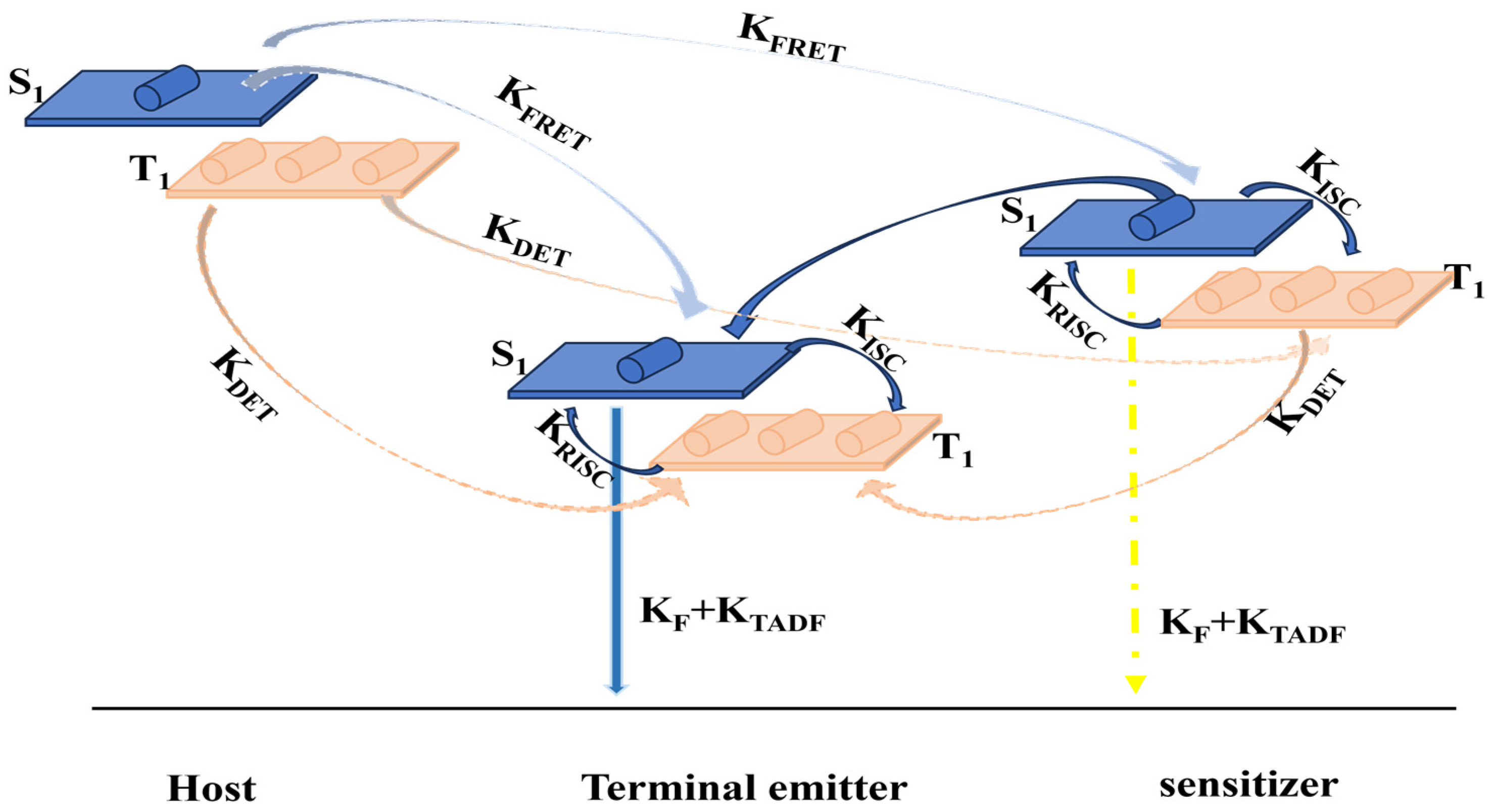
2. Development of Hyperfluorescent Organic Light-Emitting Diodes
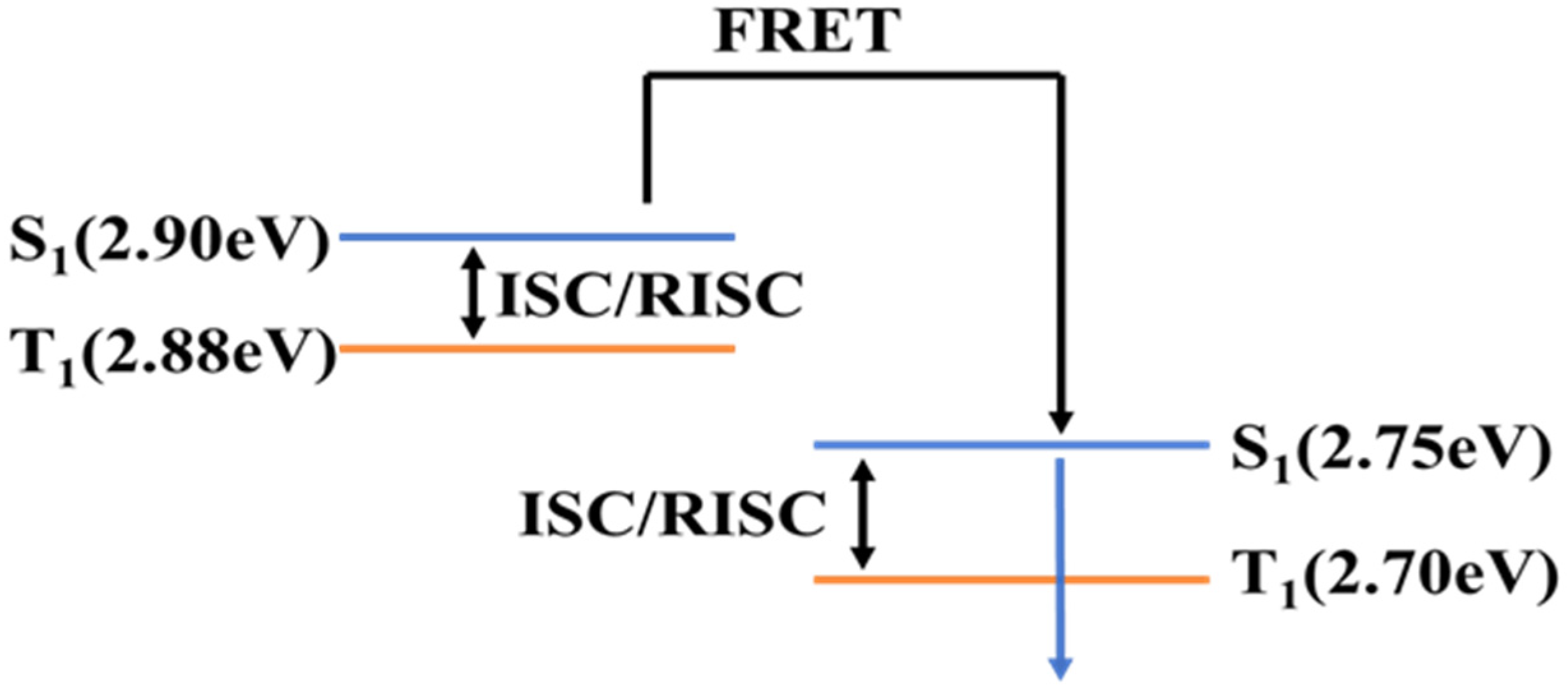
2.1. Blue Hyperfluorescent OLEDs Based on TADF-Sensitized Fluorescence
2.2. Green Hyperfluorescent OLEDs Based on TADF-Sensitized Fluorescence
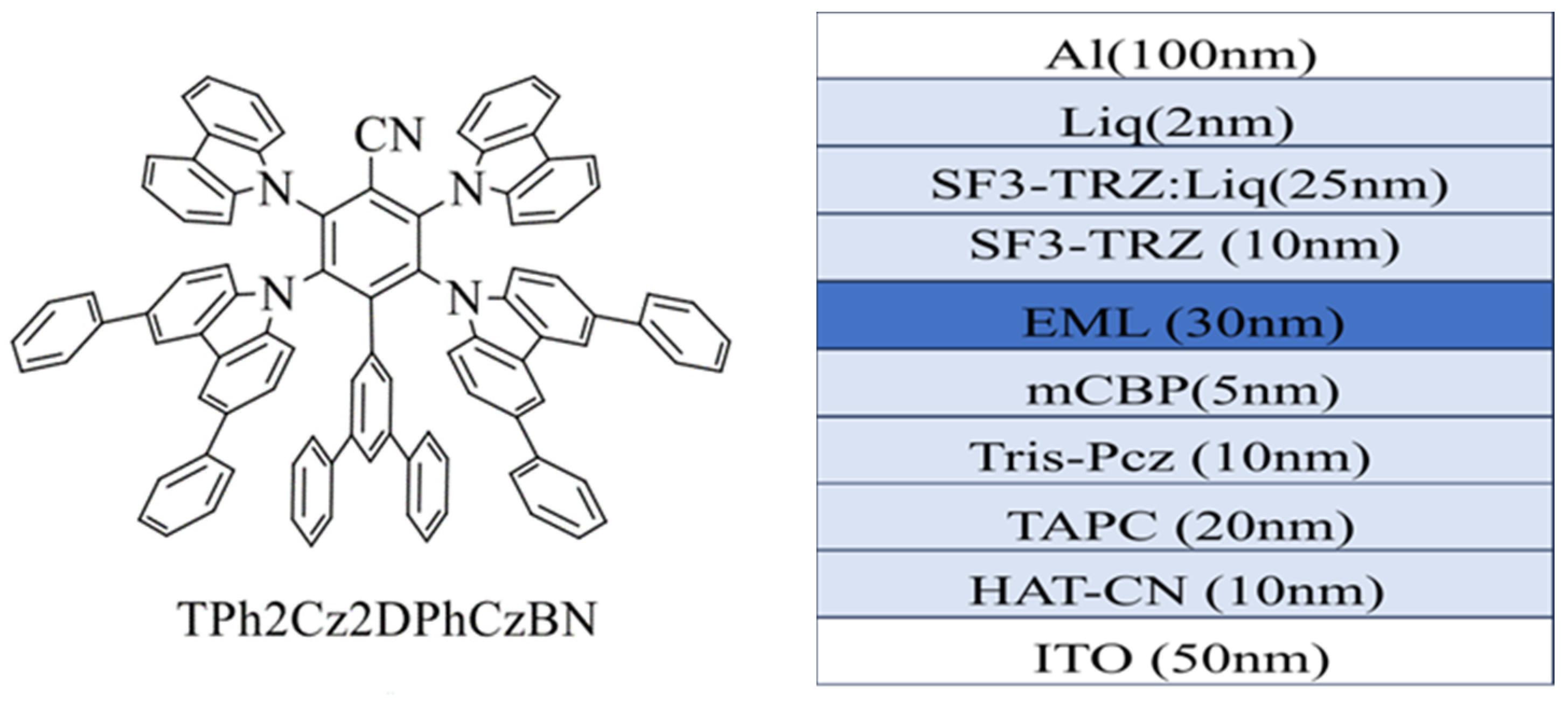
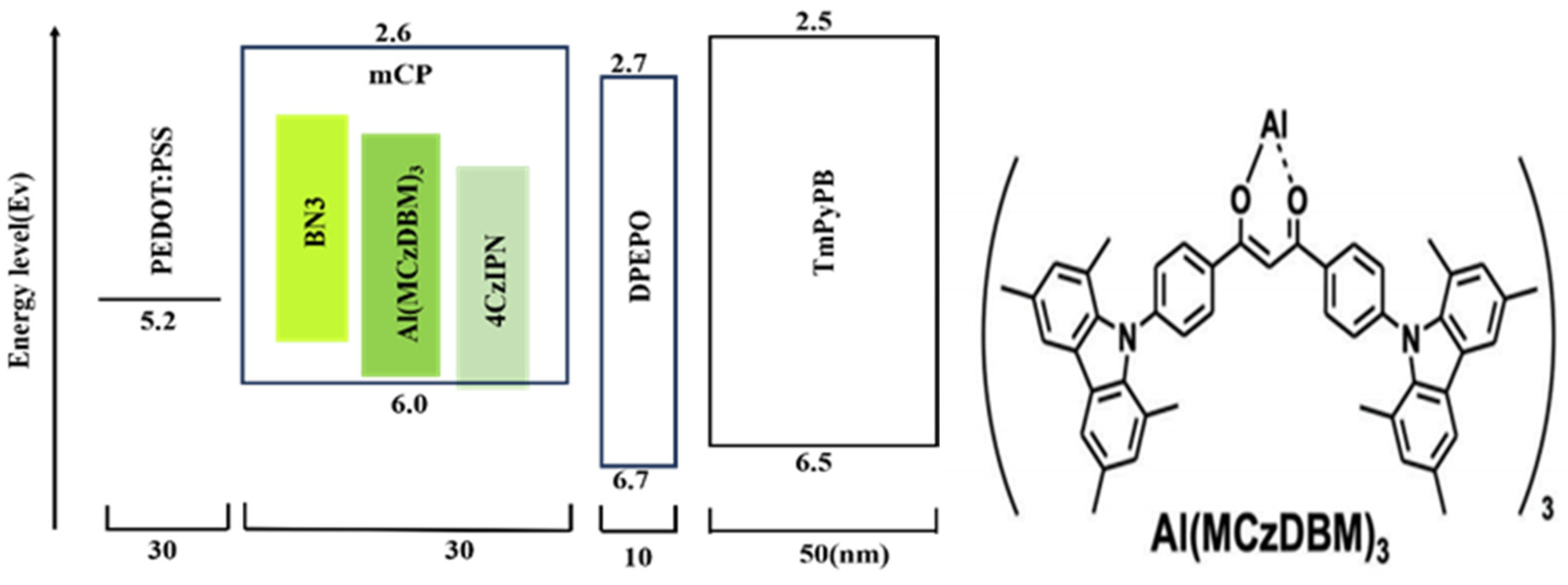
| Emitters | CE (cd A−1) a | PE (lm W−1) b | EQE (%) c | FWHM (nm) d | CIE d | Ref |
|---|---|---|---|---|---|---|
| 4CzIPN | 69.6/28.4 | 61.2/13.5 | 19.1/7.8 | 56.0 | (0.36,0.57) | [54] |
| Al(MCzDBM)3 | 83.4/66.8 | 71.6/15.8 | 21.6/9.3 | 45.0 | (0.39,0.58) | [54] |
| TTPA | 45.0/38.0 | 47.0/30.0 | 15.8/11.7 | 57.9 | (0.29,0.59) | [5] |
| m-Cz-BNCz | 57.4/27.7 | 53.7/12.8 | 15.7/7.1 | 38.0 | (0.28,0.65) | [57] |
| PhtBuPAD | 72.2/61.8 | 59.7/35.9 | 21.2/18.1 | 45.0 | (0.32,0.59) | [58] |
| 4CzIPN | -/- | -/- | 19.9/6.4 | 56.0 | (0.39,0.59) | [40] |
2.3. Red Hyperfluorescent OLEDs Based on TADF-Sensitized Fluorescence
2.4. White Hyperfluorescent OLEDs Based on TADF-Sensitized Fluorescence
3. The Future and Prospect of Hyperfluorescent Organic Light-Emitting Diodes
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Baldo, M.A.; O’Brien, D.F.; You, Y.; Shoustikov, A.; Sibley, S.; Thompson, M.E.; Forrest, S.R. Highly efficient phosphorescent emission from organic electroluminescent devices. Lett. Nat. 1998, 395, 151–154. [Google Scholar] [CrossRef]
- O’Brien, D.F.; Baldo, M.A.; Thompson, M.E.; Forrest, S.R. Improved energy transfer in electrophosphorescent devices. Appl. Phys. Lett. 1999, 74, 442–444. [Google Scholar] [CrossRef]
- Endo, A.; Sato, K.; Yoshimura, K.; Kai, T.; Kawada, A.; Miyazaki, H.; Adachi, C. Efficient up-conversion of triplet excitons into a singlet state and its application for organic light emitting diodes. Appl. Phys. Lett. 2011, 98, 083302. [Google Scholar] [CrossRef]
- Kim, J.U.; Park, I.S.; Chan, C.-Y.; Tanaka, M.; Tsuchiya, Y.; Nakanotani, H.; Adachi, C. Nanosecond-time-scale delayed fluorescence molecule for deep-blue OLEDs with small efficiency rolloff. Nat. Commun. 2020, 11, 1765. [Google Scholar] [CrossRef]
- Nakanotani, H.; Higuchi, T.; Furukawa, T.; Masui, K.; Morimoto, K.; Numata, M.; Tanaka, H.; Sagara, Y.; Yasuda, T.; Adachi, C. High-efficiency organic light-emitting diodes with fluorescent emitters. Nat. Commun. 2014, 5, 4016. [Google Scholar] [CrossRef]
- Zhang, D.; Duan, L.; Li, C.; Li, Y.; Li, H.; Zhang, D.; Qiu, Y. High-Efficiency Fluorescent Organic Light-Emitting Devices Using Sensitizing Hosts with a Small Singlet–Triplet Exchange Energy. Adv. Mater. 2014, 26, 5050–5055. [Google Scholar] [CrossRef] [PubMed]
- Adachi, J.; Kakizoe, H.; Tsang, P.K.D.; Endo, A. Hyperfluorescence;a Game Changing Technology of OLED Display. SID 2019, 50, 95–98. [Google Scholar] [CrossRef]
- Furukawa, T.; Nakanotani, H.; Inoue, M.; Adachi, C. Dual enhancement of electroluminescence efficiency and operational stability by rapid upconversion of triplet excitons in OLEDs. Sci. Rep. 2015, 5, 8429. [Google Scholar] [CrossRef]
- Byeon, S.Y.; Lee, D.R.; Yook, K.S.; Lee, J.Y. Recent Progress of Singlet-Exciton-Harvesting Fluorescent Organic Light-Emitting Diodes by Energy Transfer Processes. Adv. Mater. 2019, 31, 1803714. [Google Scholar] [CrossRef]
- Zhang, D.; Song, X.; Cai, M.; Duan, L. Blocking Energy-Loss Pathways for Ideal Fluorescent Organic Light-Emitting Diodes with Thermally Activated Delayed Fluorescent Sensitizers. Adv. Mater. 2017, 30, 1705250. [Google Scholar] [CrossRef]
- Song, W.; Lee, I.; Lee, J.Y. Host Engineering for High Quantum Efficiency Blue and White Fluorescent Organic Light-Emitting Diodes. Adv. Mater. 2015, 27, 4358–4363. [Google Scholar] [CrossRef]
- Kim, K.H.; Moon, C.K.; Sun, J.W.; Sim, B.; Kim, J.J. Triplet Harvesting by a Conventional Fluorescent Emitter Using Reverse Intersystem Crossing of Host Triplet Exciplex. Adv. Opt. Mater. 2015, 3, 895–899. [Google Scholar] [CrossRef]
- Ahn, D.H.; Jeong, J.H.; Song, J.; Lee, J.Y.; Kwon, J.H. Highly Efficient Deep Blue Fluorescent Organic Light-Emitting Diodes Boosted by Thermally Activated Delayed Fluorescence Sensitization. ACS Appl. Mater. Interfaces 2018, 10, 10246–10253. [Google Scholar] [CrossRef]
- Stavrou, K.; Franca, L.G.; Danos, A.; Monkman, A.P. Key requirements for ultraefficient sensitization in hyperfluorescence organic light-emitting diodes. Nat. Photonics 2024, 18, 554–561. [Google Scholar] [CrossRef]
- Kondo, Y.; Yoshiura, K.; Kitera, S.; Nishi, H.; Oda, S.; Gotoh, H.; Sasada, Y.; Yanai, M.; Hatakeyama, T. Narrowband deep-blue organic light-emitting diode featuring an organoboron-based emitter. Nat. Photonics 2019, 13, 678–682. [Google Scholar] [CrossRef]
- Sekitani, T.; Nakajima, H.; Maeda, H.; Fukushima, T.; Aida, T.; Hata, K.; Someya, T. Stretchable active-matrix organic light-emitting diode display using printable elastic conductors. Nat. Mater. 2009, 8, 494–499. [Google Scholar] [CrossRef]
- White, M.S.; Kaltenbrunner, M.; Głowacki, E.D.; Gutnichenko, K.; Kettlgruber, G.; Graz, I.; Aazou, S.; Ulbricht, C.; Egbe, D.A.M.; Miron, M.C.; et al. Ultrathin, highly flexible and stretchable PLEDs. Nat. Photonics 2013, 7, 811–816. [Google Scholar] [CrossRef]
- Chan, C.-Y.; Tanaka, M.; Lee, Y.-T.; Wong, Y.-W.; Nakanotani, H.; Hatakeyama, T.; Adachi, C. Stable pure-blue hyperfluorescence organic light-emitting diodes with high-efficiency and narrow emission. Nat. Photonics 2021, 15, 203–207. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, P.; Beratan, D.N. Predicting Dexter Energy Transfer Interactions from Molecular Orbital Overlaps. J. Phys. Chem. C 2020, 124, 18956–18960. [Google Scholar] [CrossRef]
- Dexter, D.L. A Theory of Sensitized Luminescence in Solids. J. Chem. Phys. 1953, 21, 836–850. [Google Scholar] [CrossRef]
- Wei, P.; Zhang, D.; Duan, L. Modulation of Förster and Dexter Interactions in Single-Emissive-Layer All-Fluorescent WOLEDs for Improved Efficiency and Extended Lifetime. Adv. Funct. Mater. 2019, 30, 1907083. [Google Scholar] [CrossRef]
- Zhao, G.; Zhou, R.; Zhang, G.; Chen, H.; Ma, D.; Tian, W.; Jiang, W.; Sun, Y. A periphery hindered strategy with a dopant and sensitizer for solution-processed red TSF-OLEDs with high color purity. J. Mater. Chem. C 2022, 10, 5230–5239. [Google Scholar] [CrossRef]
- Jung, Y.H.; Karthik, D.; Lee, H.; Maeng, J.H.; Yang, K.J.; Hwang, S.; Kwon, J.H. A New BODIPY Material for Pure Color and Long Lifetime Red Hyperfluorescence Organic Light-Emitting Diode. ACS Appl. Mater. Interfaces 2021, 13, 17882–17891. [Google Scholar] [CrossRef]
- Xie, W.; Peng, X.; Li, M.; Qiu, W.; Li, W.; Gu, Q.; Jiao, Y.; Chen, Z.; Gan, Y.; Liu, K.k.; et al. Blocking the Energy Loss of Dexter Energy Transfer in Hyperfluorescence OLEDs Via One-Step Phenyl-Fluorene Substitution of TADF Assistant Host. Adv. Opt. Mater. 2022, 10, 2200665. [Google Scholar] [CrossRef]
- Shin, D.J.; Kim, S.C.; Lee, J.Y. A reverse intersystem crossing managing assistant dopant for high external quantum efficiency red organic light-emitting diodes. J. Mater. Chem. C 2022, 10, 4821–4830. [Google Scholar] [CrossRef]
- Lee, H.; Braveenth, R.; Muruganantham, S.; Jeon, C.Y.; Lee, H.S.; Kwon, J.H. Efficient pure blue hyperfluorescence devices utilizing quadrupolar donor-acceptor-donor type of thermally activated delayed fluorescence sensitizers. Nat. Commun. 2023, 14, 419. [Google Scholar] [CrossRef]
- Lee, J.; Shin, D.J.; Jo, U.; Lee, J.Y. Reverse Intersystem Crossing Boosting Sensitizer for Ultra-High Efficiency Blue Organic Light-Emitting Diode. ACS Appl. Mater. Interfaces 2024, 16, 43786–43794. [Google Scholar] [CrossRef]
- Wang, C.; Song, X.; Li, W.; Tang, X.; Ng, M.; Chen, S.S.; Tang, W.K.; Chen, W.C.; Huo, Y.; Tang, M.C. Deuterated Multiple-Resonance Thermally Activated Delayed Fluorescence Emitter and Their Application in Vacuum-Deposited Organic Light-Emitting Diodes. Adv. Opt. Mater. 2024, 12, 2401391. [Google Scholar] [CrossRef]
- Yuan, W.; Huang, T.; Zhou, J.; Tang, M.-C.; Zhang, D.; Duan, L. High-efficiency and long-lifetime deep-blue phosphorescent OLEDs using deuterated exciplex-forming host. Nat. Commun. 2025, 16, 4446. [Google Scholar] [CrossRef]
- Chen, Y.-K.; Lei, J.; Liu, P.-C.; Lin, C.-H.; Chen, Y.-M.; Chang, W.-S.; Chen, I.C.; Hsu, L.-Y.; Wu, T.-L. Unveiling the Evolution of Afterglow in Diboraanthracene Scaffolds: From Thermally Activated Delayed Fluorescence to Room-Temperature Phosphorescence. J. Am. Chem. Soc. 2025, 147, 45603–45617. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Cheung, W.-L.; Li, S.-j.; Wang, M.; Li, W.; Wang, C.; Song, X.; Wei, G.; Song, Q.; Chen, S.S.; et al. Enhancing operational stability of OLEDs based on subatomic modified thermally activated delayed fluorescence compounds. Nat. Commun. 2023, 14, 6481. [Google Scholar] [CrossRef]
- Liu, M.; Li, C.; Duan, L.; Zhang, D. Recent Advances in Blue Multiple-Resonance Thermally Activated Delayed Fluorescence Materials and their Applications in Organic Light-Emitting Diodes. Adv. Opt. Mater. 2025, e03140. [Google Scholar] [CrossRef]
- Liu, X.; Chan, C.-Y.; Mathevet, F.; Mamada, M.; Tsuchiya, Y.; Lee, Y.-T.; Nakanotani, H.; Kobayashi, S.; Shiochi, M.; Adachi, C. Isotope Effect of Host Material on Device Stability of Thermally Activated Delayed Fluorescence Organic Light-Emitting Diodes. Small Sci. 2021, 1, 2000057. [Google Scholar] [CrossRef] [PubMed]
- Adachi, C. Third-generation organic electroluminescence materials. Jpn. J. Appl. Phys. 2014, 53, 060101. [Google Scholar] [CrossRef]
- Im, Y.; Kim, M.; Cho, Y.J.; Seo, J.-A.; Yook, K.S.; Lee, J.Y. Molecular Design Strategy of Organic Thermally Activated Delayed Fluorescence Emitters. Chem. Mater. 2017, 29, 1946–1963. [Google Scholar] [CrossRef]
- Wong, M.Y.; Zysman-Colman, E. Purely Organic Thermally Activated Delayed Fluorescence Materials for Organic Light-Emitting Diodes. Adv. Mater. 2017, 29, 1605444. [Google Scholar] [CrossRef]
- Kaji, H.; Suzuki, H.; Fukushima, T.; Shizu, K.; Suzuki, K.; Kubo, S.; Komino, T.; Oiwa, H.; Suzuki, F.; Wakamiya, A.; et al. Purely organic electroluminescent material realizing 100% conversion from electricity to light. Nat. Commun. 2015, 6, 8476. [Google Scholar] [CrossRef]
- Hatakeyama, T.; Shiren, K.; Nakajima, K.; Nomura, S.; Nakatsuka, S.; Kinoshita, K.; Ni, J.; Ono, Y.; Ikuta, T. Ultrapure Blue Thermally Activated Delayed Fluorescence Molecules: Efficient HOMO–LUMO Separation by the Multiple Resonance Effect. Adv. Mater. 2016, 28, 2777–2781. [Google Scholar] [CrossRef]
- Kim, H.J.; Yasuda, T. Narrowband Emissive Thermally Activated Delayed Fluorescence Materials. Adv. Opt. Mater. 2022, 10, 2201714. [Google Scholar] [CrossRef]
- Naveen, K.R.; Yang, H.I.; Kwon, J.H. Double boron-embedded multiresonant thermally activated delayed fluorescent materials for organic light-emitting diodes. Commun. Chem. 2022, 5, 149. [Google Scholar] [CrossRef]
- Hamze, R.; Peltier, J.L.; Sylvinson, D.; Jung, M.; Cardenas, J.; Haiges, R.; Soleilhacoup, M.; Jazzar, R.; Djurovich, P.I.; Bertrand, G.; et al. Eliminating nonradiative decay in Cu(I) emitters:>99% quantum efficiency and microsecond lifetime. Res. Artic. 2019, 363, 601–606. [Google Scholar]
- Leitl, M.J.; Krylova, V.A.; Djurovich, P.I.; Thompson, M.E.; Yersin, H. Phosphorescence versus Thermally Activated Delayed Fluorescence. Controlling Singlet–Triplet Splitting in Brightly Emitting and Sublimable Cu(I) Compounds. J. Am. Chem. Soc. 2014, 136, 16032–16038. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L.; Ying, A.; Qi, Y.; Wu, K.; Tang, Y.; Tan, Y.; Zou, Y.; Xie, G.; Gong, S.; Yang, C. Copper(I) Complex as Sensitizer Enables High-Performance Organic Light-Emitting Diodes with Very Low Efficiency Roll-Off. Adv. Funct. Mater. 2021, 31, 2106345. [Google Scholar] [CrossRef]
- Tang, R.; Xu, S.; Lam, T.L.; Cheng, G.; Du, L.; Wan, Q.; Yang, J.; Hung, F.F.; Low, K.H.; Phillips, D.L.; et al. Highly Robust CuI-TADF Emitters for Vacuum-Deposited OLEDs with Luminance up to 222 200 cd m−2 and Device Lifetimes (LT90) up to 1300 hours at an Initial Luminance of 1000 cd m−2. Angew. Chem. Int. Ed. 2022, 61, e202203982. [Google Scholar] [CrossRef]
- Sakai, Y.; Sagara, Y.; Nomura, H.; Nakamura, N.; Suzuki, Y.; Miyazaki, H.; Adachi, C. Zinc complexes exhibiting highly efficient thermally activated delayed fluorescence and their application to organic light-emitting diodes. Chem. Commun. 2015, 51, 3181–3184. [Google Scholar] [CrossRef]
- Jia, J.-H.; Liang, D.; Yu, R.; Chen, X.-L.; Meng, L.; Chang, J.-F.; Liao, J.-Z.; Yang, M.; Li, X.-N.; Lu, C.-Z. Coordination-Induced Thermally Activated Delayed Fluorescence: From Non-TADF Donor–Acceptor-Type Ligand to TADF-Active Ag-Based Complexes. Chem. Mater. 2019, 32, 620–629. [Google Scholar] [CrossRef]
- Nakao, K.; Sasabe, H.; Shibuya, Y.; Matsunaga, A.; Katagiri, H.; Kido, J. Novel Series of Mononuclear Aluminum Complexes for High-Performance Solution-Processed Organic Light-Emitting Devices. Angew. Chem. Int. Ed. 2021, 60, 6036–6041. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.-C.; Wang, K.; Shi, Y.-Z.; Cheng, Y.-C.; Lee, Y.-T.; Yu, J.; Chen, X.-K.; Adachi, C.; Zhang, X.-H. Ultrapure green organic light-emitting diodes based on highly distorted fused π-conjugated molecular design. Nat. Photonics 2023, 17, 280–285. [Google Scholar] [CrossRef]
- Hiroki, U.; Kenichi, G.; Katsuyuki, S.; Hiroko, N. Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 2012, 492, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Ishimatsu, R.; Kirino, Y.; Adachi, C.; Nakano, K.; Imato, T. Quenching Behavior of Thermally Activated Delayed Fluorescence from a Donor–Acceptor Molecule, 1,2,3,5-Tetrakis(carbazol-9-yl)-4,6-dicyanobenzene by O2. Chem. Lett. 2016, 45, 1183–1185. [Google Scholar] [CrossRef]
- Noda, H.; Chen, X.-K.; Nakanotani, H.; Hosokai, T.; Miyajima, M.; Notsuka, N.; Kashima, Y.; Brédas, J.-L.; Adachi, C. Critical role of intermediate electronic states for spin-flip processes in charge-transfer-type organic molecules with multiple donors and acceptors. Nat. Mater. 2019, 18, 1084–1090. [Google Scholar] [CrossRef]
- Jeon, S.O.; Lee, K.H.; Kim, J.S.; Ihn, S.-G.; Chung, Y.S.; Kim, J.W.; Lee, H.; Kim, S.; Choi, H.; Lee, J.Y. High-efficiency, long-lifetime deep-blue organic light-emitting diodes. Nat. Photonics 2021, 15, 208–215. [Google Scholar] [CrossRef]
- Monkman, A. Why Do We Still Need a Stable Long Lifetime Deep Blue OLED Emitter? ACS Appl. Mater. Interfaces 2021, 14, 20463–20467. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, K.; Sasabe, H.; Chiba, Y.; Yoshida, N.; Nakamura, T.; Nagasawa, K.; Sayama, Y.; Katagiri, H.; Kido, J. Ubiquitous Aluminum(III)-TADF Complex as Sensitizer for Highly Efficient Solution-Processed Hyperfluorescent OLEDs with Narrow Emission. Adv. Opt. Mater. 2024, 12, 2303303. [Google Scholar] [CrossRef]
- Samanta, P.K.; Kim, D.; Coropceanu, V.; Brédas, J.-L. Up-Conversion Intersystem Crossing Rates in Organic Emitters for Thermally Activated Delayed Fluorescence: Impact of the Nature of Singlet vs Triplet Excited States. J. Am. Chem. Soc. 2017, 139, 4042–4051. [Google Scholar] [CrossRef]
- Nemma, H.; Kori, Y.; Meguro, N.; Mimura, R.; Chiba, Y.; Kido, J.; Sasabe, H. π-Extended Phenoxazine-Based Asymmetric MR-TADF Emitters for Narrow Emission Band Green OLEDs with a Power Efficiency of 150 lm W−1, an EQE of 27%, and an LT95 of Over 3000 h at 1000 cdm−2. Adv. Opt. Mater. 2024, 13, 2402131. [Google Scholar] [CrossRef]
- Yan, L.; Chen, B.; Wang, D.; Su, N.; Zhao, L.; Wang, S.; Ding, J. Efficient solution-processed narrowband green-emitting organic light emitting diodes sensitized by a thermally activated delayed fluorescence polymer. J. Mater. Chem. C 2024, 12, 16827–16833. [Google Scholar] [CrossRef]
- Yan, L.; Su, N.; Yang, Y.; Li, X.; Sun, J.; Wang, S.; Zhao, L.; Ding, L.; Ding, J. TADF polymer enables over 20% EQE in solution-processed green fluorescent OLEDs. SmartMat 2024, 5, e1272. [Google Scholar] [CrossRef]
- Englman, R.; Jortner, J. The energy gap law for radiationless transitions in large molecules. Mol. Phys. 1970, 18, 145–164. [Google Scholar] [CrossRef]
- Wilson, J.S.; Chawdhury, N.; Al-Mandhary, M.R.A.; Younus, M.; Khan, M.S.; Raithby, P.R.; Kohler, A.; Friend, R.H. The Energy Gap Law for Triplet States in Pt-Comtaining Conjugated Polymers and Monomers. J. Am. Chem. Soc. 2001, 123, 9412–9417. [Google Scholar] [CrossRef]
- Caspar, J.V.; Kober, E.M.; Sullivan, B.P.; Meyer, T.J. Application of the Energy Gap Law to the Decay of Charge-Transfer Excited States. J. Am. Chem. Soc. 1980, 53, 2201–2204. [Google Scholar] [CrossRef]
- Zeng, W.; Zhou, T.; Ning, W.; Zhong, C.; He, J.; Gong, S.; Xie, G.; Yang, C. Realizing 22.5% External Quantum Efficiency for Solution-Processed Thermally Activated Delayed-Fluorescence OLEDs with Red Emission at 622 nm via a Synergistic Strategy of Molecular Engineering and Host Selection. Adv. Mater. 2019, 31, 1901404. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.X.; Tao, W.W.; Chen, W.C.; Xiao, Y.F.; Wang, K.; Cao, C.; Yu, J.; Li, S.; Geng, F.X.; Adachi, C.; et al. Red/Near-Infrared Thermally Activated Delayed Fluorescence OLEDs with Near 100 % Internal Quantum Efficiency. Angew. Chem. Int. Ed. 2019, 58, 14660–14665. [Google Scholar] [CrossRef]
- Sen, W.; Hu, Y.-N.; Wang, J.; Sun, D.; Wang, K.; Zhang, X.-H.; Zysman-Colman, E. Efficient orange organic light-emitting diodes employing a central aniline bridged multiresonant thermally activated delayed fluorescence emitter. J. Mater. Chem. C 2024, 12, 6177–6184. [Google Scholar] [CrossRef]
- Hung, C.-M.; Wang, S.-F.; Chao, W.-C.; Li, J.-L.; Chen, B.-H.; Lu, C.-H.; Tu, K.-Y.; Yang, S.-D.; Hung, W.-Y.; Chi, Y.; et al. High-performance near-infrared OLEDs maximized at 925 nm and 1022 nm through interfacial energy transfer. Nat. Commun. 2024, 15, 4664. [Google Scholar] [CrossRef]
- Zampetti, A.; Minotto, A.; Squeo, B.M.; Gregoriou, V.G.; Allard, S.; Scherf, U.; Chochos, C.L.; Cacialli, F. Highly Efficient Solid-State Near-infrared Organic Light-Emitting Diodes incorporating A-D-A Dyes based on α,β-unsubstituted “BODIPY” Moieties. Sci. Rep. 2017, 7, 1611. [Google Scholar] [CrossRef] [PubMed]
- Squeo, B.M.; Pasini, M. BODIPY platform: A tunable tool for green to NIR OLEDs. Supramol. Chem. 2019, 32, 56–70. [Google Scholar] [CrossRef]
- Baysec, S.; Minotto, A.; Klein, P.; Poddi, S.; Zampetti, A.; Allard, S.; Cacialli, F.; Scherf, U. Tetraphenylethylene-BODIPY aggregation-induced emission luminogens for near-infrared polymer light-emitting diodes. Sci. China Chem. 2018, 61, 932–939. [Google Scholar] [CrossRef]
- Song, X.; Zhang, D.; Zhang, Y.; Lu, Y.; Duan, L. Strategically Modulating Carriers and Excitons for Efficient and Stable Ultrapure-Green Fluorescent OLEDs with a Sterically Hindered BODIPY Dopant. Adv. Opt. Mater. 2020, 8, 2000483. [Google Scholar] [CrossRef]
- Yoon, S.J.; Kim, J.H.; Chung, W.J.; Lee, J.Y. Design Approach of Lifetime Extending Thermally Activated Delayed Fluorescence Sensitizers for Highly Efficient Fluorescence Devices. Chem.-A Eur. J. 2021, 27, 3065–3073. [Google Scholar] [CrossRef]
- Chen, D.; Cai, X.; Li, X.-L.; He, Z.; Cai, C.; Chen, D.; Su, S.-J. Efficient solution-processed red all-fluorescent organic light-emitting diodes employing thermally activated delayed fluorescence materials as assistant hosts: Molecular design strategy and exciton dynamic analysis. J. Mater. Chem. C 2017, 5, 5223–5231. [Google Scholar] [CrossRef]
- Wang, Y.-K.; Huang, C.-C.; Kumar, S.; Li, S.-H.; Dong, Z.-L.; Fung, M.-K.; Jiang, Z.-Q.; Liao, L.-S. Thermally activated delayed fluorescence sensitizer for D–A–A type emitters with orange-red light emission. J. Mater. Chem. C 2018, 6, 10030–10035. [Google Scholar] [CrossRef]
- Li, Z.; Hu, X.; Liu, G.; Tian, L.; Gao, H.; Dong, X.; Gao, T.; Cao, M.; Lee, C.-S.; Wang, P.; et al. High-Efficiency Red-Fluorescent Organic Light-Emitting Diodes with Excellent Color Purity. J. Phys. Chem. C 2021, 125, 1980–1989. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, K.H.; Lee, J.Y. Design of Thermally Activated Delayed Fluorescent Assistant Dopants to Suppress the Nonradiative Component in Red Fluorescent Organic Light-Emitting Diodes. Chem.–A Eur. J. 2019, 25, 9060–9070. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, N.; Shikita, S.; Yasuda, T. Spin-Dependent Exciton Funneling to a Dendritic Fluorophore Mediated by a Thermally Activated Delayed Fluorescence Material as an Exciton-Harvesting Host. Chem. Mater. 2017, 29, 7014–7022. [Google Scholar] [CrossRef]
- Jang, J.S.; Han, S.H.; Choi, H.W.; Yook, K.S.; Lee, J.Y. Molecular design of sensitizer to suppress efficiency loss mechanism in hyper-fluorescent organic light-emitting diodes. Org. Electron. 2018, 59, 236–242. [Google Scholar] [CrossRef]
- Cui, L.-S.; Ruan, S.-B.; Bencheikh, F.; Nagata, R.; Zhang, L.; Inada, K.; Nakanotani, H.; Liao, L.-S.; Adachi, C. Long-lived efficient delayed fluorescence organic light-emitting diodes using n-type hosts. Nat. Commun. 2017, 8, 2250. [Google Scholar] [CrossRef]
- Cui, L.S.; Xie, Y.M.; Wang, Y.K.; Zhong, C.; Deng, Y.L.; Liu, X.Y.; Jiang, Z.Q.; Liao, L.S. Pure Hydrocarbon Hosts for ≈100% Exciton Harvesting in Both Phosphorescent and Fluorescent Light-Emitting Devices. Adv. Mater. 2015, 27, 4213–4217. [Google Scholar] [CrossRef]
- Nakagawa, T.; Ku, S.-Y.; Wong, K.-T.; Adachi, C. Electroluminescence based on thermally activated delayed fluorescence generated by a spirobifluorene donor–acceptor structure. Chem. Commun. 2012, 48, 9580–9582. [Google Scholar] [CrossRef]
- Feng, Q.; Zheng, X.; Wang, H.; Zhang, H.; Qian, Y.; Tan, K.; Cao, H.; Xie, L.; Huang, W. A 9-fluorenyl substitution strategy for aromatic-imide-based TADF emitters towards efficient and stable sky blue OLEDs with nearly 30% external quantum efficiency. Mater. Adv. 2021, 2, 4000–4008. [Google Scholar] [CrossRef]
- Feng, Q.; Tan, K.; Zheng, X.; Xie, S.; Xue, K.; Bo, Y.; Zhang, H.; Lin, D.; Rao, J.; Xie, X.; et al. Simultaneous and Significant Improvements in Efficiency and Stability of Deep-Blue Organic Light Emitting Diodes through Friedel-Crafts Arylmethylation of a Fluorophore. ChemPhotoChem 2020, 4, 321–326. [Google Scholar] [CrossRef]
- Song, W.; Yook, K.S. Hyperfluorescence-based full fluorescent white organic light-emitting diodes. J. Ind. Eng. Chem. 2018, 61, 445–448. [Google Scholar] [CrossRef]
- Gao, T.; Wang, H.; Chen, Z.; Wang, Z.; Zhou, M.; Zhao, B.; Chen, F.; Zou, Q. Performance improved white full-organic light emitting diodes (WOLED) based on tricolor dual-layer of hyperfluorescence (HF). Org. Electron. 2023, 120, 106845. [Google Scholar] [CrossRef]
- Khan, F.; Urbonas, E.; Volyniuk, D.; Grazulevicius, J.V.; Mobin, S.M.; Misra, R. White hyperelectrofluorescence from solution-processable OLEDs based on phenothiazine substituted tetraphenylethylene derivatives. J. Mater. Chem. C 2020, 8, 13375–13388. [Google Scholar] [CrossRef]
- Hong, G.; Gan, X.; Leonhardt, C.; Zhang, Z.; Seibert, J.; Busch, J.M.; Bräse, S. A Brief History of OLEDs—Emitter Development and Industry Milestones. Adv. Mater. 2021, 33, 2005630. [Google Scholar] [CrossRef]
- Chen, G.; Miao, J.; Huang, X.; Zhang, Z.; Xue, Z.; Huang, M.; Li, N.; Cao, X.; Zou, Y.; Yang, C. High-power-efficiency and ultra-long-lifetime white OLEDs empowered by robust blue multi-resonance TADF emitters. Light Sci. Appl. 2025, 14, 81. [Google Scholar] [CrossRef]
- Deori, U.; Nanda, G.P.; Murawski, C.; Rajamalli, P. A perspective on next-generation hyperfluorescent organic light-emitting diodes. Chem. Sci. 2024, 15, 17739–17759. [Google Scholar] [CrossRef]

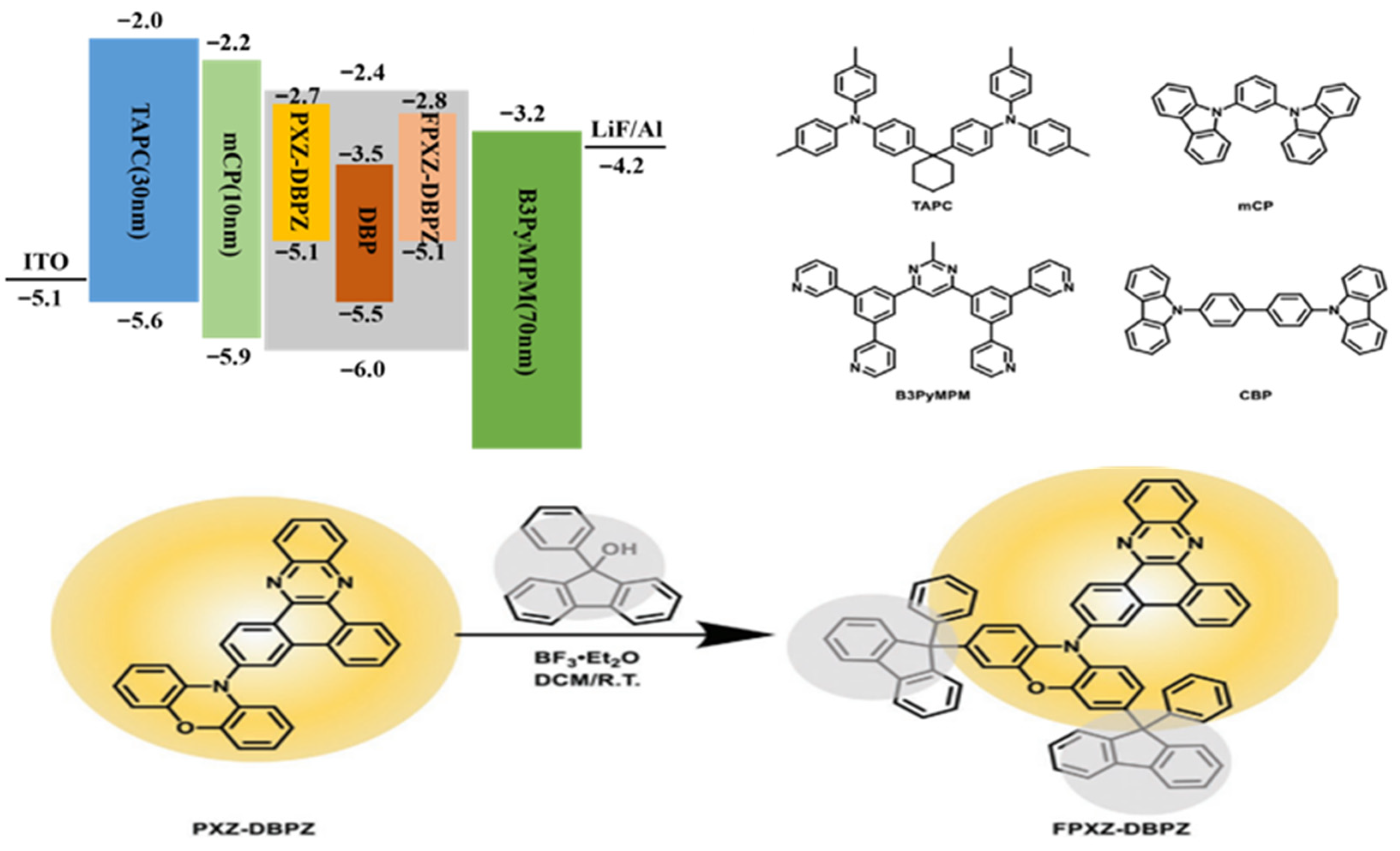
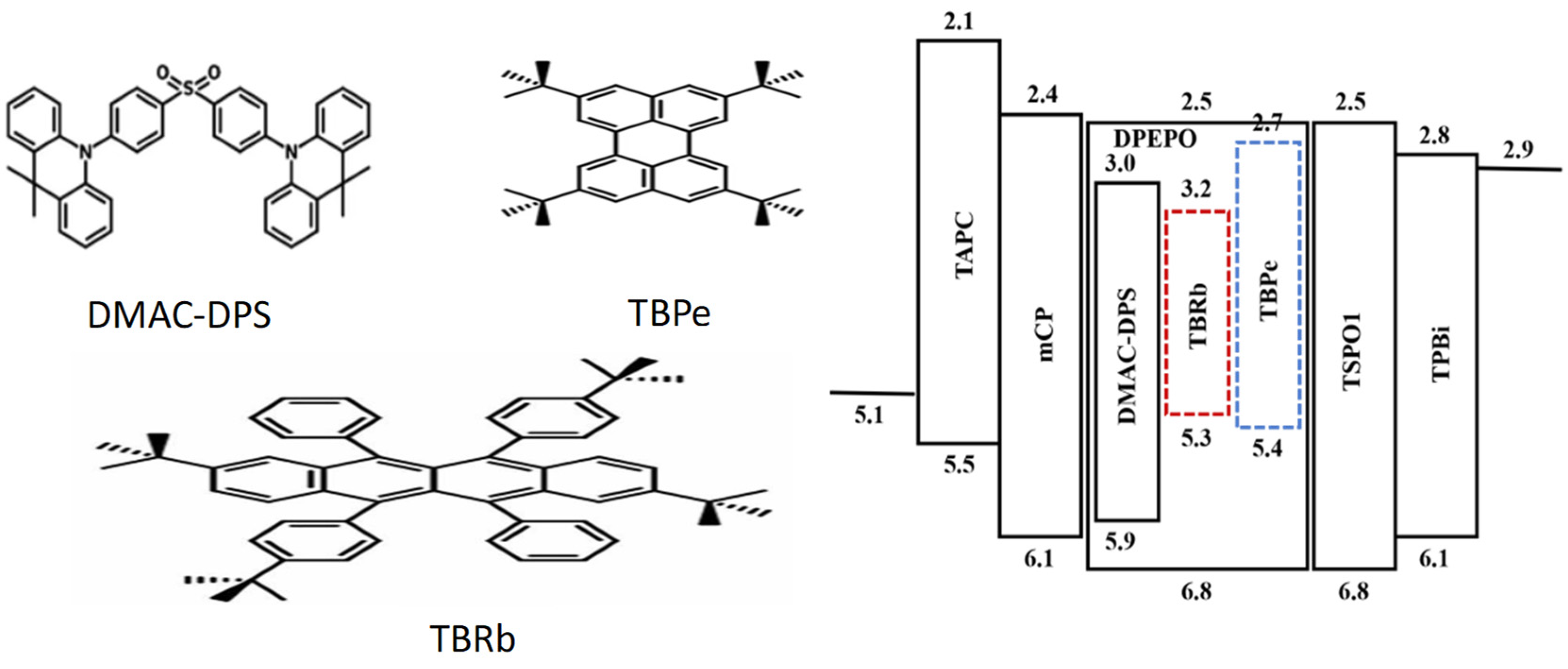
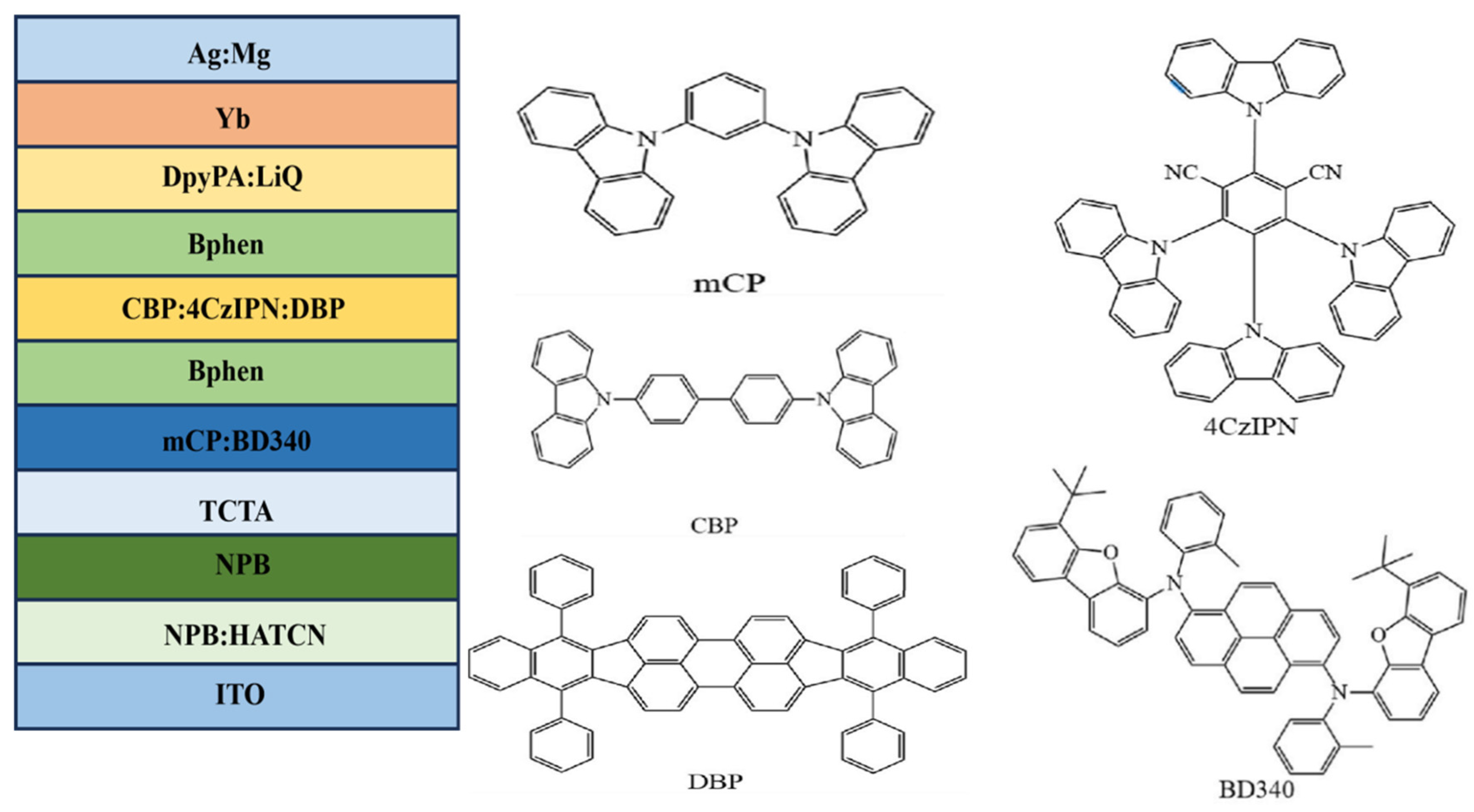
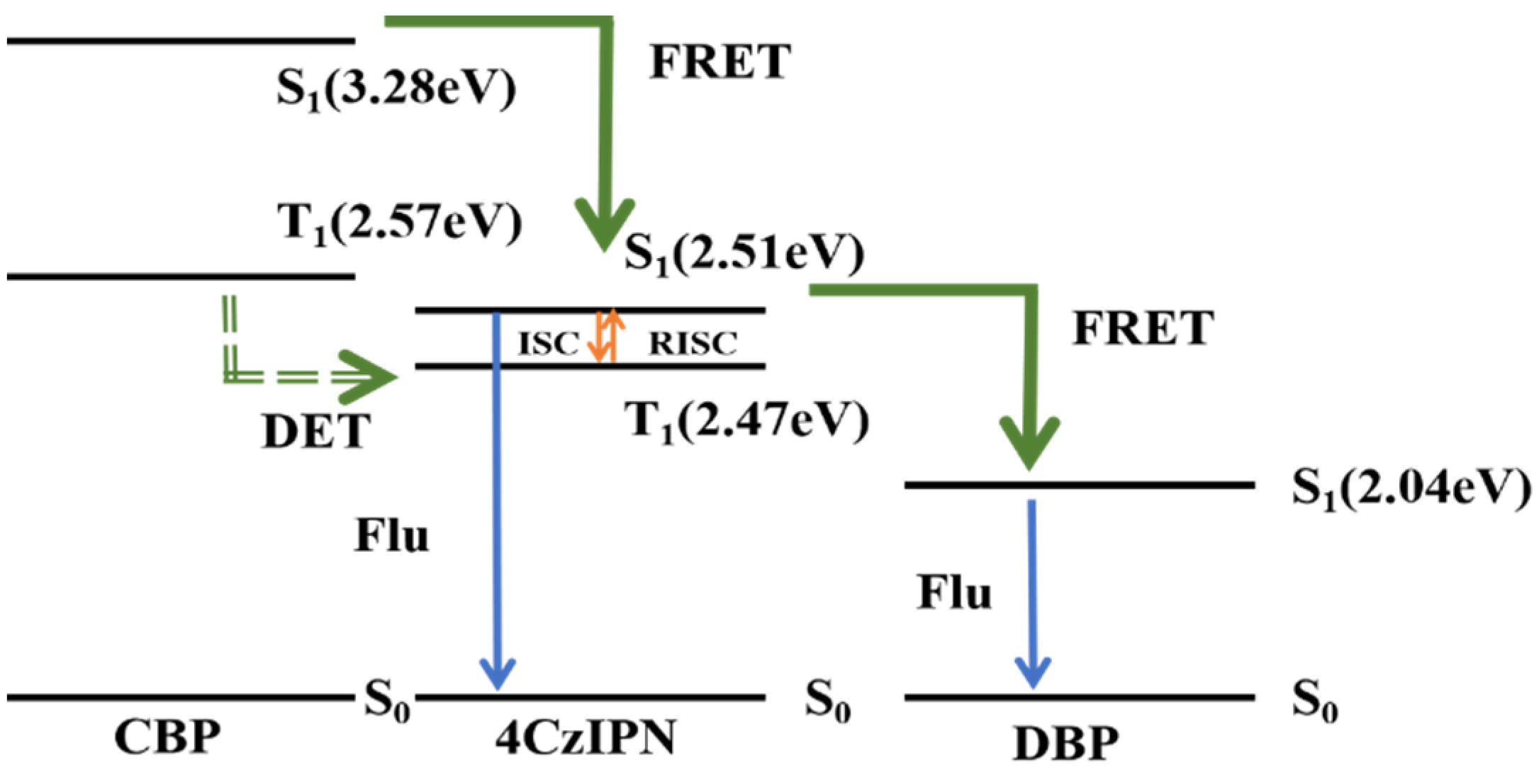
| Emitters | CE (cd A−1) a | PE (lm W−1) b | EQE (%) c | FWHM (nm) d | CIE d | Ref |
|---|---|---|---|---|---|---|
| v-DABNA | 39.0/31.0 | 41.0/16.0 | 27.0/20.0 | 18.0 | (0.15,0.20) | [18] |
| v-DABNA | 44.5/40.4 | -/- | 37.7/32.6 | 21.0 | (0.13,0.22) | [26] |
| v-DABNA | 42.0/36.6 | -/- | 43.9/37.5 | 21.0 | (0.12,0.26) | [26] |
| v-DABNA | 37.0/33.0 | 36.0/31.0 | 27.3/18.6 | 17.7 | (013,0.17) | [14] |
| p3IDCz | 38.0/26.1 | -/- | 30.7/21.8 | 16.0 | (0.13,0.16) | [27] |
| P3IDCz | 48.5/30.8 | -/- | 36.4/23.1 | 16.0 | (0.12,0.19) | [27] |
| Emitters | CE (cd A−1) a | PE (lm W−1) b | EQE (%) c | FWHM (nm) d | CIE d | Ref |
|---|---|---|---|---|---|---|
| 4tBuMB | 26.0/24.0 | -/- | 19.4/17.2 | 44.0 | (0.64,0.36) | [23] |
| 4tBuMB | 21.1/20.1 | -/- | 13.3/11.9 | 48.0 | (0.57,0.40) | [23] |
| DBP | 28.1/- | 26.0/- | 18.1/- | 57.9 | (0.61,0.38) | [24] |
| DBP | 29.6/- | 24.5/- | 15.2/- | 38.0 | (0.57,0.42) | [24] |
| DBP | 25.0/20.0 | 28.0/10.0 | 17.5/10.9 | - | (0.61,0.39) | [5] |
| DBP | 22.1/- | 27.8/1.8 | 16.9/2.6 | - | (0.65,0.35) | [73] |
| Emitters | CE (cd A−1) a | PE (lm W−1) b | EQE (%) c | FWHM (nm) d | CIE d | Ref |
|---|---|---|---|---|---|---|
| TBRb/TBPe | 29.8/24.0 | 31.1/17.3 | 12.9/10.8 | - | (0.26,0.32) | [82] |
| TBRb/TBPe | 31.1/26.1 | 31.6/17.8 | 11.2/9.7 | - | (0.35,0.40) | [82] |
| 4CzIPN/DBP/BD340 | 16.2/13.7 | 13.1/6.6 | 9.0/7.5 | - | (0.44,0.40) | [83] |
| 4CzIPN/DBP/BD340 | 22.2/8.8 | 19.2/4.3 | 10.9/4.3 | - | (0.34,0.37) | [83] |
| TFP/PTZTPE-3 | -/- | -/- | 8.2/- | - | (0.28,0.38) | [84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Li, Y.; Wang, J.; Pan, C.; Jiang, X.; Dong, H.; Wang, J.; Zhang, G. Research Progress of Hyperfluorescent Organic Electroluminescent Devices. Micromachines 2026, 17, 40. https://doi.org/10.3390/mi17010040
Li Y, Wang J, Pan C, Jiang X, Dong H, Wang J, Zhang G. Research Progress of Hyperfluorescent Organic Electroluminescent Devices. Micromachines. 2026; 17(1):40. https://doi.org/10.3390/mi17010040
Chicago/Turabian StyleLi, Yaxin, Jiaqi Wang, Chaoteng Pan, Xin Jiang, He Dong, Jin Wang, and Gang Zhang. 2026. "Research Progress of Hyperfluorescent Organic Electroluminescent Devices" Micromachines 17, no. 1: 40. https://doi.org/10.3390/mi17010040
APA StyleLi, Y., Wang, J., Pan, C., Jiang, X., Dong, H., Wang, J., & Zhang, G. (2026). Research Progress of Hyperfluorescent Organic Electroluminescent Devices. Micromachines, 17(1), 40. https://doi.org/10.3390/mi17010040







