Microfluidic Sensors Integrated with Smartphones for Applications in Forensics, Agriculture, and Environmental Monitoring
Abstract
1. Introduction
2. Recent Advancements in Fabrication of Microfluidic-Based Sensors
2.1. Chip Design
- Integration of functional components: Modern microfluidic chips often incorporate components such as valves, pumps, and sensors to enable complex operations. For instance, electrochemical sensors can be integrated into microfluidic channels for real-time detection of analytes [13].
2.2. Materials
2.2.1. Polymers
2.2.2. Glass and Silicon
2.2.3. Paper
2.2.4. Conductive Materials
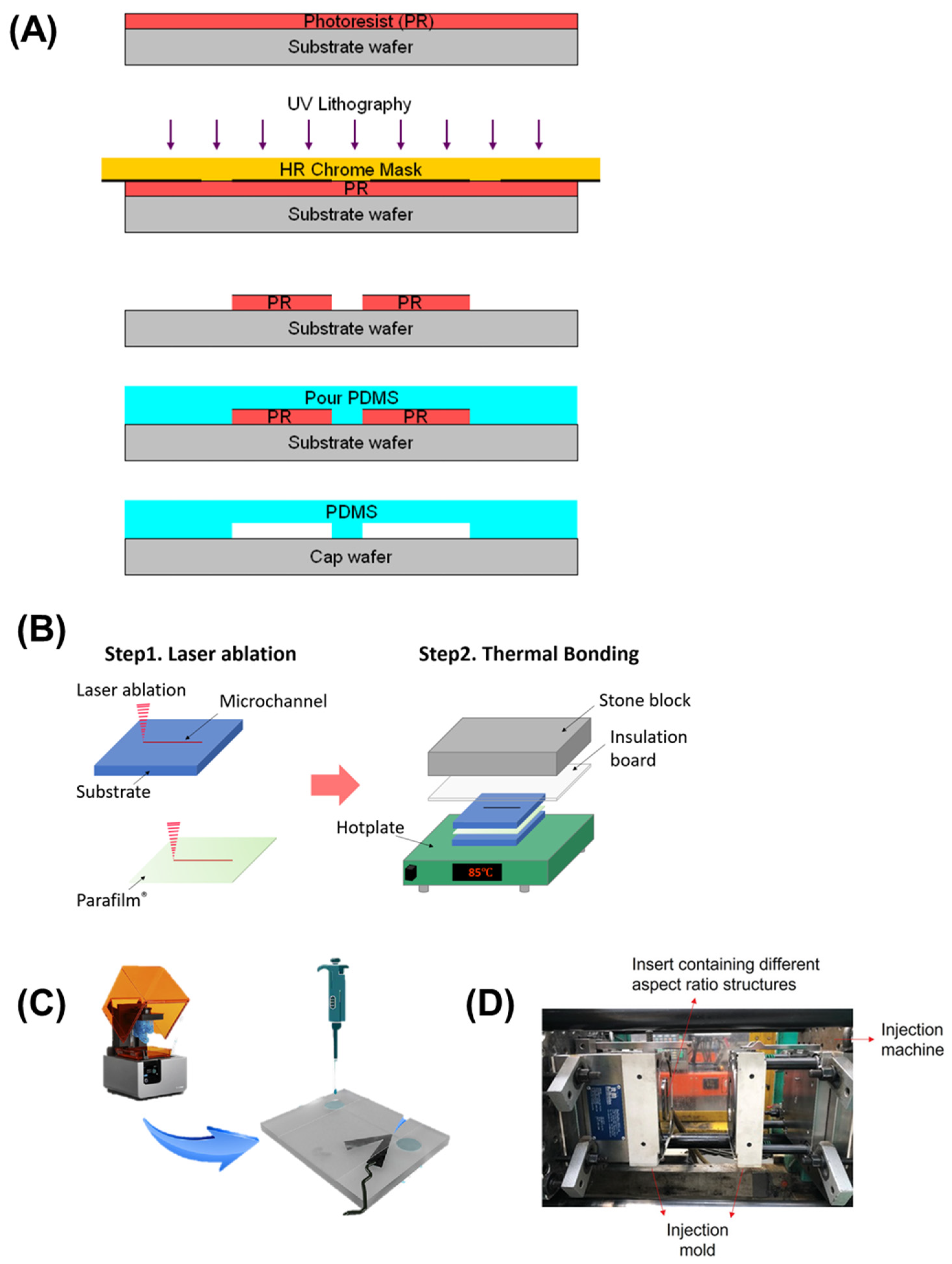
2.3. Fabrication Techniques
2.3.1. Lithographic Techniques
2.3.2. Laser Ablation
2.3.3. Three-Dimensional Printing
2.3.4. Injection Molding and Casting
3. Microfluidic Sensors for Forensic Applications
3.1. Crime Scene Analysis
3.2. Trace Evidence Analysis
3.3. Blood Pattern Analysis
3.4. DNA Analysis
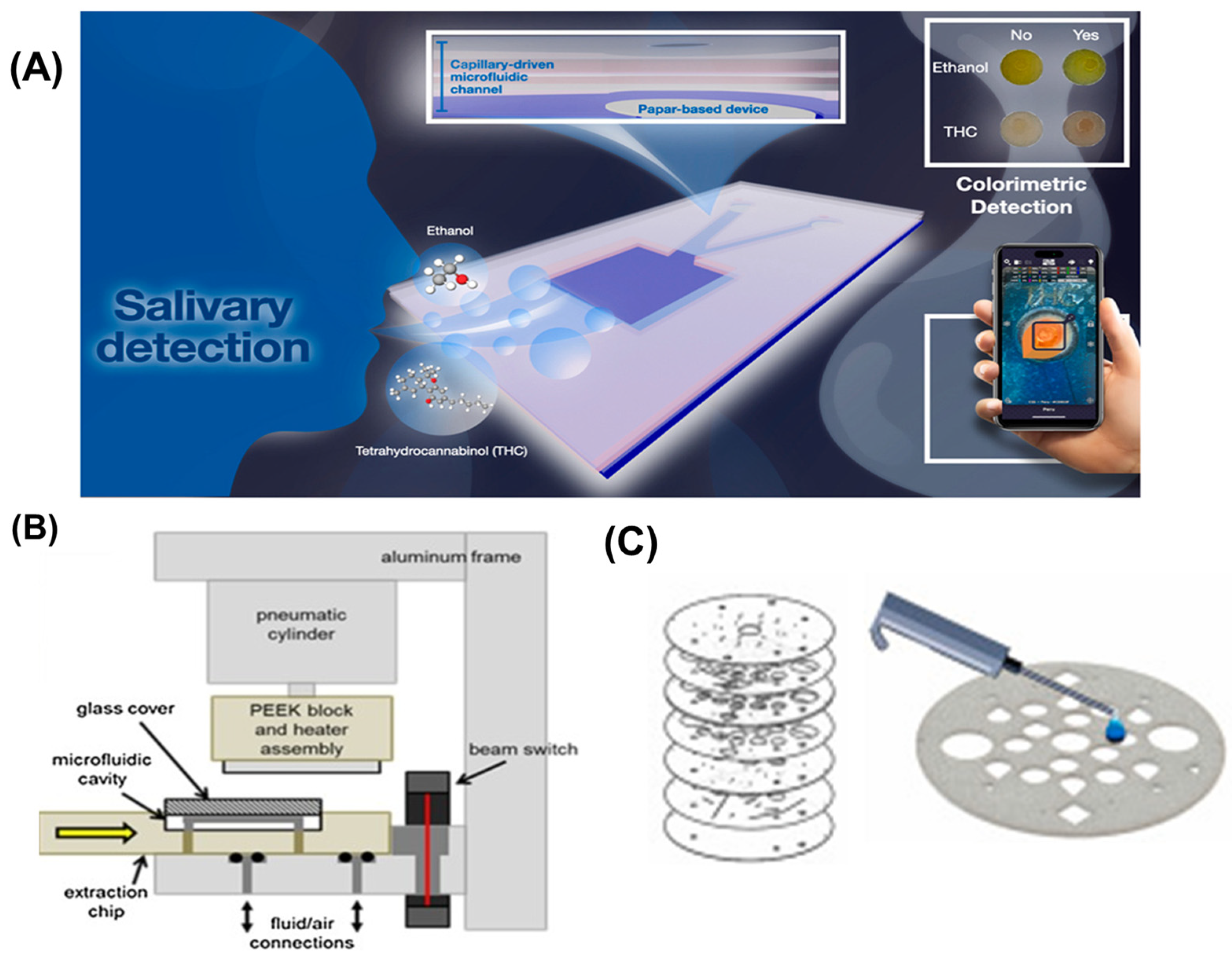
3.5. Illicit Drug Detection
3.6. Toxicological Screening
4. Microfluidic Sensors for Agricultural and Environmental Applications
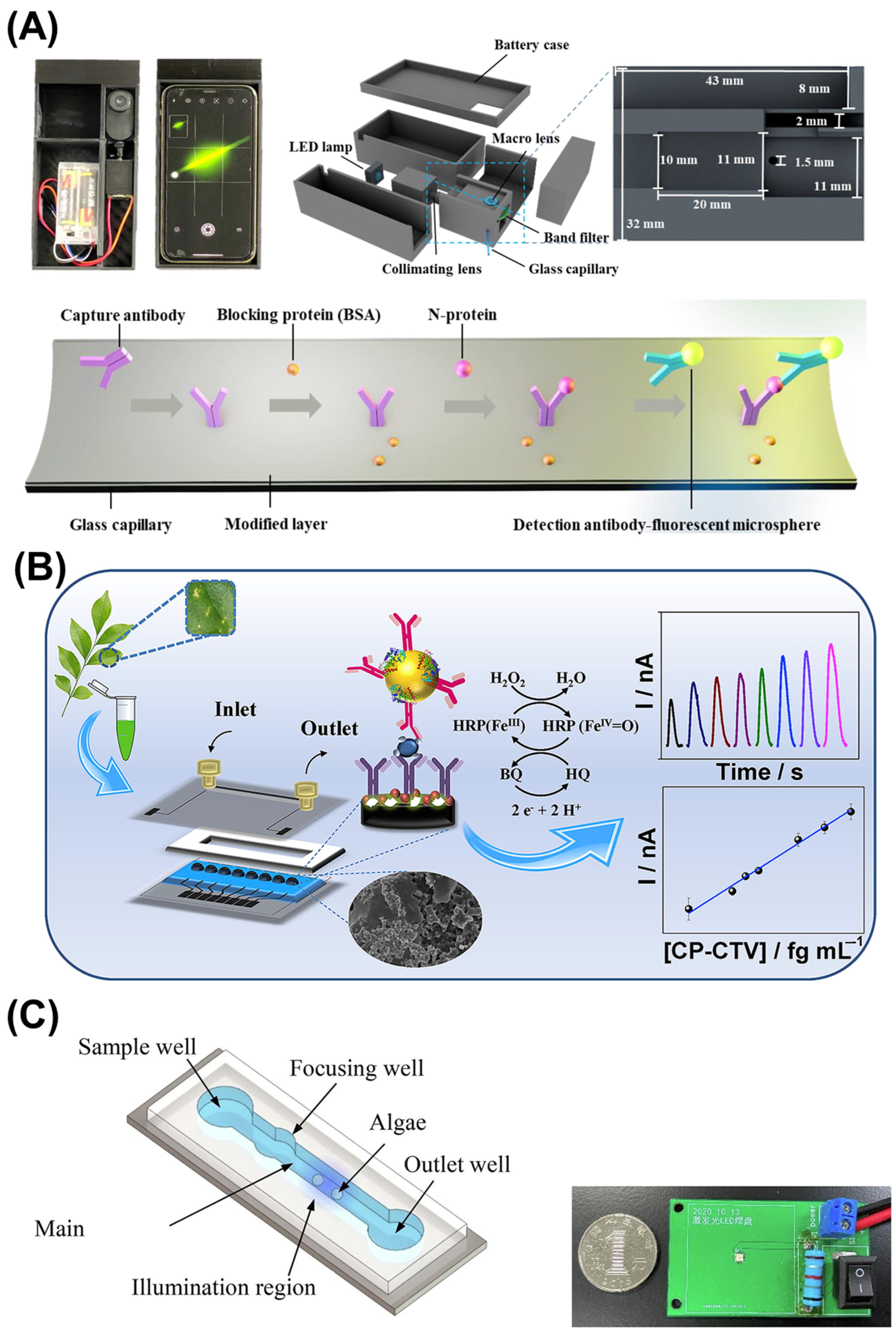
4.1. Plant Pathogen Detection
4.2. Soil Analysis
4.3. Water Quality Assessment
4.4. Environmental Monitoring
4.5. Heavy Metal Ion Detection
5. Smartphone-Based Detection Method on Microfluidic Sensors Used in Forensic, Agricultural, and Environmental Applications
5.1. Implementation of Smartphones on Microfluidic Chips
5.2. Working Principle of Smartphone Detection on Microfluidic Chips
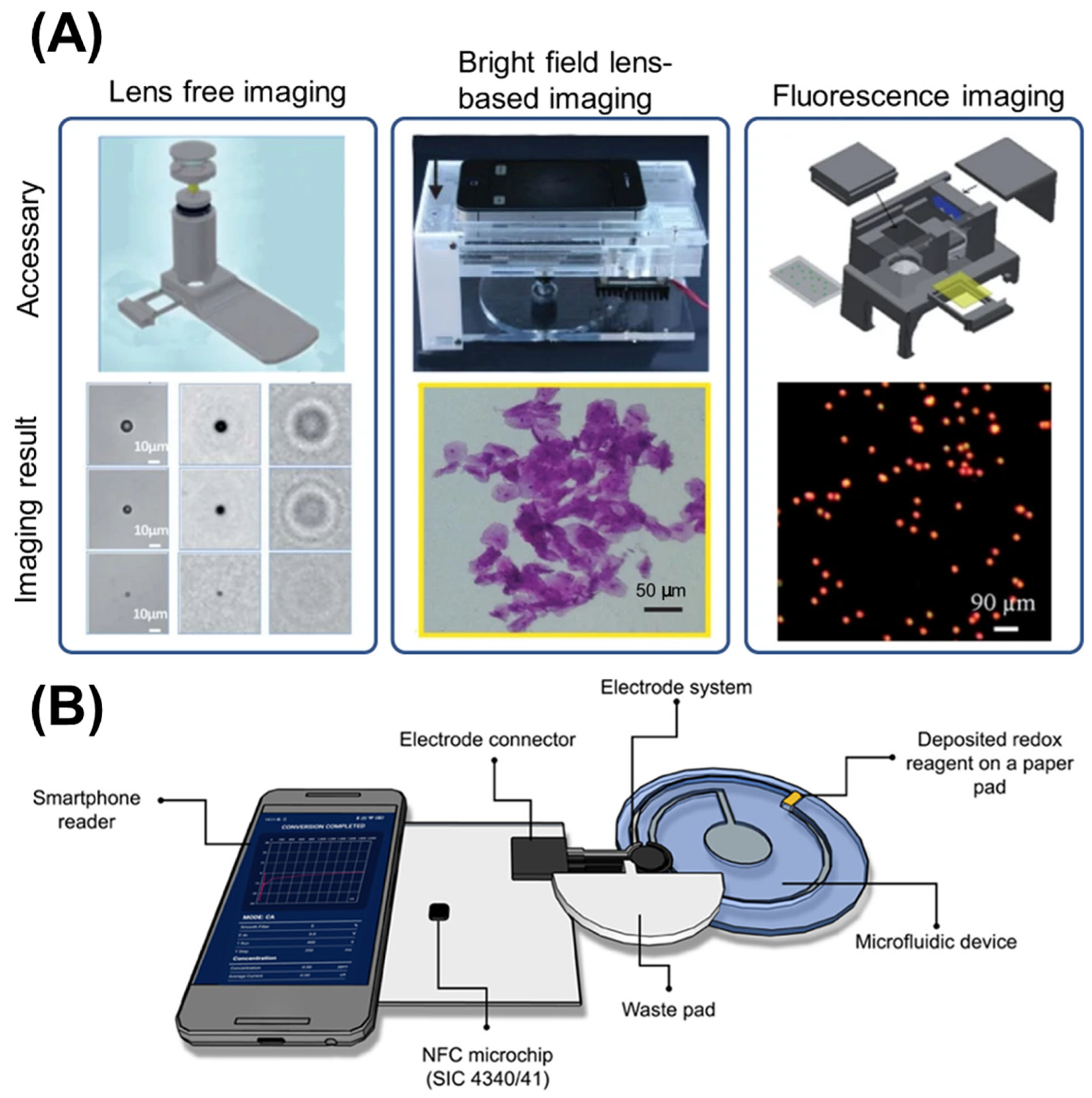
5.2.1. Optical Detection
- Colorimetric Detection: Colorimetric detection offers simplicity and accessibility, requiring minimal additional components while providing quantitative analysis through RGB or HSV color space analysis [88,120]. This method relies on color changes in the fluid, which can be quantified using the smartphone camera. For example, a pH indicator dye might change color in response to pH variations, providing a visual cue that can be analyzed quantitatively.
- Absorbance Measurement: Similar to colorimetric methods, absorbance can be measured by capturing images of the fluid with varying concentrations of colored analytes. Algorithms can be applied to determine concentration based on the intensity of color observed in captured images [82].
- Chemiluminescence: Chemiluminescence methodology involves photon generation through chemical reaction processes, with bioluminescence specifically occurring within living organisms or cellular systems. Similar to colorimetric techniques, chemiluminescence readout can be captured utilizing digital cameras and smartphone platforms, incorporating computer-based operation and data processing capabilities for quantitative biomarker analysis in portable analytical applications [121,122].
5.2.2. Electrochemical Detection
5.2.3. Electrochemiluminescence
5.3. Image Processing Techniques
5.4. Analysis Using Artificial Intelligence (AI)
5.5. Commercial Smartphone-Based Microfluidic Sensors for Forensic, Agricultural, and Environmental Applications
6. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lapins, N.; Akhtar, A.S.; Banerjee, I.; Kazemzadeh, A.; Pinto, I.F.; Russom, A. Smartphone-driven centrifugal microfluidics for diagnostics in resource limited settings. Biomed. Microdevices 2024, 26, 43. [Google Scholar] [CrossRef] [PubMed]
- Mulu, T.; Tibebe, D. Recent Advances in the Application of Microfluidic Paper Based Devices for Environment Sample Analysis. Biomed. J. Sci. Tech. Res. 2024, 59, 51491–51506. [Google Scholar] [CrossRef]
- Zhou, M.; Li, J.; Yuan, S.; Yang, X.; Lu, J.; Jiang, B. A centrifugal microfluidic system for automated detection of multiple heavy metal ions by aptamer-based colorimetric assay. Sens. Actuators B Chem. 2023, 403, 135210. [Google Scholar] [CrossRef]
- Peng, T.; Lin, X.; Yuan, S.; Zhou, M.; Jiang, B.; Jia, Y. Mixing enhancement in a straight microchannel with ultrasonically activated attached bubbles. Int. J. Heat Mass Transf. 2023, 217, 124635. [Google Scholar] [CrossRef]
- Aryal, P.; Henry, C.S. Advancements and challenges in microfluidic paper-based analytical devices: Design, manufacturing, sustainability, and field applications. Front. Lab Chip Technol. 2024, 3, 1467423. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, H.; Xu, C.; Yin, W.; Luo, L.; Liu, G.; Wang, Y. Smartphone-Integrated RPA-CRISPR-Cas12a Detection System with Microneedle Sampling for Point-of-Care Diagnosis of Potato Late Blight in Early Stage. arXiv 2025, arXiv:2506.15728. [Google Scholar] [CrossRef]
- Wang, T.; Yu, C.; Xie, X. Microfluidics for Environmental Applications, in Microfluidics in Biotechnology; Bahnemann, J., Grünberger, A., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 267–290. [Google Scholar] [CrossRef]
- Olanrewaju, A.; Beaugrand, M.; Yafia, M.; Juncker, D. Capillary microfluidics in microchannels: From microfluidic networks to capillaric circuits. Lab Chip 2018, 18, 2323–2347. [Google Scholar] [CrossRef] [PubMed]
- Suh, Y.K.; Kang, S. A Review on Mixing in Microfluidics. Micromachines 2010, 1, 82–111. [Google Scholar] [CrossRef]
- Alves, L.A.O.; Felix, J.H.d.S.; Ferreira, A.Á.M.; dos Santos, M.T.B.; da Silva, C.G.; de Castro, L.M.S.; dos Santos, J.C.S. Advances and Applications of Micro- and Mesofluidic Systems. ACS Omega 2025, 10, 12817–12836. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.R.; Hiatt, J.B.; Lu, R.; Attema, J.L.; Lobo, N.A.; Weissman, I.L.; Clarke, M.F.; Quake, S.R. Automated microfluidic chromatin immunoprecipitation from 2,000 cells. Lab Chip 2009, 9, 1365–1370. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.V.; Tice, J.D.; Apblett, C.A.; Kenis, P.J.A. Design considerations for electrostatic microvalves with applications in poly(dimethylsiloxane)-based microfluidics. Lab Chip 2012, 12, 1078–1088. [Google Scholar] [CrossRef] [PubMed]
- Al-Hujazy, R.; Collier, C.M. Design Considerations for Integration of Terahertz Time-Domain Spectroscopy in Microfluidic Platforms. Photonics 2018, 5, 5. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Tseng, T.-M.; Schlichtmann, U. Open-source interactive design platform for 3D-printed microfluidic devices. Commun. Eng. 2024, 3, 71. [Google Scholar] [CrossRef]
- Hagan, K.A.; Reedy, C.R.; Bienvenue, J.M.; Dewald, A.H.; Landers, J.P. A valveless microfluidic device for integrated solid phase extraction and polymerase chain reaction for short tandem repeat (STR) analysis. Analyst 2011, 136, 1928–1937. [Google Scholar] [CrossRef] [PubMed]
- Ajmal, R.; Zhang, W.; Liu, H.; Bai, H.; Cao, L.; Peng, B.; Li, L. Development of a Microfluidic System for Mitochondrial Extraction, Purification, and Analysis. ACS Appl. Mater. Interfaces 2025, 17, 20487–20500. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Cheng, J.; Zhang, Y.; Yao, P. A LAMP Detection System Based on a Microfluidic Chip for Pyricularia grisea. Sensors 2025, 25, 2511. [Google Scholar] [CrossRef] [PubMed]
- Tameh, M.H.; Primiceri, E.; Chiriacò, M.S.; Poltronieri, P.; Bahar, M.; Maruccio, G. Pectobacterium atrosepticum Biosensor for Monitoring Blackleg and Soft Rot Disease of Potato. Biosensors 2020, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, R.; Jin, Z.; Fan, Y.; Zhou, X.; Zhang, Y. Injection molding and characterization of PMMA-based microfluidic devices. Microsyst. Technol. 2019, 26, 1317–1324. [Google Scholar] [CrossRef]
- Xiao, B.; Wang, M.; Zhang, J.; Wang, N.; Fu, W.; Chen, H.; Wang, H.; Li, L.; Pang, X.; Liu, C.; et al. Rapid DNA extraction and microfluidic LAMP system in portable equipment for GM crops detection. Sens. Actuators B Chem. 2024, 411, 135716. [Google Scholar] [CrossRef]
- Agha, A.; Waheed, W.; Alamoodi, N.; Mathew, B.; Alnaimat, F.; Abu-Nada, E.; Abderrahmane, A.; Alazzam, A. A Review of Cyclic Olefin Copolymer Applications in Microfluidics and Microdevices. Macromol. Mater. Eng. 2022, 307, 2200053. [Google Scholar] [CrossRef]
- Han, J.P.; Sun, J.; Wang, L.; Liu, P.; Zhuang, B.; Zhao, L.; Liu, Y.; Li, C.X. The Optimization of Electrophoresis on a Glass Microfluidic Chip and its Application in Forensic Science. J. Forensic Sci. 2017, 62, 1603–1612. [Google Scholar] [CrossRef] [PubMed]
- Aralekallu, S.; Boddula, R.; Singh, V. Development of glass-based microfluidic devices: A review on its fabrication and biologic applications. Mater. Des. 2022, 225, 111517. [Google Scholar] [CrossRef]
- Nielsen, J.B.; Hanson, R.L.; Almughamsi, H.M.; Pang, C.; Fish, T.R.; Woolley, A.T. Microfluidics: Innovations in Materials and Their Fabrication and Functionalization. Anal. Chem. 2019, 92, 150–168. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Gao, W.; Yin, J.; Liang, L.; Zou, J.; Jin, Q. Microfluidic sensor integrated with nanochannel liquid conjunct Ag/AgCl reference electrode for trace Pb(II) measurement. Anal. Chim. Acta 2021, 1164, 338511. [Google Scholar] [CrossRef] [PubMed]
- Harshey, A.; Kumar, A.; Kumar, A.; Das, T.; Nigam, K.; Srivastava, A. Focusing the intervention of paper-based microfluidic devices for the forensic investigative purposes. Microfluid. Nanofluidics 2023, 27, 65. [Google Scholar] [CrossRef]
- Yu, L.; Shi, Z.Z. Microfluidic paper-based analytical devices fabricated by low-cost photolithography and embossing of Parafilm®. Lab Chip 2015, 15, 1642–1645. [Google Scholar] [CrossRef] [PubMed]
- Lisowski, P.; Zarzycki, P.K. Microfluidic Paper-Based Analytical Devices (μPADs) and Micro Total Analysis Systems (μTAS): Development, Applications and Future Trends. Chromatographia 2013, 76, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Baldo, M.A.; Messina, G.A.; Sanz, M.I.; Raba, J. Microfluidic Immunosensor with Micromagnetic Beads Coupled to Carbon-Based Screen-Printed Electrodes (SPCEs) for Determination of Botrytis cinerea in Tissue of Fruits. J. Agric. Food Chem. 2010, 58, 11201–11206. [Google Scholar] [CrossRef] [PubMed]
- Julich, S.; Riedel, M.; Kielpinski, M.; Urban, M.; Kretschmer, R.; Wagner, S.; Fritzsche, W.; Henkel, T.; Möller, R.; Werres, S. Development of a lab-on-a-chip device for diagnosis of plant pathogens. Biosens. Bioelectron. 2011, 26, 4070–4075. [Google Scholar] [CrossRef] [PubMed]
- Gale, B.K.; Jafek, A.R.; Lambert, C.J.; Goenner, B.L.; Moghimifam, H.; Nze, U.C.; Kamarapu, S.K. A Review of Current Methods in Microfluidic Device Fabrication and Future Commercialization Prospects. Inventions 2018, 3, 60. [Google Scholar] [CrossRef]
- Scott, S.M.; Ali, Z. Fabrication Methods for Microfluidic Devices: An Overview. Micromachines 2021, 12, 319. [Google Scholar] [CrossRef] [PubMed]
- Prabhakarpandian, B.; Shen, M.-C.; Pant, K.; Kiani, M.F. Microfluidic devices for modeling cell–cell and particle–cell interactions in the microvasculature. Microvasc. Res. 2011, 82, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Christoffersson, J.; Mandenius, C.-F. Fabrication of a Microfluidic Cell Culture Device Using Photolithographic and Soft Lithographic Techniques. In Cell-Based Assays Using iPSCs for Drug Development and Testing; Mandenius, C.-F., Ross, J.A., Eds.; Springer: New York, NY, USA, 2019; pp. 227–233. [Google Scholar] [CrossRef]
- Koo, C.; Malapi-Wight, M.; Kim, H.S.; Cifci, O.S.; Vaughn-Diaz, V.L.; Ma, B.; Kim, S.; Abdel-Raziq, H.; Ong, K.; Jo, Y.-K.; et al. Development of a Real-Time Microchip PCR System for Portable Plant Disease Diagnosis. PLoS ONE 2013, 8, e82704. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wang, T.; Wang, Y.; Zeng, S.; Ho, Y.-P.; Ho, H.-P. Rapid Prototyping of Multi-Functional and Biocompatible Parafilm®-Based Microfluidic Devices by Laser Ablation and Thermal Bonding. Micromachines 2023, 14, 656. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Johnson, Z.T.; Sanborn, D.; Hjort, R.G.; Garland, N.T.; Soares, R.R.A.; Van Belle, B.; Jared, N.; Li, J.; Jing, D.; et al. Tuning the Structure, Conductivity, and Wettability of Laser-Induced Graphene for Multiplexed Open Microfluidic Environmental Biosensing and Energy Storage Devices. ACS Nano 2021, 16, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.M.d.C.; Griveau, S.; Bedioui, F.; Orlye, F.D.; da Silva, J.A.F.; Varenne, A. Stereolithography based 3D-printed microfluidic device with integrated electrochemical detection. Electrochim. Acta 2022, 407, 139888. [Google Scholar] [CrossRef]
- Yamashita, R.A.; Carvalho, R.M.; Petroni, J.M.; Pedão, E.R.; Guerbas, F.M.R.; Tronchini, M.P.; Ferreira, V.S.; de Melo, E.I.; da Silva, R.A.B.; Lucca, B.G. 3D-printed microfluidic thread device with integrated detector: A green and portable tool for amperometric detection of fungicide benzovindiflupyr in forensic samples. Microchem. J. 2022, 182, 107853. [Google Scholar] [CrossRef]
- March, G.; Nguyen, T.D.; Piro, B. Modified Electrodes Used for Electrochemical Detection of Metal Ions in Environmental Analysis. Biosensors 2015, 5, 241–275. [Google Scholar] [CrossRef] [PubMed]
- McLennan, H.J.; Blanch, A.J.; Wallace, S.J.; Ritter, L.J.; Heinrich, S.L.; Gardner, D.K.; Dunning, K.R.; Gauvin, M.J.; Love, A.K.; Thompson, J.G. Nano-liter perfusion microfluidic device made entirely by two-photon polymerization for dynamic cell culture with easy cell recovery. Sci. Rep. 2023, 13, 562. [Google Scholar] [CrossRef]
- Dzikonski, D.; Bekker, E.; Zamboni, R.; Ciechanska, D.; Schwab, A.; Denz, C.; Imbrock, J. Hybrid Microfluidic Chip Design with Two-Photon Polymerized Protein-Based Hydrogel Microstructures for Single Cell Experiments. Adv. Mater. Technol. 2025, 10, 2401571. [Google Scholar] [CrossRef]
- Baltima, A.; Panagopoulou, H.; Economou, A.; Kokkinos, C. 3D-printed fluidic electrochemical microcell for sequential injection/stripping analysis of heavy metals. Anal. Chim. Acta 2021, 1159, 338426. [Google Scholar] [CrossRef] [PubMed]
- Attia, U.M.; Marson, S.; Alcock, J.R. Micro-injection moulding of polymer microfluidic devices. Microfluid. Nanofluidics 2009, 7, 1–28. [Google Scholar] [CrossRef]
- Etxeberria, L.; Badiola, J.H.; Astigarraga, M.; Garitaonandia, F.; Zaldua, A.M. Comparison between Injection Molding (IM) and Injection-Compression Molding (ICM) for Mass Manufacturing of Thermoplastic Microfluidic Devices. Macromol. Mater. Eng. 2023, 309, 2300231. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Ayachit, N.H.; Aminabhavi, T.M. Biosensors and Microfluidic Biosensors: From Fabrication to Application. Biosensors 2022, 12, 543. [Google Scholar] [CrossRef] [PubMed]
- Alhalaili, B.; Popescu, I.N.; Rusanescu, C.O.; Vidu, R. Microfluidic Devices and Microfluidics-Integrated Electrochemical and Optical (Bio)Sensors for Pollution Analysis: A Review. Sustainability 2022, 14, 12844. [Google Scholar] [CrossRef]
- Celetti, G. Two-Photon Polymerization 3D Printing for Microfluidics. EDEN TECH. Available online: https://eden-microfluidics.com/news-events/two-photon-polymerization-3d-printing-for-microfluidics-application-note/ (accessed on 9 July 2025).
- McGoldrick, L.K.; Halámek, J. Recent Advances in Noninvasive Biosensors for Forensics, Biometrics, and Cybersecurity. Sensors 2020, 20, 5974. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.K.; Tharmavaram, M.; Pandey, G. Conventional Technologies in Forensic Science. In Technology in Forensic Science; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 17–34. [Google Scholar] [CrossRef]
- Morillas, Á.V.; Suhling, K.; Frascione, N. Unlocking the potential of forensic traces: Analytical approaches to generate investigative leads. Sci. Justice 2022, 62, 310–326. [Google Scholar] [CrossRef] [PubMed]
- Petherick, W.; Rowan, A. Chapter 3—Physical Evidence and the Crime Scene. In Applied Crime Analysis; Petherick, W., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 39–61. [Google Scholar] [CrossRef]
- Upadhyay, M.; Shrivastava, P.; Verma, K.; Joshi, B. Recent advancements in identification and detection of saliva as forensic evidence: A review. Egypt. J. Forensic Sci. 2023, 13, 17. [Google Scholar] [CrossRef]
- Srisomwat, C.; Bawornnithichaiyakul, N.; Khonyoung, S.; Tiyapongpattana, W.; Butcha, S.; Youngvises, N.; Chailapakul, O. Unveiling the potential of the capillary-driven microfluidic paper-based device integrated with smartphone-based for simultaneously colorimetric salivary ethanol and Δ9-tetrahydrocannabinol analysis. Talanta 2024, 280, 126770. [Google Scholar] [CrossRef] [PubMed]
- Haag, M.G.; Haag, L.C. Chapter 12—Trace Evidence Considerations Associated with Firearms. In Shooting Incident Reconstruction, 2nd ed.; Haag, M.G., Haag, L.C., Eds.; Academic Press: San Diego, CA, USA, 2011; pp. 207–217. [Google Scholar] [CrossRef]
- DiMaio, V.; Dana, S.; Taylor, W.; Ondrusek, J. Use of Scanning Electron Microscopy and Energy Dispersive X-Ray Analysis (SEM-EDXA) in Identification of Foreign Material on Bullets. J. Forensic Sci. 1987, 32, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Sacco, M.A.; Gualtieri, S.; Santos, A.; Mendes, B.; Raffaele, R.; Tarallo, A.P.; Verrina, M.C.; Ranno, F.; Monterossi, M.D.; Ricci, P.; et al. Scanning Electron Microscopy Techniques in the Analysis of Gunshot Residues: A Literature Review. Appl. Sci. 2025, 15, 2634. [Google Scholar] [CrossRef]
- Sultana, N.; Gunning, S.; Furst, S.J.; Garrard, K.P.; Dow, T.A.; Vinueza, N.R. Direct analysis of textile dyes from trace fibers by automated microfluidics extraction system coupled with Q-TOF mass spectrometer for forensic applications. Forensic Sci. Int. 2018, 289, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Home, P.H.; Norman, D.G.; Williams, M.A. Software for the trajectory analysis of blood-drops: A systematic review. Forensic Sci. Int. 2021, 328, 110992. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.; Stern, H.S. Towards a likelihood ratio approach for bloodstain pattern analysis. Forensic Sci. Int. 2022, 341, 111512. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Wang, W.S.; Vanapalli, S.A. Microfluidic viscometers for shear rheology of complex fluids and biofluids. Biomicrofluidics 2016, 10, 043402. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Zhbanov, A.; Yang, S. Microfluidic Systems for Blood and Blood Cell Characterization. Biosensors 2022, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Alford, R.L.; Caskey, C.T. DNA analysis in forensics, disease and animal/plant identification. Curr. Opin. Biotechnol. 1994, 5, 29–33. [Google Scholar] [CrossRef] [PubMed]
- de Roo, R.; Mapes, A.; van Cooten, M.; van Hooff, B.; Kneppers, S.; Kokshoorn, B.; Valkenburg, T.; de Poot, C. Introducing a Rapid DNA Analysis Procedure for Crime Scene Samples Outside of the Laboratory—A Field Experiment. Sensors 2023, 23, 4153. [Google Scholar] [CrossRef] [PubMed]
- Reedy, C.R.; Hagan, K.A.; Marchiarullo, D.J.; Dewald, A.H.; Barron, A.; Bienvenue, J.M.; Landers, J.P. A modular microfluidic system for deoxyribonucleic acid identification by short tandem repeat analysis. Anal. Chim. Acta 2011, 687, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Al Balawi, A.N.; Yusof, N.A.; Kamaruzaman, S.; Mohammad, F.; Wasoh, H.; Al Abbosh, K.F.; Al-Lohedan, H.A. Synthesis, Characterization, and Application of Poly(4,4′-Cyclohexylidene Bisphenol Oxalate) for Solid-Phase Extraction of DNA. BioMed Res. Int. 2019, 2019, 7064073. [Google Scholar] [CrossRef] [PubMed]
- Carthy, É.; Hughes, B.; Higgins, E.; Early, P.; Merne, C.; Walsh, D.; Parle-McDermott, A.; Kinahan, D.J. Automated solid phase DNA extraction on a lab-on-a-disc with two-degrees of freedom instrumentation. Anal. Chim. Acta 2023, 1280, 341859. [Google Scholar] [CrossRef] [PubMed]
- Reifenberger, G.C.; Thomas, B.A.; Rhodes, D.V.L. Comparison of DNA Extraction and Amplification Techniques for Use with Engorged Hard-Bodied Ticks. Microorganisms 2022, 10, 1254. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Wang, H.; Cao, Y.; Huang, Y.; Hu, Y.; Zhou, Y.; Wei, Y.; Li, X.; Hou, T.; Wang, Y.; et al. Evaluation of microfluidics-based droplet PCR combined with multiplex STR system in forensic science. Electrophoresis 2021, 43, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Zaya, D.N.; Ashley, M.V. Plant Genetics for Forensic Applications. In Plant DNA Fingerprinting and Barcoding: Methods and Protocols; Sucher, N.J., Hennell, J.R., Carles, M.C., Eds.; Humana Press: Totowa, NJ, USA, 2012; pp. 35–52. [Google Scholar] [CrossRef]
- Guijt, R.M.; Armstrong, J.P.; Candish, E.; Lefleur, V.; Percey, W.J.; Shabala, S.; Hauser, P.C.; Breadmore, M.C. Microfluidic chips for capillary electrophoresis with integrated electrodes for capacitively coupled conductivity detection based on printed circuit board technology. Sens. Actuators B Chem. 2011, 159, 307–313. [Google Scholar] [CrossRef]
- Gou, T.; Hu, J.; Wu, W.; Ding, X.; Zhou, S.; Fang, W.; Mu, Y. Smartphone-based mobile digital PCR device for DNA quantitative analysis with high accuracy. Biosens. Bioelectron. 2018, 120, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Al-Hetlani, E. Forensic drug analysis and microfluidics. Electrophoresis 2013, 34, 1262–1272. [Google Scholar] [CrossRef] [PubMed]
- Qiang, W.; Zhai, C.; Lei, J.; Song, C.; Zhang, D.; Sheng, J.; Ju, H. Disposable microfluidic device with ultraviolet detection for highly resolved screening of illicit drugs. Analyst 2009, 134, 1834–1839. [Google Scholar] [CrossRef] [PubMed]
- Bruijns, B.; Veciana, A.; Tiggelaar, R.; Gardeniers, H. Cyclic Olefin Copolymer Microfluidic Devices for Forensic Applications. Biosensors 2019, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Manousi, N.; Samanidou, V. Green sample preparation of alternative biosamples in forensic toxicology. Sustain. Chem. Pharm. 2021, 20, 100388. [Google Scholar] [CrossRef]
- Kusano, M.; Sakamoto, Y.; Natori, Y.; Miyagawa, H.; Tsuchihashi, H.; Ishii, A.; Zaitsu, K. Development of ‘Quick-DB forensic’: A total workflow from QuEChERS-dSPE method to GC–MS/MS quantification of forensically relevant drugs and pesticides in whole blood. Forensic Sci. Int. 2019, 300, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Celio, L.; Ottaviani, M.; Cancelliere, R.; Di Tinno, A.; Panjan, P.; Sesay, A.M.; Micheli, L. Microfluidic Flow Injection Immunoassay System for Algal Toxins Determination: A Case of Study. Front. Chem. 2021, 9, 626630. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Musso, N.; Grasso, M.; Costantino, A.; Lazzarino, G.; Tascedda, F.; Gulisano, M.; Lunte, S.M.; Caraci, F. Microfluidics as a Novel Tool for Biological and Toxicological Assays in Drug Discovery Processes: Focus on Microchip Electrophoresis. Micromachines 2020, 11, 593. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, M. On-Chip Liquid Chromatography. Encyclopedia 2022, 2, 617–624. [Google Scholar] [CrossRef]
- Le, D.T.; Vu, N.T. Progress of loop-mediated isothermal amplification technique in molecular diagnosis of plant diseases. Appl. Biol. Chem. 2017, 60, 169–180. [Google Scholar] [CrossRef]
- Thaitrong, N.; Charlermroj, R.; Himananto, O.; Seepiban, C.; Karoonuthaisiri, N.; Willson, R.C. Implementation of Microfluidic Sandwich ELISA for Superior Detection of Plant Pathogens. PLoS ONE 2013, 8, e83231. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Chen, T.; Gao, J.; Dong, C.; Wong, A.H.-H.; Jia, Y.; Mak, P.-I.; Deng, C.-X.; Martins, R.P. A digital microfluidic system for loop-mediated isothermal amplification and sequence specific pathogen detection. Sci. Rep. 2017, 7, 14586. [Google Scholar] [CrossRef] [PubMed]
- Natsuhara, D.; Takishita, K.; Tanaka, K.; Kage, A.; Suzuki, R.; Mizukami, Y.; Saka, N.; Nagai, M.; Shibata, T. A Microfluidic Diagnostic Device Capable of Autonomous Sample Mixing and Dispensing for the Simultaneous Genetic Detection of Multiple Plant Viruses. Micromachines 2020, 11, 540. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Qi, J.; Shen, Y.; Yang, N.; Liu, S.; Wang, A.; Wang, C.; Tang, J. Collection, nucleic acid release, amplification, and visualization platform for rapid field detection of rice false smut. Lab Chip 2023, 23, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Shymanovich, T.; Saville, A.C.; Paul, R.; Wei, Q.; Ristaino, J.B. Rapid Detection of Viral, Bacterial, Fungal, and Oomycete Pathogens on Tomatoes with Microneedles, LAMP on a Microfluidic Chip, and Smartphone Device. Phytopathology® 2024, 114, 1975–1983. [Google Scholar] [CrossRef] [PubMed]
- Jammes, F.C.; Maerkl, S.J. How single-cell immunology is benefiting from microfluidic technologies. Microsyst. Nanoeng. 2020, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Yang, Y.; Zhao, G.; Wang, H.; Dai, Y.; Huang, X. A Low-Cost Microfluidic-Based Detection Device for Rapid Identification and Quantification of Biomarkers-Based on a Smartphone. Biosensors 2023, 13, 753. [Google Scholar] [CrossRef] [PubMed]
- Freitas, T.A.; Proença, C.A.; Baldo, T.A.; Materón, E.M.; Wong, A.; Magnani, R.F.; Faria, R.C. Ultrasensitive immunoassay for detection of Citrus tristeza virus in citrus sample using disposable microfluidic electrochemical device. Talanta 2019, 205, 120110. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Reddy, K.R.; Kakani, V.G.; Reddy, V.R. Nitrogen deficiency effects on plant growth, leaf photosynthesis, and hyperspectral reflectance properties of sorghum. Eur. J. Agron. 2005, 22, 391–403. [Google Scholar] [CrossRef]
- Dutta, P.; Kumari, A.; Mahanta, M.; Upamanya, G.K.; Heisnam, P.; Borua, S.; Kaman, P.K.; Mishra, A.K.; Mallik, M.; Muthukrishnan, G.; et al. Nanotechnological approaches for management of soil-borne plant pathogens. Front. Plant Sci. 2023, 14, 1136233. [Google Scholar] [CrossRef] [PubMed]
- Kung, C.-T.; Hou, C.-Y.; Wang, Y.-N.; Fu, L.-M. Microfluidic paper-based analytical devices for environmental analysis of soil, air, ecology and river water. Sens. Actuators B Chem. 2019, 301, 126855. [Google Scholar] [CrossRef]
- Tobiszewski, M.; Vakh, C. Analytical applications of smartphones for agricultural soil analysis. Anal. Bioanal. Chem. 2023, 415, 3703–3715. [Google Scholar] [CrossRef] [PubMed]
- Khongpet, W.; Pencharee, S.; Puangpila, C.; Hartwell, S.K.; Lapanantnoppakhun, S.; Jakmunee, J. Exploiting an automated microfluidic hydrodynamic sequential injection system for determination of phosphate. Talanta 2018, 177, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Cahill, D.M. Detection, identification and disease diagnosis of soilborne pathogens. Australas. Plant Pathol. 1999, 28, 34–44. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, K.; Yan, H.; Liu, C.; Zhu, X.; Chen, B. Microfluidics as an Emerging Platform for Exploring Soil Environmental Processes: A Critical Review. Environ. Sci. Technol. 2022, 56, 711–731. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Xie, R.; Liu, D.; Liu, Y.; Liang, R. Ecological Responses of Soil Microbial Communities to Heavy Metal Stress in a Coal-Based Industrial Region in China. Microorganisms 2023, 11, 1392. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Chan, H.T.; Chen, Q.; Chen, H. Application of Nanotechnology in Analysis and Removal of Heavy Metals in Food and Water Resources. Nanomaterials 2021, 11, 1792. [Google Scholar] [CrossRef]
- Cui, W.; Ren, Z.; Song, Y.; Ren, C.L. Development and potential for point-of-care heavy metal sensing using microfluidic systems: A brief review. Sens. Actuators A Phys. 2022, 344, 113733. [Google Scholar] [CrossRef]
- Rosen, R. Mass spectrometry for monitoring micropollutants in water. Curr. Opin. Biotechnol. 2007, 18, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Zulkifli, S.N.; Rahim, H.A.; Lau, W.-J. Detection of contaminants in water supply: A review on state-of-the-art monitoring technologies and their applications. Sens. Actuators B Chem. 2018, 255, 2657–2689. [Google Scholar] [CrossRef] [PubMed]
- Karle, M.; Vashist, S.K.; Zengerle, R.; von Stetten, F. Microfluidic solutions enabling continuous processing and monitoring of biological samples: A review. Anal. Chim. Acta 2016, 929, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Chang, H.; Jiang, H.; Wang, P.; Li, N.; Song, Y.; Li, D. Smartphone based microfluidic lab-on-chip device for real-time detection, counting and sizing of living algae. Measurement 2021, 187, 110304. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, X.; Xiang, W.; Wang, T.; Otis, C.; Sarge, L.; Lei, Y.; Li, B. Forward-Looking Roadmaps for Long-Term Continuous Water Quality Monitoring: Bottlenecks, Innovations, and Prospects in a Critical Review. Environ. Sci. Technol. 2022, 56, 5334–5354. [Google Scholar] [CrossRef] [PubMed]
- Filippidou, M.K.; Kanaris, A.I.; Aslanidis, E.; Rapesi, A.; Tsounidi, D.; Ntouskas, S.; Skotadis, E.; Tsekenis, G.; Tsoukalas, D.; Tserepi, A.; et al. Integrated Plastic Microfluidic Device for Heavy Metal Ion Detection. Micromachines 2023, 14, 1595. [Google Scholar] [CrossRef] [PubMed]
- Shahvar, A.; Shamsaei, D.; Saraji, M.; Arab, N.; Alijani, S. Microfluidic-based liquid-liquid microextraction in combination with smartphone-based on-chip detection for the determination of copper in biological, environmental, and food samples. Microchem. J. 2021, 160, 105655. [Google Scholar] [CrossRef]
- Ghaseminasab, K.; Aletaha, N.; Hasanzadeh, M. Smartphone-assisted microfluidic and spectrophotometric recognition of hydrazine: A new platform towards rapid analysis of carcinogenic agents and environmental technology. RSC Adv. 2023, 13, 3575–3585. [Google Scholar] [CrossRef] [PubMed]
- Aryal, P.; Hefner, C.; Martinez, B.; Henry, C.S. Microfluidics in environmental analysis: Advancements, challenges, and future prospects for rapid and efficient monitoring. Lab Chip 2024, 24, 1175–1206. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Jang, A.; MacKnight, E.T.; Wu, P.-M.; Do, J.; Shim, J.S.; Bishop, P.L.; Ahn, C.H. An On-Site Heavy Metal Analyzer With Polymer Lab-on-a-Chips for Continuous Sampling and Monitoring. IEEE Sens. J. 2009, 9, 586–594. [Google Scholar] [CrossRef]
- Xiao, M.; Liu, Z.; Xu, N.; Jiang, L.; Yang, M.; Yi, C. A Smartphone-Based Sensing System for On-Site Quantitation of Multiple Heavy Metal Ions Using Fluorescent Carbon Nanodots-Based Microarrays. ACS Sens. 2020, 5, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Punnoy, P.; Aryal, P.; Hefner, C.E.; Brack, E.; Rodthongkum, N.; Potiyaraj, P.; Henry, C.S. Smartphone-assisted dual-sided capillary microfluidic device for multiplex detection of heavy metals and nutrients in drinking water. Anal. Chim. Acta 2025, 1356, 344031. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wang, M. A Portable Smartphone-Based Sensing System Using a 3D-Printed Chip for On-Site Biochemical Assays. Sensors 2018, 18, 4002. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Peretz-Soroka, H.; Liu, Y.; Lin, F. Novel developments in mobile sensing based on the integration of microfluidic devices and smartphones. Lab Chip 2016, 16, 943–958. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Xing, Y.; Zhang, Y.; Zhang, P.; He, Y.; Ghamsari, F.; Ramasubramanian, M.K.; Wang, Y.; Ai, H.; Oberholzer, J. Smartphone-microfluidic fluorescence imaging system for studying islet physiology. Front. Endocrinol. 2022, 13, 1039912. [Google Scholar] [CrossRef] [PubMed]
- Brás, E.J.S.; Fortes, A.M.; Chu, V.; Fernandes, P.; Conde, J.P. Microfluidic device for the point of need detection of a pathogen infection biomarker in grapes. Analyst 2019, 144, 4871–4879. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Xu, L.; Koh, D.; Nyayapathi, N.; Oh, K.W. Various On-Chip Sensors with Microfluidics for Biological Applications. Sensors 2014, 14, 17008–17036. [Google Scholar] [CrossRef] [PubMed]
- Yoo, W.S.; Kang, K.; Kim, J.G.; Yoo, Y. Image-Based Quantification of Color and Its Machine Vision and Offline Applications. Technologies 2023, 11, 49. [Google Scholar] [CrossRef]
- Parker, R.W.; Wilson, D.J.; Mace, C.R. Open software platform for automated analysis of paper-based microfluidic devices. Sci. Rep. 2020, 10, 11284. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, Y.; Zhou, M.; Han, Y.; Zhang, M.; Gao, Z.; Liu, Z.; Chen, P.; Du, W.; Zhang, X.; et al. Smartphone-based platforms implementing microfluidic detection with image-based artificial intelligence. Nat. Commun. 2023, 14, 1341. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Li, C.; Wang, M.; Cao, H.; Ye, T.; Hao, L.; Wu, X.; Yin, F.; Yu, J.; Xu, F. Low-cost, portable, on-site fluorescent detection of As(III) by a paper-based microfluidic device based on aptamer and smartphone imaging. Microchim. Acta 2023, 190, 109. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Li, F.; Zhang, W.; Shen, W.; Yang, D.; Bian, Z.; Cui, H. Chemiluminescence Immunoassays for Simultaneous Detection of Three Heart Disease Biomarkers Using Magnetic Carbon Composites and Three-Dimensional Microfluidic Paper-Based Device. Anal. Chem. 2019, 91, 13006–13013. [Google Scholar] [CrossRef] [PubMed]
- Calabretta, M.M.; Zangheri, M.; Calabria, D.; Lopreside, A.; Montali, L.; Marchegiani, E.; Trozzi, I.; Guardigli, M.; Mirasoli, M.; Michelini, E. Paper-Based Immunosensors with Bio-Chemiluminescence Detection. Sensors 2021, 21, 4309. [Google Scholar] [CrossRef] [PubMed]
- Boonkaew, S.; Szot-Karpińska, K.; Niedziółka-Jönsson, J.; de Marco, A.; Jönsson-Niedziółka, M. NFC Smartphone-Based Electrochemical Microfluidic Device Integrated with Nanobody Recognition for C-Reactive Protein. ACS Sens. 2024, 9, 3066–3074. [Google Scholar] [CrossRef] [PubMed]
- Lafleur, J.P.; Senkbeil, S.; Jensen, T.G.; Kutter, J.P. Gold nanoparticle-based optical microfluidic sensors for analysis of environmental pollutants. Lab Chip 2012, 12, 4651–4656. [Google Scholar] [CrossRef] [PubMed]
- Azinheiro, S.; Kant, K.; Shahbazi, M.-A.; Garrido-Maestu, A.; Prado, M.; Dieguez, L. A smart microfluidic platform for rapid multiplexed detection of foodborne pathogens. Food Control 2020, 114, 107242. [Google Scholar] [CrossRef]
- Ge, L.; Yu, J.; Ge, S.; Yan, M. Lab-on-paper-based devices using chemiluminescence and electrogenerated chemiluminescence detection. Anal. Bioanal. Chem. 2014, 406, 5613–5630. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Khoshfetrat, S.M.; Kabiri, S.; Dorraji, P.S.; Larijani, B.; Omidfar, K. Electrochemiluminescence paper-based screen-printed electrode for HbA1c detection using two-dimensional zirconium metal-organic framework/Fe3O4 nanosheet composites decorated with Au nanoclusters. Microchim. Acta 2021, 188, 296. [Google Scholar] [CrossRef] [PubMed]
- Apichai, S.; Saenjum, C.; Pattananandecha, T.; Phojuang, K.; Wattanakul, S.; Kiwfo, K.; Jintrawet, A.; Grudpan, K. Cost-Effective Modern Chemical Sensor System for Soil Macronutrient Analysis Applied to Thai Sustainable and Precision Agriculture. Plants 2021, 10, 1524. [Google Scholar] [CrossRef] [PubMed]
- Ngugi, L.C.; Abelwahab, M.; Abo-Zahhad, M. Recent advances in image processing techniques for automated leaf pest and disease recognition—A review. Inf. Process. Agric. 2021, 8, 27–51. [Google Scholar] [CrossRef]
- Wenwen, M.; Zixin, H.; Zha, L.; Dongfeng, S.; Zijun, G.; Jian, H.; Park, B.; Yingjian, W. Image-free Hu invariant moment measurement by single-pixel detection. Opt. Laser Technol. 2024, 181, 111581. [Google Scholar] [CrossRef]
- Vashist, S.K.; van Oordt, T.; Schneider, E.M.; Zengerle, R.; von Stetten, F.; Luong, J.H. A smartphone-based colorimetric reader for bioanalytical applications using the screen-based bottom illumination provided by gadgets. Biosens. Bioelectron. 2015, 67, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Talebjedi, B.; Heydari, M.; Taatizadeh, E.; Tasnim, N.; Li, I.T.S.; Hoorfar, M. Neural Network-Based Optimization of an Acousto Microfluidic System for Submicron Bioparticle Separation. Front. Bioeng. Biotechnol. 2022, 10, 878398. [Google Scholar] [CrossRef] [PubMed]
- Dupuit, V.; Briançon-Marjollet, A.; Delacour, C. Portrait of intense communications within microfluidic neural networks. Sci. Rep. 2023, 13, 12306. [Google Scholar] [CrossRef] [PubMed]
- Negiş, H. Using Models and Artificial Neural Networks to Predict Soil Compaction Based on Textural Properties of Soils under Agriculture. Agriculture 2023, 14, 47. [Google Scholar] [CrossRef]
- Yang, N.; Chen, C.; Li, T.; Li, Z.; Zou, L.; Zhang, R.; Mao, H. Portable Rice Disease Spores Capture and Detection Method Using Diffraction Fingerprints on Microfluidic Chip. Micromachines 2019, 10, 289. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mao, H.; Zhang, X.; Liu, Y.; Du, X. A Rapid Detection Method for Tomato Gray Mold Spores in Greenhouse Based on Microfluidic Chip Enrichment and Lens-Less Diffraction Image Processing. Foods 2021, 10, 3011. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Chen, B.; Fu, E.S.; Yan, H. Computer vision meets microfluidics: A label-free method for high-throughput cell analysis. Microsyst. Nanoeng. 2023, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Pouyanfar, N.; Harofte, S.Z.; Soltani, M.; Siavashy, S.; Asadian, E.; Ghorbani-Bidkorbeh, F.; Keçili, R.; Hussain, C.M. Artificial intelligence-based microfluidic platforms for the sensitive detection of environmental pollutants: Recent advances and prospects. Trends Environ. Anal. Chem. 2022, 34, e00160. [Google Scholar] [CrossRef]
- Singhal, C.M.; Kaushik, V.; Awasthi, A.; Zalke, J.B.; Palekar, S.; Rewatkar, P.; Srivastava, S.K.; Kulkarni, M.B.; Bhaiyya, M.L. Deep Learning-Enhanced Portable Chemiluminescence Biosensor: 3D-Printed, Smartphone-Integrated Platform for Glucose Detection. Bioengineering 2025, 12, 119. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.-F.; Podder, S.; Chen, P.-Y. Microsystem Advances through Integration with Artificial Intelligence. Micromachines 2023, 14, 826. [Google Scholar] [CrossRef] [PubMed]
- Pennacchio, A.; Giampaolo, F.; Piccialli, F.; Cuomo, S.; Notomista, E.; Spinelli, M.; Amoresano, A.; Piscitelli, A.; Giardina, P. A machine learning-enhanced biosensor for mercury detection based on an hydrophobin chimera. Biosens. Bioelectron. 2022, 196, 113696. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Yu, N.; Tian, D.; Wang, Y.; Li, S.; Li, Z. Toward Embedded Sensing Automation and Miniaturization for Portable Smart Cost-Effective Algae Monitor. IEEE Sens. J. 2020, 21, 5230–5239. [Google Scholar] [CrossRef]
- Rateni, G.; Dario, P.; Cavallo, F. Smartphone-Based Food Diagnostic Technologies: A Review. Sensors 2017, 17, 1453. [Google Scholar] [CrossRef] [PubMed]
- Nelis, J.; Tsagkaris, A.; Dillon, M.; Hajslova, J.; Elliott, C. Smartphone-based optical assays in the food safety field. TrAC Trends Anal. Chem. 2020, 129, 115934. [Google Scholar] [CrossRef] [PubMed]
- Bazyar, H. On the Application of Microfluidic-Based Technologies in Forensics: A Review. Sensors 2023, 23, 5856. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, X.; Liu, Q.; Zhao, W.; Liu, S.; Sui, G. Microfluidic System for Rapid Detection of Airborne Pathogenic Fungal Spores. ACS Sens. 2018, 3, 2095–2103. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Yu, N.; Zhou, G.; Wu, Y.; Wang, C. A wireless multi-channel low-cost lab-on-chip algae culture monitor AIoT system for algae farm. Comput. Electron. Agric. 2022, 193, 106647. [Google Scholar] [CrossRef]
- Peters, K.L.; Corbin, I.; Kaufman, L.M.; Zreibe, K.; Blanes, L.; McCord, B.R. Simultaneous colorimetric detection of improvised explosive compounds using microfluidic paper-based analytical devices (μPADs). Anal. Methods 2014, 7, 63–70. [Google Scholar] [CrossRef]
- Lopez-Ruiz, N.; Curto, V.F.; Erenas, M.M.; Benito-Lopez, F.; Diamond, D.; Palma, A.J.; Capitan-Vallvey, L.F. Smartphone-Based Simultaneous pH and Nitrite Colorimetric Determination for Paper Microfluidic Devices. Anal. Chem. 2014, 86, 9554–9562. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Dubey, S.K.; Goel, S. IoT enabled microfluidic colorimetric detection platform for continuous monitoring of nitrite and phosphate in soil. Comput. Electron. Agric. 2022, 195, 106856. [Google Scholar] [CrossRef]
- Placer, L.; Lavilla, I.; Pena-Pereira, F.; Bendicho, C. A 3D microfluidic paper-based analytical device with smartphone-assisted colorimetric detection for iodine speciation in seaweed samples. Sens. Actuators B Chem. 2022, 377, 133109. [Google Scholar] [CrossRef]
- Xiang, X.; Song, M.; Xu, X.; Lu, J.; Chen, Y.; Chen, S.; He, Y.; Shang, Y. Microfluidic Biosensor Integrated with Signal Transduction and Enhancement Mechanism for Ultrasensitive Noncompetitive Assay of Multiple Mycotoxins. Anal. Chem. 2023, 95, 7993–8001. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Shi, X.; Zhou, S.; Tian, J.; Cao, R. Smartphone-based paper microfluidic detection implementing a versatile quick response code conversion strategy. Sens. Actuators B Chem. 2024, 406, 135393. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, J.; Wu, J.; Wang, L.; Yao, C.; Hu, J.; Zhang, H. A microfluidic paper-based fluorescent sensor integrated with a smartphone platform for rapid on-site detection of omethoate pesticide. Food Chem. 2024, 463, 141205. [Google Scholar] [CrossRef] [PubMed]
- Krauss, S.T.; Remcho, T.P.; Lipes, S.M.; Aranda, R.; Maynard, H.P.; Shukla, N.; Li, J.; Tontarski, R.E.; Landers, J.P. Objective Method for Presumptive Field-Testing of Illicit Drug Possession Using Centrifugal Microdevices and Smartphone Analysis. Anal. Chem. 2016, 88, 8689–8697. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; Kang, D.; Xue, Y.; Lee, S.; Pielak, R.M.; Kim, J.; Hwang, T.; Min, S.; Banks, A.; Bastien, P.; et al. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci. Transl. Med. 2016, 8, 366ra165. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Jalal, U.M.; Im, S.B.; Ko, S.; Shim, J.S. A smartphone-based optical platform for colorimetric analysis of microfluidic device. Sens. Actuators B Chem. 2017, 239, 52–59. [Google Scholar] [CrossRef]
- Ansari, N.; Lodha, A.; Pandya, A.; Menon, S.K. Determination of cause of death using paper-based microfluidic device as a colorimetric probe. Anal. Methods 2017, 9, 5632–5639. [Google Scholar] [CrossRef]
- Aymerich, J.; Márquez, A.; Terés, L.; Muñoz-Berbel, X.; Jiménez, C.; Domínguez, C.; Serra-Graells, F.; Dei, M. Cost-effective smartphone-based reconfigurable electrochemical instrument for alcohol determination in whole blood samples. Biosens. Bioelectron. 2018, 117, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.-C.; Yang, R.-J.; Ju, W.-J.; Fu, L.-M. Microfluidic paper-based platform for whole blood creatinine detection. Chem. Eng. J. 2018, 348, 117–124. [Google Scholar] [CrossRef]
- Mathaweesansurn, A.; Thongrod, S.; Khongkaew, P.; Phechkrajang, C.M.; Wilairat, P.; Choengchan, N. Simple and fast fabrication of microfluidic paper-based analytical device by contact stamping for multiple-point standard addition assay: Application to direct analysis of urinary creatinine. Talanta 2020, 210, 120675. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.; Sáez-Hernández, R.; Armenta, S.; Morales-Rubio, A.; Cervera, M. 3D-printed portable device for illicit drug identification based on smartphone-imaging and artificial neural networks. Talanta 2024, 276, 126217. [Google Scholar] [CrossRef] [PubMed]
| Ref. | Year | Target Molecules | Sample Type | Sensing Technique | Chip Fabrication | Smartphone Integration | LOD | Reaction Time | Accuracy |
|---|---|---|---|---|---|---|---|---|---|
| [149] | 2014 | Nitrite (NO2−) | Water | Colorimetric | Paper-based microfluidics; reagents deposited on patterned cellulose | Camera captures coloration; Custom app processes hue and saturation | 0.52 mg/L | Rapid | - |
| [146] | 2018 | Aspergillus niger spores | Whole spores | Immunofluorescence using antibody-conjugated microspheres | PDMS-based chips (soft lithography) | No—fluorescence microscopy was used; Potentially adaptable for smartphone | 300 spores/m3 | 2–3 h, incl. enrichment | >90% |
| [103] | 2022 | Live algae | Water | Optical detection | PDMS microchannel (soft lithography) | Camera captures images; Custom app counts algae | 500 μm algae/s | Real-time | 92% |
| [150] | 2022 | Nitrite (NO2−) and phosphate (PO43−) ions | Soil samples | Colorimetric | PDMS-based chip (soft lithography) from UV-laser patterned mold | Smartphone powers the device; Bluetooth transfers data; app for near-range monitoring; cloud server for long-range access | 0.33 µM NO2−; 0.75 µM PO43− | 2–3 min NO2−; ~10 min PO43− | CV < 5% |
| [147] | 2022 | Microalga (Scenedesmus quadricauda) | Whole algal cells | Lensless imaging with CMOS sensor and neural network image segmentation | PDMS microfluidic chip (soft lithography); embedded with a lensless CMOS image sensor | No; suggested but not implemented; Bluetooth transfers data | - | Real-time | 96.35% |
| [151] | 2023 | Iodide (I−) and Iodate (IO3−) | Seaweed extracts | Colorimetric | 3D origami paper microfluidics (wax printing) | Camera captures the green channel intensity; ImageJ or app process images | 9.8 µM I−; 0.6 µM IO3− | 1 min | RSD 1.7% I−; 3.3% IO3− |
| [152] | 2023 | Mycotoxins AFB1, OTA, ZEN, FB1, T-2, and DON | Food samples | CRISPR/Cas12a system with quantum dots | PDMS (soft lithography) | Camera captures the fluorescence signals; images are analyzed via color model | 1.4–3.9 fg/mL | 40 min | CV < 5% 88.8–110% correlation with HPLC |
| [153] | 2024 | pH, ascorbic acid (AA), and 5-hydroxymethylfurfural (HMF) | Water samples | Colorimetric | Three-layer QR-coded paper microfluidics | Camera scans QR code and captures images; custom app quantify coloration | 5.56 ppm AA; 6.73 ppm HMF; 0.1 pH units | 10 min | CV < 10% |
| [86] | 2024 | Tomato pathogens: Alternaria spp., Xanthomonas perforans, Phytophthora infestans, tomato spotted wilt virus (TSWV) | Genomic DNA and RNA | Loop-mediated isothermal amplification (LAMP); fluorescence and colorimetric readouts | PDMS-based microfluidic chip (soft lithography) using a 3D-printed mold | Camera captures fluorescence images; ImageJ analyzes the images | 1 pg DNA | 30 min | 90–100% |
| [154] | 2025 | Omethoate pesticide | Extracts from spinach, wheat, tap water | Aptamer-based fluorescence sensing | Laser-printed two-layered paper chip | Camera captures images; app with a CNN regression model quantifies fluorescence | 0.16 nM | <10 min | R2 = 0.9964 |
| [148] | 2014 | Nitrate, nitrite, chlorate, perchlorate, ammonium, TNT, RDX, PETN, TATP, urea nitrate, H2O2 | Whole explosive compounds | Colorimetric | Wax printing on chromatography paper | Visual color changes assessed by eye or optionally scanned via color densitometry | 0.39 to 19.8 mg | >10 min | - |
| [155] | 2016 | Cocaine and methamphetamine | Powdered drug samples | Colorimetric | Centrifugal microfluidic device from polyester-toner layers | Camera images reaction zones; images are analyzed via hue and saturation color spaces | 0.25 (cocaine) and 0.75 (meth.) mg/mL | - | - |
| [156] | 2016 | Sweat biomarkers: chloride, lactate, glucose, pH | Sweat | Colorimetric | Soft, stretchable PDMS-based elastomer | Camera images detection zones; NFC transmits data | 10–100 mM chloride | - | - |
| [157] | 2017 | Blood hematocrit | Whole blood samples | Colorimetric | Laser-cut polymer double-sided tape adhered to PMMA substrate | Camera captures blood images; app processes and analyzes images | 0.1% hematocrit | 1 min | Sensitive across 10–65% range |
| [158] | 2017 | Psychoactive drug: alprazolam (ALP) | Blood and vitreous humor | Colorimetric | Paper-based microfluidics with silver nanoparticles | Camera quantifies coloration | 10 ng/mL | - | - |
| [159] | 2018 | Ethanol | Whole blood samples | Enzymatic detection (Aox and HRP) | Silicon microfabrication; Microfabricated Pt electrodes; Laser-cut PMMA | USB powers the device and acquire data from micro-potentiostat | 0.0375 g/L | <5 min | Error < 0.009% |
| [160] | 2018 | Creatinine | Whole blood | Colorimetric | Paper-based microfluidics (wax printing) | Camera detects coloration; app processes data | - | 5 min | - |
| [161] | 2020 | Human urinary creatinine | Urine | Colorimetric | Paper-based microfluidics (contact stamping) | Camera captures color images; ImageJ analyzes red-to-green (R/G) intensity ratio | - | - | RSD = 2.1% 98.1–104% correlation with HPLC |
| [162] | 2024 | Illicit drugs: cocaine, methamphetamine, MDMA, amphetamine, synthetic cathinones, pyrrolidine, methylenedioxy derivatives | Crushed or dissolved drug samples | Colorimetric | Microwell device (3D printing) | Camera captures colorations in microwells; RGB values processed using an artificial neural network (ANN) | μg range | <5 min | >83.4% sensitivity; 100% specificity |
| [54] | 2024 | Ethanol and Δ9-THC | Saliva | Colorimetric | Paper-based microfluidics (wax printing) | Camera mages detection zones; app quantifies colorations | - | 40 min | Recovery 98–102% ethanol and 95–105% THC; RSD < 5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loima, T.; Yoon, J.-Y.; Kaarj, K. Microfluidic Sensors Integrated with Smartphones for Applications in Forensics, Agriculture, and Environmental Monitoring. Micromachines 2025, 16, 835. https://doi.org/10.3390/mi16070835
Loima T, Yoon J-Y, Kaarj K. Microfluidic Sensors Integrated with Smartphones for Applications in Forensics, Agriculture, and Environmental Monitoring. Micromachines. 2025; 16(7):835. https://doi.org/10.3390/mi16070835
Chicago/Turabian StyleLoima, Tadsakamon, Jeong-Yeol Yoon, and Kattika Kaarj. 2025. "Microfluidic Sensors Integrated with Smartphones for Applications in Forensics, Agriculture, and Environmental Monitoring" Micromachines 16, no. 7: 835. https://doi.org/10.3390/mi16070835
APA StyleLoima, T., Yoon, J.-Y., & Kaarj, K. (2025). Microfluidic Sensors Integrated with Smartphones for Applications in Forensics, Agriculture, and Environmental Monitoring. Micromachines, 16(7), 835. https://doi.org/10.3390/mi16070835








