A Disposable Dopamine Sensor Based on Oxidized Cellulose Nanofibril-Modified SPCE
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Apparatus
2.3. Production of TOCNFs
2.4. TOCNF-Modification of the Working Electrode
3. Results and Discussion
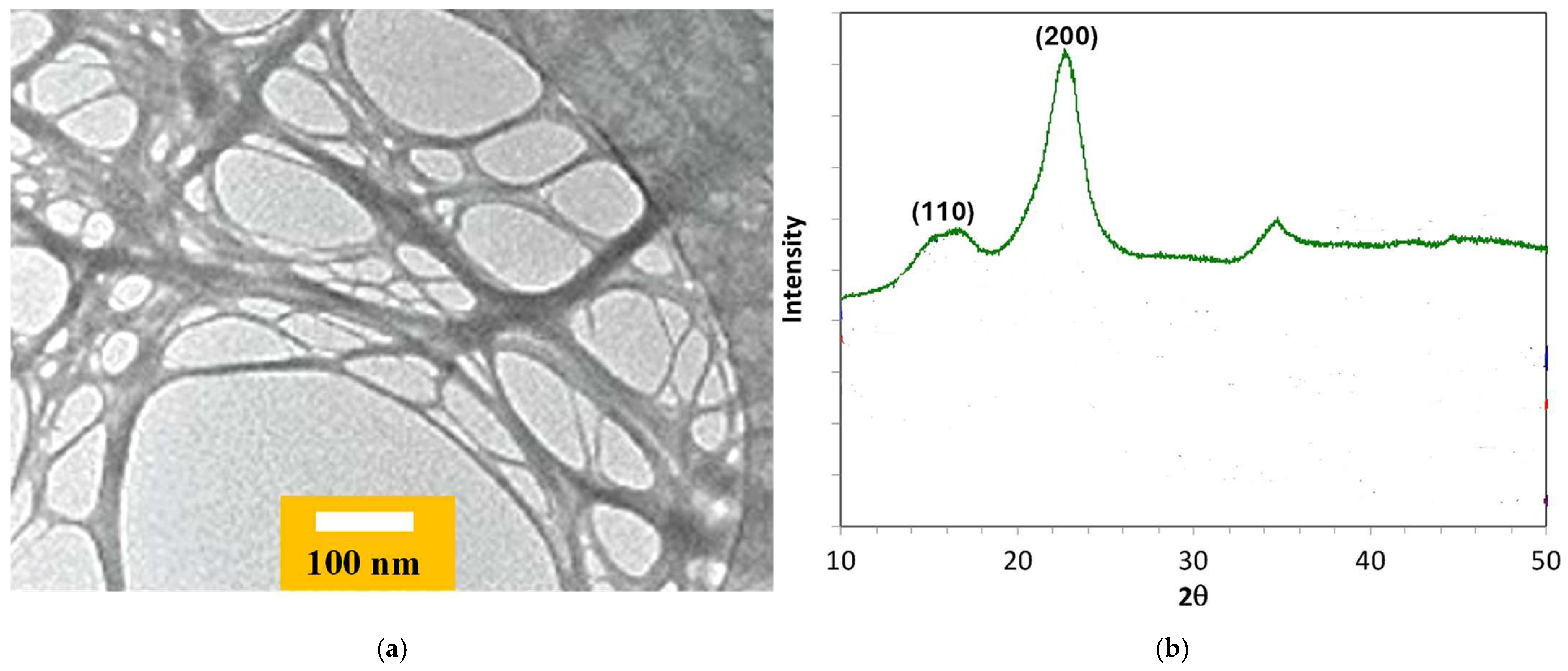
3.1. Morphological Characterization of TOCNFs
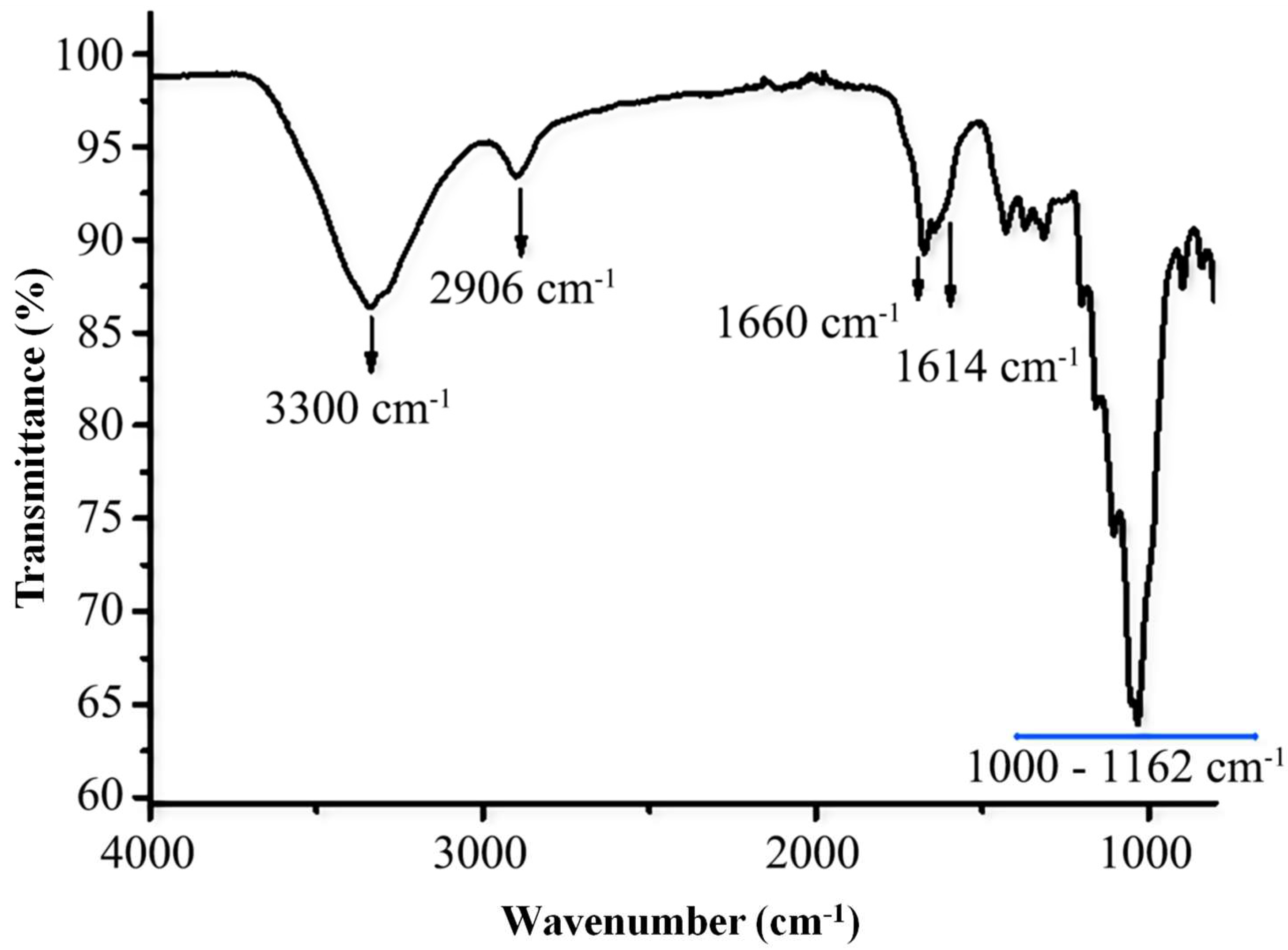
3.2. FTIR Characterization of TOCNFs
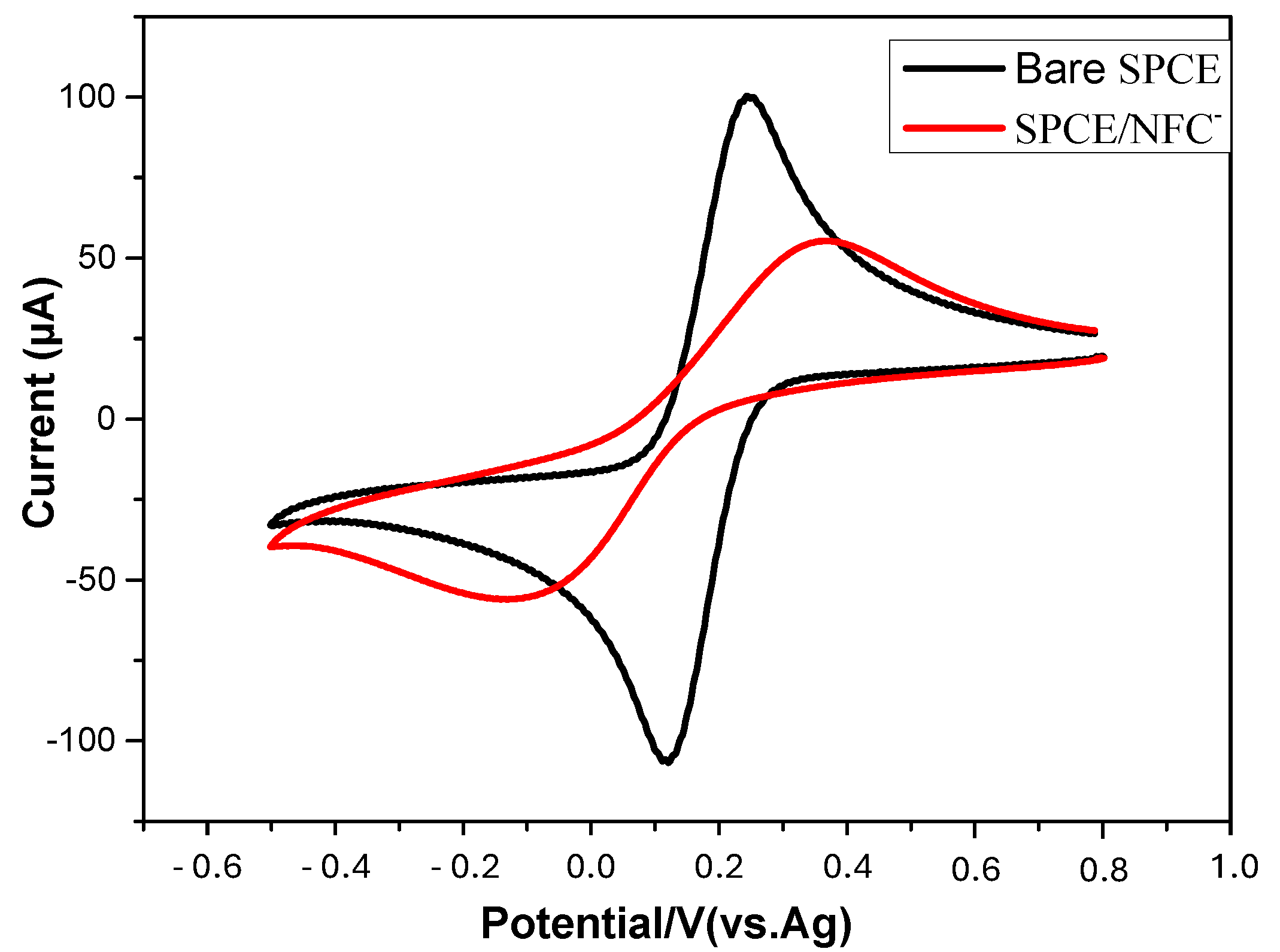
3.3. Electrochemical Characterization of TOCNF-Modified SPCE
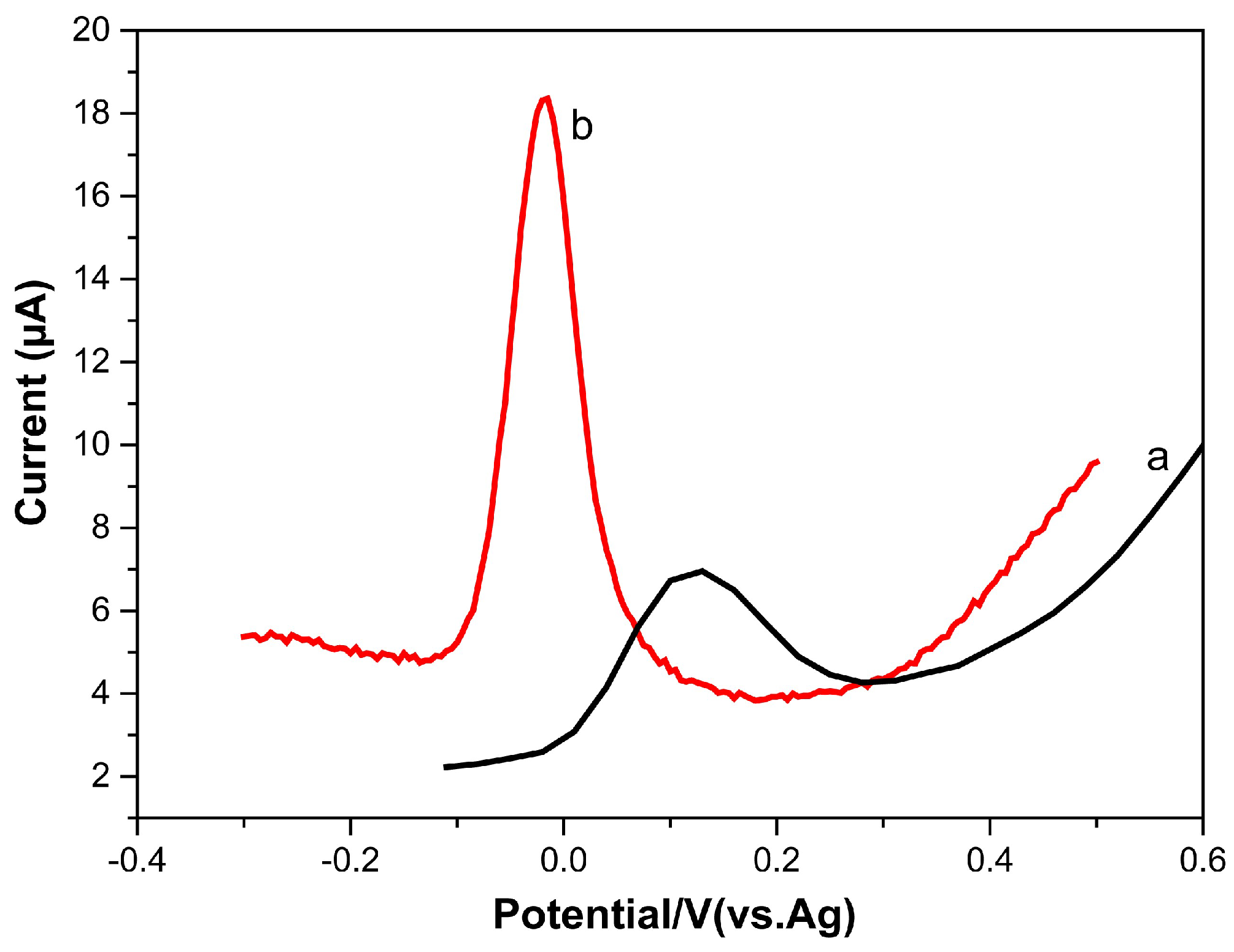
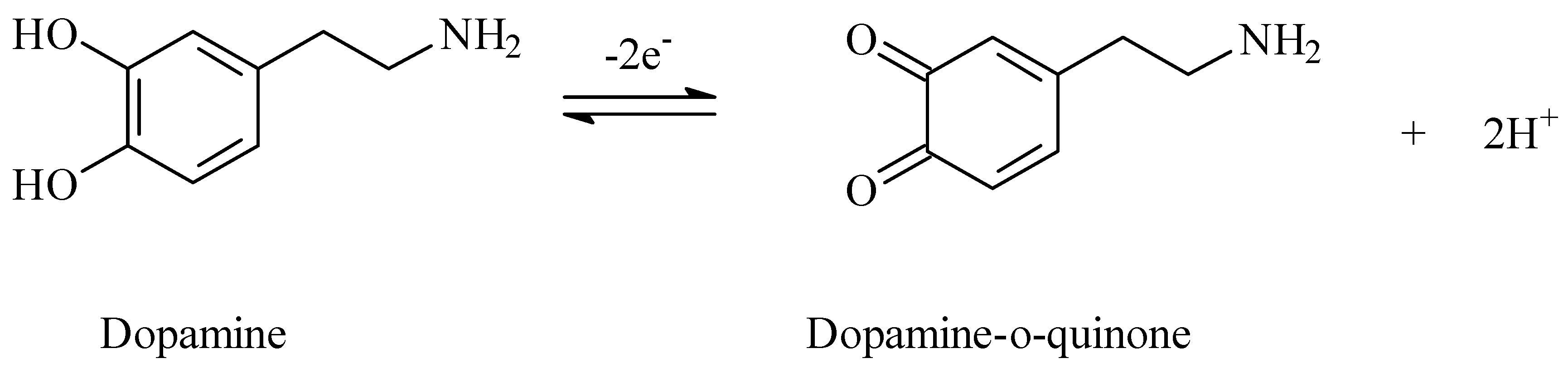
3.4. Electrochemical Behavior of Dopamine
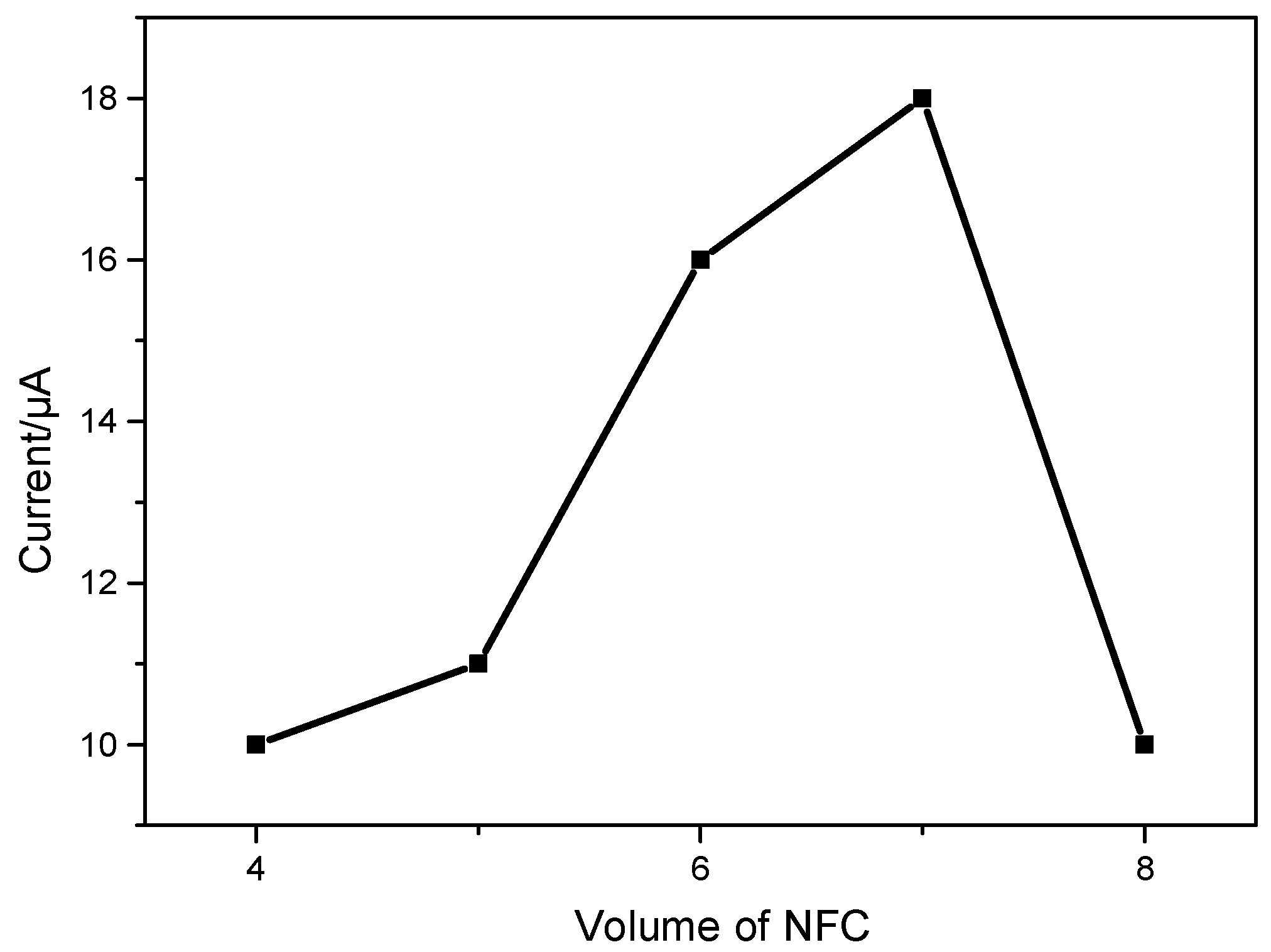
3.4.1. Effect of the Modified Amount of TOCNFs
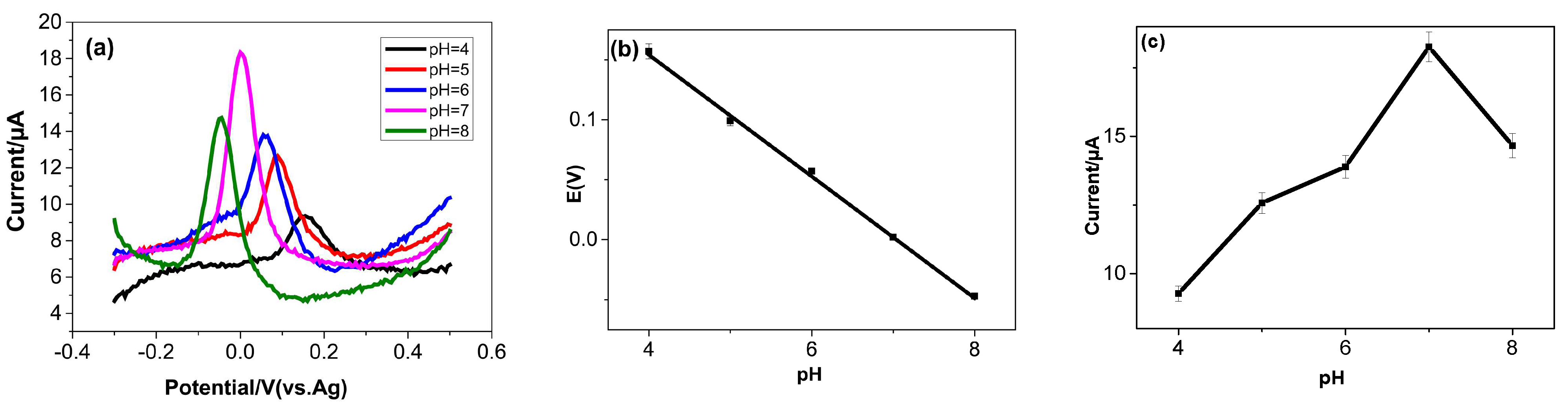
3.4.2. Influence of pH
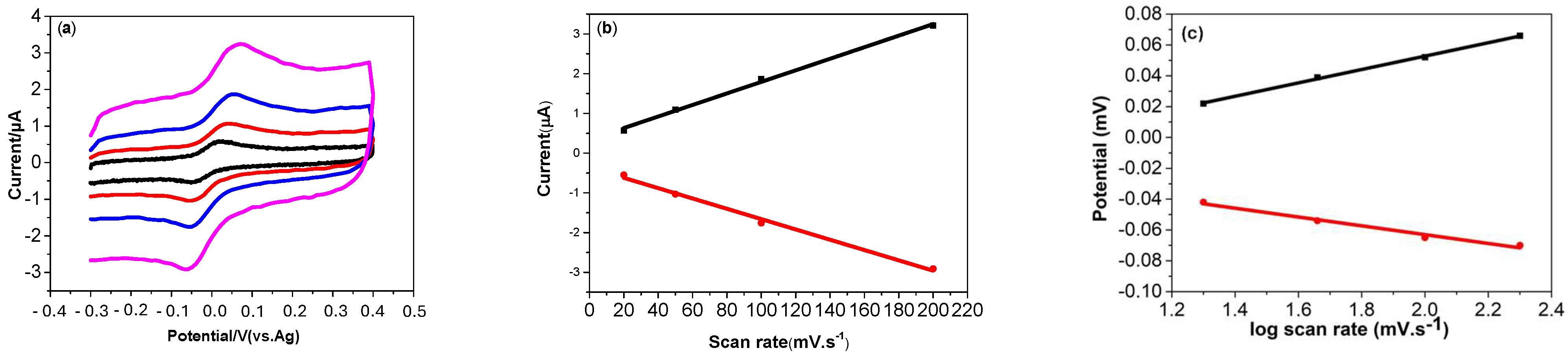
3.4.3. Effect of Scan Rate
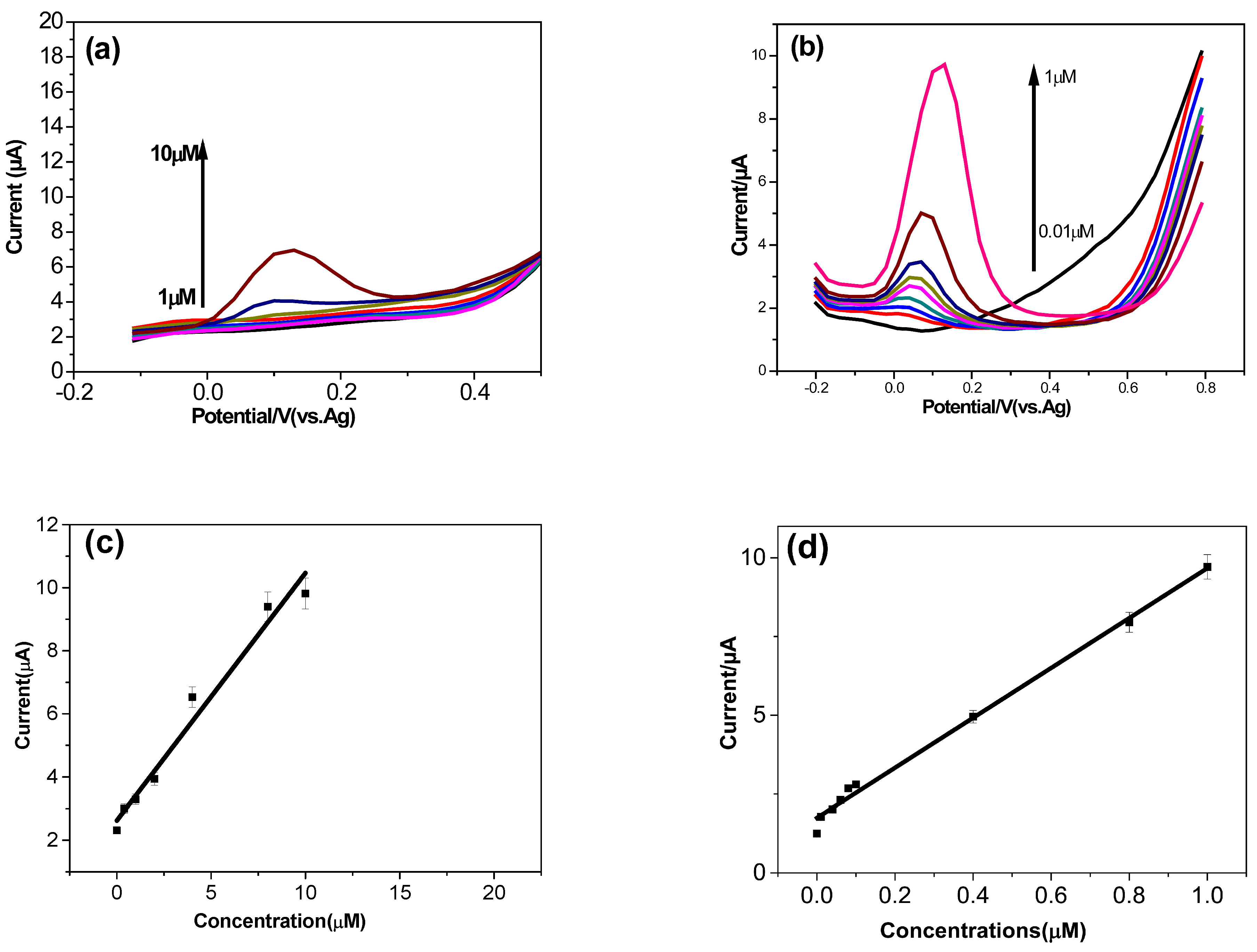
3.5. Analytical Performance of the Dopamine Sensor
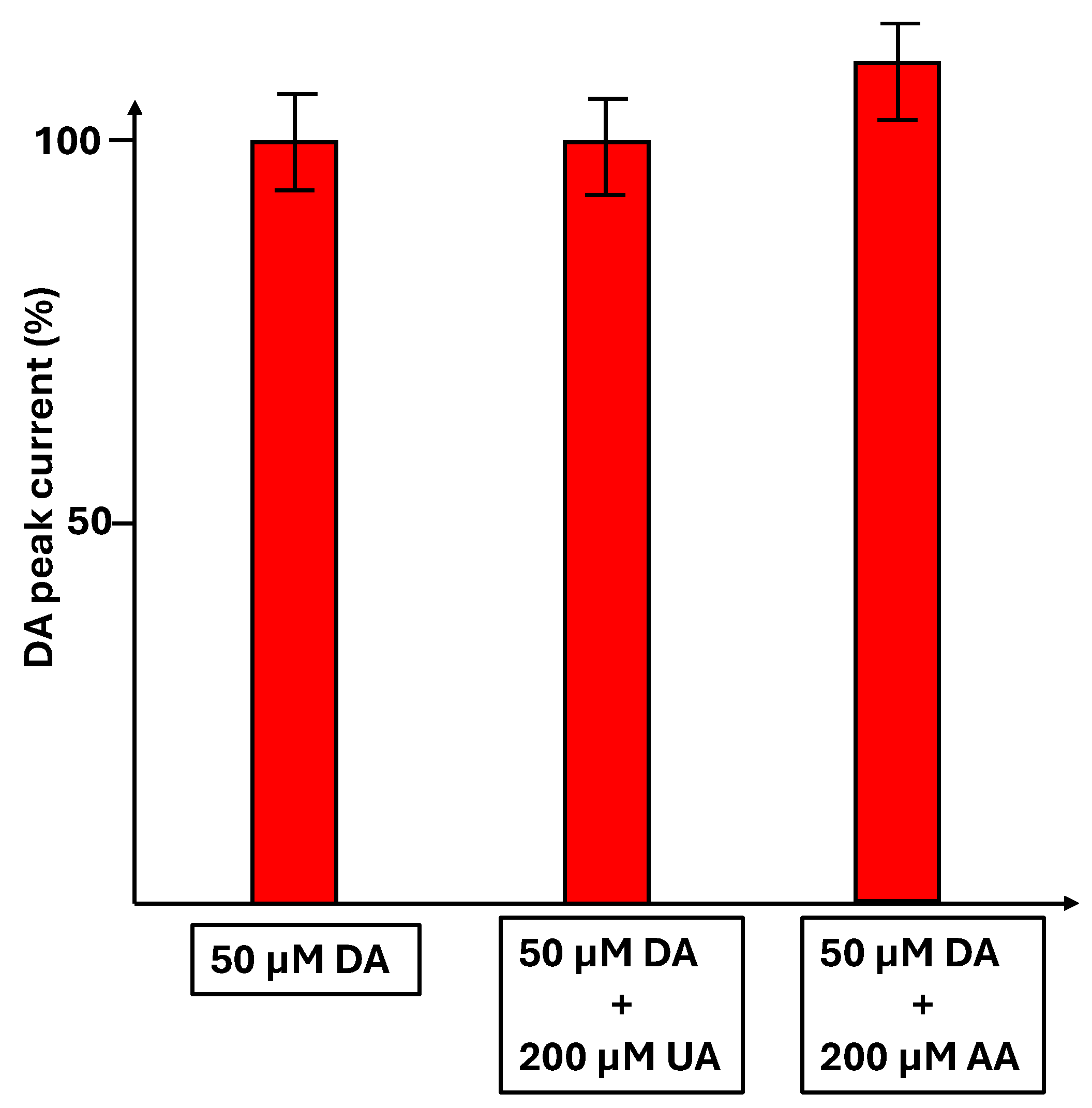
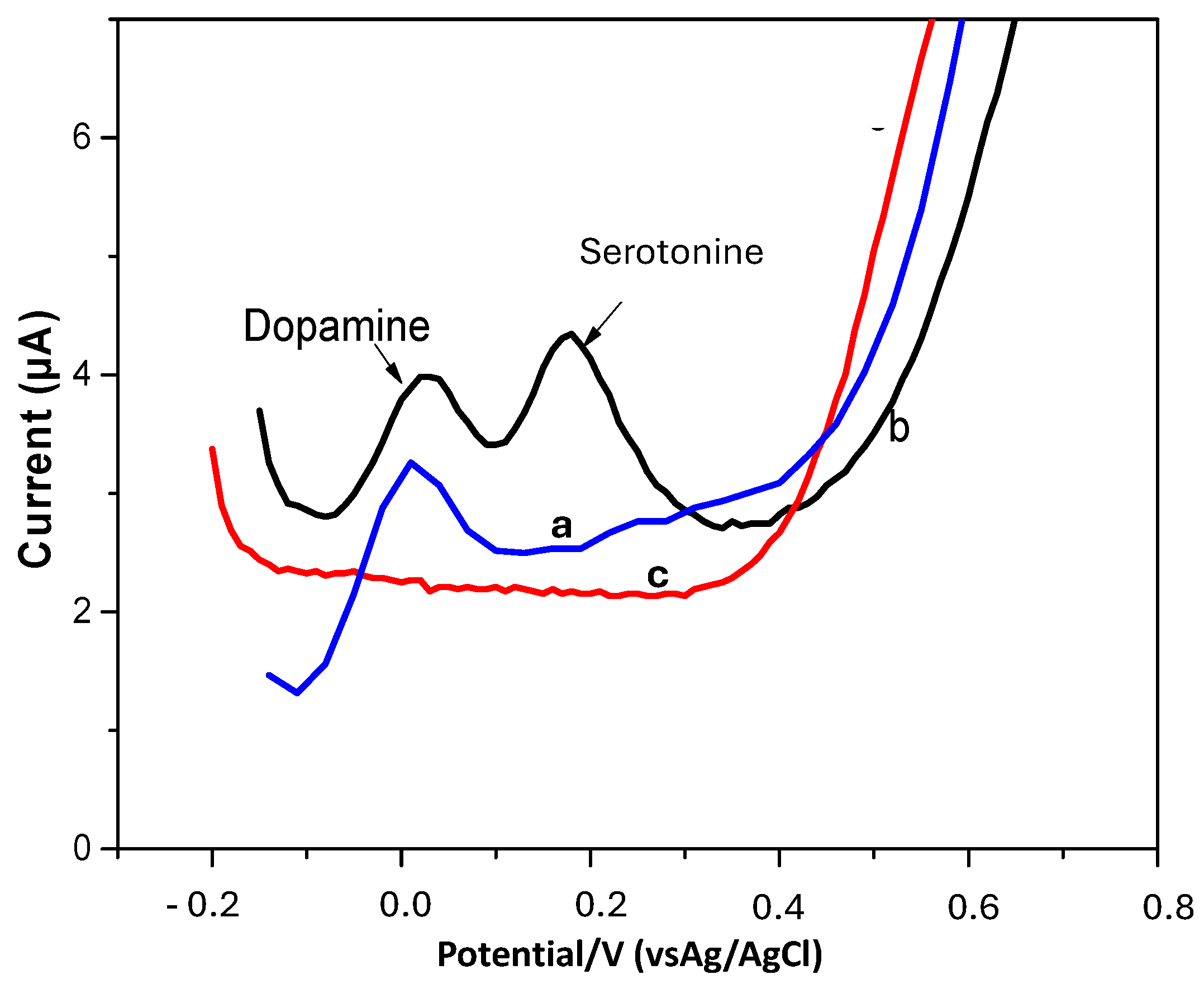
3.6. Detection of Dopamine in the Presence of Uric Acid and Ascorbic Acid and Another Neurotransmitter, Serotonin
3.7. Analysis of Real Biological Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, D.; Lee, S.; Piao, Y. Electrochemical Determination of Dopamine and Acetaminophen Using Activated Graphene-Nafion Modified Glassy Carbon Electrode. J. Electroanal. Chem. 2017, 794, 221–228. [Google Scholar] [CrossRef]
- Fang, J.; Xie, Z.; Wallace, G.; Wang, X. Co-Deposition of Carbon Dots and Reduced Graphene Oxide Nanosheets on Carbon-Fiber Microelectrode Surface for Selective Detection of Dopamine. Appl. Surf. Sci. 2017, 412, 131–137. [Google Scholar] [CrossRef]
- Yu, H.; Jiang, J.; Zhang, Z.; Wan, G.; Liu, Z.; Chang, D.; Pan, H. Preparation of Quantum Dots CdTe Decorated Graphene Composite for Sensitive Detection of Uric Acid and Dopamine. Anal. Biochem. 2017, 519, 92–99. [Google Scholar] [CrossRef]
- Klein, M.O.; Battagello, D.S.; Cardoso, A.R.; Hauser, D.N.; Bittencourt, J.C.; Correa, R.G. Dopamine: Functions, Signaling, and Association with Neurological Diseases. Cell. Mol. Neurobiol. 2019, 39, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Lakard, S.; Pavel, I.-A.; Lakard, B. Electrochemical Biosensing of Dopamine Neurotransmitter: A Review. Biosensors 2021, 11, 179. [Google Scholar] [CrossRef] [PubMed]
- Gaudry, D.; Carlsen, S.; Khemani, L.; Miotto, J.; Alkalay, D. Determination of the Dopamine Agonist CGS 15855A in Human Plasma by Gas Chromatography/Mass Spectrometry. Anal. Lett. 1989, 22, 2307–2321. [Google Scholar] [CrossRef]
- Li, N.; Guo, J.; Liu, B.; Yu, Y.; Cui, H.; Mao, L.; Lin, Y. Determination of Monoamine Neurotransmitters and Their Metabolites in a Mouse Brain Microdialysate by Coupling High-Performance Liquid Chromatography with Gold Nanoparticle-Initiated Chemiluminescence. Anal. Chim. Acta 2009, 645, 48–55. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Y.; Li, X.; Guo, X.; Zhang, B.; Jia, X.; Dai, B. A Simple, Fast and Low-Cost Turn-on Fluorescence Method for Dopamine Detection Using in Situ Reaction. Anal. Chim. Acta 2016, 944, 51–56. [Google Scholar] [CrossRef]
- Gao, W.; Qi, L.; Liu, Z.; Majeed, S.; Kitte, S.A.; Xu, G. Efficient Lucigenin/Thiourea Dioxide Chemiluminescence System and Its Application for Selective and Sensitive Dopamine Detection. Sens. Actuators B Chem. 2017, 238, 468–472. [Google Scholar] [CrossRef]
- Fayemi, O.E.; Adekunle, A.S.; Kumara Swamy, B.E.; Ebenso, E.E. Electrochemical Sensor for the Detection of Dopamine in Real Samples Using Polyaniline/NiO, ZnO, and Fe3O4 Nanocomposites on Glassy Carbon Electrode. J. Electroanal. Chem. 2018, 818, 236–249. [Google Scholar] [CrossRef]
- Fayemi, O.E.; Baskar, R.; Adekunle, A.S.; Sherif, E.M.; Ebenso, E.E. SPEEK/ZnO Nanocomposite Modified Gold Electrode for Electrochemical Detection of Dopamine. Electroanalysis 2020, 32, 2713–2722. [Google Scholar] [CrossRef]
- Manbohi, A.; Ahmadi, S.H. Sensitive and Selective Detection of Dopamine Using Electrochemical Microfluidic Paper-Based Analytical Nanosensor. Sens. Bio-Sens. Res. 2019, 23, 100270. [Google Scholar] [CrossRef]
- Zablocka, I.; Wysocka-Zolopa, M.; Winkler, K. Electrochemical Detection of Dopamine at a Gold Electrode Modified with a Polypyrrole–Mesoporous Silica Molecular Sieves (MCM-48) Film. Int. J. Mol. Sci. 2018, 20, 111. [Google Scholar] [CrossRef]
- Adams, R.N. Probing Brain Chemistry with Electroanalytical Techniques. Anal. Chem. 1976, 48, 1126A–1138A. [Google Scholar] [CrossRef]
- Raj, C.R.; Tokuda, K.; Ohsaka, T. Electroanalytical Applications of Cationic Self-Assembled Monolayers: Square-Wave Voltammetric Determination of Dopamine and Ascorbate. Bioelectrochemistry 2001, 53, 183–191. [Google Scholar] [CrossRef]
- Fabregat, G.; Córdova-Mateo, E.; Armelin, E.; Bertran, O.; Alemán, C. Ultrathin Films of Polypyrrole Derivatives for Dopamine Detection. J. Phys. Chem. C 2011, 115, 14933–14941. [Google Scholar] [CrossRef]
- Jiang, L.; Xie, Q.; Li, Z.; Li, Y.; Yao, S. A Study on Tannic Acid-Doped Polypyrrole Films on Gold Electrodes for Selective Electrochemical Detection of Dopamine. Sensors 2005, 5, 199–208. [Google Scholar] [CrossRef]
- Kalimuthu, P.; John, S.A. Electropolymerized Film of Functionalized Thiadiazole on Glassy Carbon Electrode for the Simultaneous Determination of Ascorbic Acid, Dopamine and Uric Acid. Bioelectrochemistry 2009, 77, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Oztekin, Y.; Tok, M.; Bilici, E.; Mikoliunaite, L.; Yazicigil, Z.; Ramanaviciene, A.; Ramanavicius, A. Copper Nanoparticle Modified Carbon Electrode for Determination of Dopamine. Electrochim. Acta 2012, 76, 201–207. [Google Scholar] [CrossRef]
- Yan, J.; Liu, S.; Zhang, Z.; He, G.; Zhou, P.; Liang, H.; Tian, L.; Zhou, X.; Jiang, H. Simultaneous Electrochemical Detection of Ascorbic Acid, Dopamine and Uric Acid Based on Graphene Anchored with Pd–Pt Nanoparticles. Colloids Surf. B Biointerfaces 2013, 111, 392–397. [Google Scholar] [CrossRef]
- Reddy, S.; Kumara Swamy, B.E.; Jayadevappa, H. CuO Nanoparticle Sensor for the Electrochemical Determination of Dopamine. Electrochim. Acta 2012, 61, 78–86. [Google Scholar] [CrossRef]
- Elhag, S.; Ibupoto, Z.H.; Liu, X.; Nur, O.; Willander, M. Dopamine Wide Range Detection Sensor Based on Modified Co3O4 Nanowires Electrode. Sens. Actuators B Chem. 2014, 203, 543–549. [Google Scholar] [CrossRef]
- Goyal, R.N.; Gupta, V.K.; Bachheti, N.; Sharma, R.A. Electrochemical Sensor for the Determination of Dopamine in Presence of High Concentration of Ascorbic Acid Using a Fullerene-C60 Coated Gold Electrode. Electroanalysis 2008, 20, 757–764. [Google Scholar] [CrossRef]
- Kim, Y.-R.; Bong, S.; Kang, Y.-J.; Yang, Y.; Mahajan, R.K.; Kim, J.S.; Kim, H. Electrochemical Detection of Dopamine in the Presence of Ascorbic Acid Using Graphene Modified Electrodes. Biosens. Bioelectron. 2010, 25, 2366–2369. [Google Scholar] [CrossRef] [PubMed]
- Tofanica, B.-M.; Mikhailidi, A.; Fortună, M.E.; Rotaru, R.; Ungureanu, O.C.; Ungureanu, E. Cellulose Nanomaterials: Characterization Methods, Isolation Techniques, and Strategies. Crystals 2025, 15, 352. [Google Scholar] [CrossRef]
- Kalia, S.; Boufi, S.; Celli, A.; Kango, S. Nanofibrillated Cellulose: Surface Modification and Potential Applications. Colloid Polym. Sci. 2014, 292, 5–31. [Google Scholar] [CrossRef]
- Boufi, S.; González, I.; Delgado-Aguilar, M.; Tarrès, Q.; Pèlach, M.À.; Mutjé, P. Nanofibrillated Cellulose as an Additive in Papermaking Process: A Review. Carbohydr. Polym. 2016, 154, 151–166. [Google Scholar] [CrossRef]
- Jebali, Z.; Nabili, A.; Majdoub, H.; Boufi, S. Cellulose Nanofibrils (CNFs) from Ammophila Arenaria, a Natural and a Fast Growing Grass Plant. Int. J. Biol. Macromol. 2018, 107, 530–536. [Google Scholar] [CrossRef]
- Taleat, Z.; Khoshroo, A.; Mazloum-Ardakani, M. Screen-Printed Electrodes for Biosensing: A Review (2008–2013). Microchim. Acta 2014, 181, 865–891. [Google Scholar] [CrossRef]
- Lavanya, N.; Sekar, C.; Ficarra, S.; Tellone, E.; Bonavita, A.; Leonardi, S.G.; Neri, G. A Novel Disposable Electrochemical Sensor for Determination of Carbamazepine Based on Fe Doped SnO2 Nanoparticles Modified Screen-Printed Carbon Electrode. Mater. Sci. Eng. C 2016, 62, 53–60. [Google Scholar] [CrossRef]
- Durairaj, V.; Wester, N.; Etula, J.; Laurila, T.; Lehtonen, J.; Rojas, O.J.; Pahimanolis, N.; Koskinen, J. Multiwalled Carbon Nanotubes/Nanofibrillar Cellulose/Nafion Composite-Modified Tetrahedral Amorphous Carbon Electrodes for Selective Dopamine Detection. J. Phys. Chem. C 2019, 123, 24826–24836. [Google Scholar] [CrossRef]
- Durairaj, V.; Liljeström, T.; Wester, N.; Engelhardt, P.; Sainio, S.; Wilson, B.P.; Li, P.; Kontturi, K.S.; Tammelin, T.; Laurila, T.; et al. Role of Nanocellulose in Tailoring Electroanalytical Performance of Hybrid Nanocellulose/Multiwalled Carbon Nanotube Electrode. Cellulose 2022, 29, 9217–9233. [Google Scholar] [CrossRef]
- Al Kiey, S.A.; Khalil, A.M.; Kamel, S. Insight into TEMPO-Oxidized Cellulose-Based Composites as Electrochemical Sensors for Dopamine Assessment. Int. J. Biol. Macromol. 2023, 239, 124302. [Google Scholar] [CrossRef] [PubMed]
- Bourigua, S.; Boussema, F.; Jebali, Z.; Barhoumi, H.; Majdoub, H.; Maaref, A.; Jaffrezic-Renault, N. A Glassy Carbon Electrode Modified with Cellulose Nanofibrils from Ammophila arenaria for the Sensitive Detection of L-Trytophan. J. Sens. Technol. 2024, 14, 35–50. [Google Scholar] [CrossRef]
- Annu; Sharma, S.; Nitin, A.; Jain, R. Cellulose Fabricated Pencil Graphite Sensor for the Quantification of Hazardous Herbicide Atrazine. Diam. Relat. Mater. 2020, 105, 107788. [CrossRef]
- Zhang, B.; Wu, Q.; Li, B.; Tang, X.; Ju, F.; Yang, Q.; Wang, Q.; Lang, Y. Electrochemical Detection of Dopamine in Real Samples by an Indium Tin Oxide-Coated Glass Electrode Modified with Carbon Nanotubes. Int. J. Electrochem. Sci. 2020, 15, 137–148. [Google Scholar] [CrossRef]
- Mohammadi, N.; Najafi, M.; Bahrami Adeh, M. Highly defective mesoporous carbon—Ionic liquid paste electrode as sensitive voltammetric sensor for determination of chlorogenic acid in herbal extracts. Sens. Actuators B Chem. 2017, 243, 838–846. [Google Scholar] [CrossRef]
- Laviron, E. General Expression of the Linear Potential Sweep Voltammogram in the Case of Diffusionless Electrochemical Systems. J. Electroanal. Chem. Interfacial Electrochem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Thomas, J.; Anitha, P.K.; Thomas, T.; Thomas, N. The Influence of B-Site Cation in LaBO3 (B = Fe, Co, Ni) Perovskites on the Nanomolar Sensing of Neurotransmitters. Sens. Actuators B Chem. 2021, 332, 129362. [Google Scholar] [CrossRef]
| Electrode | Method | Detection Limit (nM) | Linear Range (µM) | Reference |
|---|---|---|---|---|
| Sulfated CNF/MWCNT-modified taC | DPV | 65 | 0.05–100 | [30] |
| TOCNF/graphene/AgNP-modified GCE | DPV | 0.5 | 0.005–250 | [32] |
| TOCNF-modified SPCE | DPV | 10 | 0.01–100 | This work |
| Sample | Added (µM) | Found (µM) | Recovery (%) |
|---|---|---|---|
| Urine | 0.06 | 0.07 ± 0.03 | 116 |
| 0.10 | 0.11 ± 0.02 | 100 | |
| 0.60 | 0.61 ± 0.01 | 101 | |
| 1 | 0.98 ± 0.01 | 98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boussema, F.; Bourigua, S.; Jebali, Z.; Majdoub, H.; Jaffrezic-Renault, N.; Ben Halima, H. A Disposable Dopamine Sensor Based on Oxidized Cellulose Nanofibril-Modified SPCE. Micromachines 2025, 16, 743. https://doi.org/10.3390/mi16070743
Boussema F, Bourigua S, Jebali Z, Majdoub H, Jaffrezic-Renault N, Ben Halima H. A Disposable Dopamine Sensor Based on Oxidized Cellulose Nanofibril-Modified SPCE. Micromachines. 2025; 16(7):743. https://doi.org/10.3390/mi16070743
Chicago/Turabian StyleBoussema, Feriel, Sondes Bourigua, Zayneb Jebali, Hatem Majdoub, Nicole Jaffrezic-Renault, and Hamdi Ben Halima. 2025. "A Disposable Dopamine Sensor Based on Oxidized Cellulose Nanofibril-Modified SPCE" Micromachines 16, no. 7: 743. https://doi.org/10.3390/mi16070743
APA StyleBoussema, F., Bourigua, S., Jebali, Z., Majdoub, H., Jaffrezic-Renault, N., & Ben Halima, H. (2025). A Disposable Dopamine Sensor Based on Oxidized Cellulose Nanofibril-Modified SPCE. Micromachines, 16(7), 743. https://doi.org/10.3390/mi16070743







