Material Removal on Hydrogen-Terminated Diamond Surface via AFM Tip-Based Local Anodic Oxidation
Abstract
1. Introduction
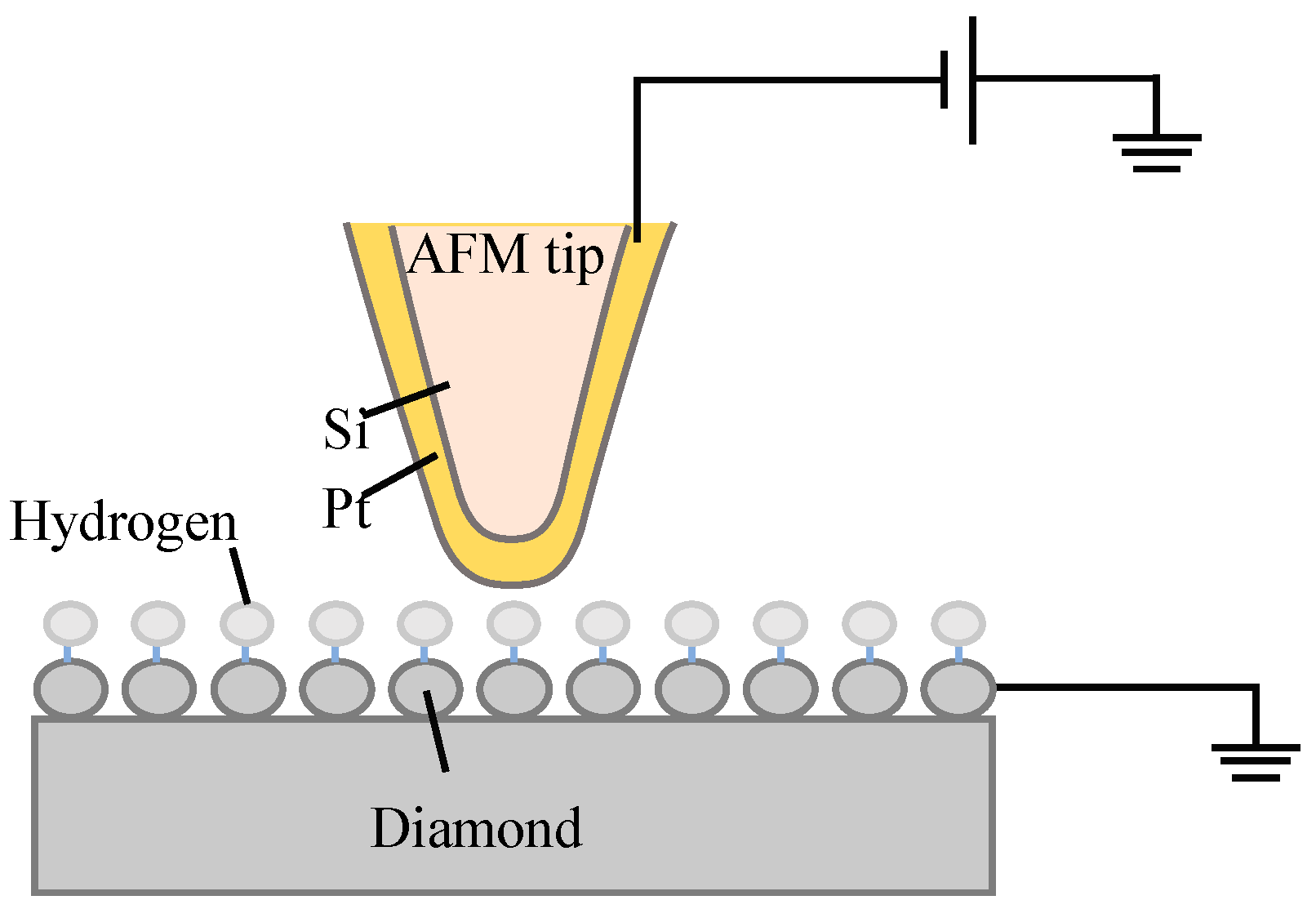
2. Materials and Methods
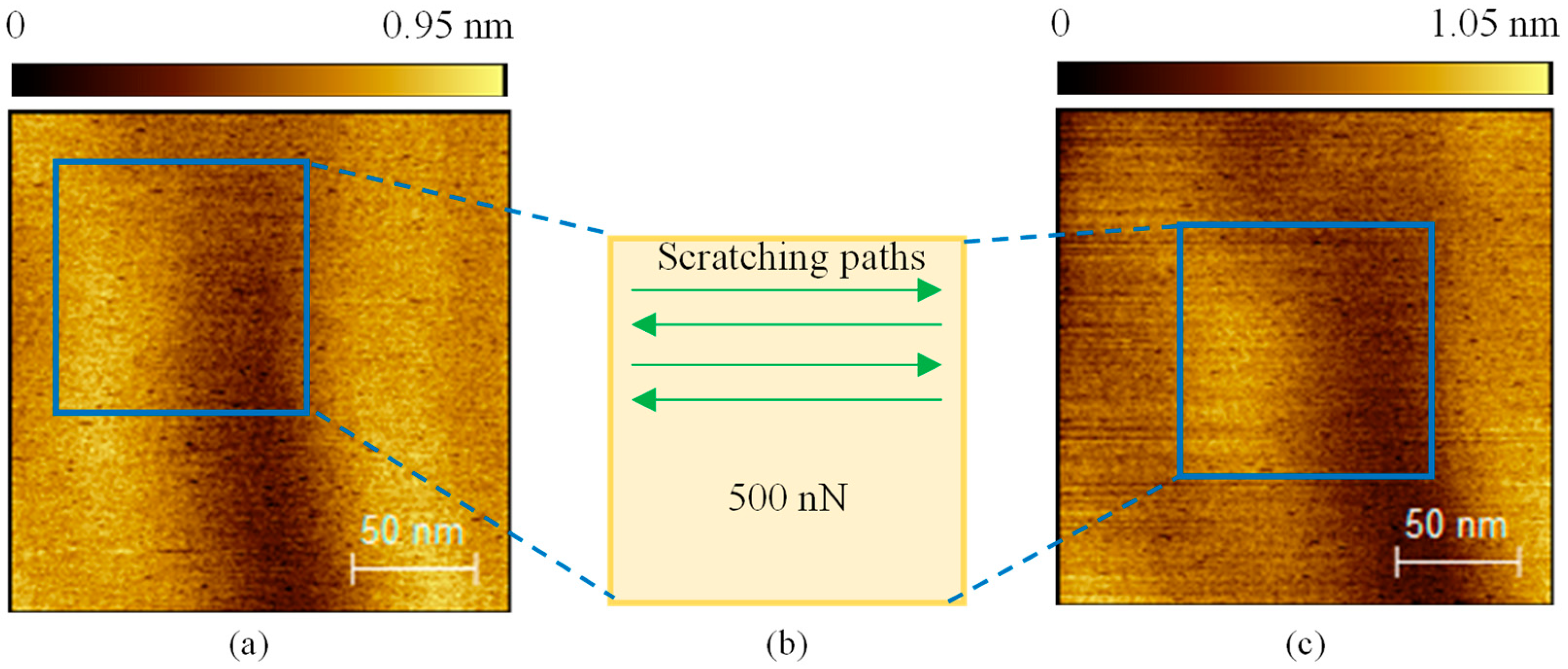
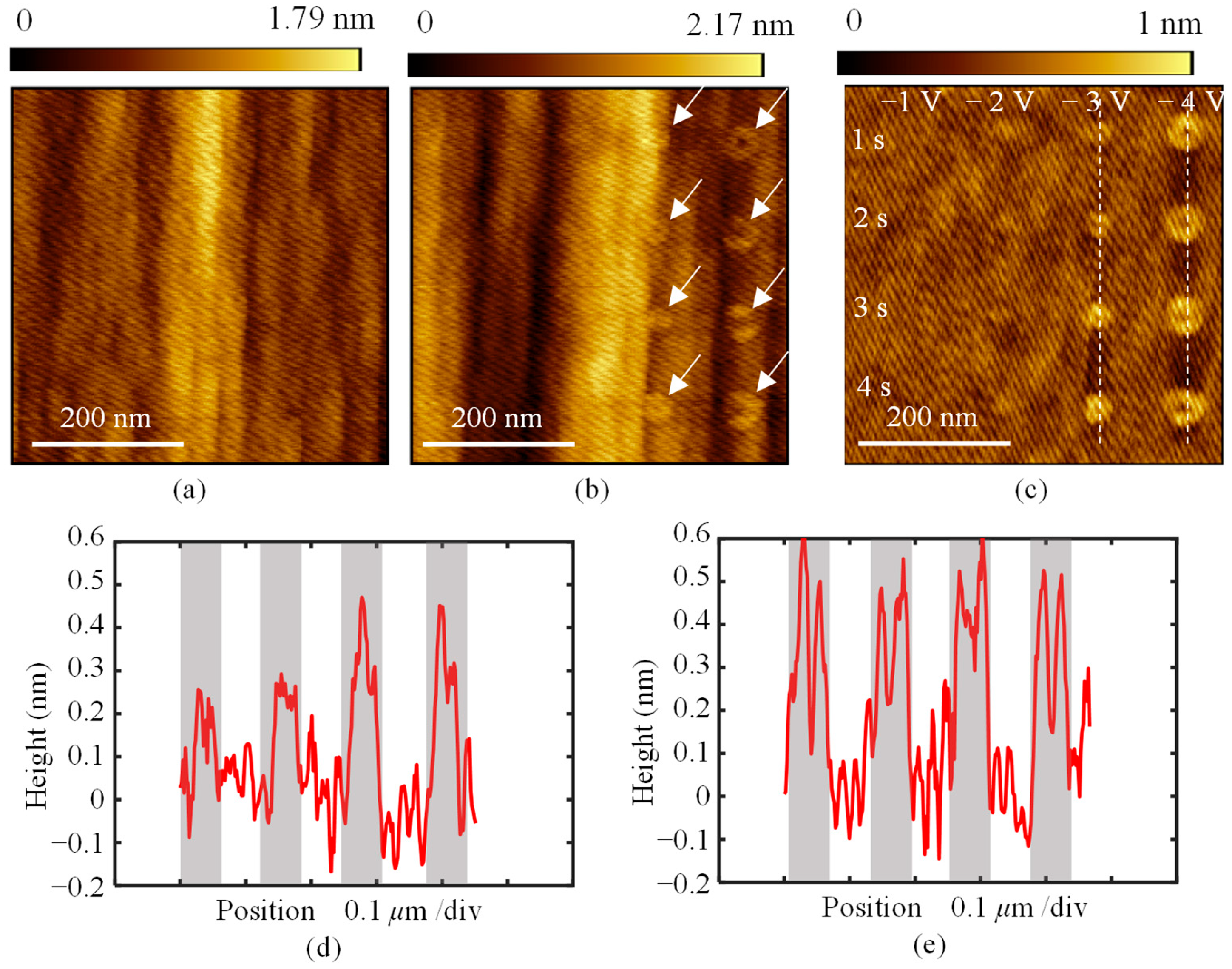
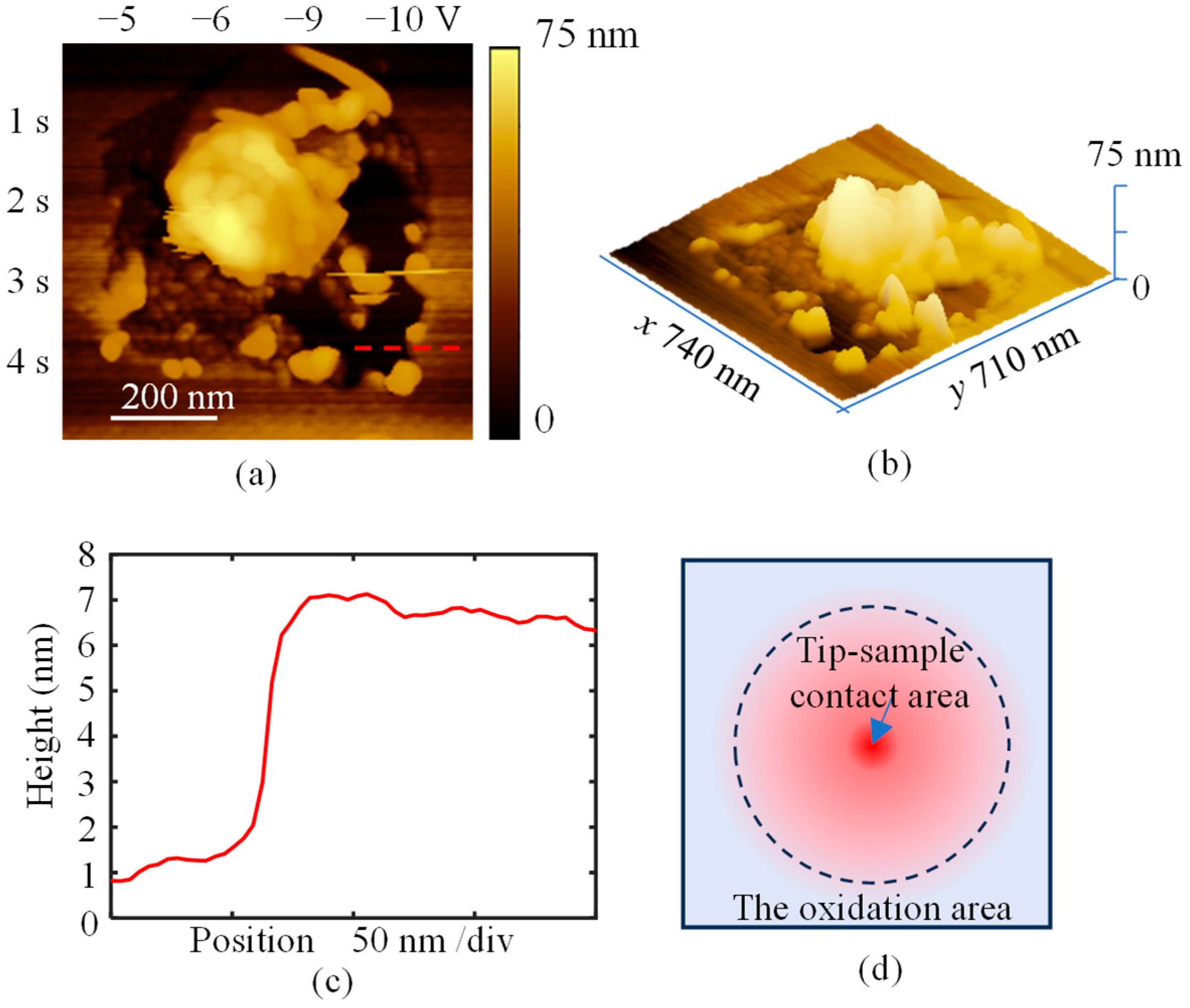
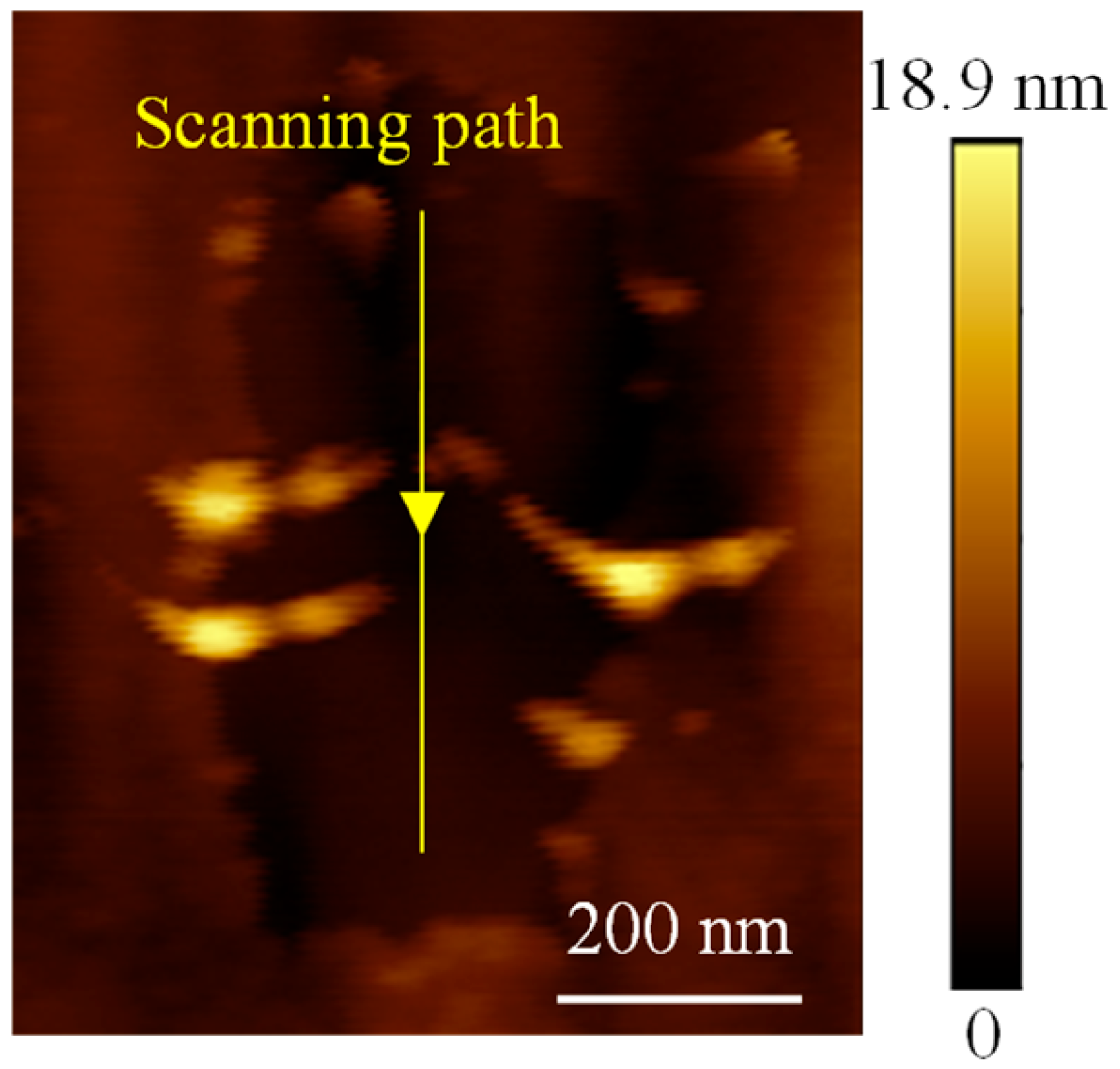
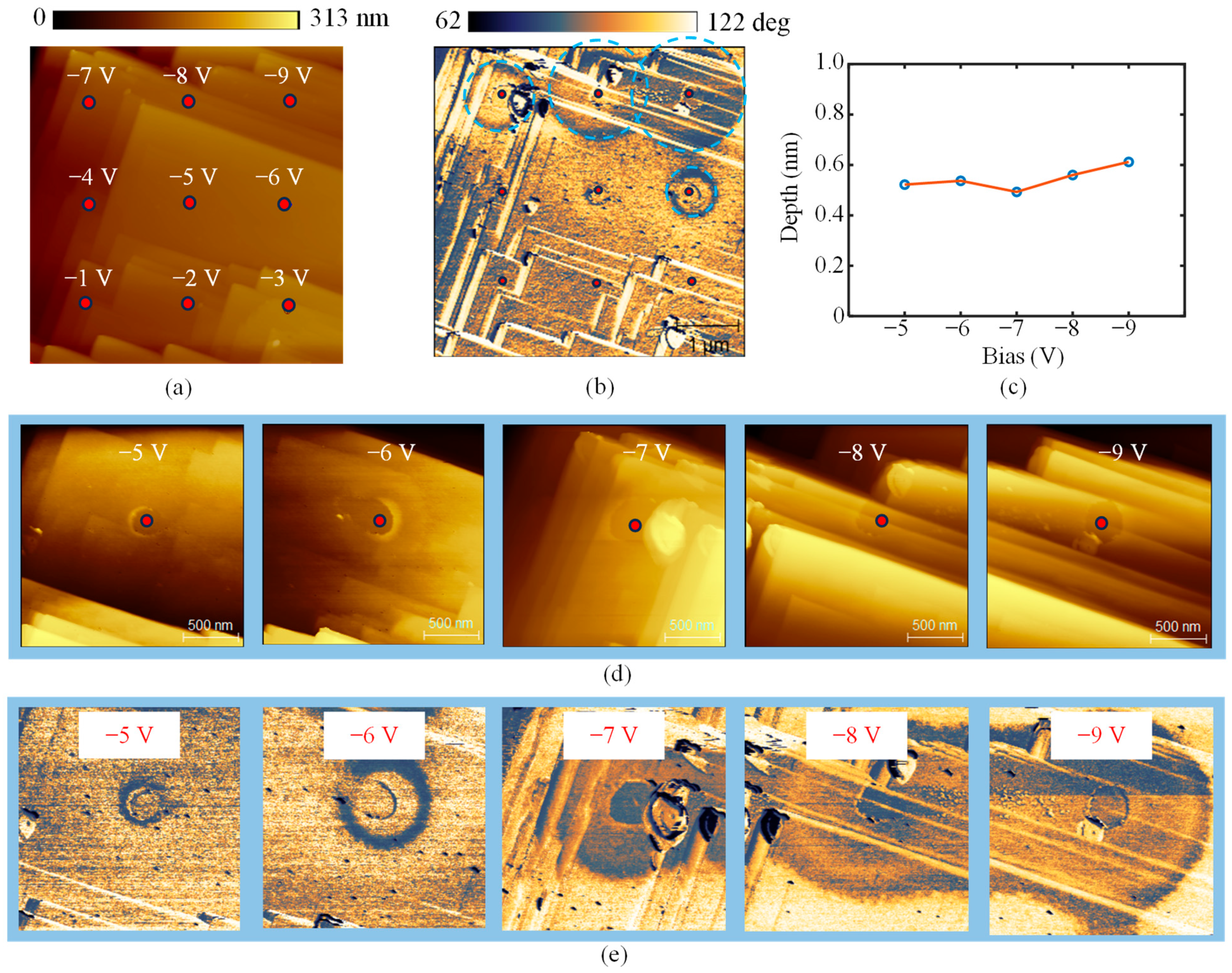
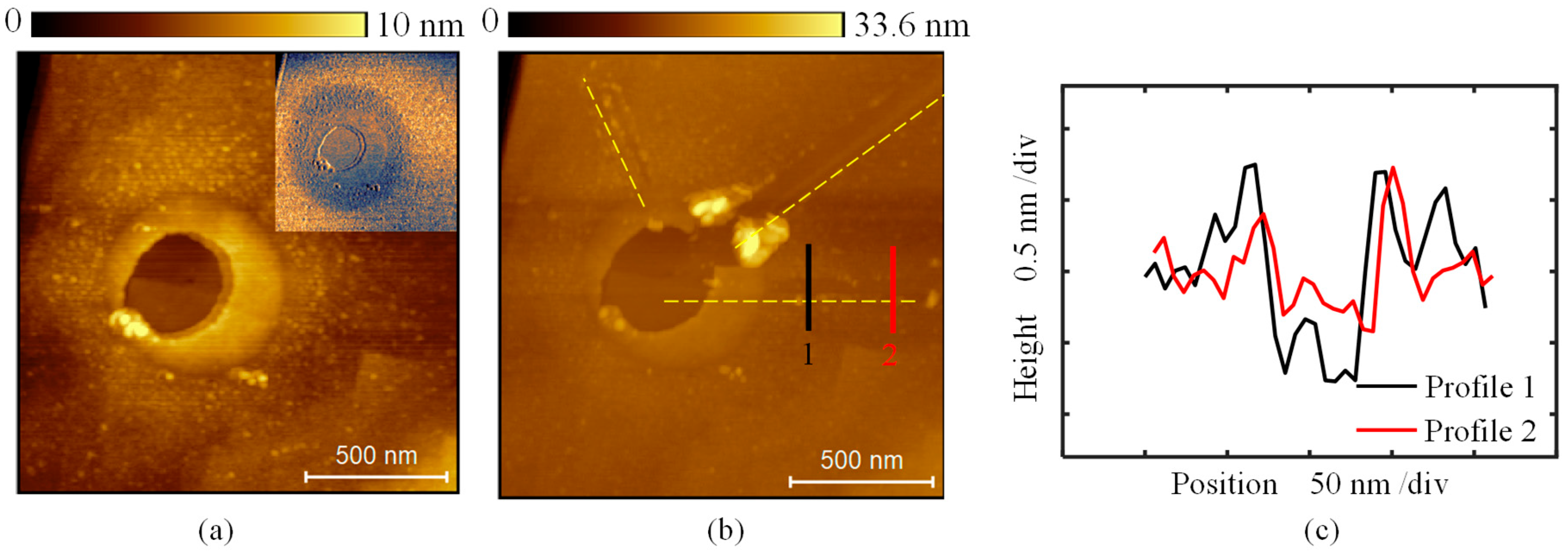
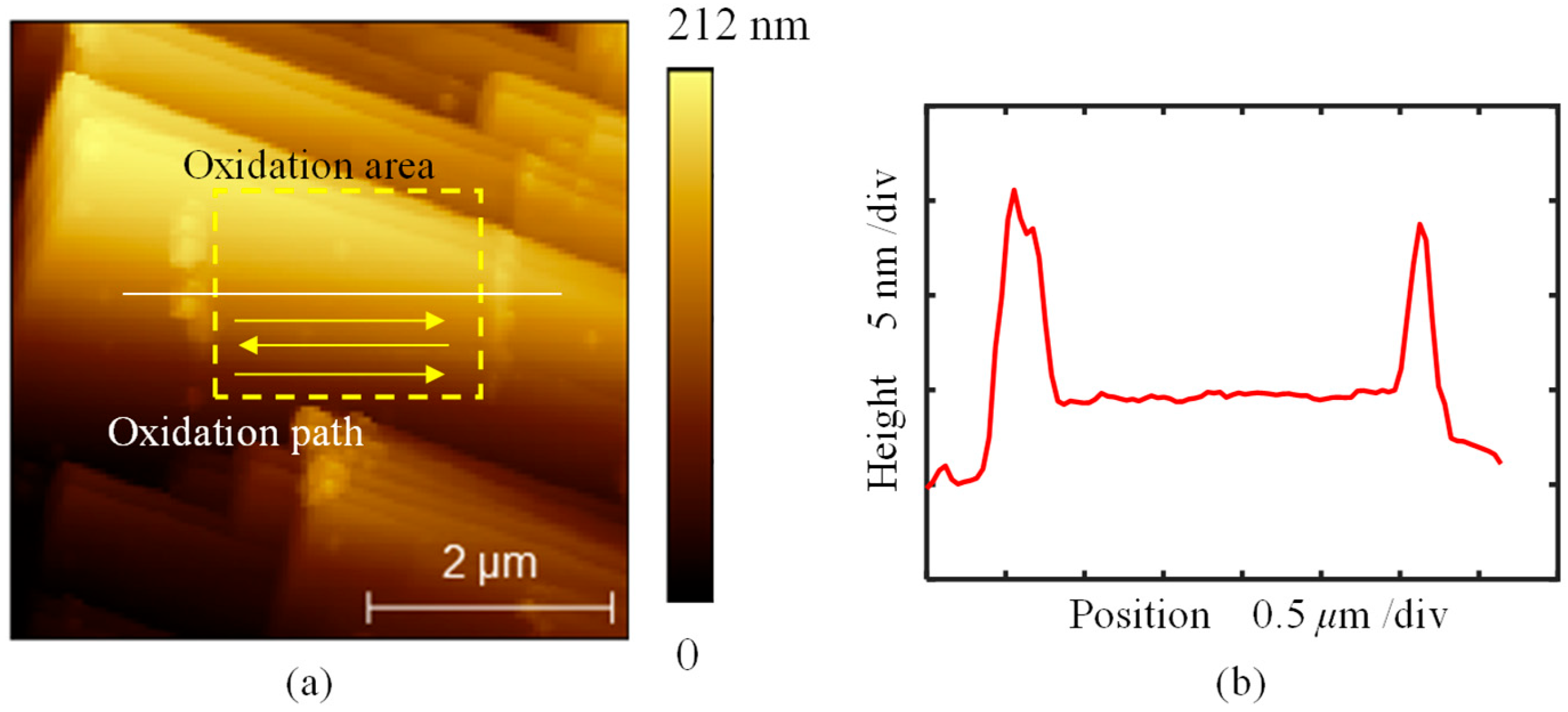
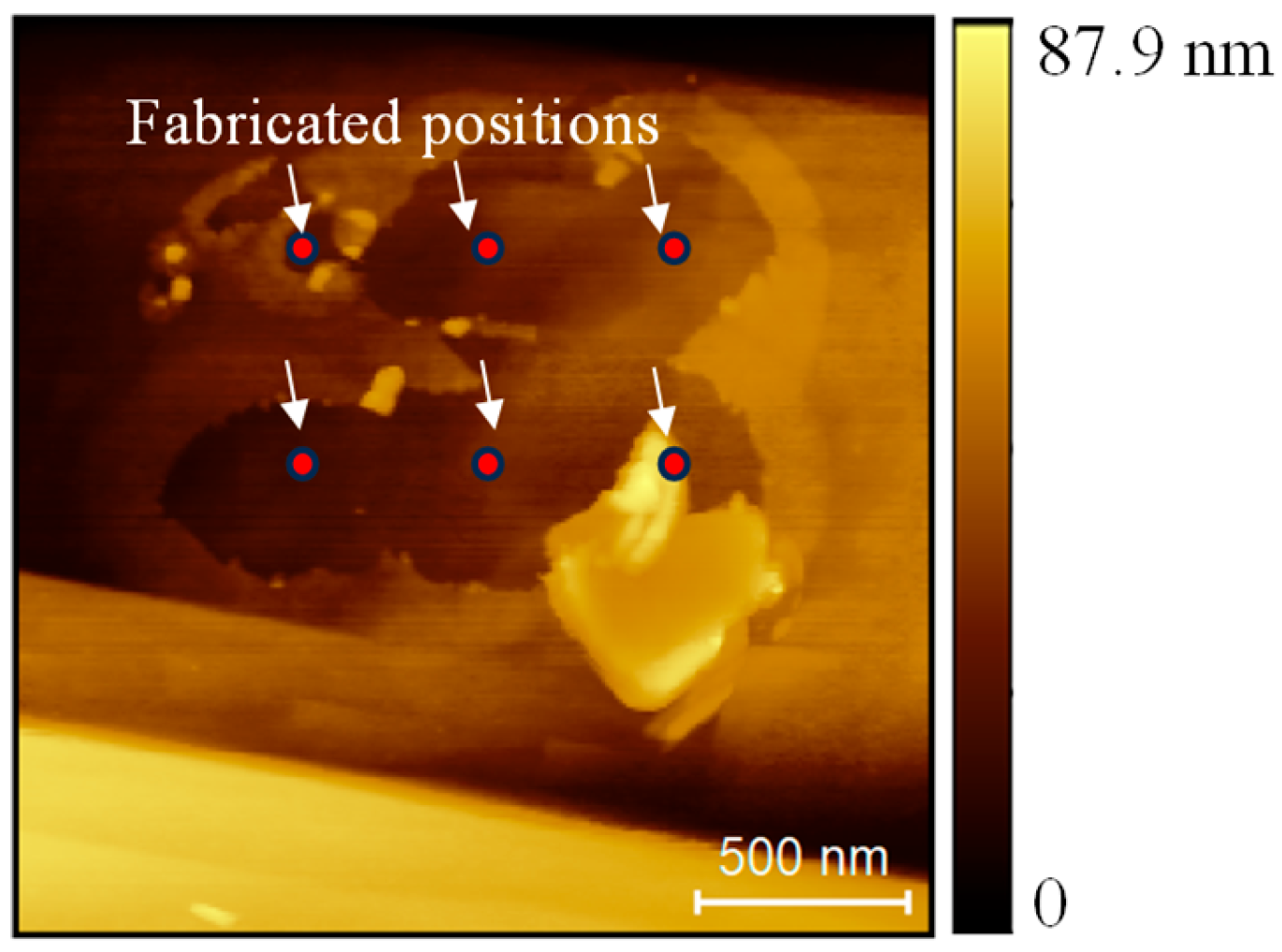
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFM | atomic force microscope |
| MOFET | metal-oxide-semiconductor field-effect transistor (MOFET) |
| 2DHG | 2D hole gas |
| CVD | chemical vapor deposition (CVD) |
| RHEED | reflection high-energy electron diffraction (RHEED) |
References
- Cheng, L.; Zhu, S.; Ouyang, X.; Zheng, W. Bandgap evolution of diamond. Diam. Relat. Mater. 2023, 132, 109638. [Google Scholar] [CrossRef]
- Inyushkin, A.V.; Taldenkov, A.N.; Ralchenko, V.G.; Bolshakov, A.P.; Koliadin, A.V.; Katrusha, A.N. Thermal conductivity of high purity synthetic single crystal diamonds. Phys. Rev. B 2018, 97, 144305. [Google Scholar] [CrossRef]
- Portier, A.; Donatini, F.; Dauvergne, D.; Gallin-Martel, M.L.; Pernot, J. Carrier Mobility up to 106 cm2 V−1 s−1 Measured in Single-Crystal Diamond by the Time-of-Flight Electron-Beam-Induced-Current Technique. Phys. Rev. Appl. 2023, 20, 024037. [Google Scholar] [CrossRef]
- Matsumoto, T.; Kato, H.; Oyama, K.; Makino, T.; Ogura, M.; Takeuchi, D.; Inokuma, T.; Tokuda, N.; Yamasaki, S. Inversion channel diamond metal-oxide-semiconductor field-effect transistor with normally off characteristics. Sci. Rep. 2016, 6, 31585. [Google Scholar] [CrossRef] [PubMed]
- Bürgler, B.; Sjolander, T.F.; Brinza, O.; Tallaire, A.; Achard, J.; Maletinsky, P. All-optical nuclear quantum sensing using nitrogen-vacancy centers in diamond. npj Quantum Inf. 2023, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Fei, H.; Sang, D.; Zou, L.; Ge, S.; Yao, Y.; Fan, J.; Wang, C.; Wang, Q. Research progress of optoelectronic devices based on diamond materials. Front. Phys. 2023, 11, 1226374. [Google Scholar] [CrossRef]
- Takagi, Y.; Shiraishi, K.; Kasu, M.; Sato, H. Mechanism of hole doping into hydrogen terminated diamond by the adsorption of inorganic molecule. Surf. Sci. 2013, 609, 203–206. [Google Scholar] [CrossRef]
- Crawford, K.G.; Maini, I.; Macdonald, D.A.; Moran, D.A.J. Surface transfer doping of diamond: A review. Prog. Surf. Sci. 2021, 96, 100613. [Google Scholar] [CrossRef]
- Fu, Y.; Xu, R.; Xu, Y.; Zhou, J.; Wu, Q.; Kong, Y.; Zhang, Y.; Chen, T.; Yan, B. Characterization and Modeling of Hydrogen-Terminated MOSFETs With Single-Crystal and Polycrystalline Diamond. IEEE Electron Device Lett. 2018, 39, 1704–1707. [Google Scholar] [CrossRef]
- Rivero, P.; Shelton, W.; Meunier, V. Surface properties of hydrogenated diamond in the presence of adsorbates: A hybrid functional DFT study. Carbon 2016, 110, 469–479. [Google Scholar] [CrossRef]
- Chen, Z.; Fu, Y.; Kawarada, H.; Xu, Y. Microwave diamond devices technology: Field-effect transistors and modeling. Int. J. Numer. Model. Electron. Netw. Devices Fields 2020, 34, e2800. [Google Scholar] [CrossRef]
- Luo, X.; Gao, J.; Xie, W.; Hasan, R.M.M.; Qin, Y. Flexible single-step fabrication of programmable 3D nanostructures by pulse-modulated local anodic oxidation. CIRP Ann. 2023, 72, 177–180. [Google Scholar] [CrossRef]
- Lorenzoni, M.; Torre, B. Scanning probe oxidation of SiC, fabrication possibilities and kinetics considerations. Appl. Phys. Lett. 2013, 103, 163109. [Google Scholar] [CrossRef]
- Rezek, B.; Nebel, C.E.; Stutzmann, M. Hydrogenated diamond surfaces studied by atomic and Kelvin force microscopy. Diam. Relat. Mater. 2004, 13, 740–745. [Google Scholar] [CrossRef]
- Wang, T.; Boer-Duchemin, E.; Tranvouez, E.; Cartwright, R.; Comtet, G.; Dujardin, G.; Mayne, A.J. Low voltage fabrication of sub-nanometer insulating layers on hydrogenated diamond. J. Appl. Phys. 2011, 110, 034311. [Google Scholar] [CrossRef]
- Toma, C.; Volodin, A.; Bogdan, G.; Deferme, W.; Haenen, K.; Nesladek, M.; Van Haesendonck, C. Tip voltage controlled local modification of hydrogenated diamond surface with an atomic force microscope. Phys. Status Solidi A-Appl. Mater. Sci. 2007, 204, 2920–2924. [Google Scholar] [CrossRef]
- Hofmann, M.; Lenk, C.; Ivanov, T.; Rangelow, I.W.; Reum, A.; Ahmad, A.; Holz, M.; Manske, E. Field emission from diamond nanotips for scanning probe lithography. J. Vac. Sci. Technol. B 2018, 36, 06JL02. [Google Scholar] [CrossRef]
- Tang, P.; Yu, B.; Guo, J.; Song, C.; Qian, L. Maskless micro/nanofabrication on GaAs surface by friction-induced selective etching. Nanoscale Res. Lett. 2014, 9, 59. [Google Scholar] [CrossRef]
- Rezek, B.; Nebel, C.E. Electronic properties of plasma hydrogenated diamond surfaces: A microscopic study. Diam. Relat. Mater. 2006, 15, 1374–1377. [Google Scholar] [CrossRef]
- Geis, M.W.; Fedynyshyn, T.H.; Plaut, M.E.; Wade, T.C.; Wuorio, C.H.; Vitale, S.A.; Varghese, J.O.; Grotjohn, T.A.; Nemanich, R.J.; Hollis, M.A. Chemical and semiconducting properties of NO2-activated H-terminated diamond. Diam. Relat. Mater. 2018, 84, 86–94. [Google Scholar] [CrossRef]
- Kaibara, Y.; Sugata, K.; Tachiki, M.; Umezawa, H.; Kawarada, H. Control wettability of the hydrogen-terminated diamond surface and the oxidized diamond surface using an atomic force microscope. Diam. Relat. Mater. 2003, 12, 560–564. [Google Scholar] [CrossRef]
- Loh, K.P.; Xie, X.N.; Lim, Y.H.; Teo, E.J.; Zheng, J.C.; Ando, T. Surface oxygenation studies on (100)-oriented diamond using an atom beam source and local anodic oxidation. Surf. Sci. 2002, 505, 93–114. [Google Scholar] [CrossRef]
- Enriquez, J.I.; Muttaqien, F.; Michiuchi, M.; Inagaki, K.; Geshi, M.; Hamada, I.; Morikawa, Y. Oxidative etching mechanism of the diamond (100) surface. Carbon 2021, 174, 36–51. [Google Scholar] [CrossRef]
- Tachiki, M.; Kaibara, Y.; Sumikawa, Y.; Shigeno, M.; Kanazawa, H.; Banno, T.; Soup Song, K.; Umezawa, H.; Kawarada, H. Characterization of locally modified diamond surface using Kelvin probe force microscope. Surf. Sci. 2005, 581, 207–212. [Google Scholar] [CrossRef]
- Manfredotti, C.; Vittone, E.; Paolini, C.; Bianco, L.; Fizzotti, F.; Giudice, A.L.; Olivero, P. Control of Hydrogenation Patterning for CVD Diamond Substrates by AFM Local Anodic Oxidation. Surf. Eng. 2003, 19, 441–446. [Google Scholar] [CrossRef]
- Yianni, S.A.; Hofmann, M.; Schenk, A.K.; Reuter, C.; Rangelow, I.W.; Pakes, C.I. Mask-less nano-structuring of hydrogen terminated diamond using localized field emission scanning probe lithography (FE-SPL). Appl. Phys. Lett. 2022, 120, 093503. [Google Scholar] [CrossRef]
- Simon, P.; Beck, P.; Rathi, A.; Stutzmann, M.; Garrido, J.A. Photocurrent spectroscopy of in-plane surface conductive diamond homostructures. Phys. Rev. B 2020, 101, 205306. [Google Scholar] [CrossRef]
- Xu, J.; Lu, K.; Fan, D.; Wang, Y.; Xu, S.; Kubo, M. Different Etching Mechanisms of Diamond by Oxygen and Hydrogen Plasma: A Reactive Molecular Dynamics Study. J. Phys. Chem. C 2021, 125, 16711–16718. [Google Scholar] [CrossRef]
- Tamayo, J.; García, R. Deformation, Contact Time, and Phase Contrast in Tapping Mode Scanning Force Microscopy. Langmuir 1996, 12, 4430–4435. [Google Scholar] [CrossRef]
- Kramer, A.; Liashkovich, I.; Ludwig, Y.; Shahin, V. Atomic force microscopy visualises a hydrophobic meshwork in the central channel of the nuclear pore. Pflügers Arch.-Eur. J. Physiol. 2008, 456, 155–162. [Google Scholar] [CrossRef]
- Tachiki, M.; Fukuda, T.; Sugata, K.; Seo, H.; Umezawa, H.; Kawarada, H. Nanofabrication on Hydrogen-Terminated Diamond Surfaces by Atomic Force Microscope Probe-Induced Oxidation. Jpn. J. Appl. Phys. 2000, 39, 4631. [Google Scholar] [CrossRef]

| Conditions | Chamber Cleaning | Sample I | Sample II |
|---|---|---|---|
| Temperature (°C) | 850 | 800 | 800 |
| Gas pressure (Torr) | 80 | 80 | 80 |
| Microwave power (W) | 2500 | 3000 | 2500 |
| Etching time (min) | 90 | 60 | 180 |
| Gas flow (sccm) | 1000 | 1000 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Cao, Z.-H.; Li, Z.; Chen, Y.-L. Material Removal on Hydrogen-Terminated Diamond Surface via AFM Tip-Based Local Anodic Oxidation. Micromachines 2025, 16, 981. https://doi.org/10.3390/mi16090981
Tang J, Cao Z-H, Li Z, Chen Y-L. Material Removal on Hydrogen-Terminated Diamond Surface via AFM Tip-Based Local Anodic Oxidation. Micromachines. 2025; 16(9):981. https://doi.org/10.3390/mi16090981
Chicago/Turabian StyleTang, Jinyan, Zhong-Hao Cao, Zhongwei Li, and Yuan-Liu Chen. 2025. "Material Removal on Hydrogen-Terminated Diamond Surface via AFM Tip-Based Local Anodic Oxidation" Micromachines 16, no. 9: 981. https://doi.org/10.3390/mi16090981
APA StyleTang, J., Cao, Z.-H., Li, Z., & Chen, Y.-L. (2025). Material Removal on Hydrogen-Terminated Diamond Surface via AFM Tip-Based Local Anodic Oxidation. Micromachines, 16(9), 981. https://doi.org/10.3390/mi16090981






