3D-Printed Multi-Stimulus-Responsive Hydrogels: Fabrication and Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Gel/SA-TA Bioinks
2.2. Characterization of Gel/SA-TA Bioinks
2.3. Three-Dimensional Printing Gel/SA-TA Bioink
2.4. Characterization of Gel/SA-TA Bioink Printing Samples
2.5. Preparation of Gel/SA-TA@Fe3+ Bioinks
2.6. Characterization of Gel/SA-TA@Fe3+ Bioink Printing Samples
2.7. Measurement of Gel/SA-TA@Fe3+ Impedance Feedback Signals in Response to Deformable and Infrared Stimuli
3. Results and Discussion
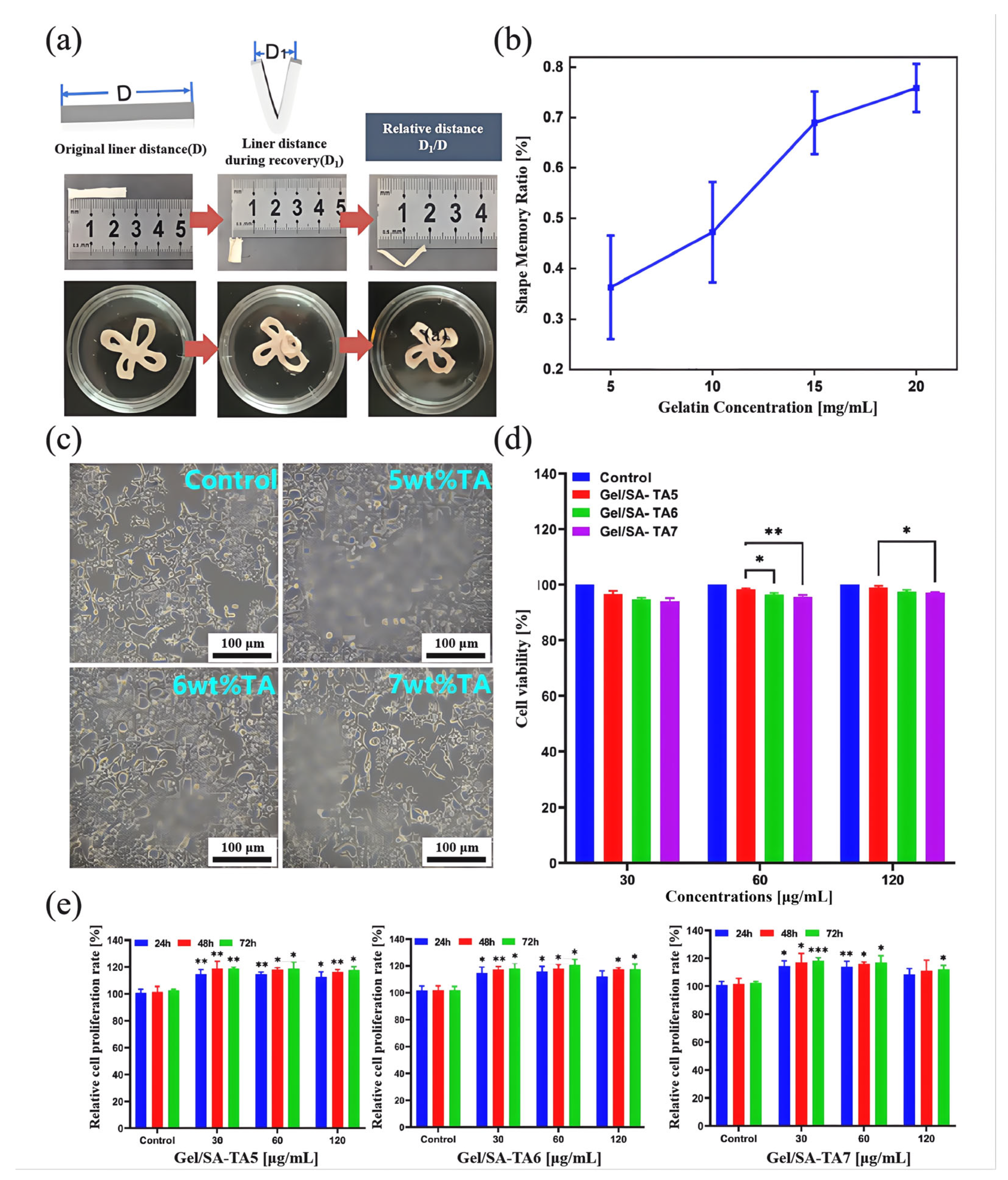
3.1. Performance of Gel/SA-TA Bioprinting Ink
3.2. Performance of Gel/SA-TA Bioink Printing Samples
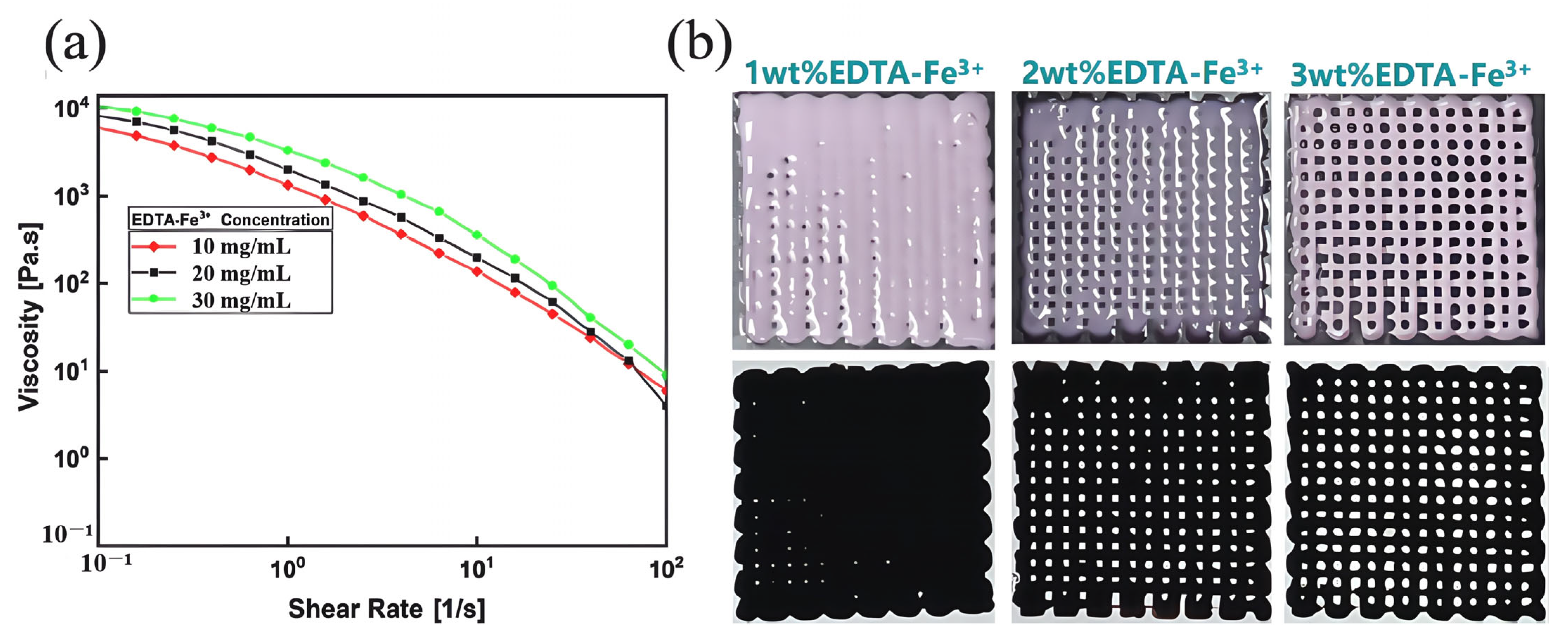
3.3. Performance of Gel/SA-TA@Fe3+ Bioprinting Ink
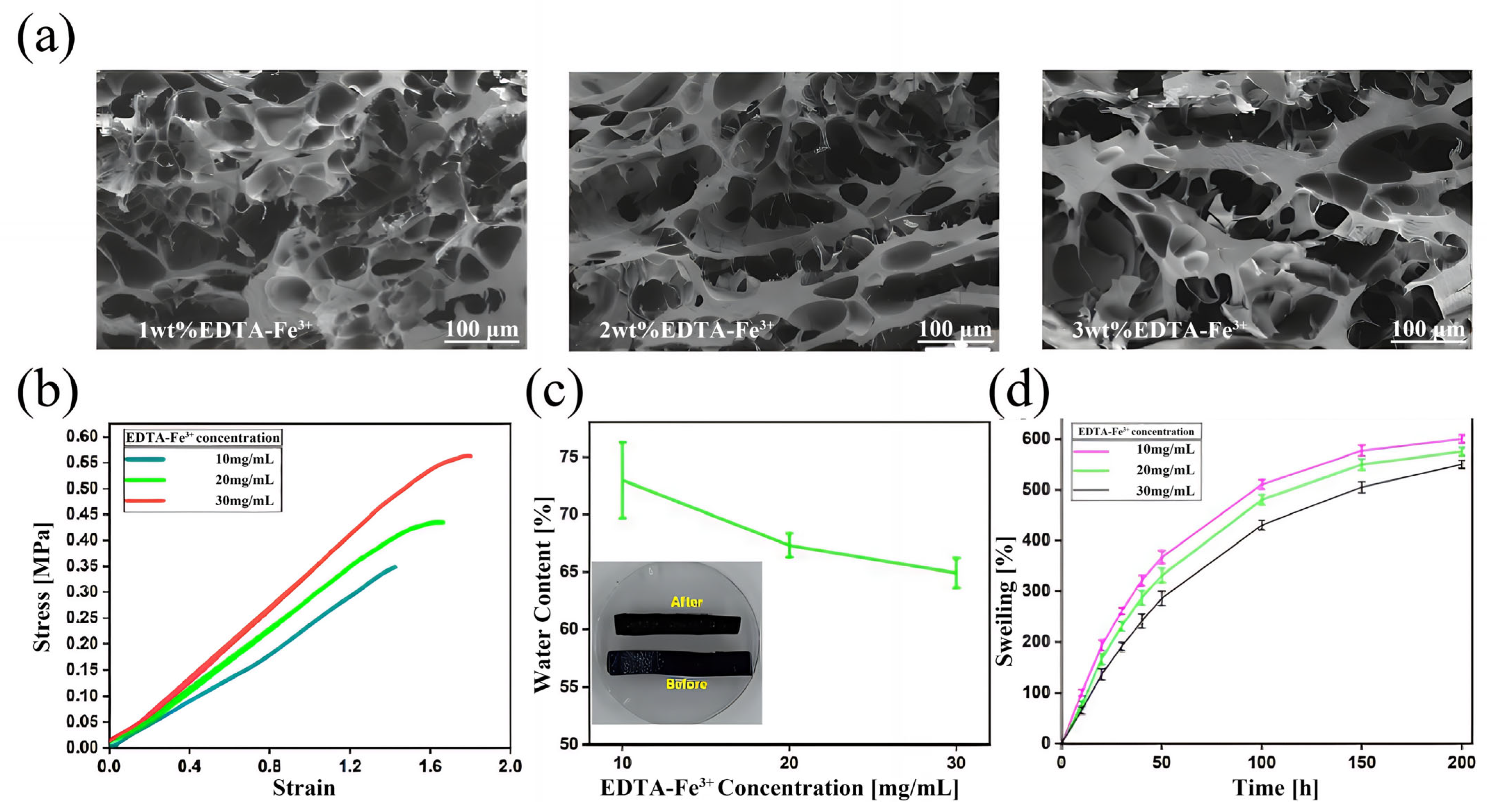
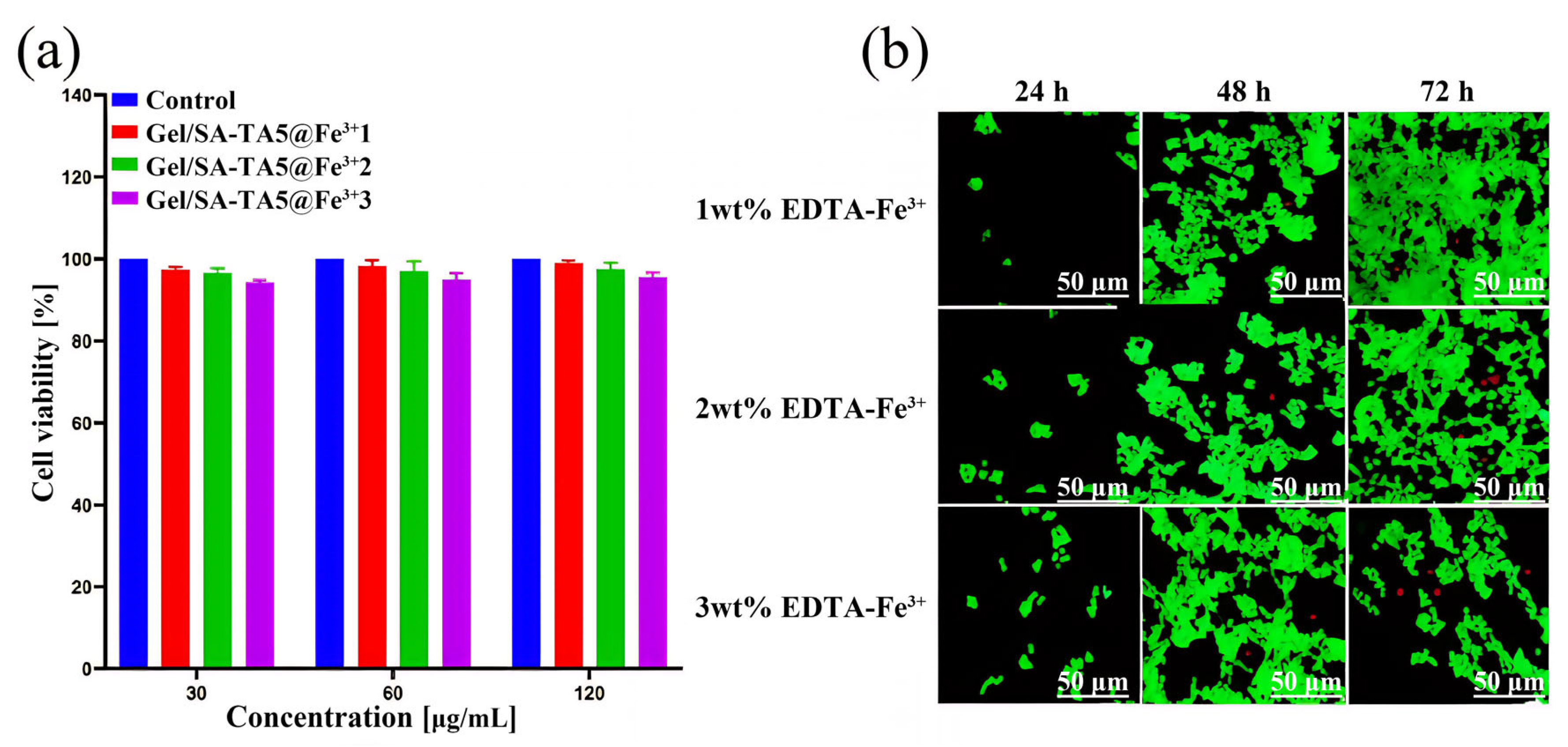
3.4. Performance of Gel/SA-TA@Fe3+ Bioink Printing Samples
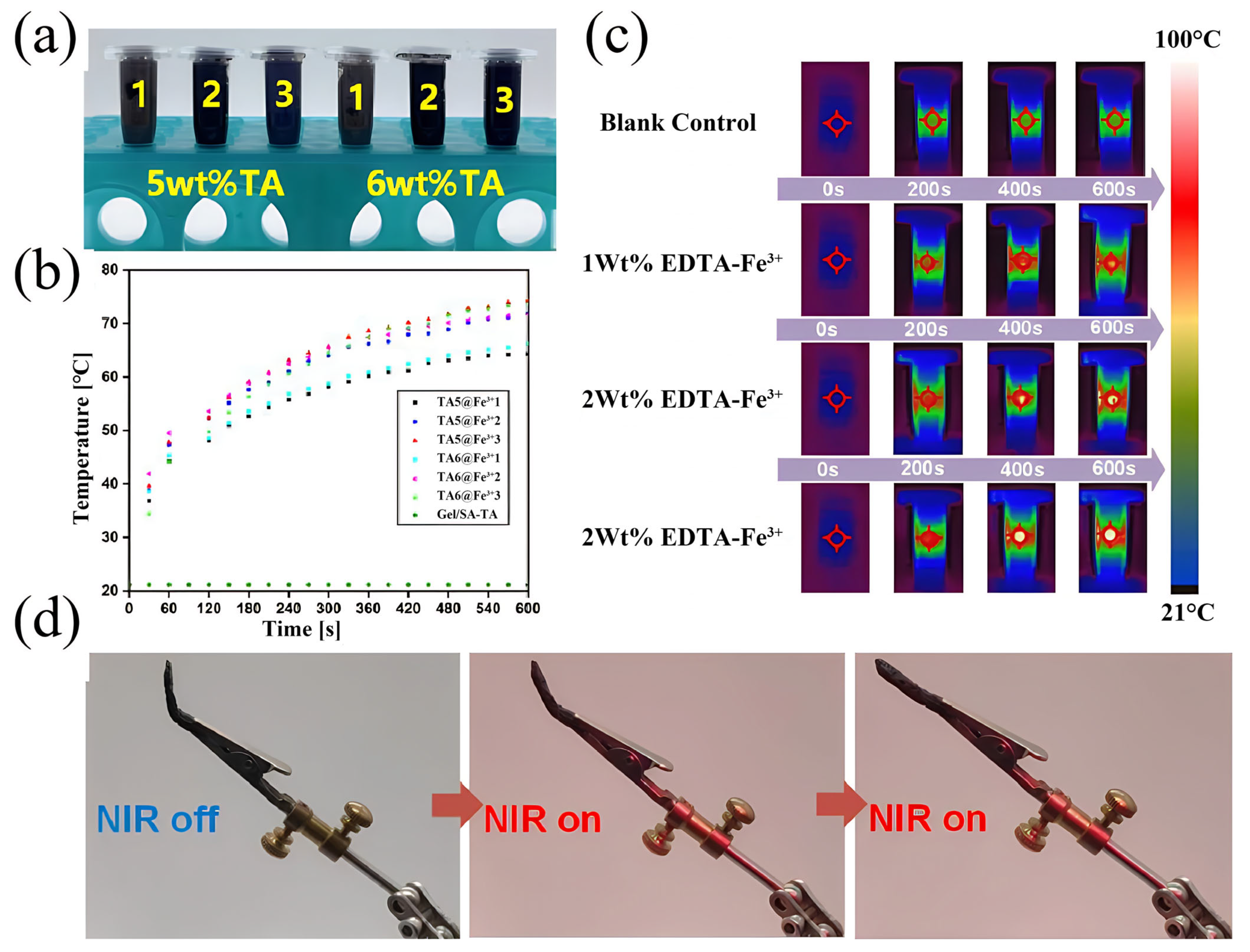
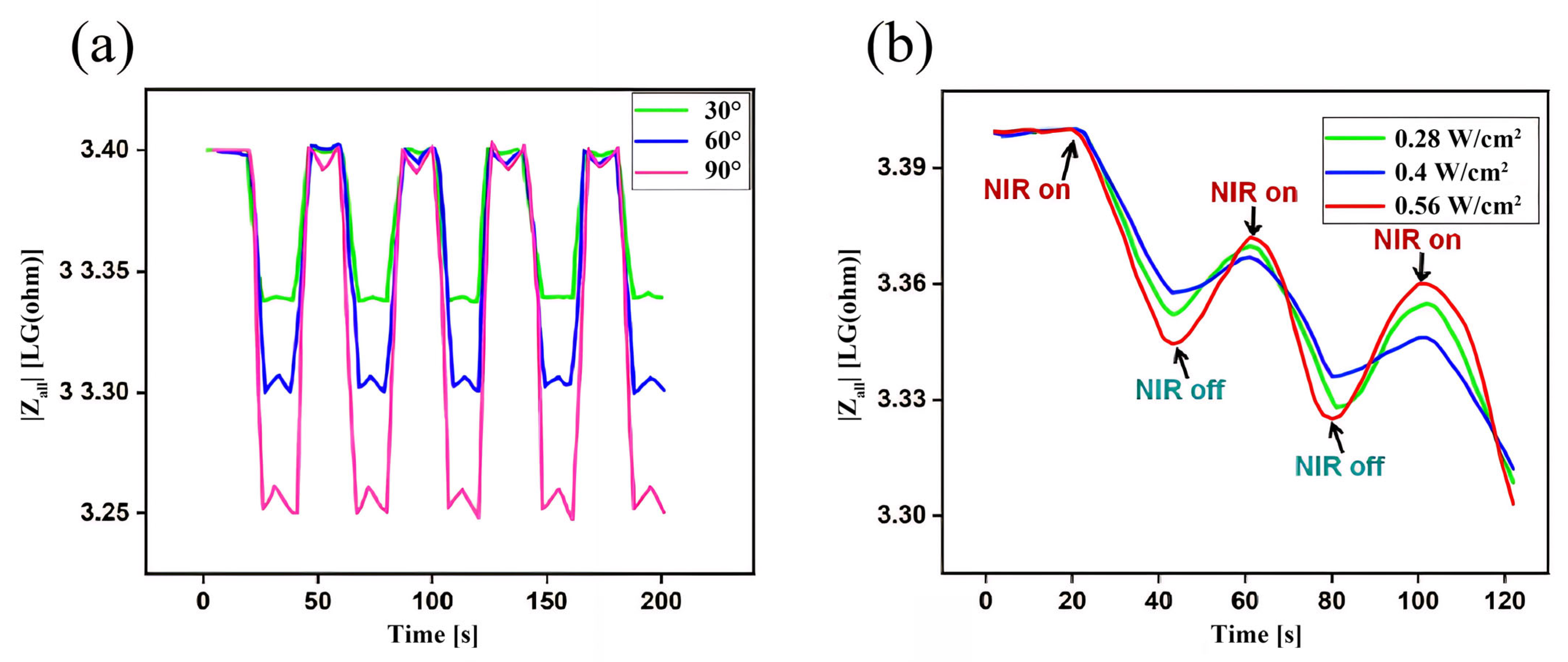
3.5. Analysis of Gel/SA-TA@Fe3+ Impedance Feedback Signals in Response to Deformable and Infrared Stimuli
4. Conclusions
- TA introduces shear-thinning characteristics to the hydrogel system during viscosity changes, improving the preparation efficiency while ensuring the accuracy of the printed Gel/SA-TA 3D models. Furthermore, TA’s ability to generate intermolecular forces with the Gel/SA matrix results in a 6.6-fold increase in tensile modulus compared with pure Gel/SA hydrogel. The resulting composite also demonstrates good cytocompatibility and a positive effect on 293T cell proliferation.
- Adding Fe3+ provides the Gel/SA-TA hydrogel system with a dual stimulus–response to NIR and temperature. Given the strong metal coordination between TA and Fe3+, the tensile strength of the Gel/SA-TA@Fe3+ hydrogel is also significantly improved compared with the Gel/SA-TA hydrogel. Meanwhile, the Gel/SA-TA@Fe3+ hydrogel also exhibits good cytocompatibility.
- The sensing mechanism of the hydrogels was explored, and the impedance of the Gel/SA-TA@Fe3+ hydrogel was smaller and more stable at a high frequency. When the hydrogel is actually used as a flexible sensor, the working voltage value should be set at 0.02 V. Finally, the Gel/SA-TA@Fe3+ hydrogel had good NIR stimulus–response performance and deformation–response performance, which is of great significance for the real-time monitoring of vital signs and human movement in the daily use of hydrogels in biomedical devices.
- In future studies, more comprehensive mechanical property tests (such as compression, bending, robustness, and durability tests) and more extensive primary cell experiments (such as other primary cells and stem cell experiments) will enhance the exhaustiveness of this research.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DIW | Direct ink writing |
| AM | Additive manufacturing |
| SA | Sodium alginate |
| TA | Tannic acid |
| NIR | Near-infrared |
| EDTA | Ethylene diamine tetra acetic acid |
References
- Liu, R.; Liu, Y.; Fu, S.; Jin, K.; Ma, J.; Wan, Y.; Tian, Y. Humidity Adaptive Antifreeze Hydrogel Sensor for Intelligent Control and Human-Computer Interaction. Small 2025, 20, 202308092. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, Y.; Hong, W. Bamboo-Inspired, Environmental Friendly PDMS/Plant Fiber Composites-Based Capacitive Flexible Pressure Sensors by Origami for Human–Machine Interaction. ACS Sustain. Chem. Eng. 2024, 12, 4835–4845. [Google Scholar] [CrossRef]
- Gong, Y.; Zhang, K.; Lei, I.M.; Wang, Y.; Zhong, J. Advances in Piezoelectret Materials-Based Bidirectional Haptic Communication Devices. Adv. Mater. 2024, 36, 2405308. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Kong, Q.; Li, S.; Dai, K.; Attia, N.F.; Wang, H.; Wang, J.; Jiang, S. Wearable flexible tactile triboelectric sensor Constructed with 3D printing for material perception. Chem. Eng. J. 2025, 516, 163924. [Google Scholar] [CrossRef]
- Lin, M.; Zheng, Z.; Yang, L.; Luo, M.; Fu, L.; Lin, B.; Xu, C. A High-Performance, Sensitive, Wearable Multifunctional Sensor Based on Rubber/CNT for Human Motion and Skin Temperature Detection. Adv. Mater. 2022, 34, 202107309. [Google Scholar] [CrossRef]
- Wu, S.; Zhao, Y.; Wang, J.; Zhao, X.; Zhai, W.; Zhu, X.; Wan, P.; Cui, M.; Gao, L.; Dai, K. All-nanofiber self-powered electronic skin with electrospinning humidity-controlled structure for human muscle detection during fitness. Compos. Commun. 2025, 57, 102421. [Google Scholar] [CrossRef]
- Yan, Y.; Zheng, J.; Zhang, Q.; Li, Y.; Li, G.; Zhang, Z.; Wang, P.; Wu, W.; Zhai, J.; Xu, Y. Ultrafast piezoresistive flexible pressure sensor for vibration and sound detection with a bandwidth over 20 kHz. Chem. Eng. J. 2025, 517, 164221. [Google Scholar] [CrossRef]
- Chen, H.; Zhuo, F.; Zhou, J.; Liu, Y.; Zhang, J.; Dong, S.; Liu, X.; Elmarakbi, A.; Duan, H.; Fu, Y. Advances in graphene-based flexible and wearable strain sensors. Chem. Eng. J. 2023, 25, 464. [Google Scholar] [CrossRef]
- Mao, Z.; Suzuki, S.; Nabae, H.; Miyagawa, S.; Suzumori, K.; Maeda, S. Machine learning-enhanced soft robotic system inspired by rectal functions to investigate fecal incontinence. Bio-Des. Manuf. 2025, 8, 482–494. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, X.; Li, D.; Ma, Z.; Liu, Z.; Bai, X.; Mao, Z. Predicting flow status of a flexible rectifier using cognitive computing. Expert Syst. Appl. 2025, 264, 125878. [Google Scholar] [CrossRef]
- Ma, G.; Wang, J.; Di, T.; Fu, Y.; Wang, Z.; Hao, Y.; Niu, F.; Wu, D. Effect of heat treatment on microstructure, phase transformation behavior and shape memory effect of NiTi shape memory alloy manufactured by additive manufacturing. J. Alloys Compd. 2025, 1, 181340. [Google Scholar] [CrossRef]
- Ozair, H.; Baluch, A.H.; Rehman, M.A.; Wadood, A. Shape memory hybrid composites. ACS Omega 2022, 7, 36052–36069. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, F.; Abdiryim, T.; Liu, X. Stimuli-responsive hydrogels as promising platforms for soft actuators. Mater. Today Phys. 2024, 40, 101281. [Google Scholar] [CrossRef]
- Gao, S.; Zhou, A.; Wang, J.; Li, F.; Cao, B. A tunable temperature-responsive and tough platform for controlled drug delivery. New J. Chem. 2021, 45, 13056–13063. [Google Scholar] [CrossRef]
- Yin, H.; Liu, F.; Abdiryim, T.; Liu, X. Self-healing hydrogels: From synthesis to multiple applications. ACS Mater. Lett. 2023, 5, 1787–1830. [Google Scholar] [CrossRef]
- Xiao, S.; Zhao, Y.; Jin, S.; He, Z.; Duan, G.; Gu, H.; Xu, H.; Cao, X.; Ma, C.; Wu, J. Regenerable bacterial killing–releasing ultrathin smart hydrogel surfaces modified with zwitterionic polymer brushes. E-Polymers 2022, 22, 719–732. [Google Scholar] [CrossRef]
- Wu, Q.; Ma, C.; Chen, L.; Sun, Y.; Wei, X.; Ma, C.; Zhao, H.; Yang, X.; Ma, X.; Zhang, C.; et al. A tissue paper/hydrogel composite light-responsive biomimetic actuator fabricated by in situ polymerization. Polymers 2022, 14, 5454. [Google Scholar] [CrossRef]
- Bai, N.; Wang, L.; Wang, Q.; Deng, J.; Wang, Y.; Lu, P.; Huang, J.; Li, G.; Zhang, Y.; Yang, J. Graded intrafillable architecture-based iontronic pressure sensor with ultra-broad-range high sensitivity. Nat. Commun. 2020, 11, 209. [Google Scholar] [CrossRef]
- Gorissen, B.; Van Hoof, C.; Reynaerts, D.; De Volder, M. SU8 etch mask for patterning PDMS and its application to flexible fluidic microactuators. Microsyst. Nanoeng. 2016, 2, 16045. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, B.; Ye, T.; Gao, W.; Pei, G.; Luo, J.; Deng, J.; Yuan, W. One-step fabrication of flexible bioinspired superomniphobic surfaces. ACS Appl. Mater. Interfaces 2022, 14, 39665–39672. [Google Scholar] [CrossRef]
- Almohammed, S.; Alruwaili, M.; Reynaud, E.G.; Redmond, G.; Rodriguez, B.J. 3D-printed peptide-hydrogel nanoparticle composites for surface-enhanced raman spectroscopy sensing. ACS Appl. Nano Mater. 2019, 2, 5029–5034. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Wei, Q.; Wang, Y.; Li, M.; Li, D.; Zhang, L. A 3D printable, highly stretchable, self-healing hydrogel-based sensor based on polyvinyl alcohol/sodium tetraborate/sodium alginate for human motion monitoring. Int. J. Biol. Macromol. 2022, 219, 1216–1226. [Google Scholar] [CrossRef] [PubMed]
- Guan, R.; Zheng, H.; Liu, Q.; Ou, K.T.; Li, D.S.; Fan, J.; Fu, Q.; Sun, Y. DIW 3D printing of hybrid magnetorheological materials for application in soft robotic grippers. Compos. Sci. Technol. 2022, 223, 109409. [Google Scholar] [CrossRef]
- Yang, G.; Sun, Y.; Qin, L.; Li, M.; Ou, K.; Fang, J.; Fu, Q. Direct-ink-writing (DIW) 3D printing functional composite materials based on supra-molecular interaction. Compos. Sci. Technol. 2021, 215, 109013. [Google Scholar] [CrossRef]
- Cong, C.; Wang, R.; Zhu, W.; Zheng, X.; Sun, F.; Wang, X.; Jiang, F.; Sang, W.J.; Lim, S.; Kim, S.H. Self-powered strain sensing devices with wireless transmission: DIW-printed conductive hydrogel electrodes featuring stretchable and self-healing properties. J. Colloid Interface Sci. 2025, 678, 588–598. [Google Scholar] [CrossRef]
- Ouyang, L.; Yao, R.; Chen, X.; Na, J.; Sun, W. 3D printing of HEK 293FT cell-laden hydrogel into macroporous constructs with high cell viability and normal biological functions. Biofabrication 2015, 7, 015010. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Y.; Wang, T.; Sun, W.; Tong, Z. Ultrafast and programmable shape memory hydrogel of gelatin soaked in tannic acid solution. ACS Appl. Mater. Interfaces 2020, 12, 46701–46709. [Google Scholar] [CrossRef]
- Neffe, A.T.; Lwenberg, C.; Julich-Gruner, K.K.; Behl, M.; Lendlein, A. Thermally-induced shape-memory behavior of degradable gelatin-based networks. Int. J. Mol. Sci. 2021, 22, 5892. [Google Scholar] [CrossRef]
- Zhao, L.; Sha, H.; Liu, Y.; Yao, B.; Fu, X. Tuning alginate-gelatin bioink properties by varying solvent and their impact on stem cell behavior. Sci. Rep. 2018, 8, 8020. [Google Scholar]
- Zhang, A.; Wang, F.; Chen, L.; Wei, X.; Jiang, S. 3D printing hydrogels for actuators: A review. Chin. Chem. Lett. 2021, 32, 2923–2932. [Google Scholar] [CrossRef]
- Ahlfeld, T.; Köhler, T.; Czichy, C.; Lode, A.; Gelinsky, M. A methylcellulose hydrogel as support for 3D plotting of complex shaped calcium phosphate scaffolds. Gels 2018, 4, 68. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tan, Y.J.; Leong, K.F.; Li, L. 3D bioprinting of highly thixotropic alginate/methylcellulose hydrogel with strong interface bonding. ACS Appl. Mater. Interfaces 2017, 9, 20086–20097. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, R. Fabrication and properties of multifunctional sodium alginate/MXene/nano-hydroxyapatite hydrogel for potential bone defect repair. J. Mater. Res. Technol. 2025, 36, 913–921. [Google Scholar] [CrossRef]
- Dou, L.; Li, B.; Kai, Z.; Xin, C.; Hu, H. Physical properties and antioxidant activity of gelatin-sodium alginate edible films with tea polyphenols. Int. J. Biol. Macromol. 2018, 118, 1377–1383. [Google Scholar] [CrossRef]
- Zhang, G.-Q.; Zha, L.-S.; Zhou, M.-H.; Ma, J.-H.; Liang, B.-R. Rapid deswelling of sodium alginate/poly(N-isopropylacrylamide) semi-interpenetrating polymer network hydrogels in response to temperature and pH changes. Colloid Polym. Sci. 2005, 4, 431–438. [Google Scholar] [CrossRef]
- El-Ghaffar, M.A.; Hashem, M.S.; El-Awady, M.K.; Rabie, A.M. Ph-sensitive sodium alginate hydrogels for riboflavin controlled release. Carbohydr. Polym. 2012, 89, 667–675. [Google Scholar] [CrossRef]
- Li, S.; Wang, D.; Wang, G.; Huang, L.; Zhou, S.; Sun, X.; Zhao, R.; Yao, T.; Zhao, K.; Chen, R. TA/PVP hydrogel coating enhanced photo-Fenton membranes with antifouling and self-cleaning properties for efficient oil–water emulsion separation. Sep. Purif. Technol. 2025, 360, 131252. [Google Scholar]
- Tang, Z.; Yang, J.; Li, S.; Wu, Z.; Mondal, A.K. Anti-swellable, stretchable, self-healable, shape-memory and supramolecular conductive TA-based hydrogels for amphibious motion sensors. Eur. Polym. J. 2024, 211, 113034. [Google Scholar] [CrossRef]
- Ma, C.; Peng, S.; Chen, L.; Cao, X.; Sun, Y.; Chen, L.; Yang, L.; Ma, C.; Liu, Q.; Liu, Z. Anisotropic Bi-layer hydrogel actuator with pH-responsive colorchanging and photothermal-responsive shape-changing Bi-functional synergy. Gels 2023, 9, 438. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, M.; He, X.; Huang, L.; Zhang, Y.; Ren, B.; Zhong, M.; Chang, Y.; Yang, J.; Zheng, J. Dual salt- and thermoresponsive programmable bilayer hydrogel actuators with pseudo-interpenetrating double-network structures. ACS Appl. Mater. Interfaces 2018, 10, 21642–21653. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, K.; Wu, B.; Lu, J.; Jian, Y.; Xue, Y.; Yang, X.; Zhang, J.; Chen, T. Programmatically regulating morphological evolution of inert polymeric hydrogels using anchored large-deformable muscle. Chem. Mater. 2022, 34, 6582–6592. [Google Scholar] [CrossRef]
- Han, Z.; Wang, P.; Mao, G.; Yin, T.; Yang, W. Dual pH-responsive hydrogel actuator for lipophilic drug delivery. ACS Appl. Mater. Interfaces 2020, 12, 12010–12017. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, J.; Ren, J.; Qu, X. 3D Graphene Oxide polymer Hydrogel: Near-infrared Light-triggered Active Scaffold for Reversible Cell Capture and on-demand Release. Adv. Mater. 2013, 25, 6737–6743. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Chen, L.; Wang, Y.; Sun, Y.; Ma, C.; Yang, X.; Jiang, S.; Duan, G. An electrospinning anisotropic hydrogel with remotely-controlled photo-responsive deformation and long-range navigation for synergist actuation. Chem. Eng. J. 2022, 433, 134258. [Google Scholar] [CrossRef]
- He, Z.; Zhou, Z.; Yuan, W. Highly adhesive, stretchable, and antifreezing hydrogel with excellent mechanical properties for sensitive motion sensors and temperature-/humidity-driven actuators. ACS Appl. Mater. Interfaces 2022, 14, 38205–38215. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, J.; Wang, T.; Sun, W.; Tong, Z. Multiple Shape Memory, Self-Healable, and Supertough PAA-GO-Fe3+ Hydrogel. Macromol. Mater. Eng. 2017, 302, 1600359. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Wang, J.; Li, X.; Chen, W. Fe3+, NIR light and thermal responsive triple network composite hydrogel with multi-shape memory effect. Compos. Sci. Technol. 2021, 106, 108653. [Google Scholar] [CrossRef]
- Shen, J.; Dai, Y.; Xia, F.; Zhang, X. Polyacrylamide/EDTA-modified chitosan/graphene oxide hydrogels as an adsorbent and supercapacitor for sustainable applications. Sustain. Mater. Technol. 2023, 36, e00586. [Google Scholar] [CrossRef]
- Cheng, L.; Tang, Q.; Zhang, Y.; Cheng, X.; Miao, A.; Su, J.; Wu, S.; Niu, F.; Zhang, L.; Duan, Y. Three-Dimensional Printed Multiresponsive Structures of Smart Hydrogel. 3D Print. Addit. Manuf. 2022, 11, 1–14. [Google Scholar] [CrossRef]
- Biswal, D.; Hilt, J.Z. Analysis of Oxygen Inhibition in Photopolymerizations of Hydrogel Micropatterns Using FTIR Imaging. Macromolecules 2009, 42, 973–979. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Razak, S.I.A.; Hassan, A.; Qureshi, S.Q.; Stojanović, G.M.; Ihsan-Ul-Haq. Multifunctional Arabinoxylan-functionalized-Graphene Oxide Based Composite Hydrogel for Skin Tissue Engineering. Front. Bioeng. Biotechnol. 2022, 10, 865059. [Google Scholar] [CrossRef] [PubMed]
- Habib, A.M.; Khoda, B. Rheological analysis of bio-ink for 3D bio-printing processes. J. Manuf. Process. 2022, 76, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, Y.; Zhao, C.; Cheng, J.; Liao, J. High tensile and antifreeze polyacrylamide hydrogel strain sensor suitable for human motion monitoring under low-temperature conditions. Sens. Actuators A Phys. 2025, 387, 116467. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Y.; Liu, H.; Song, J.; Wang, J.; Shu, J.; Li, Z.; Huang, Y.; Huang, Y. CPMOH Hydrogel with High Tensile Properties and Environmental Resistance Benefits from Intermolecular Hydrogen Bonding for ECG Monitoring. Compos. Struct. 2025, 354, 118820. [Google Scholar] [CrossRef]
- Bacakova, L.; Filova, E.; Parizek, M.; Ruml, T.; Svorcik, V. Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol. Adv. 2011, 29, 739–767. [Google Scholar] [CrossRef]
- Veskovi, A.; Nakarada, U.; Bijeli, A.P. A novel methodology for hydrogel water content determination by EPR: The basis for real-time monitoring of controlled drug release and hydrogel swelling and degradation. Polym. Test. 2021, 98, 107187. [Google Scholar] [CrossRef]
- ISO 10993-5:2009/Amd 1:2018; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Chen, G.; Huang, J.; Gu, J.; Peng, S.; Hou, L. Highly tough supramolecular double network hydrogel electrolytes for an artificial flexible and low-temperature tolerant sensor. J. Mater. Chem. A 2020, 8, 6776–6784. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, J.; Wang, Z.; Wu, J.; Meng, G.; Liu, Z.; Guo, X. One-pot fabrication of triple-network structure hydrogels with high-strength and self-healing properties. Mater. Lett. 2017, 207, 53–56. [Google Scholar] [CrossRef]
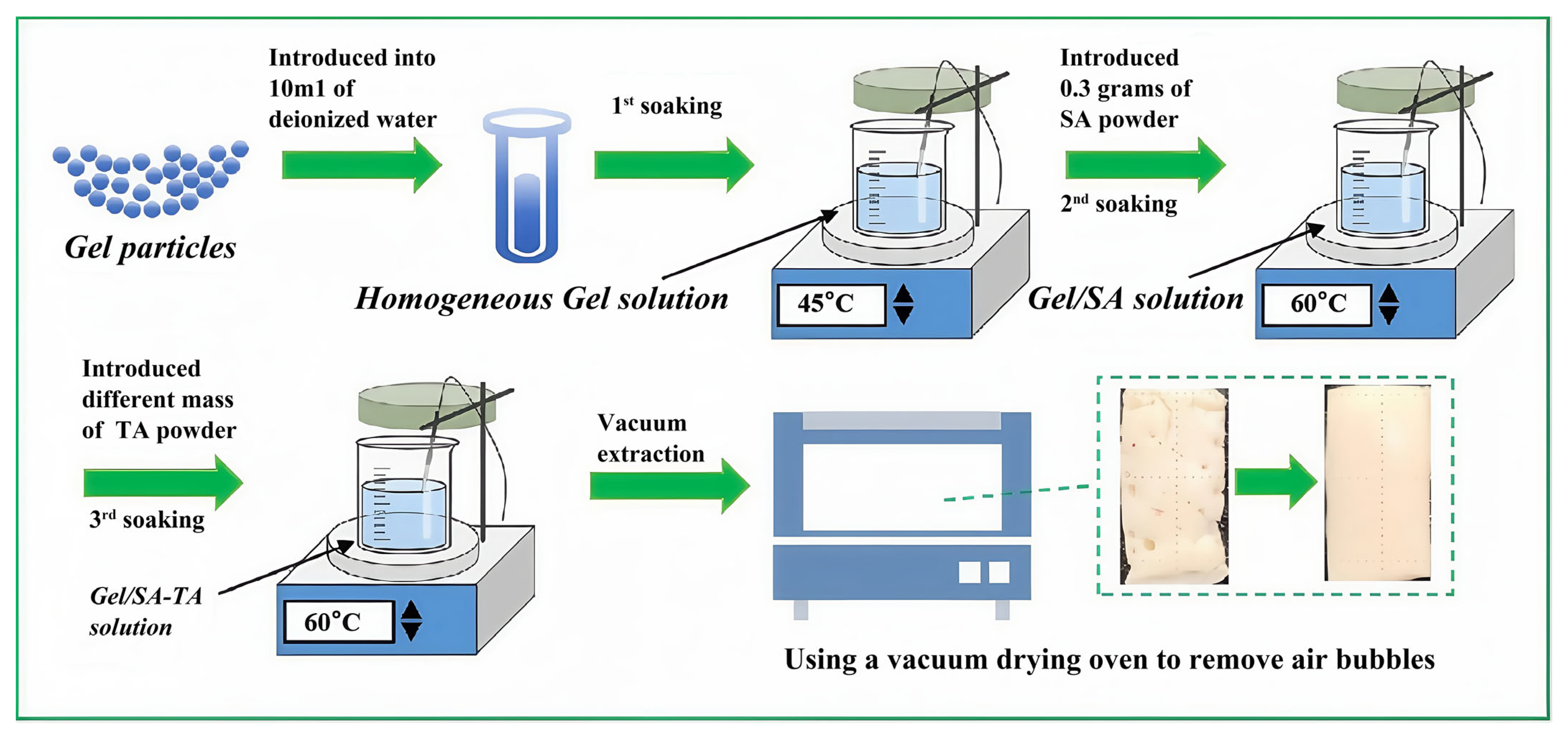
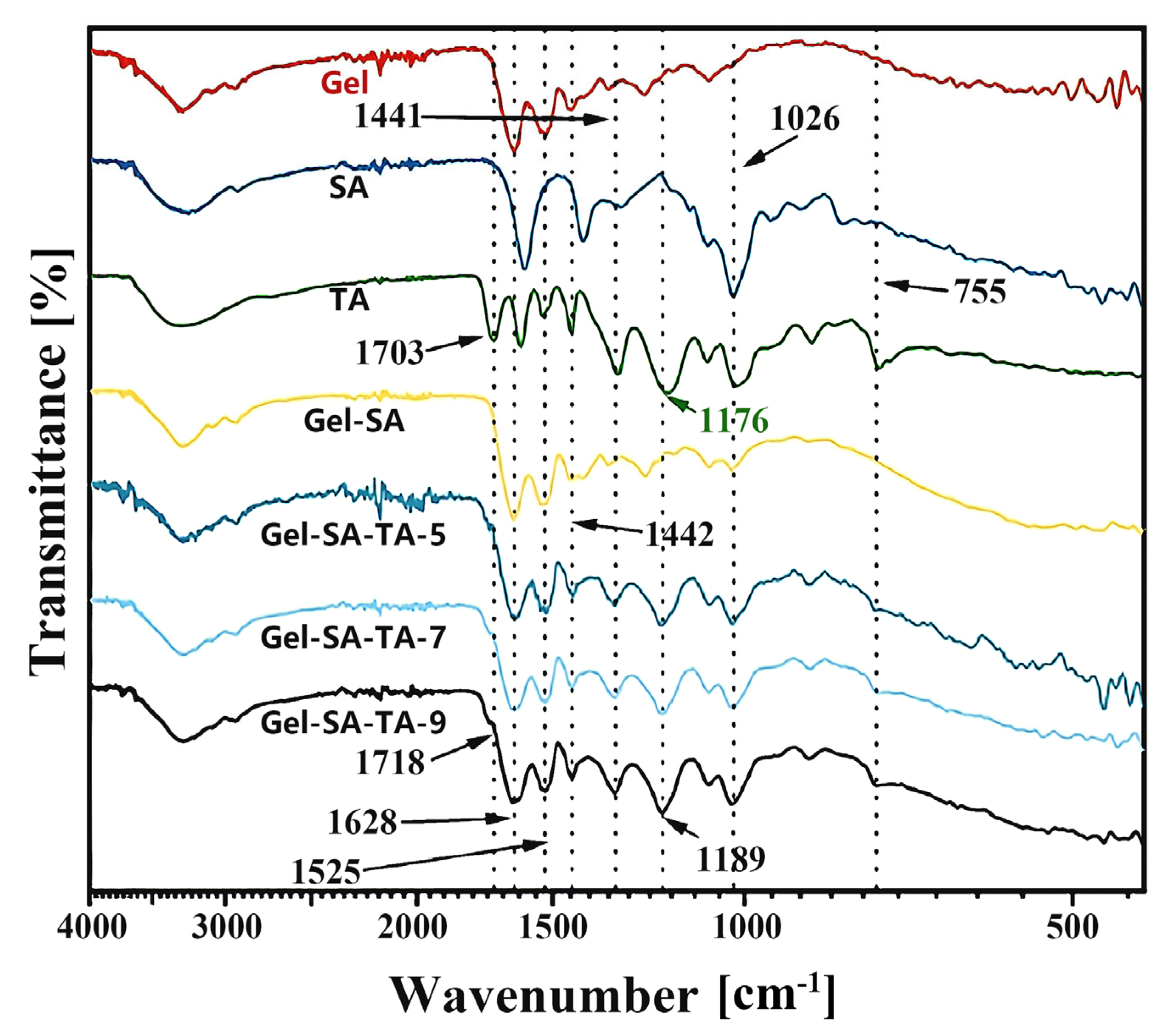
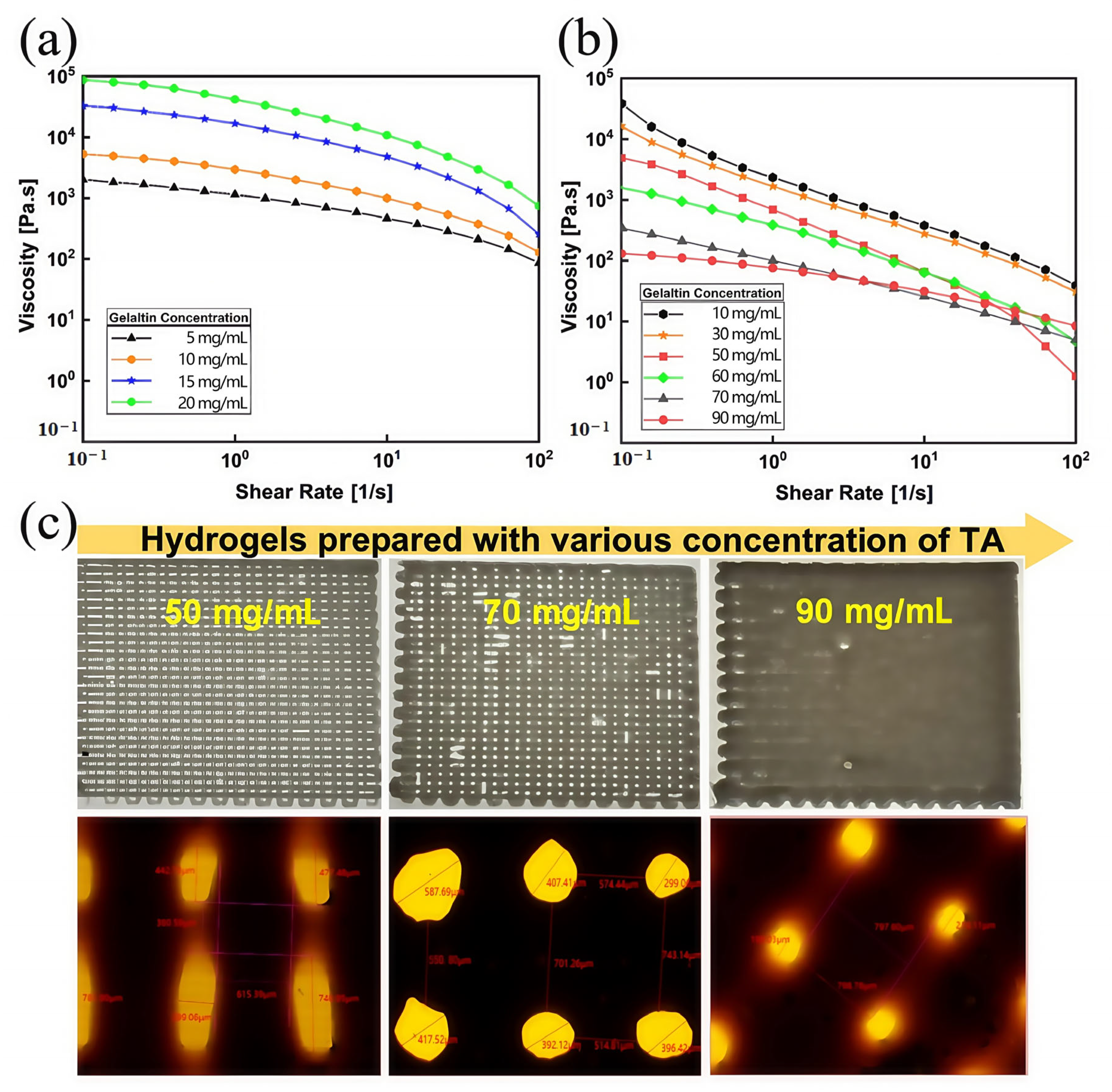
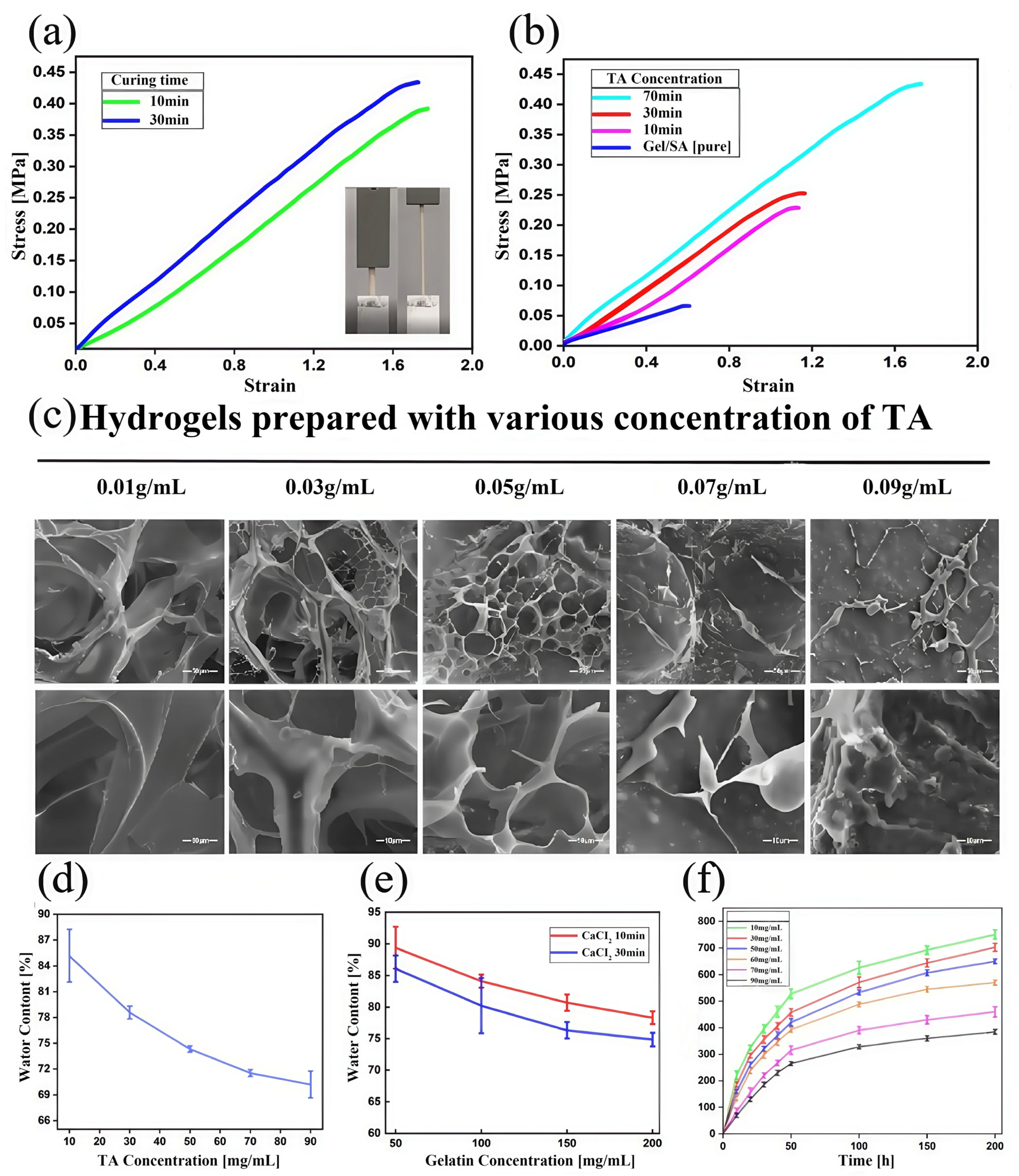

| No. | SA (wt%) | Gel (wt%) | TA (wt%) |
|---|---|---|---|
| 1 | 3 | 5 | \ |
| 2 | 3 | 10 | \ |
| 3 | 3 | 15 | \ |
| 4 | 3 | 20 | 1 |
| 5 | 3 | 20 | 3 |
| 6 | 3 | 20 | 5 |
| 7 | 3 | 20 | 6 |
| 8 | 3 | 20 | 7 |
| 9 | 3 | 20 | 9 |
| No. | SA (wt%) | Gel (wt%) | TA (wt%) | EDTA-FeNa (wt%) |
|---|---|---|---|---|
| 1 | 3 | 20 | 5 | 1 |
| 2 | 3 | 20 | 5 | 2 |
| 3 | 3 | 20 | 5 | 3 |
| 4 | 3 | 20 | 6 | 1 |
| 5 | 3 | 20 | 6 | 2 |
| 6 | 3 | 20 | 6 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Ma, Z.; Tang, Q.; Yang, R. 3D-Printed Multi-Stimulus-Responsive Hydrogels: Fabrication and Characterization. Micromachines 2025, 16, 788. https://doi.org/10.3390/mi16070788
Wu J, Ma Z, Tang Q, Yang R. 3D-Printed Multi-Stimulus-Responsive Hydrogels: Fabrication and Characterization. Micromachines. 2025; 16(7):788. https://doi.org/10.3390/mi16070788
Chicago/Turabian StyleWu, Jinzhe, Zhiyuan Ma, Qianqian Tang, and Runhuai Yang. 2025. "3D-Printed Multi-Stimulus-Responsive Hydrogels: Fabrication and Characterization" Micromachines 16, no. 7: 788. https://doi.org/10.3390/mi16070788
APA StyleWu, J., Ma, Z., Tang, Q., & Yang, R. (2025). 3D-Printed Multi-Stimulus-Responsive Hydrogels: Fabrication and Characterization. Micromachines, 16(7), 788. https://doi.org/10.3390/mi16070788







