Enhanced Photoelectrochemical Performance of BiVO4 Photoanodes Co-Modified with Borate and NiFeOx
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Instruments
2.3. Preparation of BiVO4 Electrodes
2.4. Preparation of B/BiVO4/NiFeOx Photoelectrodes
2.5. PEC Characterization and Evaluation Methods of Photoanodes
3. Results and Analysis
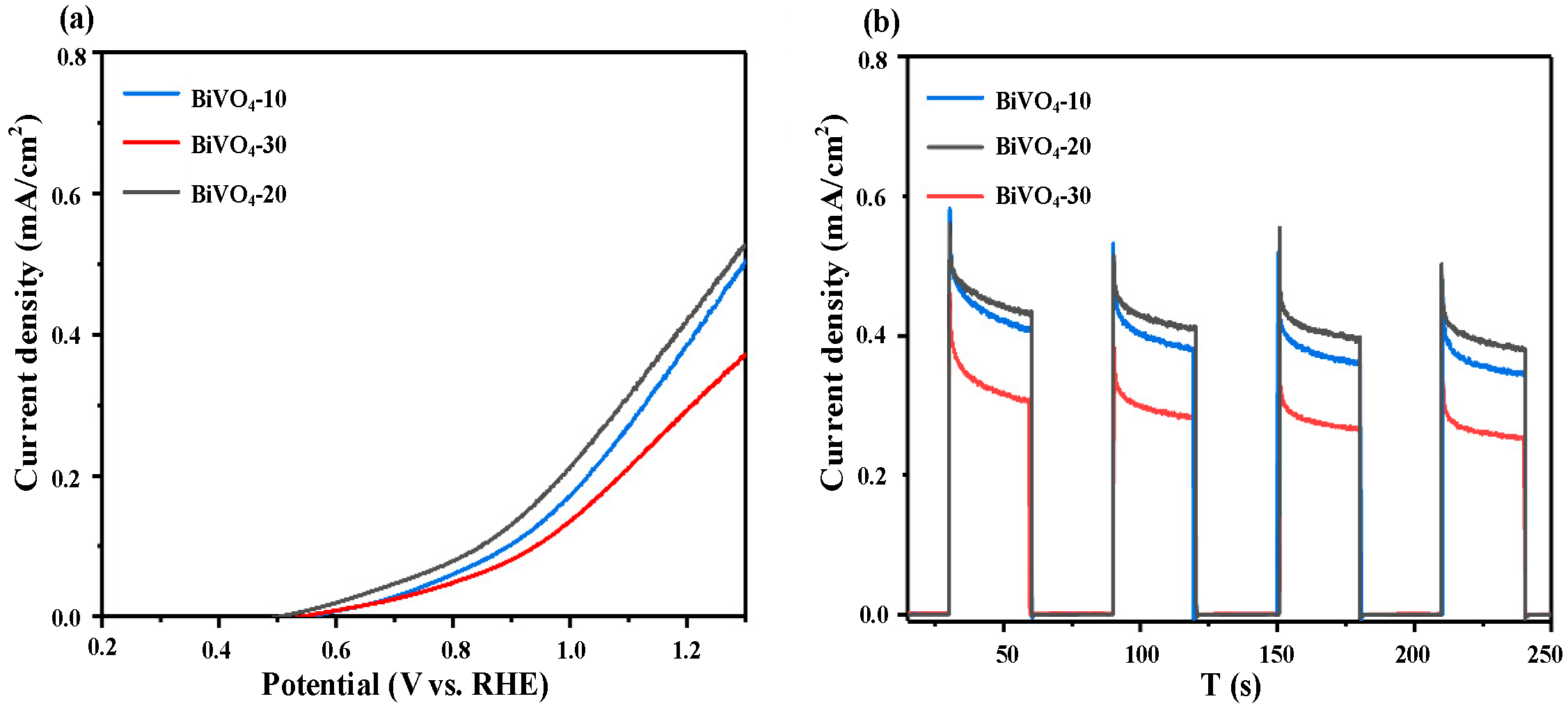
3.1. Characterization of BiVO4 Photoanode
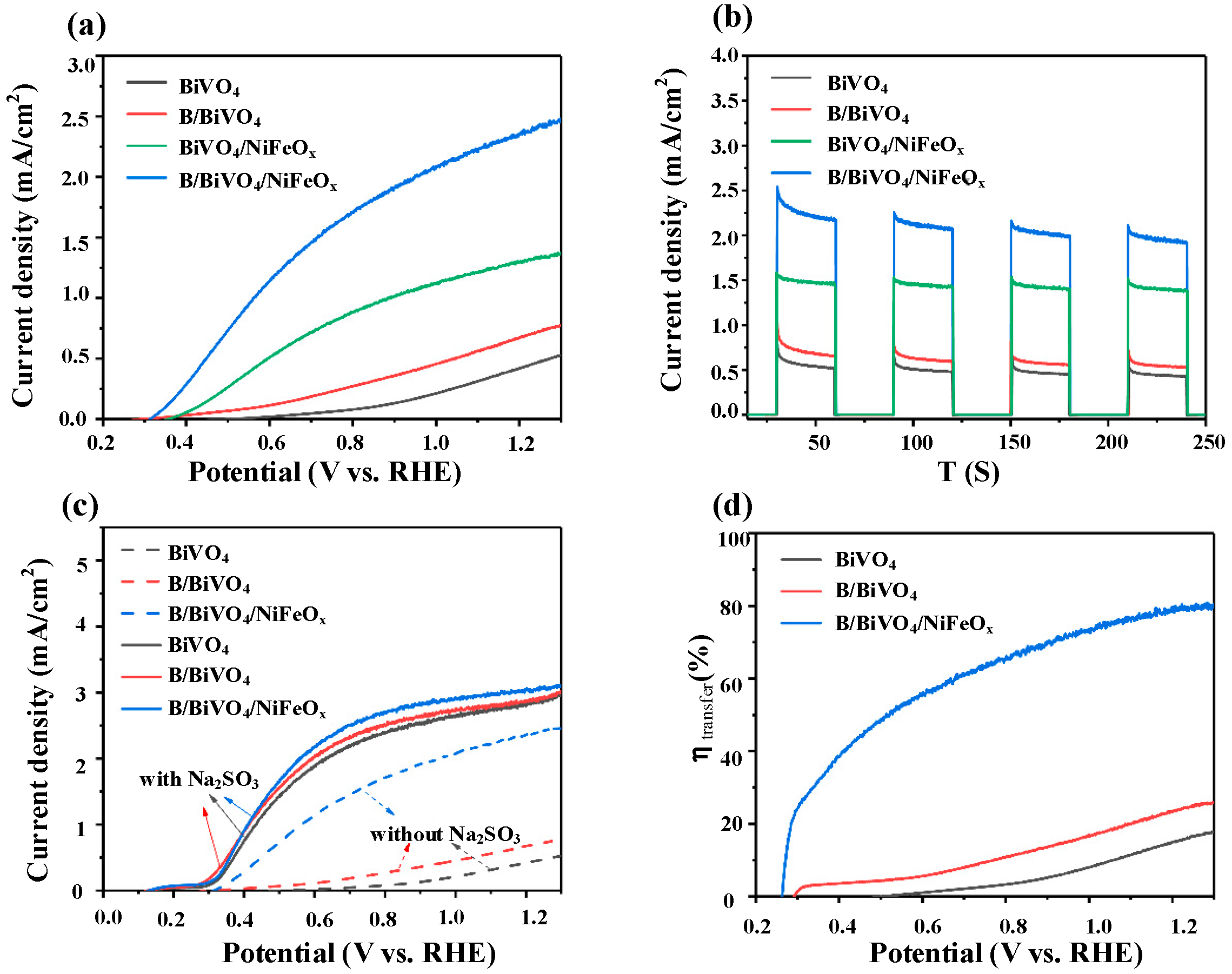
3.2. Characterization of B/BiVO4/NiFeOx; Photoelectrodes
3.2.1. Morphological Structure Analysis
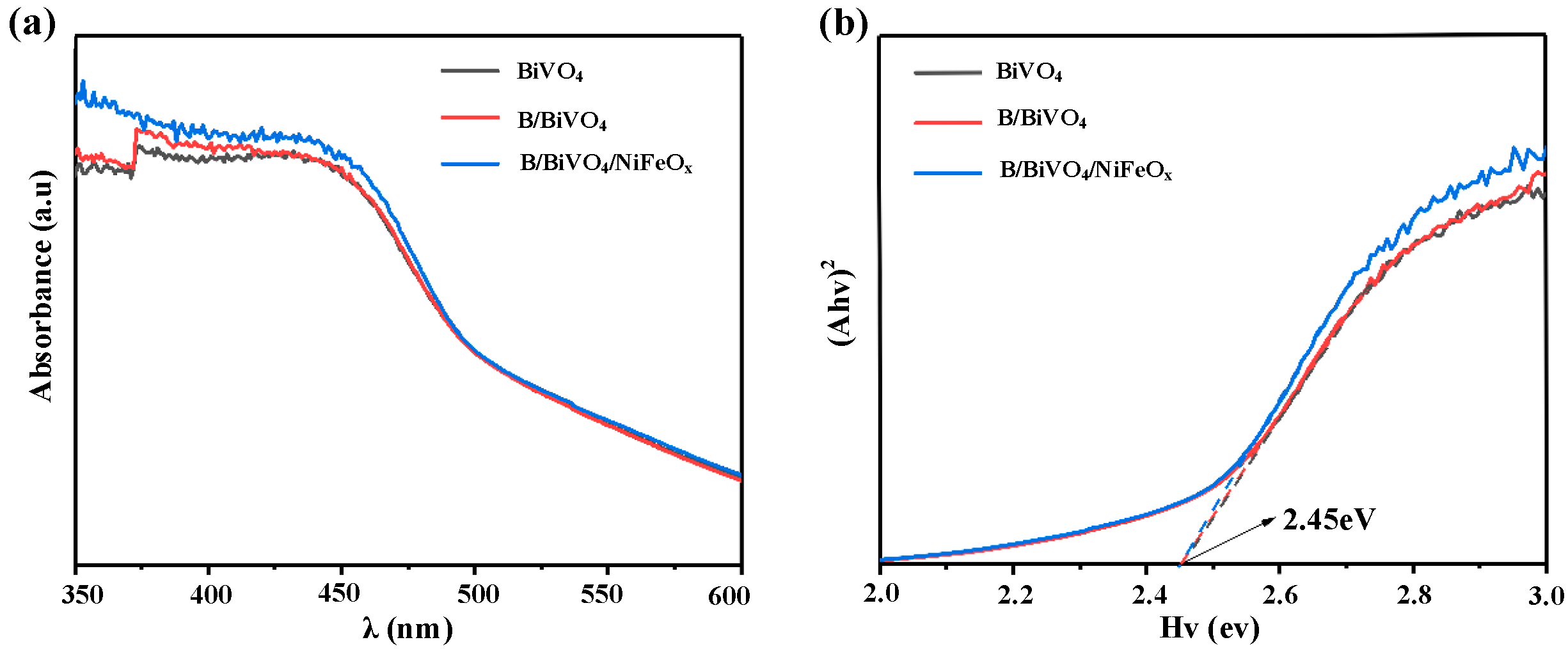
3.2.2. Optical Property Analysis
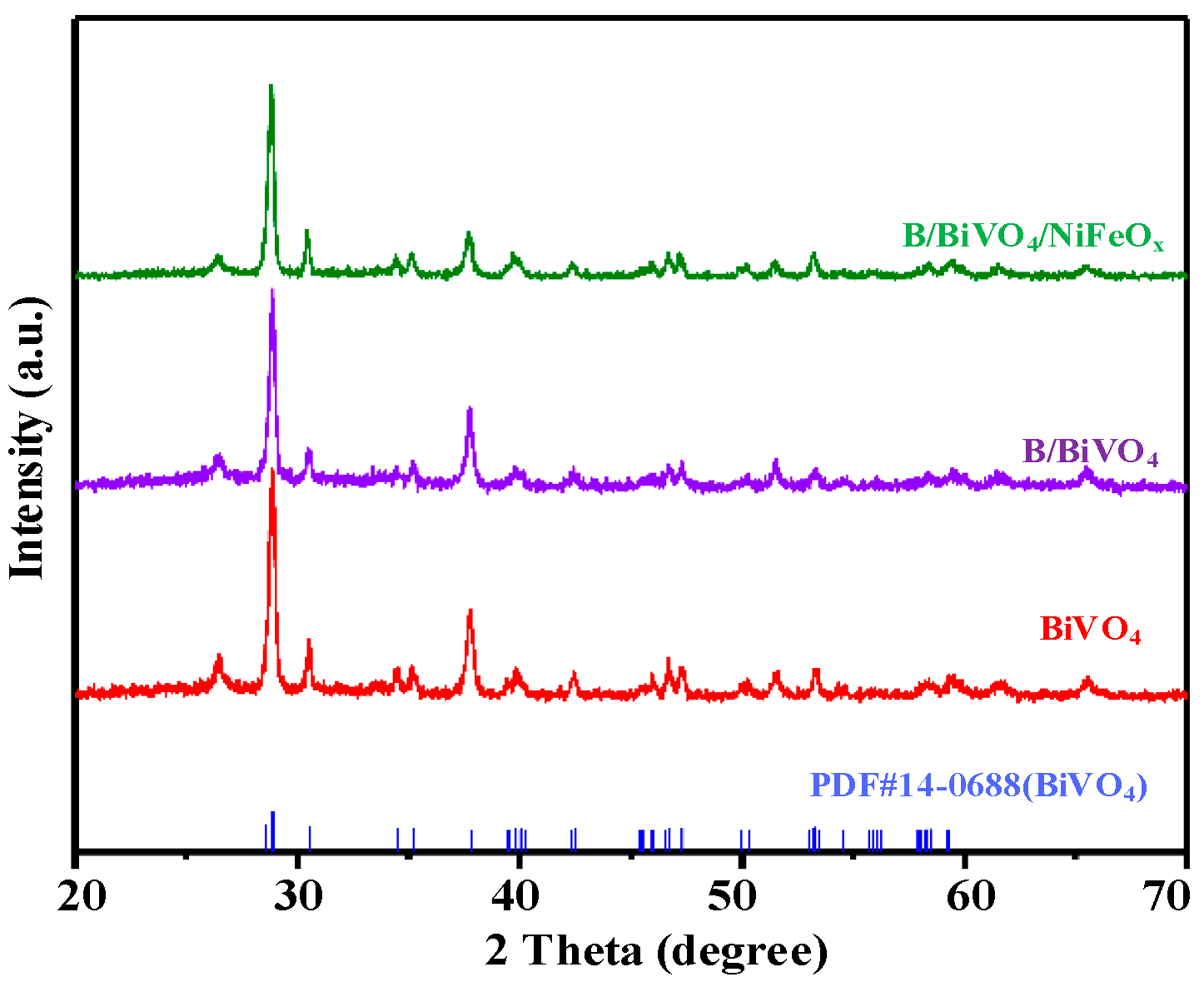
3.2.3. Crystal Structure Analysis
3.2.4. Elemental Composition Analysis
- Enhanced electronic conductivity—Oxygen vacancies introduce donor-like states near the conduction band, thereby improving charge carrier mobility and facilitating electron transport within the semiconductor.
- Improved surface reaction kinetics—Oxygen-deficient sites can act as active centers for water oxidation, promoting the adsorption and activation of OH− intermediates and accelerating the OER.
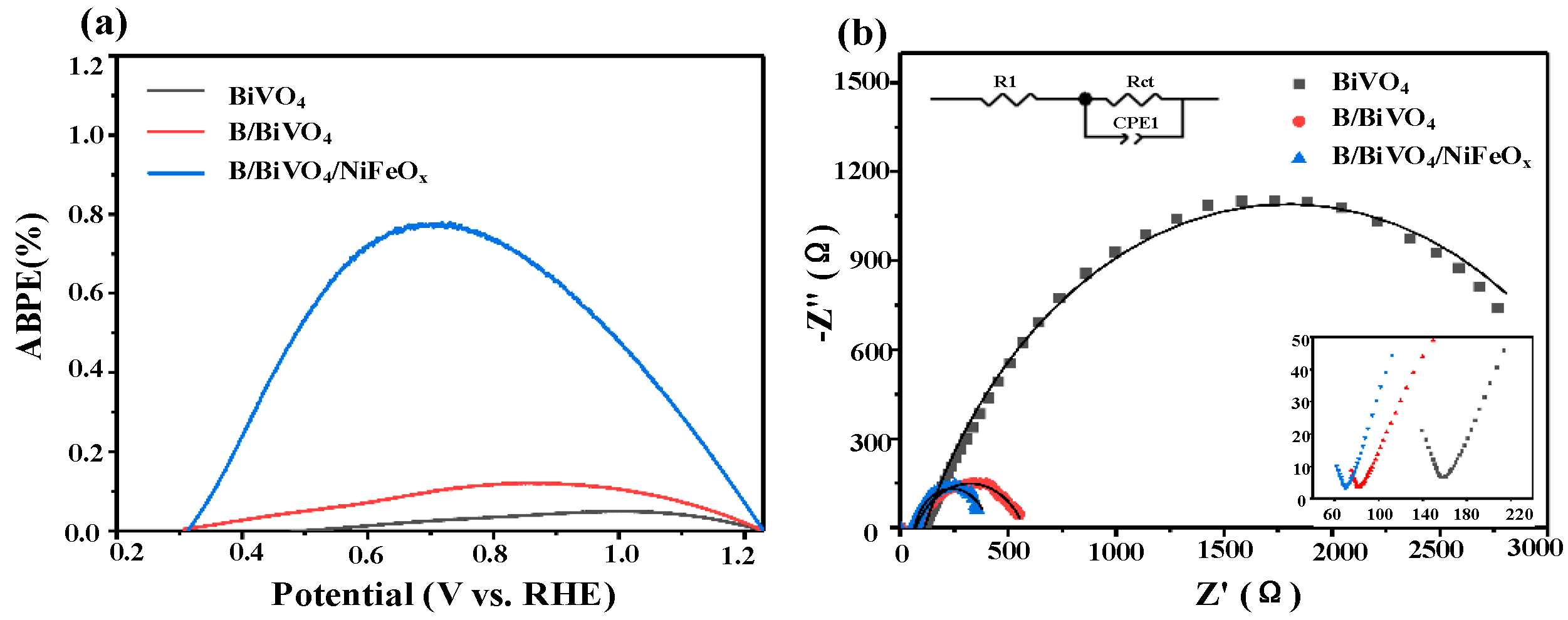
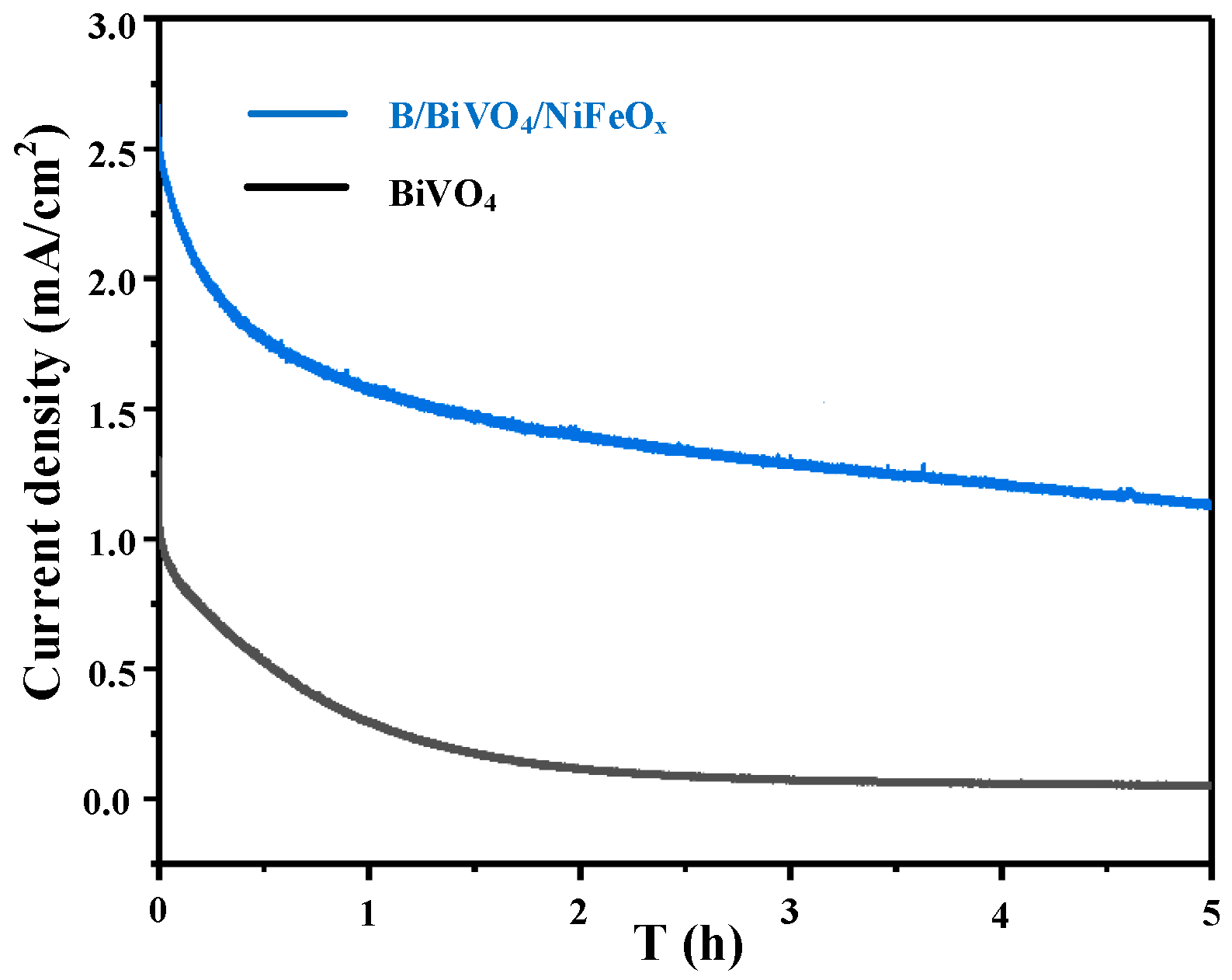
3.3. Electrochemical Performance Analysis
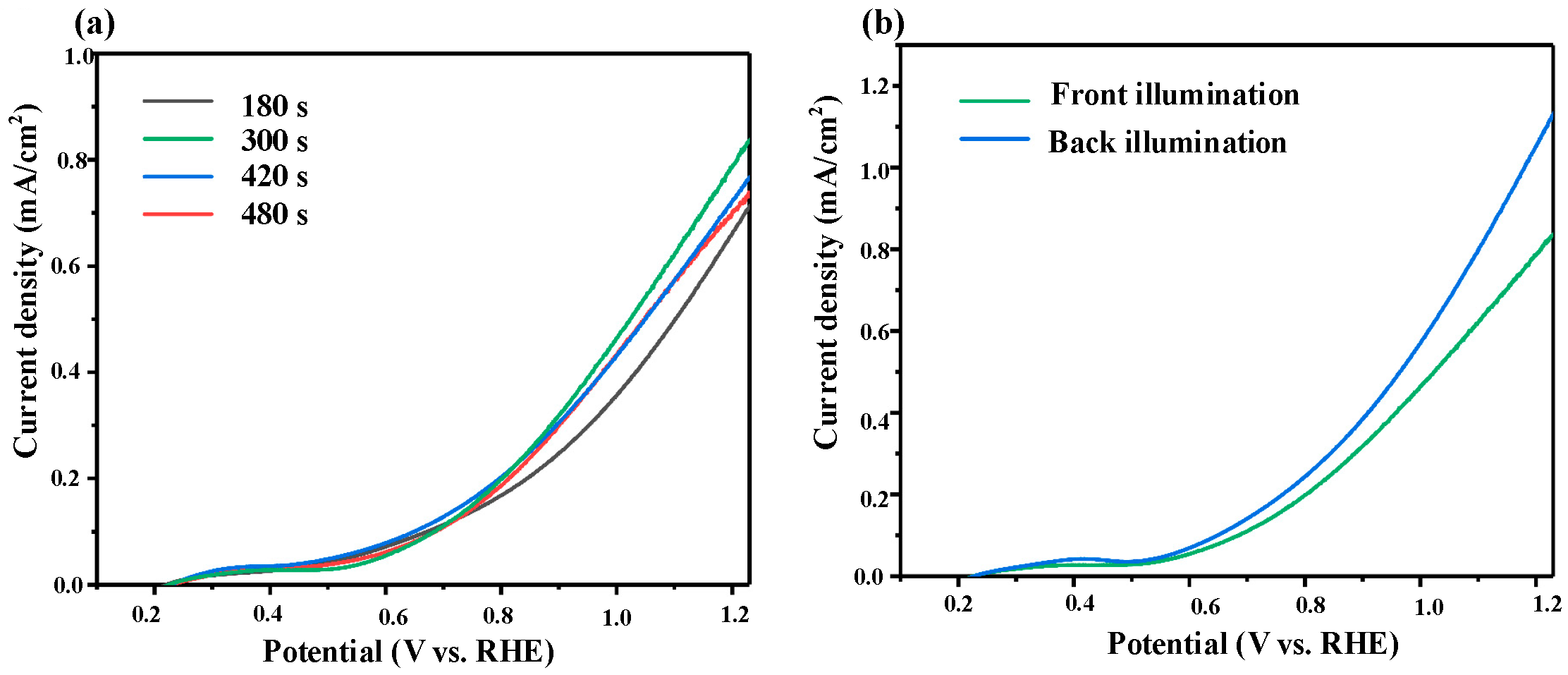
3.3.1. PEC Performance
3.3.2. Electrochemical Impedance Spectroscopy (EIS)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, P.B.; Li, X.Y.; Tang, T.; Cheng, Y.; Wang, Y.; Yang, Y.Q.; Liu, L.; Li, Y.W.; Li, M.; Xiao, J.R.; et al. Modularized Cathode with Neural Network Topology for High Rate and Fault-Tolerant Lithium–Sulfur Batteries. Adv. Mater. 2025, 37, 2504908. [Google Scholar] [CrossRef]
- He, H.C.; Xiong, Y.L.; Xiao, H.; Han, T.; Guo, Y.J.; Li, J.H.; Chen, Q.W.; Zhang, Y.H.; Du, J.Y.; Ke, G.L. Photothermal-enhanced Aolar Water Oxidation on NiO/Amorphous Carbon/BiVO4 and CoOx/Amorphous Carbon/BiVO4 Photoanodes. Catal. Sci. Technol. 2023, 13, 5776–5784. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Huang, Y.H.; Wang, J.N.; Wei, X.M.; Ma, F. The Influences of Nb and N Dopants on Elastic, Electronic and Optical Properties of Monoclinic BiVO4. Mater. Res. Express 2019, 6, 115911. [Google Scholar] [CrossRef]
- He, H.C.; Zhou, Y.; Ke, G.L.; Zhong, X.H.; Yang, M.J.; Bian, L.; Lv, K.L.; Dong, F.Q. Improved Surface Charge Transfer in MoO3/BiVO4 Heterojunction Film for Photoelectrochemical Water Oxidation. Electrochim. Acta 2017, 257, 181–191. [Google Scholar] [CrossRef]
- Ma, D.Q.; Wang, W.Y.; Wang, Q.Z.; Wang, Q.Z.; Dai, Y.L.; Zhu, K.; Xu, H.C.; Yuan, C.; Dong, P.Y.; Xi, X.G. A Novel Visible-Light-Driven Z-scheme C3N5/BiVO4 Heterostructure with Enhanced Photocatalytic Degradation Performance. Environ. Sci. Pollut. Res. 2024, 31, 19687–19698. [Google Scholar] [CrossRef]
- Choe, H.R.; Kim, J.H.; Ma, A.; Jung, H.; Kim, H.Y.; Nam, K.M. Understanding Reaction Kinetics by Tailoring Metal Co-catalysts of the BiVO4 Photocatalyst. ACS Omega 2019, 4, 16597–16602. [Google Scholar] [CrossRef]
- Chen, L.M.; Yu, Y.F.; Wu, M.; Huang, J.H.; Liu, Y.N.; Liu, X.H.; Qiu, G.Z. Synthesis of Hollow BiVO4/Ag Composite Microspheres and Their Photocatalytic and Surface-Enhanced Raman Scattering Properties. ChemPlusChem 2015, 80, 871–877. [Google Scholar] [CrossRef]
- Wu, Q.F.; Bao, S.Y.; Tian, B.Z.; Xiao, Y.F.; Zhang, J.L. Double-Diffusion-based Synthesis of BiVO4 Mesoporous Single Crystals with Enhanced Photocatalytic Activity for Oxygen Evolution. Chem. Commun. 2016, 52, 7478–7481. [Google Scholar] [CrossRef]
- Lee, H.; Choi, J.H.; Jung, K.; Lim, H.Y.; Choi, D.G.; Jung, J.Y.; Jeong, J.H.; Kim, T.W.; Park, J.H.; Lee, J. Large-area, Regular, Periodically Ordered, and Au-Nanoparticle-Decorated 1-D BiVO4/WO3 Nanorods as Photoanodes with Enhanced Photoelectrochemical Performance. J. Ind. Eng. Chem. 2023, 125, 325–335. [Google Scholar] [CrossRef]
- Vishlaghi, M.B.; Kahraman, A.; Österbacka, N.; Usman, E.; Erdem, E.; Alphan Sennaroğlu, A.; Wiktor, J.; Kaya, S. Accelerating Water Oxidation on BiVO4 Photoanodes via Surface Modification with Co Dopants. J. Mater. Chem. A 2023, 11, 16648–16658. [Google Scholar] [CrossRef]
- Kim, T.W.; Choi, K.S. Nanoporous BiVO4 Photoanodes with Dual-Layer Oxygen Evolution Catalysts for Solar Water Splitting. Science 2014, 343, 990–994. [Google Scholar] [CrossRef]
- Yang, N.C.; Zhang, S.A.; Xiao, Y.J.; Qi, Y.; Bao, Y.F.; Xu, P.; Jin, S.Y.; Zhang, F.X. Insight into the Key Restriction of BiVO4 Photoanodes Prepared by Pyrolysis Method for Scalable Preparation. Angew. Chem.-Int. Edit. 2023, 62, e202308729. [Google Scholar] [CrossRef]
- Saxena, S.; Verma, A.; Asha, K.; Biswas, N.K.; Srivastav, A.; Satsangi, V.R.; Shrivastav, R.; Dass, S. BiVO4/Fe2O3/ZnFe2O4 Triple Heterojunction for an Enhanced PEC Performance for Hydrogen Generation. RSC Adv. 2022, 12, 12552–12563. [Google Scholar] [CrossRef]
- Arunachalam, M.; Kanase, R.S.; Badiger, J.G.; Sayed, S.A.; Ahn, K.S.; Ha, J.S.; Ryu, S.W.; Kang, S.H. Durable Bias-Free Solar Water-Splitting Cell Composed of n+p-Si/Nb2O5/NiPt Photocathode and W:BiVO4/NiCo(O-OH)2 Photoanode. Chem. Eng. J. 2023, 474, 145242. [Google Scholar] [CrossRef]
- Pedroni, M.; Chiarello, G.L.; Haghshenas, N.; Canetti, M.; Ripamonti, D.; Selli, E.; Vassallo, E. Bismuth Vanadate Photoanodes for Water Splitting Deposited by Radio Frequency Plasma Reactive Co-sputtering. J. Vac. Sci. Technol. B 2020, 38, 012203. [Google Scholar] [CrossRef]
- Sun, M.; Yuan, C.; Gao, R.T. A Bridging Coordination of Urea Tailoring Metal Hydroxides Oxygen Evolution Catalysts Promotes Stable Solar Water Splitting. Chem. Eng. J. 2021, 426, 131062. [Google Scholar] [CrossRef]
- Naing, M.T.; Hwang, J.B.; Lee, J.; Kim, Y.; Jung, Y.; Lee, S.H. Synergistic Effect of Co Doping and Borate Impregnation on BiVO4 Photoanode for Efficient Photoelectrochemical Water Splitting. Int. J. Hydrog. Energy 2024, 55, 234–242. [Google Scholar] [CrossRef]
- Ratnayake, S.P.; Ren, J.W.; Van Embden, J.; McConville, C.F.; Gaspera, E.D. SILAR Deposition of Bismuth Vanadate Photoanodes for Photoelectrochemical Water Splitting. J. Mater. Chem. A 2021, 9, 25641–25650. [Google Scholar] [CrossRef]
- Guo, W.L.; Tang, D.; Mabayoje, O.; Wygant, B.R.; Xiao, P.; Zhang, Y.H.; Mullins, C.B. A Simplified Successive Ionic Layer Adsorption and Reaction (s-SILAR) Method for Growth of Porous BiVO4 Thin Films for Photoelectrochemical Water Oxidation. J. Electrochem. Soc. 2017, 164, H119–H125. [Google Scholar] [CrossRef]
- Meng, Q.J.; Zhang, B.B.; Fan, L.Z.; Liu, H.D.; Valvo, M.; Edström, K.; Cuartero, M.; Marco, R.; Crespo, G.A.; Sun, L.C. Efficient BiVO4 Photoanodes by Postsynthetic Treatment: Remarkable Improvements in Photoelectrochemical Performance from Facile Borate Modification. Angew. Chem.-Int. Edit. 2019, 58, 19027–19033. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, L.; Qu, S.Y.; Du, A.J.; Tang, J.W.; Ng, Y.H. Polaron-Mediated Transport in BiVO4 Photoanodes for Solar Water Oxidation. ACS Energy Lett. 2023, 8, 2177–2184. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, L.; Du, A.J.; Irani, R.; Krol, R.; Abdi, F.F.; Ng, Y.H. Low-Bias Photoelectrochemical Water Splitting via Mediating Trap States and Small Polaron Hopping. Nat. Commun. 2022, 13, 6231. [Google Scholar] [CrossRef]
- Wang, S.C.; He, T.W.; Chen, P.; Du, A.J.; Ostrikov, K.; Huang, W.; Wang, L.Z. In Situ Formation of Oxygen Vacancies Achieving Near-Complete Charge Separation in Planar BiVO4 Photoanodes. Adv Mater. 2020, 32, 2001385. [Google Scholar] [CrossRef]
- Seong, C.; Mane, P.; Bae, H.; Lee, S.; Kang, S.H.; Ryu, S.W.; Ha, J.S. Simple Fabrication of BiVO4 Thin Films Synthesized by Modified SILAR Method: Effect of Film Thickness. J. Electrochem. Soc. 2022, 169, 016501. [Google Scholar] [CrossRef]
- Zafeiropoulos, G.; Varadhan, P.; Johnson, H.; Kamphuis, L.; Pandiyan, A.; Kinge, S.; Sanden, M.C.M.; Tsampas, M.N. Rational Design of Photoelectrodes for the Fully Integrated Polymer Electrode Membrane-Photoelectrochemical Water-Splitting System: A Case Study of Bismuth Vanadate. ACS Appl. Energy Mater. 2021, 4, 9600–9610. [Google Scholar] [CrossRef]
- Xiufang He, X.F.; Marken, F.; Vertova, A.; Minguzzi, A. Roles of Oxygen Vacancies in Layered Double Hydroxides-based Catalysts for Wastewater Remediation: Fundamentals and Prospects. J. Environ. Manag. 2025, 385, 125583. [Google Scholar] [CrossRef]
- Wang, S.C.; Chen, P.; Bai, Y.; Yun, J.H.; Liu, G.; Wang, L.Z. New BiVO4 Dual Photoanodes with Enriched Oxygen Vacancies for Efficient Solar-Driven Water Splitting. Adv. Mater. 2018, 30, 1800486. [Google Scholar] [CrossRef]
- Mohamed, S.K.; Bashat, A.M.A.; Hassan, H.M.A.; Ismail, N.; Rouby, W.M.A.E. Optimizing the Performance of Auy/Nix/TiO2 NTs Photoanodes for Photoelectrochemical Water Splitting. RSC Adv. 2023, 13, 14018–14032. [Google Scholar] [CrossRef]
- Pihosh, Y.; Turkevych, I.; Mawatari, K.; Asai, T.; Hisatomi, T.; Uemura, J.; Tosa, M.; Shimamura, K.; Kubota, J.; Domen, K.; et al. Nanostructured WO3/BiVO4 Photoanodes for Efficient Photoelectrochemical Water Splitting. Small 2014, 10, 3692–3699. [Google Scholar] [CrossRef]
- Sun, H.H.; Hua, W.; Liang, S.Y.; Li, Y.Y.; Wang, J.G. A Self-Adaptive Semiconductor-Liquid Junction for Highly Active and Stable Solar Water Splitting. J. Mater. Chem. A 2022, 10, 20414–20423. [Google Scholar] [CrossRef]
- Zhang, J.N.; Huang, Y.C.; Lu, X.Y.; Yang, J.D.; Tong, Y.X. Enhanced BiVO4 Photoanode Photoelectrochemical Performance via Borate Treatment and a NiFeOx Cocatalyst. ACS Sustain. Chem. Eng. 2021, 9, 8306–8314. [Google Scholar] [CrossRef]
- Sim, U.; Moon, J.; An, J.; Kang, J.H.; Jerng, S.E.; Moon, J.; Cho, S.P.; Hong, B.H.; Nam, K.T. N-doped Graphene Quantum Sheets on Silicon Nanowire Photocathodes for Hydrogen Production. Energy Environ. Sci. 2015, 8, 1329–1338. [Google Scholar] [CrossRef]
- She, H.D.; Jiang, M.; Yue, P.F.; Huang, J.W.; Wang, L.; Li, J.Z.; Zhu, G.Q.; Wang, Q.Z. Metal (Ni2+/Co2+) Sulfides Modified BiVO4 for Effective Improvement in Photoelectrochemical Water Splitting. J. Colloid Interface Sci. 2019, 549, 80–88. [Google Scholar] [CrossRef]
- Liu, C.H.; Luo, H.; Xu, Y.; Wang, W.C.; Liang, Q.; Mitsuzaki, N.; Chen, Z.D. Cobalt-Phosphate-Modified Mo:BiVO4 Mesoporous Photoelectrodes for Enhanced Photoelectrochemical Water Splitting. J. Mater. Sci. 2019, 54, 10670–10683. [Google Scholar] [CrossRef]

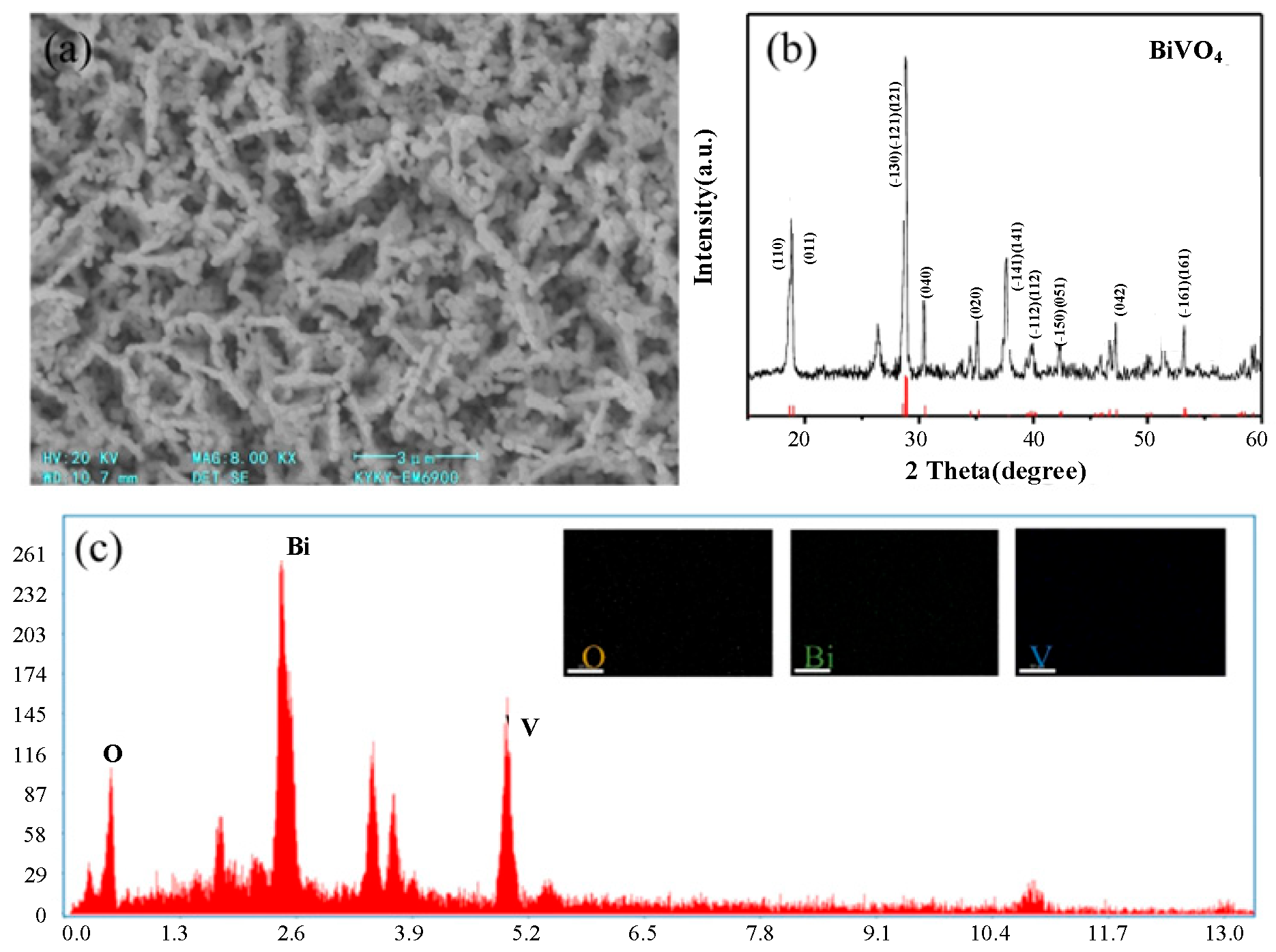


| Instrument | Model | Manufacturer |
|---|---|---|
| Precision Electronic Balance | SQP | Sartorius Scientific Instruments Co., Ltd., Beijing, China |
| High-Resolution X-ray Diffractometer | Panalytical | Malvern Panalytical, Almelo, The Netherlands |
| Scanning Electron Microscope | TESCAN MIRA LMS | TESCAN (China) Co., Ltd., Brno, Czech Republic/Shanghai, China |
| X-ray Diffractometer | MiniFlex-600 | Rigaku Corporation, Tokyo, Japan |
| UV-Visible Spectrophotometer | UV-3600 PLUS | Shimadzu Corporation, Kyoto, Japan |
| X-ray Photoelectron Spectrometer | Thermo Scientific K-Alpha | Thermo Fisher Scientific, Waltham, MA, USA |
| Xenon Lamp | PLS-SXE300+ | Beijing Pofilai Technology Co., Ltd., Beijing, China |
| Muffle Furnace | KSL-1100X | Hefei Kejing Material Technology Co., Ltd., Hefei, China |
| Electrochemical Workstation | CHI 760E | Shanghai Chenhua Instruments Co., Ltd., Shanghai, China |
| Digital Constant Temperature Magnetic Stirrer | 85-2 | Changzhou Yuexin Instrument Manufacturing Co., Ltd., Changzhou, China |
| pH Tester | PH-10 | Lichen Instruments, Shanghai, China |
| Elements | Weight Percentage (%) | Atomic Percentage (%) |
|---|---|---|
| B | 24.12 | 57.6 |
| O | 16.04 | 25.89 |
| Bi | 34.63 | 4.28 |
| V | 15.13 | 7.67 |
| Fe | 5.83 | 2.70 |
| Ni | 4.24 | 1.87 |
| Total | 99.99 | 99.99 |
| Photoelectrode | R1 (Ω) | Rct (Ω) |
|---|---|---|
| BiVO4 | 111.3 | 3370 |
| B/BiVO4 | 70.69 | 503.4 |
| B/BiVO4/NiFeOx | 66.25 | 343.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, S.; Cheng, Y.; Zhou, T.; Li, S.; Xie, D.; Li, X. Enhanced Photoelectrochemical Performance of BiVO4 Photoanodes Co-Modified with Borate and NiFeOx. Micromachines 2025, 16, 866. https://doi.org/10.3390/mi16080866
Cheng S, Cheng Y, Zhou T, Li S, Xie D, Li X. Enhanced Photoelectrochemical Performance of BiVO4 Photoanodes Co-Modified with Borate and NiFeOx. Micromachines. 2025; 16(8):866. https://doi.org/10.3390/mi16080866
Chicago/Turabian StyleCheng, Siqiang, Yun Cheng, Taoyun Zhou, Shilin Li, Dong Xie, and Xinyu Li. 2025. "Enhanced Photoelectrochemical Performance of BiVO4 Photoanodes Co-Modified with Borate and NiFeOx" Micromachines 16, no. 8: 866. https://doi.org/10.3390/mi16080866
APA StyleCheng, S., Cheng, Y., Zhou, T., Li, S., Xie, D., & Li, X. (2025). Enhanced Photoelectrochemical Performance of BiVO4 Photoanodes Co-Modified with Borate and NiFeOx. Micromachines, 16(8), 866. https://doi.org/10.3390/mi16080866






