Abstract
Photobiomodulation (PBM) harnesses near-infrared (NIR) light to stimulate cellular processes, offering non-invasive treatment options for a range of conditions, including chronic wounds, inflammation, and neurological disorders. NIR light-emitting diodes (LEDs) are emerging as safer and more scalable alternatives to conventional lasers, but optimizing their performance for clinical use remains a challenge. This perspective explores the latest advances in NIR-emitting materials, spanning Group III–V, IV, and II–VI semiconductors, organic small molecules, polymers, and perovskites, with an emphasis on their applicability to PBM. Particular attention is given to the promise of perovskite LEDs, including lead-free and lanthanide-doped variants, for delivering narrowband, tunable NIR emission. Furthermore, we examine photonic and plasmonic engineering strategies that enhance light extraction, spectral precision, and device efficiency. By integrating advances in materials science and nanophotonics, it is increasingly feasible to develop flexible, biocompatible, and high-performance NIR LEDs tailored for next-generation therapeutic applications.
1. Introduction
Photobiomodulation (PBM) is a non-invasive, non-thermal therapeutic approach that employs non-ionizing light to stimulate beneficial biological processes at the cellular and tissue levels. Near-infrared (NIR) light-emitting diodes (LEDs) have emerged as particularly effective tools for PBM, because they combine deep tissue penetration with precise cellular-level interaction and minimal side effects. PBM operates through photon absorption by chromophores such as cytochrome c oxidase (COX), which enhances cellular metabolism, reduces inflammation, and promotes tissue regeneration [1,2,3]. This therapeutic approach typically employs LEDs and lasers that emit light across the visible (400–700 nm) and NIR (700–1350 nm) spectra [4,5,6], thereby linking fundamental photobiological mechanisms to practical clinical outcomes. NIR wavelengths, especially NIR I (700–1000 nm), are the most effective one for clinical applications such as chronic wound healing, transcranial neurostimulation, and retinal disease healing [4,7,8].
In addition to therapeutic benefits, NIR light allows precise monitoring and modulation of cellular activity with subcellular spatial resolution and sub-millisecond temporal accuracy [9]. Its ability to penetrate deeply into tissues with minimal scattering and photodamage enables researchers to study dynamic cellular processes, manipulate neural or biochemical pathways, and achieve targeted therapeutic interventions. These characteristics translate directly into clinical advantages, including dermatology, neurology, and ophthalmology. Collectively, these properties highlight the unique potential of NIR LEDs to bridge fundamental photobiological effects with clinical applications.
Realizing this potential requires the development of novel NIR-emitting materials that are efficient, biocompatible, and environmentally friendly. Advanced NIR light sources optimized for interaction with biological systems can expand PBM to new clinical and biomedical applications. By integrating innovations in materials, precise light delivery, and mechanistic understanding, NIR light-based approaches create a seamless pathway from fundamental research to transformative precision medicine.
Traditional inorganic LEDs, based on Group III–V, IV, and II–VI semiconductors, provide high radiative efficiency but are limited by high production costs, rigidity, and toxicity [6]. Emerging technologies that utilize small molecules and conjugated polymers offer flexible and low-cost alternatives, although stability and efficiency in the NIR region remain challenging [10]. Perovskite LEDs, particularly those that use lead halide nanocrystals, exhibit excellent performance in the visible spectrum due to their high photoluminescence quantum yield (PLQY) and tunable emission [11,12]. However, extending these advantages to NIR emission is still challenging, which has driven the development of lead-free alternatives such as tin-based perovskites, double perovskites, and lanthanide-doped halides [11,12,13].
Beyond material innovations, photonic and plasmonic engineering can further optimize NIR LED performance. Nanostructured electrodes, optical cavities, and surface plasmon coupling improve light outcoupling, spectral precision, and thermal management [14,15]. Taken together, advances in both materials and photonic design provide a comprehensive strategy to enhance NIR LEDs for PBM, as seen in the roadmap of Figure 1. The convergence of optoelectronics and biomedicine in this perspective represents a transformative opportunity for next-generation therapeutic technologies.
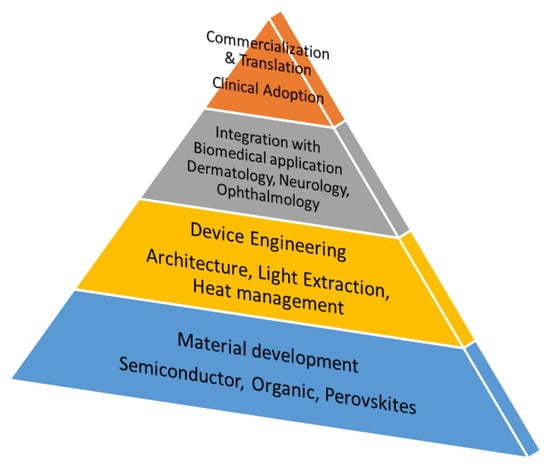
Figure 1.
Roadmap for photobiomodulation (PBM) based on the material development and device engineering.
2. NIR Emission for Photobiomodulation
2.1. Biological Basis of PBM
Understanding the biological basis of PBM is essential before discussing device design. PBM employs red (630–700 nm), NIR-I (700–1000 nm), and NIR-II (1000–1350 nm) to modulate cellular activity without thermal damage. However, in our discussion, we would like to limit our discussion to NIR-I as it is the region with the most benefits for clinical and preclinical studies, including wound healing, reduced inflammation, pain relief, brain therapy, and retinal diseases [16]. The primary mechanism involves the absorption of photons by COX, leading to photodissociation of nitric oxide, enhanced electron transport, and increased ATP production [2,16]. These events promote cell proliferation, regulate cytokine expression, and improve perfusion. The “optical window” (700–950 nm) is especially effective for tissue penetration, with 800–950 nm wavelengths most suitable for deeper targets [2,17].
2.2. Clinical Applications
Clinical evidence highlights PBM’s broad therapeutic potential across dermatology, neurology, and ophthalmology, see Figure 2a. In the brain, transcranial PBM has shown promise in Alzheimer’s disease, traumatic brain injury, and ischemic stroke, where mechanisms include vasodilation, enhanced neurogenesis, mitochondrial support, and antiapoptotic signaling that contribute to better neural recovery [8]. In dermatology and wound healing, PBM accelerates tissue repair by stimulating re-epithelialization, suppressing pro-inflammatory cytokines such as IL-1 and TNF-, and promoting angiogenesis and extracellular matrix remodeling, leading to faster closure of chronic ulcers and improved scar quality [16,17]. In ophthalmology, PBM represents a non-invasive strategy for retinal diseases, with clinical studies reporting gains in visual acuity and reduced pathological changes in age-related macular degeneration and diabetic macular edema [18,19,20]. Preclinical and early clinical evidence also supports the benefits in retinopathy of prematurity, amblyopia beyond the critical period, retinitis pigmentosa, and methanol-induced retinal toxicity [21,22,23,24]. Taken together, these findings position PBM as a versatile and cost-effective adjunctive therapy with translational relevance in the major domains of medicine, while underscoring the need for standardized protocols and large-scale trials to validate long-term efficacy.
2.3. Light Sources: Lasers vs. LEDs
PBM primarily employs lasers and LEDs as light sources. As illustrated in Figure 2b, lasers generate a focused coherent beam that enables deep tissue penetration and precise fluence delivery, whereas LEDs emit broader and less intense light. A single LED provides wide but shallow illumination, while an LED array produces overlapping beams that enhance scattering and create larger regions of high photon density. These different LED configurations are used in PBM to target different therapeutic applications. Although lasers offer superior coherence and penetration, LEDs are generally preferred for their safety, affordability, and suitability for large-area or wearable treatments. LEDs also minimize thermal and ocular risks, making them particularly advantageous for long-term or self-administered use. Recent developments in flexible, skin-conformal LED arrays further support efficient, low-cost, and personalized PBM therapies [7].
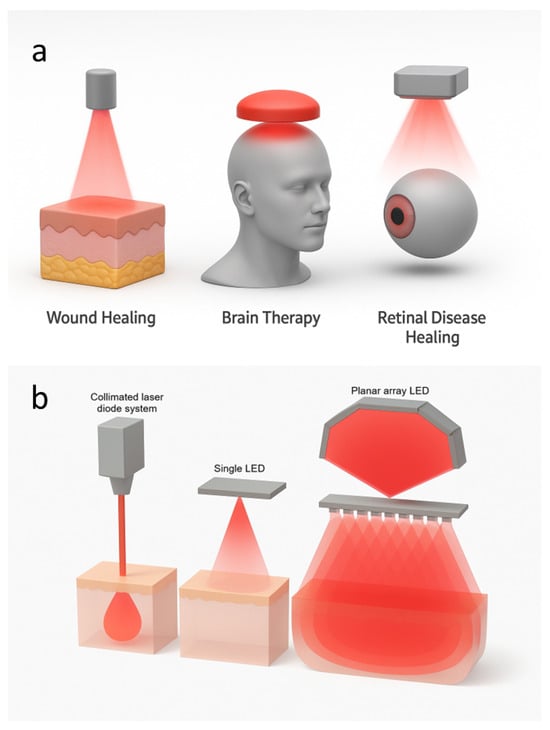
Figure 2.
Near-infrared (NIR) PBM using light-based systems. (a) Applications of light therapy, including wound healing, brain therapy, and retinal disease healing. (b) Comparison of light delivery methods: a collimated laser diode, a single light-emitting diode (LED), and a planar array LED.
2.4. Device Metrics and Dosimetry
Reproducible PBM requires linking biological outcomes with measurable device parameters. Key metrics include irradiance (), the optical power per unit area [25]; radiant exposure (), the cumulative dose defined as [26]; and duty cycle (%), the fraction of time a source emits within each pulse cycle [25]. These quantities provide a common framework for translating clinical prescriptions into engineering design choices.
2.5. Translating Clinical Protocols into Device Parameters
Clinical PBM protocols are typically reported using wavelength, fluence, irradiance, treatment time, and duty cycle. In contrast, device engineers manipulate emitter density, panel area, diode drive current, and pulsing schemes. Establishing a mapping between these domains is critical for safety and reproducibility. For example, a clinical protocol specifying 810 nm at 50 mW/cm2 for 2 min of continuous-wave (CW) illumination can be implemented by tuning diode current, emitter spacing, and optical layout. If pulsed-wave (PW) operation is used (e.g., 50% duty cycle), either the session time or instantaneous irradiance must be doubled to deliver the same radiant exposure. Because PBM outcomes follow a biphasic dose–response, both under-dosing and over-dosing risk reducing therapeutic benefit [27].
Table 1 illustrates the diversity of PBM protocols across medical domains. In neurology, dosing spans from low-fluence CW delivery in acute stroke (808 nm, 1.2 J/cm2) [28] to higher fluences in TBI (810–980 nm, 14.8–28.3 J/cm2 with PW at 10 Hz) [29], and very high doses in depressive disorders (810 nm, 60 J/cm2) [30]. In wound care, PBM protocols vary by depth and severity: superficial burns use 3 J/cm2 at 785–830 nm [31], dermal wounds employ 1–6 J/cm2 at 810–830 nm [2], and deep diabetic wounds require pulsed 904 nm light at high irradiance (up to 18.3 J/cm2) [5,32]. Retinal applications typically employ near-infrared light between 780 and 830 nm to avoid photothermal damage. Clinical studies demonstrate improvements in visual acuity and scotoma reduction in age-related macular degeneration [18], while additional reports show benefits in amblyopia and retinitis pigmentosa [22,23]. Together, these data emphasize how wavelength, fluence, and duty cycle must be carefully tuned not only to tissue depth and pathology but also to the optical safety constraints of the retina.

Table 1.
Representative PBM clinical and preclinical studies for wound healing, neurological, psychiatric, and retinal conditions. An asterisk (*) indicates animal studies outside ophthalmology.
2.6. Thermal Load and Worked Example
Thermal management is a central engineering challenge. Heating depends on irradiance, wavelength, beam profile, and duty cycle. For example, 980 nm sources typically generate more heat than 810 nm sources, and Gaussian beams concentrate hotspots compared to flat-top beams [33]. Furthermore, as seen on Table 2, we consider a diode with radiance and Lambertian emission (half-angle 60°) illuminating 1 cm2. The irradiance is (31,000 mW/cm2). With a 50% duty cycle, the average irradiance is 16,000 mW/cm2. Over 60 s, this corresponds to 940 J/cm2, far above therapeutic ranges. Such calculations highlight the need to balance duty cycle, beam divergence, and exposure duration to ensure safety.

Table 2.
Worked example converting radiance and angular profile to skin-plane irradiance and radiant exposure.
2.7. Challenges and Limitations
Despite progress, obstacles remain. Figure 3 illustrates device-level challenges such as the limited operational stability of NIR-emitting materials, particularly perovskite LEDs at high current densities. Accurate control of wavelength, irradiance, and dosage is also critical; deviations from prescribed values can compromise efficacy or cause adverse effects [2]. Superpulsed NIR LEDs (e.g., 904 nm nanosecond-scale pulses) represent one strategy to improve penetration while reducing thermal load.
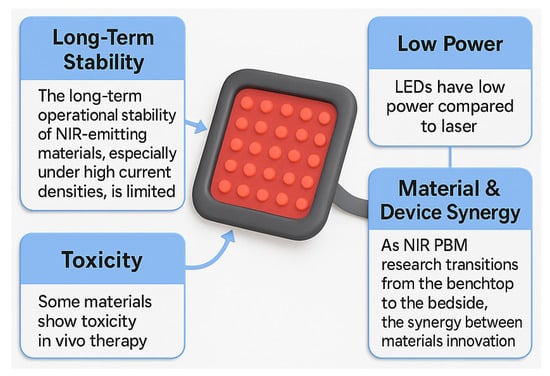
Figure 3.
Obstacles in NIR LED for PBM.
2.8. Future Directions
Integrating biological understanding, clinical validation, and quantitative dosimetry will guide the development of standardized PBM devices. Emerging technologies such as perovskite-based NIR LEDs and wearable platforms with biosensor feedback promise personalized closed-loop therapies. Progress will require collaboration between materials science, device engineering, and medicine to fully realize the therapeutic potential of PBM.
3. NIR LED Emissive Materials
This section consists of four major classes of materials for NIR LEDs in PBM applications: conventional semiconductors, small molecules, polymers, and perovskites. Figure 4 provides an overview of NIR LED materials.
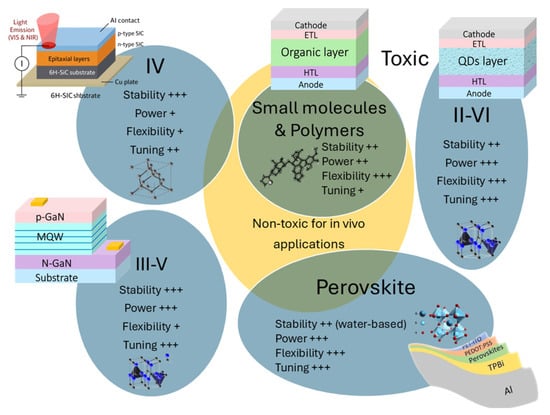
Figure 4.
Types of NIR LED emissive materials. Several factors including stability, power, flexibility, tuning, and toxicity as depicted in Figure 3 are considered.
3.1. Conventional Semiconductors (Groups III–V, IV, II–VI)
First, group III–V semiconductors such as GaAs, InGaAs, and AlGaAs exhibit direct bandgaps and high radiative efficiencies across 700–950 nm [34,35], resulting in large power. These materials are used in high-power NIR LEDs for clinical applications, including wound healing and neurostimulation [36]. However, epitaxial growth on rigid substrates limits their applicability to flexible and wearable devices [37]. Then, group IV semiconductors (e.g., SiC) have indirect bandgaps, making them inefficient emitters under standard conditions [38]. Nonetheless, strain engineering and nanostructuring have improved SiC-based NIR LEDs, particularly for telecom and bioimaging applications [38,39]. Finally, group II–VI semiconductors, such as CdSe, ZnTe, and CdHgSe, can be tuned for NIR emission via quantum dot engineering and alloying [40,41]. This latest semiconductor offers the best integration with the flexible polymer substrate, but has worse stability compared to the other two. For tuning, similar to III–V semiconductors with pnictogen anions, II–VI materials can be tuned by adjusting the composition of chalcogenide anions, respectively.
3.2. Small-Molecule and Polymer LEDs
Organic NIR LEDs based on small molecules and conjugated polymers offer flexibility, low-cost fabrication, and biocompatibility. Small-molecule NIR OLEDs utilize donor–acceptor architectures to achieve emissions beyond 700 nm [11]. Despite progress, nonradiative losses and limited stability remain challenges. Molecular engineering approaches such as backbone rigidification and aggregation suppression have improved an external quantum efficiency (EQE) up to 10% in the 800–850 nm range [42,43,44].
Polymer-based NIR LEDs are well-suited for large-area, skin-conformal devices. Low-bandgap copolymers incorporating benzodithiophene, diketopyrrolopyrrole, or isoindigo units enable emissions between 700 and 950 nm [45]. Techniques like orthogonal solvent processing and host–guest blending improve morphology and device stability [46,47]. Though the current EQE (4%) is modest, they align with the low irradiance levels (100 mW/cm2) required for PBM [48]. However, the best EQE of polymer-based NIR LED currently relies on interfacial energy transfer, which is approximately 20% [49].
Both small molecules and polymers are significantly more flexible than group III–V and group IV semiconductors, but they are less stable in comparison. In terms of wavelength tuning, these organic emitters exhibit broadband emission due to their molecular transitions, making them less straightforward than the inorganic semiconductors, as their emission properties depend heavily on the ligands. However, this behavior could change if they are doped with lanthanides.
3.3. Perovskite LEDs
Perovskite-based materials have emerged as versatile candidates for NIR LEDs owing to their tunable bandgaps, high PLQY, and facile fabrication through solution processing [50,51]. Some progresses in perovskite NIR LEDs were already discussed by Liu et al. [9] while they are depicted in Figure 5. In lead-halide perovskites such as CsPbI3 and FAPbI3, NIR emission typically arises from band-edge recombination between the conduction band minimum and valence band maximum, yielding narrowband luminescence in the 700–800 nm range [52,53]. Tin-based analogs, such as CsSnI3, extend the emission toward 950 nm, though the instability of Sn2+ due to oxidation remains a critical issue [54]. Emission wavelength and device efficiency can be further tuned through dimensional engineering, compositional alloying, and surface passivation strategies [9].
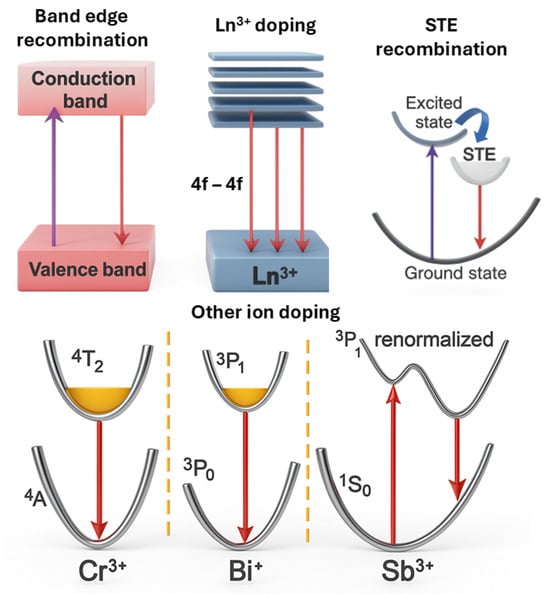
Figure 5.
Conceptual drawings of the mechanisms driving NIR emission in perovskite materials. The purple, red, and blue arrows correspond to the absorption, emission, and energy transfer process, respectively.
Lanthanide ion doping (Ln3+) (e.g., Yb3+, Nd3+, Er3+) introduces sharp atomic-like NIR emissions through 4f transitions, typically in the 900–1600 nm range [55]. Quantum cutting mechanisms in Yb3+-doped systems have yielded the PLQY that exceed 100% [56]. However, PLQY values above 100% obtained under photoluminescence excitation do not directly imply high EQE under electroluminescence. Efficient EL requires not only radiative efficiency at Yb3+ centers but also effective carrier injection and transfer to these sites. Without optimized carrier pathways, the EQE of devices can remain much lower than the PLQY. Co-doping and host lattice optimization, including lead-free alternatives like Cs2AgBiBr6, have shown promise in mitigating toxicity while preserving NIR performance [57]. However, the intrinsically low absorption cross-sections of 4f transitions necessitate sensitizer ions or energy transfer mechanisms to enable efficient excitation [55].
In addition to narrowband emitters, broadband NIR emission can be achieved through self-trapped exciton (STE) recombination, as observed in vacancy-ordered or low-dimensional perovskites like DFPD2CsBiI6 and Bmpip2SnI4 [58,59]. These STEs arise from strong electron–phonon coupling and lattice distortion, offering broad emission suitable for diffuse illumination in PBM or imaging [60]. Optimizing lattice rigidity and minimizing nonradiative losses are key to improving the PLQY in such systems.
Moreover, doping with transition or heavy metal ions, such as Cr3+, Bi+/Bi3+, or
Sb3+, creates localized states in the bandgap, enabling additional NIR transitions. Cr3+-doped double perovskites have achieved emissions from 958 to 1010 nm with a PLQY up to 23% [61,62]. Bi+ and Sb3+ dopants offer emissions in the 900–1015 nm range with efficiencies as high as 70% [9,63].
Perovskite NIR LEDs based on these mechanisms have reached an EQE exceeding 20% in some band-edge-emission devices [64], and continued progress in co-doping and hybrid emission strategies could further expand the spectral range and application space [58,59,65]. Perovskite materials exhibit similar NIR LED properties to those of small molecules and polymers, see Figure 4. However, like III–V and II–VI semiconductors, their emission wavelength can be easily tuned by adjusting the composition of halide ions. Although the emission remains broad, incorporating lanthanide dopants may help in achieving more precise wavelength tuning.
Despite rapid progress, perovskite NIR LEDs still face key bottlenecks, including nonradiative recombination at defect sites, ion migration, and limited operational lifetimes relative to III–V devices [54,64]. Unencapsulated devices often show T5 values of only a few to tens of hours, though improved interfaces and compositions have extended lifetimes of 100 to 300 h [12,54]. For clinical applications of PBM, stability and encapsulation are critical, with >1000 h under 85 °C/85% RH recognized as a benchmark [66,67]. Hybrid polymer–inorganic encapsulation shows promise, but scalable solutions are needed [66]. Photonic strategies such as photonic crystals or microlens arrays can increase outcoupling by 1.5–2×, highlighting their essential role [68,69].
3.4. Non-Toxic Emitters
Toxicity remains a major consideration for in vivo applications, see Figure 4. Most III–V and II–VI semiconductor materials [70] are toxic due to the presence of As, Cd, or Hg, while group IV materials such as SiC are generally considered safe, though some derivatives or fabrication processes may pose risks [39]. In contrast, small-molecule and polymer LEDs are largely composed of carbon-based compounds and are thus more biocompatible [66]. However, their limited optical power output can restrict their effectiveness in PBM applications.
3.5. Comparative Performance Analysis
For PBM applications, device metrics must extend beyond those typically considered for display and lighting. Critical parameters include the emission peak () within the optical therapeutic window, spectral full-width at half maximum (FWHM), external quantum efficiency (EQE), wall-plug efficiency (WPE), and the radiance or irradiance at the treatment plane. Reliability under operation is assessed by the current density at EQE roll-off (), spectral drift during continuous drive (S), and the device half-lifetime () under clinically relevant test conditions. Additionally, for wearable PBM systems, the surface temperature rise (ΔT) during operation is a key safety and comfort parameter.
Table 3 summarizes the reported performance of representative LED device classes suitable for NIR-PBM. The values consolidate data from recent literature on PBM light sources [10,11,12,67,71,72] and emerging optoelectronic materials, together with device physics evaluations from display and lighting research.

Table 3.
Comparison of different LED material classes for NIR photobiomodulation (700–1000 nm). Parameters: emission peak (), full-width at half maximum (FWHM), external quantum efficiency (EQE), wall-plug efficiency (WPE), radiance/irradiance at treatment plane (Rad./Ir.) in (mW/cm2) †, current density at EQE roll-off (), spectral drift (S) in (A/cm2) ‡, with CW test conditions, surface temperature rise (), and references (Ref.).
Although III–V semiconductors remain the benchmark for efficiency and operational stability, their rigid and expensive fabrication limits widespread deployment in wearable devices. Group IV silicon-based emitters offer CMOS compatibility yet are fundamentally limited in radiative efficiency. Groups II–VI quantum dot LEDs and perovskite LEDs deliver attractive spectral control and solution-processability, though both face challenges in stability. Organic small-molecule and polymer LEDs enable mechanical flexibility but generally suffer from low efficiency and short operational lifetimes. Looking forward, the most promising strategies may involve hybrid integration, combining stable inorganic emitters (III–V or II–VI) with lightweight flexible substrates, or advancing perovskite and QLED technologies for cost-effective, spectrally precise, and large-area PBM systems.
From all materials, we believe that perovskites offer a promising path forward, combining the solution processability and the long spectral tunability while lead-free-based perovskites have improved safety profiles. Systems such as Cs2AgBiBr6 and Cs2NaInCl6 doped with lanthanide or transition metal ions have shown the potential for NIR emission without toxicity concerns [9]. Nonetheless, the field of lead-free perovskite NIR LEDs remains in its infancy, with considerable room for performance optimization. Ongoing material development, including improvements in phase stability, PLQY, and energy transfer efficiency, is expected to unlock their full potential for safe and effective PBM.
4. Photonic, Plasmonic and Optoelectronic Engineering for NIR LEDs
Photonic, plasmonic, and optoelectronic engineering strategies are vital to enhancing the performance of NIR LEDs, particularly for PBM applications, as seen in Figure 6. These approaches aim to overcome limitations such as nonradiative recombination, emitter aggregation, and energy-gap law constraints [73]. Nanostructures like photonic crystals and plasmonic arrays can localize electromagnetic fields at the nanoscale, enhancing spontaneous emission via near-field interactions [74,75,76,77,78]. Complementary strategies, including dipole orientation alignment [79,80] and light outcoupling designs [68], further mitigate optical losses and improve the external EQE.
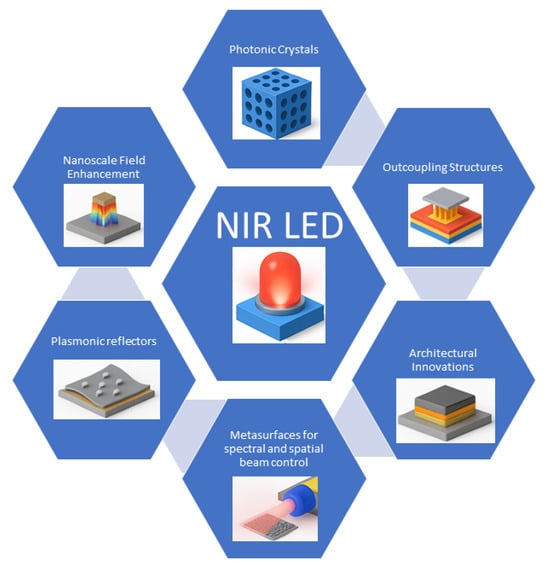
Figure 6.
Key components for photonic, plasmonic, and optoelectronic engineering of NIR LEDs.
Photonic crystals, developed as waveguides, light extraction surfaces, cavities for lasers, and biosensing elements, have been adapted for NIR LED platforms to achieve optical feedback and spectral refinement [81]. Similarly, III–V based light sources integrated with photonic crystal cavities present opportunities for narrowband and directional PBM sources [82,83]. On the emitter front, ZnF2 shell passivation of II–VI and III–V quantum dots significantly enhances the PLQY by reducing surface trap states [84]. Coupling this with in situ photo-crosslinked transport layers leads to better charge balance and device stability, with the reported EQE exceeding 20% [84].
Plasmonic enhancements using embedded nanostructures, such as silver nanoparticles in flexible polymers, recycle internally reflected photons without sacrificing mechanical compliance [74,85]. These features are particularly beneficial for wearable or implantable PBM systems, where conformability is essential. Recent innovations in metasurfaces offer precise spatial and spectral control of NIR emission. Engineered sub-wavelength resonator arrays enable beam shaping and polarization tuning, while chiral plasmonic ceramics facilitate circularly polarized light emission, aiding selectivity for PBM in neuro stimulation [86,87,88].
Plasmonic heating and spin-antenna concepts have also been reported in related optoelectronic systems, and in the context of NIR LED-stack geometries, such plasmonic nanostructures can both enhance light extraction and influence thermal load within wearable arrays, where the emitter–plasmon distance critically determines the balance between radiative enhancement and heat dissipation [89]. Likewise, spin-antenna effects can be understood in engineered cavity–emitter alignment within layered LED stacks [90], promoting directional emission and spectral selectivity for PBM applications. In parallel, the broader field of NIR optoelectronics contributes additional insights into device performance optimization. Miao et al. [69] demonstrated that microcavity top-emission architectures significantly boost light outcoupling efficiency in perovskite LEDs, reaching an EQE of 20.2%. Their angular emission analysis confirmed the role of optical interference effects in maximizing photon extraction. Similarly, Zhang et al. emphasized the outcoupling bottleneck as a major challenge even when the internal quantum efficiency approaches unity.
Device speed and bandwidth are also essential for advanced PBM systems. Takeda et al. demonstrated photonic crystals that achieve a modulation bandwidth of 17.8 GHz, support 25 GHz direct modulation, and operate with ultralow energy making it exceptionally efficient and fast for on-chip or short-range optical interconnect applications [82]. Such technology can be adapted to NIR LEDs for fast-switching PBM applications, e.g., neurostimulation. We expect that for the future, the convergence of photonic, plasmonic, and optoelectronic engineering will drive the development of high-performance NIR LEDs for PBM. From metasurfaces to microcavity designs, these advancements enable not only enhanced emission but also intelligent and personalized therapeutic capabilities.
5. Concluding Remarks and Perspectives
NIR LEDs hold tremendous promise for advancing non-invasive PBM, owing to their ability to deliver therapeutic light to deep tissues with minimal side effects [1,2]. As PBM continues to demonstrate efficacy in treating conditions ranging from chronic wounds to neurodegenerative diseases [4,17], the demand for efficient, safe, and flexible NIR emitters is growing rapidly. In this context, perovskite-based NIR LEDs have emerged as a compelling alternative to traditional inorganic semiconductors and organic emitters. Lead halide perovskites offer narrowband emission and high photoluminescence quantum yields, while tin-based, lanthanide-doped, and vacancy-ordered perovskites extend emission into clinically relevant NIR wavelengths [12,13,58]. However, material instability, particularly under operational stress, remains a persistent barrier. Further research into oxidation-resistant compositions and passivation strategies will be essential for device longevity [54,59]. The integration of photonic and plasmonic engineering represents a synergistic pathway to overcome the intrinsic limitations of NIR materials. Optical cavity designs, dipole alignment, and plasmonic nanostructures have already shown promising enhancements in light outcoupling and emission intensity [14,74,80]. In the future, metasurfaces, anisotropic nanostructures, and thermally adaptive photonic platforms offer exciting frontiers for dynamic spectral control [86,91]. From a translational perspective, biocompatibility and toxicity remain central concerns for clinical deployment. Recent lead-free perovskites are gaining attention as safer alternatives, but their performance in long-term and in vivo settings is not yet fully validated [57,66]. Comprehensive toxicological studies and scalable synthesis methods are critical next steps toward clinical integration. In summary, the convergence of advanced materials science, photonic engineering, and biomedical research is catalyzing the development of next-generation NIR LEDs tailored for PBM. Interdisciplinary collaboration will be pivotal in transforming laboratory-scale innovations into practical, wearable, and patient-friendly therapeutic tools. Future work should prioritize materials stability, device reliability, spectral specificity, and biocompatibility to fully unlock the therapeutic potential of engineered NIR light.
Funding
S.M. acknowledges the support by the European Commission and the Polish National Science Centre (NCN) under the Marie Skłodowska-Curie COFUND grant (POLONEZ BIS 2) under Agreement No: UMO-2022/45/P/ST3/04170. M.D.B. acknowledges the supports from CINTILIGHT (no. 0005/2024/UB) and the NCN under grant OPUS-24 no. 2022/47/B/ST5/01966.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors acknowledge the constant support of Łukasiewicz PORT in the completion of this work.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Lee, S.Y.; Jeon, S.; Kwon, Y.W.; Kwon, M.; Kang, M.S.; Seong, K.Y.; Park, T.E.; Yang, S.Y.; Han, D.W.; Hong, S.W.; et al. Combinatorial wound healing therapy using adhesive nanofibrous membrane equipped with wearable LED patches for photobiomodulation. Sci. Adv. 2022, 8, e1646. [Google Scholar] [CrossRef]
- Yadav, A.; Gupta, A. Noninvasive red and near-infrared wavelength-induced photobiomodulation: Promoting impaired cutaneous wound healing. Photodermatol. Photoimmunol. Photomed. 2017, 33, 4–13. [Google Scholar] [CrossRef]
- Purushothuman, S.; Johnstone, D.M.; Nandasena, C.; Mitrofanis, J.; Stone, J. Photobiomodulation with near infrared light mitigates Alzheimer’s disease-related pathology in cerebral cortex–evidence from two transgenic mouse models. Alzheimer’s Res. Ther. 2014, 6, 2. [Google Scholar] [CrossRef]
- Min, P.K.; Goo, B.L. 830 nm light emitting diode low level light therapy (LED-LLLT) enhances wound healing: A preliminary study. Lasers Surg. Med. 2013, 22, 43–49. [Google Scholar] [CrossRef]
- Oyebode, O.; Houreld, N.N.; Abrahamse, H. Photobiomodulation in Diabetic Wound Healing: A Review of Red and Near-infrared Wavelength Applications. Cell Biochem. Funct. 2021, 39, 596–612. [Google Scholar] [CrossRef] [PubMed]
- Cha, G.D.; Kim, D.; Kim, D.C. Wearable and Implantable Light-Emitting Diodes and Their Biomedical Applications. Korean J. Chem. Eng. 2024, 41, 1–24. [Google Scholar] [CrossRef]
- Felician, M.C.P.; Belotto, R.; Tardivo, J.P.; Baptista, M.S.; Martins, W.K. Photobiomodulation: Cellular, molecular, and clinical aspects. J. Photochem. Photobiol. B 2023, 17, 100197. [Google Scholar] [CrossRef]
- Nairuz, T.; Cho, S.; Lee, J.H. Photobiomodulation Therapy on Brain: Pioneering an Innovative Approach to Revolutionize Cognitive Dynamics. Cells 2024, 13, 966. [Google Scholar] [CrossRef]
- Liu, Y.; Di Stasio, F.; Bi, C.; Zhang, J.; Xia, Z.; Shi, Z.; Manna, L. Near-Infrared Light Emitting Metal Halides: Materials, Mechanisms, and Applications. Adv. Mater. 2024, 36, 2312482. [Google Scholar] [CrossRef]
- Triana, M.A.; Restrepo, A.A.; Lanzafame, R.J.; Palomaki, P.; Dong, Y. Quantum dot light-emitting diodes as light sources in photomedicine: Photodynamic therapy and photobiomodulation. J. Phys. Mater. 2020, 3, 032002. [Google Scholar] [CrossRef]
- Xiong, W.; Zhang, C.; Fang, Y.; Peng, M.; Sun, W. Progresses and Perspectives of Near-Infrared Emission Materials with “Heavy Metal-Free” Organic Compounds for Electroluminescence. Polymers 2023, 15, 98. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, Z.; Zhang, J.; He, Y.; Dai, J.; Li, X.; Shi, Z.; Manna, L. Light-Emitting Diodes Based on Metal Halide Perovskite and Perovskite Related Nanocrystals. Adv. Mater. 2025, 37, 2415606. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Jung, J.G.; Lee, Y.J.; Park, M.H. Lead-Free Halide Perovskite Nanocrystals for Light-Emitting Diodes. Materials 2023, 16, 6317. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Zhou, J.; Yan, F.; Gao, M.; Tang, J.; Huang, L.; Luo, Y. Unlocking the potential of photobiomodulation therapy for brain neurovascular coupling: The biological effects and medical applications. J. Cereb. Blood Flow Metab. 2025, 45, 800–830. [Google Scholar] [CrossRef]
- Jeon, Y.; Choi, H.R.; Kwon, J.H.; Choi, S.; Nam, K.M.; Park, K.C.; Choi, K.C. Sandwich-structure transferable free-form OLEDs for wearable and disposable skin wound photomedicine. Light Sci. Appl. 2019, 8, 114. [Google Scholar] [CrossRef]
- Desmet, K.D.; Paz, D.A.; Corry, J.J.; Eells, J.T.; Wong-Riley, M.T.; Henry, M.M.; Buchmann, E.V.; Connelly, M.P.; Dovi, J.V.; Liang, H.L.; et al. Clinical and Experimental Applications of NIR-LED Photobiomodulation. Photomed. Laser Surg. 2006, 24, 121–128. [Google Scholar] [CrossRef]
- Yokomizo, S.; Kopp, T.; Roessing, M.; Morita, A.; Lee, S.; Cho, S.; Ogawa, E.; Komai, E.; Inoue, K.; Fukushi, M.; et al. Near-Infrared II Photobiomodulation Preconditioning Ameliorates Stroke Injury via Phosphorylation of eNOS. Stroke 2024, 55, 1641–1649. [Google Scholar] [CrossRef]
- Ivandic, B.; Ivandic, T. Low-level laser therapy improves vision in patients with age-related macular degeneration. Photomed. Laser Surg. 2008, 26, 241–245. [Google Scholar] [CrossRef]
- Tang, J.; Herda, A.; Kern, T. Photobiomodulation in the treatment of patients with non-center-involving diabetic macular oedema. Br. J. Ophthalmol. 2014, 98, 1013–1015. [Google Scholar] [CrossRef]
- Geneva, I.I. Photobiomodulation for the treatment of retinal diseases: A review. Int. J. Ophthalmol. 2016, 9, 145–152. [Google Scholar] [CrossRef]
- Natoli, R.; Valter, K.; Barbosa, M.; Dahlstrom, J.; Rutar, M.; Kent, A.; Provis, J. 670 nm photobiomodulation as a novel protection against retinopathy of prematurity: Evidence from oxygen induced retinopathy models. PLoS ONE 2013, 8, e72135. [Google Scholar] [CrossRef]
- Ivandic, B.; Ivandic, T. Low-level laser therapy improves visual acuity in adolescent and adult patients with amblyopia. Photomed. Laser Surg. 2012, 30, 167–171. [Google Scholar] [CrossRef]
- Ivandic, B.; Ivandic, T. Low-level laser therapy improves vision in a patient with retinitis pigmentosa. Photomed. Laser Surg. 2014, 32, 181–184. [Google Scholar] [CrossRef]
- Eells, J.; Henry, M.; Summerfelt, P.; Wong-Riley, M.; Buchmann, E.; Kane, M.; Whelan, N.; Whelan, H. Therapeutic photobiomodulation for methanol-induced retinal toxicity. Proc. Natl. Acad. Sci. USA 2003, 100, 3439–3444. [Google Scholar] [CrossRef]
- Martins, M.D.; Marques, M.M.; Esteves-Pereira, T.C.; Arany, P.R. Basic Principles of Physics in Photobiomodulation. In Photobiomodulation Therapy in Oral Medicine: Evidence-Based Clinical Protocols; Springer Nature: Cham, Switzerland, 2025; pp. 3–10. [Google Scholar] [CrossRef]
- IUPAC. Compendium of Chemical Terminology, 5th ed.; The “Gold Book”; IUPAC: Research Triangle Park, NC, USA, 2025; p. 409. [Google Scholar] [CrossRef]
- Zein, R.; Selting, W.; Hamblin, M. Review of light parameters and photobiomodulation efficacy: Dive into complexity. J. Biomed. Opt. 2018, 23, 120901. [Google Scholar] [CrossRef] [PubMed]
- Lampl, Y.; Zivin, J.A.; Fisher, M.; Lew, R.; Welin, L.; Dahlof, B.; Borenstein, P.; Andersson, B.; Perez, J.; Caparo, C.; et al. Infrared Laser Therapy for Ischemic Stroke: A New Treatment Strategy. Stroke 2007, 38, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Morries, L.; Cassano, P.; Henderson, T. Treatments for traumatic brain injury with emphasis on transcranial near-infrared laser phototherapy. Neuropsychiatr. Dis. Treat. 2015, 11, 2159. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, F.; Johnston, A.L.; Ravichandran, C.; Polcari, A.; Teicher, M.H.; Webb, R.H.; Hamblin, M.R. Psychological benefits 2 and 4 weeks after a single treatment with near infrared light to the forehead: A pilot study of 10 patients with major depression and anxiety. Behav. Brain Funct. 2009, 5, 46. [Google Scholar] [CrossRef]
- Rathnakar, B.; Rao, B.S.S.; Prabhu, V.; Chandra, S.; Rai, S.; Rao, A.C.K.; Sharma, M.; Gupta, P.K.; Mahato, K.K. Photo-biomodulatory response of low-power laser irradiation on burn tissue repair in mice. Photodermatol. Photoimmunol. Photomed. 2016, 33, 4–13. [Google Scholar] [CrossRef]
- Gupta, A.; Keshri, G.K.; Yadav, A.; Gola, S.; Chauhan, S.; Salhan, A.K.; Bala Singh, S. Superpulsed (Ga-As, 904 nm) low-level laser therapy (LLLT) attenuates inflammatory response and enhances healing of burn wounds. J. Biophotonics 2015, 8, 489–501. [Google Scholar] [CrossRef]
- Cronshaw, M.; Parker, S.; Grootveld, M.; Lynch, E. Photothermal Effects of High-Energy Photobiomodulation Therapies: An In Vitro Investigation. Biomedicines 2023, 11, 1634. [Google Scholar] [CrossRef] [PubMed]
- Sze, S.M.; Ng, K.K. Physics of Semiconductor Devices, 3rd ed.; Wiley-Interscience: Hoboken, NJ, USA, 2006. [Google Scholar] [CrossRef]
- Haggren, T.; Tan, H.H.; Jagadish, C. III–V Thin Films for Flexible, Cost-Effective, and Emerging Applications in Optoelectronics and Photonics. Acc. Mater. Res. 2023, 4, 1046–1056. [Google Scholar] [CrossRef]
- Zeinalvand Farzin, B.; Seyedein Ardebili, S.B.; Kang, T.I.; Kim, J.S.; Nguyen, P.D.; Lee, S.J. Photoreflectance Analysis of InAsPSb/InGaAs Multi-Quantum Well LED Structures with Different Well/Barrier Numbers. Photonics 2024, 11, 277. [Google Scholar] [CrossRef]
- Zhao, C.; Li, Z.; Tang, T.; Sun, J.; Zhan, W.; Xu, B.; Sun, H.; Jiang, H.; Liu, K.; Qu, S.; et al. Novel III-V semiconductor epitaxy for optoelectronic devices through two-dimensional materials. Prog. Quantum Electron. 2021, 76, 100313. [Google Scholar] [CrossRef]
- Fuchs, F.; Soltamov, V.; Väth, S.; Baranov, P.; Mokhov, E.; Astakhov, G.; Dyakonov, V. Silicon carbide light-emitting diode as a prospective room temperature source for single photons. Sci. Rep. 2013, 3, 1637. [Google Scholar] [CrossRef]
- Jain, J.; Hryciw, A.; Baer, T.; Miller, D.; Brongersma, M.; Howe, R. A micromachining-based technology for enhancing germanium light emission via tensile strain. Nat. Photonics 2012, 6, 398–405. [Google Scholar] [CrossRef]
- Bera, D.; Qian, L.; Tseng, T.K.; Holloway, P.H. Quantum Dots and Their Multimodal Applications: A Review. Materials 2010, 3, 2260–2345. [Google Scholar] [CrossRef]
- Prudnikau, A.; Roshan, H.; Paulus, F.; Martín-García, B.; Hübner, R.; Bahmani Jalali, H.; De Franco, M.; Prato, M.; Di Stasio, F.; Lesnyak, V. Efficient Near-Infrared Light-Emitting Diodes Based on CdHgSe Nanoplatelets. Adv. Funct. Mater. 2024, 34, 2310067. [Google Scholar] [CrossRef]
- Hu, X.; Mi, J.; Qin, A.; Zhu, C.; Chen, Z.; Yang, Z.; Huang, W. Retrofitting NIR-II absorbing organic semiconducting fluorophores for reinvigorating deep-tissue fluorescence bioimaging. Coord. Chem. Rev. 2025, 545, 216992. [Google Scholar] [CrossRef]
- dos Santos, P.L.; Stachelek, P.; Takeda, Y.; Pander, P. Recent advances in highly-efficient near infrared OLED emitters. Mater. Chem. Front. 2024, 8, 1731–1766. [Google Scholar] [CrossRef]
- Cho, H.H.; Gorgon, S.; Londi, G.; Giannini, S.; Cho, C.; Ghosh, P.; Tonnelé, C.; Casanova, D.; Olivier, Y.; Baikie, T.; et al. Efficient near-infrared organic light-emitting diodes with emission from spin doublet excitons. Nat. Photonics 2024, 18, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Kamya, E.; Lu, Z.; Cao, Y.; Pei, R. Effective design of organic luminogens for near-infrared-II fluorescence imaging and photo-mediated therapy. J. Mater. Chem. B 2022, 10, 9770–9788. [Google Scholar] [CrossRef] [PubMed]
- Burns, S.; Macleod, J.; Do, T.T.; Sonar, P.; Yambem, S. Effect of thermal annealing Super Yellow emissive layer on efficiency of OLEDs. Sci. Rep. 2017, 7, 40805. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sachnik, O.; van der Zee, B.; Thakur, K.; Ramanan, C.; Wetzelaer, G.J.A.H.; Blom, P.W.M. Universal Electroluminescence at Voltages below the Energy Gap in Organic Light-Emitting Diodes. Adv. Opt. Mater. 2021, 9, 2101149. [Google Scholar] [CrossRef]
- Zhang, R.; Qu, J. The Mechanisms and Efficacy of Photobiomodulation Therapy for Arthritis: A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 14293. [Google Scholar] [CrossRef]
- Hung, C.M.; Wang, S.F.; Chao, W.C.; Li, J.L.; Chen, B.H.; Lu, C.H.; Tu, K.Y.; Yang, S.D.; Hung, W.Y.; Chi, Y.; et al. High-performance near-infrared OLEDs maximized at 925 nm and 1022 nm through interfacial energy transfer. Nat. Commun. 2024, 15, 4664. [Google Scholar] [CrossRef]
- Birowosuto, M.D. Photonics Gets a Makeover: The New Era of Perovskite Devices. Micromachines 2025, 16, 832. [Google Scholar] [CrossRef]
- Mahato, S.; Roy, B.; Bose, S.; Sangwan, S.K.; Das, N.C.; Birowosuto, M.D.; Ray, S.K. Atomically Precise Ruddlesden–Popper Faults Induced Enhanced Emission in Ligand Stabilized Mixed Halide Perovskites. Adv. Mater. 2025, 37, e03680. [Google Scholar] [CrossRef]
- Zhao, X.; Tan, Z.K. Large-area near-infrared perovskite light-emitting diodes. Nat. Photonics 2020, 14, 215–218. [Google Scholar] [CrossRef]
- Xie, C.; Zhao, X.; Ong, E.; Tan, Z.K. Transparent near-infrared perovskite light-emitting diodes. Nat. Commun. 2020, 11, 4213. [Google Scholar] [CrossRef]
- Yuan, F.; Folpini, G.; Liu, T.; Singh, U.; Treglia, A.; Lim, J.W.M.; Klarbring, J.; Simak, S.; Abrikosov, I.; Sum, T.C.; et al. Bright and stable near-infrared lead-free perovskite light-emitting diodes. Nat. Photonics 2024, 18, 170–176. [Google Scholar] [CrossRef]
- Hebbink, G.A.; Grave, L.; Woldering, L.A.; Reinhoudt, D.N.; van Veggel, F.C.J.M. Unexpected Sensitization Efficiency of the Near-Infrared Nd3+, Er3+, and Yb3+ Emission by Fluorescein Compared to Eosin and Erythrosin. J. Phys. Chem. A 2003, 107, 2483–2491. [Google Scholar] [CrossRef]
- Loh, S.M.; Jing, Y.; Sum, T.C.; Bruno, A.; Mhaisalkar, S.G.; Blundell, S.A. Mechanism of Quantum Cutting in Yb-Doped CsPbCl3. J. Phys. Chem. Lett. 2025, 16, 2295–2300. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, F.; Guo, K.; Horn, J.; Sorrentino, R.; Conforto, G.; Lamberti, F.; Brescia, R.; Drago, F.; Prato, M.; He, Z.; et al. Lanthanide-Induced Photoluminescence in Lead-Free Cs2AgBiBr6 Bulk Perovskite: Insights from Optical and Theoretical Investigations. J. Phys. Chem. Lett. 2020, 11, 8893–8900. [Google Scholar] [CrossRef] [PubMed]
- Bai, T.; Wang, X.; He, Y.; Wei, H.; Su, Y.; Chen, J. Turning Self-Trapped Exciton Emission to Near-Infrared Region in Thermochromism Zero-Dimensional Hybrid Metal Halides. Adv. Opt. Mater. 2023, 11, 2301110. [Google Scholar] [CrossRef]
- Sun, P.P.; Kripalani, D.R.; Hao, M.; Chi, W.; Li, W.; Zhou, K. Emissive Nature and Molecular Behavior of Zero-Dimensional Organic–Inorganic Metal Halides Bmpip2MX4. J. Phys. Chem. Lett. 2020, 11, 5234–5240. [Google Scholar] [CrossRef]
- Timmers, H.; Kowligy, A.; Lind, A.; Cruz, F.C.; Nader, N.; Silfies, M.; Ycas, G.; Allison, T.K.; Schunemann, P.G.; Papp, S.B.; et al. Molecular fingerprinting with bright, broadband infrared frequency combs. Optica 2018, 5, 727–732. [Google Scholar] [CrossRef]
- Fu, X.; Li, H.; Yue, H.; Li, Z.; Feng, J.; Zhang, H. Cr3+/Yb3+ Codoped Cs2NaInCl6 Double Perovskites for Near-Infrared Light-Emitting Diodes. Inorg. Chem. 2025, 64, 8782–8791. [Google Scholar] [CrossRef]
- Jeevaraj, M.; Sivaganesh, D.; Saravanakumar, S.; Asath Bahadur, S.; Sudhahar, S.; Krishna Kumar, M. Broadband near infrared emission in Cr3+: Cs2AgBiCl6 double perovskite halides. Opt. Mater. 2023, 143, 114294. [Google Scholar] [CrossRef]
- Arfin, H.; Kshirsagar, A.S.; Kaur, J.; Mondal, B.; Xia, Z.; Chakraborty, S.; Nag, A. ns2 Electron (Bi3+ and Sb3+) Doping in Lead-Free Metal Halide Perovskite Derivatives. Chem. Mater. 2020, 32, 10255–10267. [Google Scholar] [CrossRef]
- Lee, T.W. Over a Decade of Progress in Metal-Halide Perovskite Light-Emitting Diodes. Adv. Mater. 2025, 37, 2508542. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Zhang, X.; Yin, W.; Wang, Y.; Wang, H.; Lu, M.; Li, Z.; Gu, Z.; Yu, W.W. Yb3+ and Yb3+/Er3+ doping for near-infrared emission and improved stability of CsPbCl3 nanocrystals. J. Mater. Chem. C 2018, 6, 10101–10105. [Google Scholar] [CrossRef]
- Guo, K.; Righetto, M.; Minotto, A.; Zampetti, A.; Cacialli, F. Non-toxic near-infrared LEDs. iScience 2021, 24, 102545. [Google Scholar] [CrossRef] [PubMed]
- Heiskanen, V.; Hamblin, M.R. Photobiomodulation: Lasers vs. light emitting diodes? Photochem. Photobiol. Sci. 2018, 17, 1003–1017. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Cheng, L.; Li, Y.; Li, W.; Chen, J.; Lee, S.; Tang, J. High-Efficiency Perovskite Light-Emitting Diodes with Synergetic Outcoupling Enhancement. Adv. Mater. 2019, 31, e1901517. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Cheng, L.; Zou, W.; Gu, L.; Zhang, J.; Guo, Q.; Peng, Q.; Xu, M.; He, Y.; Zhang, S.; et al. Microcavity top-emission perovskite light-emitting diodes. Light Sci. Appl. 2020, 9, 89. [Google Scholar] [CrossRef]
- Medintz, I.L.; Uyeda, H.T.; Goldman, E.R.; Mattoussi, H. Quantum Dot Bioconjugates for Imaging, Labelling and Sensing. Nat. Mater. 2005, 4, 435–446. [Google Scholar] [CrossRef]
- Hamblin, M.R. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2018, 5, 337–361. [Google Scholar] [CrossRef]
- Cronshaw, M.; Parker, S.; Hamadah, O.; Arnabat-Dominguez, J.; Grootveld, M. Photobiomodulation LED Devices for Home Use: Design, Function and Potential: A Pilot Study. Dent. J. 2025, 13, 76. [Google Scholar] [CrossRef]
- Yin, W.; Zhang, X.; Yang, X.; Rogach, A.L.; Zheng, W. Emitter structure design of near-infrared quantum dot light-emitting devices. Mater. Today 2023, 67, 446–467. [Google Scholar] [CrossRef]
- Makowski, M.; Ye, W.; Kowal, D.; Maddalena, F.; Mahato, S.; Amrillah, Y.T.; Zajac, W.; Witkowski, M.E.; Drozdowski, K.J.; Nathaniel; et al. Scaling Up Purcell-Enhanced Self-Assembled Nanoplasmonic Perovskite Scintillators into the Bulk Regime. Adv. Mater. 2025, 37, e2417874. [Google Scholar] [CrossRef]
- Ye, W.; Yong, Z.; Go, M.; Kowal, D.; Maddalena, F.; Tjahjana, L.; Wang, H.; Arramel, A.; Dujardin, C.; Birowosuto, M.D.; et al. The Nanoplasmonic Purcell Effect in Ultrafast and High-Light-Yield Perovskite Scintillators. Adv. Mater. 2024, 36, 2309410. [Google Scholar] [CrossRef] [PubMed]
- Yusof, N.N.; Hashim, S.; Ghoshal, S.K.; Azlan, M.N.; Zaid, M.H.M.; Boukhris, I.; Kebaili, I. Spectrographic analysis of zinc-sulfate-magnesium-phosphate glass containing neodymium ions: Impact of silver–gold nanoparticles plasmonic coupling. J. Lumin. 2022, 242, 118571. [Google Scholar] [CrossRef]
- Bae, J.Y.; Nam, K.H.; Jeong, C.B.; Kim, G.H.; Chang, K.S. Numerical analysis for characterization of the gold nanorod mediated-plasmonic heating with temporary NIR laser radiation for superficial breast cancer therapy. In Proceedings of the SPIE-The International Society for Optical Engineering, San Diego, CA, USA, 28 August–1 September 2016; Volume 9921, p. 992129. [Google Scholar] [CrossRef]
- Jaffe, T.; Sorias, O.; Gal, L.; Kalish, R.; Orenstein, M. Plasmonic ‘templar cross’ antennas for subwavelength addressing of spin states in diamonds. In Proceedings of the 2016 IEEE Photonics Conference, IPC 2016, Waikoloa, HI, USA, 2–6 October 2016; pp. 204–205. [Google Scholar] [CrossRef]
- Song, N.; Xue, J.; Chen, Y.; Xin, X.; Wu, J.; Liang, N.; Ye, L.; Zhai, T.; Wang, Z. Meniscus-Guided Molecular Alignment for High-Efficiency Solution-Processed DR/NIR-OLEDs. Adv. Opt. Mater. 2025, e01346. [Google Scholar] [CrossRef]
- Ly, K.T.; Chen-Cheng, R.W.; Lin, H.W.; Shiau, Y.J.; Liu, S.H.; Chou, P.T.; Tsao, C.S.; Huang, Y.C.; Chi, Y. Near-infrared organic light-emitting diodes with very high external quantum efficiency and radiance. Nat. Photonics 2017, 11, 63–68. [Google Scholar] [CrossRef]
- Kumela, A.G.; Gemta, A.B.; Hordofa, A.K.; Birhanu, R.; Mekonnen, H.D.; Sherefedin, U.; Weldegiorgis, K. A Review on Hybridization of Plasmonic and Photonic Crystal Biosensors for Effective Cancer Cell Diagnosis. Nanoscale Adv. 2023, 5, 6382–6399. [Google Scholar] [CrossRef]
- Takeda, K.; Sato, T.; Shinya, A.; Nozaki, K.; Kobayashi, W.; Taniyama, H.; Notomi, M.; Hasebe, K.; Kakitsuka, T.; Matsuo, S. Few-fJ/bit data transmissions using directly modulated lambda-scale embedded active region photonic-crystal lasers. Nat. Photonics 2013, 7, 569–575. [Google Scholar] [CrossRef]
- Birowosuto, M.D.; Yokoo, A.; Zhang, G.; Tateno, K.; Kuramochi, E.; Taniyama, H.; Takiguchi, M.; Notomi, M. Movable high-Q nanoresonators realized by semiconductor nanowires on a Si photonic crystal platform. Nat. Mater. 2014, 13, 279–285. [Google Scholar] [CrossRef]
- Li, H.; Zhang, W.; Bian, Y.; Ahn, T.K.; Shen, H.; Ji, B. ZnF2-Assisted Synthesis of Highly Luminescent InP/ZnSe/ZnS Quantum Dots for Efficient and Stable Electroluminescence. Nano Lett. 2022, 22, 4067–4073. [Google Scholar] [CrossRef]
- Khan, A.U.; Guo, Y.; Chen, X.; Liu, G. Spectral-selective plasmonic polymer nanocomposites across the visible and near-infrared. ACS Nano 2019, 13, 4255–4266. [Google Scholar] [CrossRef]
- Amirhosseini, S.A.; Maram, D.K. Quality factor engineering in NIR optical metasurfaces using Ge2Sb2Te5 and graphene. In Proceedings of the IEEE Sensors, Kobe, Japan, 20–23 October 2024; pp. 1–4. [Google Scholar] [CrossRef]
- Hasan, R.; Mollah, A.; Utsob, S. High-Q quasi-BIC metasurfaces for optical trapping of nanoparticles. Opt. Contin. 2025, 4, 1104–1117. [Google Scholar] [CrossRef]
- Shao, X.; Zhu, C.; Kumar, P.; Wang, Y.; Lu, J.; Cha, M.; Yao, L.; Cao, Y.; Mao, X.; Heinz, H.; et al. Voltage-modulated untwist deformations and multispectral optical effects from ion intercalation into chiral ceramic nanoparticles. Adv. Mater. 2023, 35, 2206956. [Google Scholar] [CrossRef]
- Liang, Z.; Sun, J.; Jiang, Y.; Jiang, L.; Chen, X. Plasmonic Enhanced Optoelectronic Devices. Plasmonics 2014, 9, 859–866. [Google Scholar] [CrossRef]
- Koleják, P.; Lezier, G.; Vala, D.; Mathmann, B.; Halagačka, L.; Gelnárová, Z.; Dusch, Y.; Lampin, J.F.; Tiercelin, N.; Postava, K.; et al. Maximizing the Electromagnetic Efficiency of Spintronic Terahertz Emitters. Adv. Photon. Res. 2024, 5, 2400064. [Google Scholar] [CrossRef]
- Zavidovskiy, I.A.; Martynov, I.V.; Tselikov, D.I.; Syuy, A.V.; Popov, A.A.; Novikov, S.M.; Kabashin, A.V.; Arsenin, A.V.; Tselikov, G.I.; Volkov, V.S.; et al. Leveraging femtosecond laser ablation for tunable near-infrared optical properties in MoS2-Gold nanocomposites. Nanomaterials 2024, 14, 1961. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).