Etched Tungsten Oxide Modified with Au for Quick Xylene Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Synthesis
2.2. Materials Characterization
2.3. Gas Sensor Fabrication and Measurement
3. Results
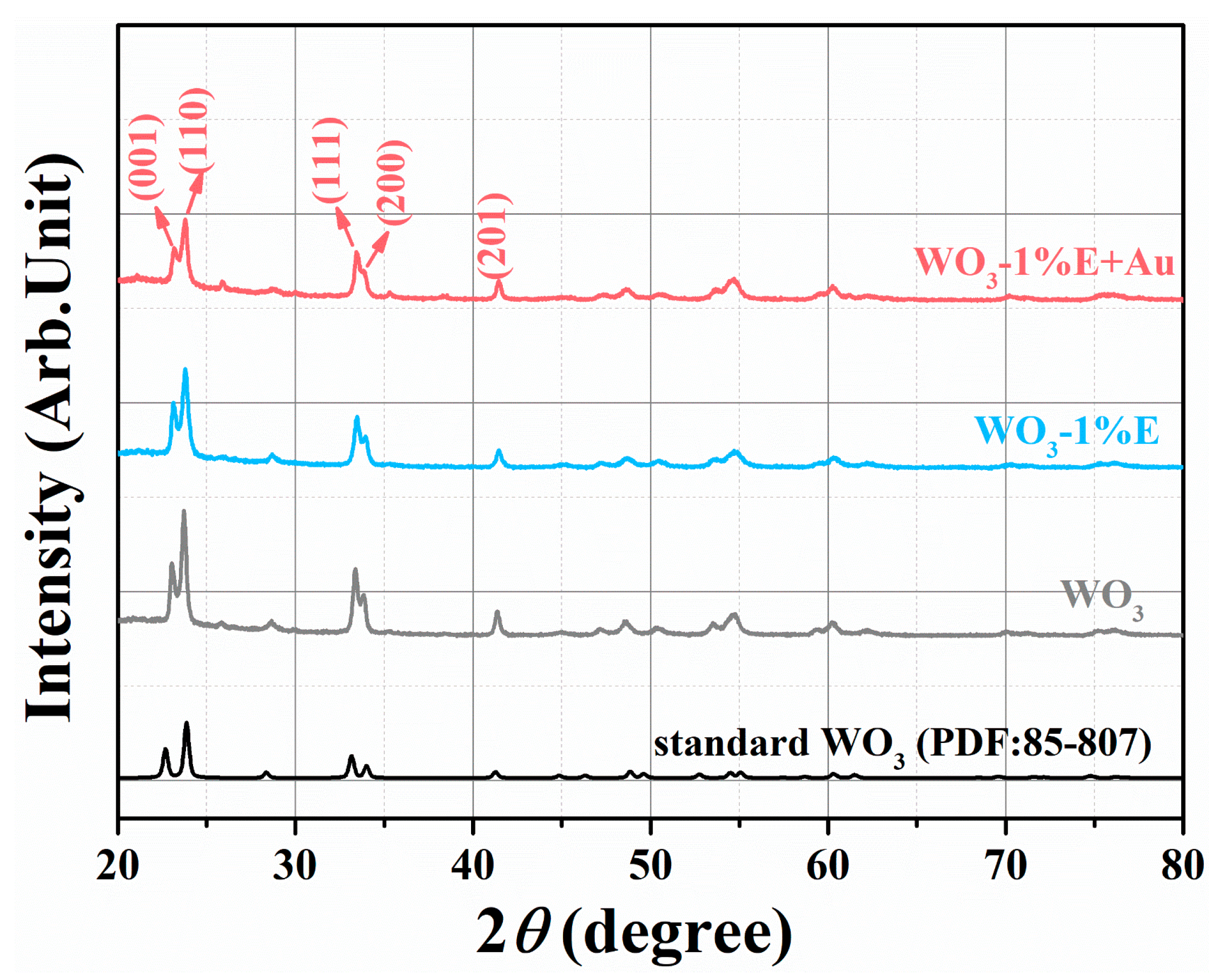
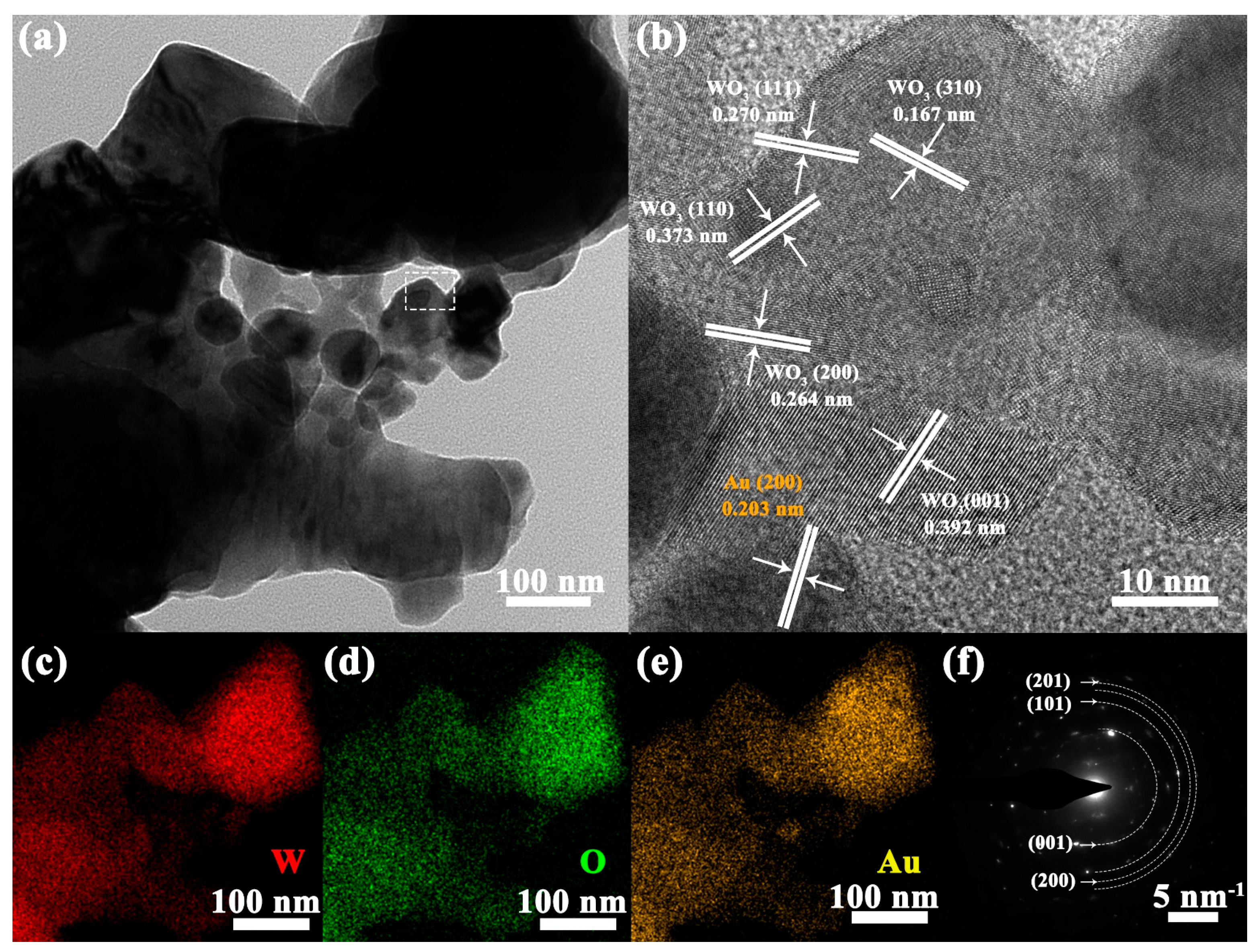
3.1. Structural and Morphological Characteristics
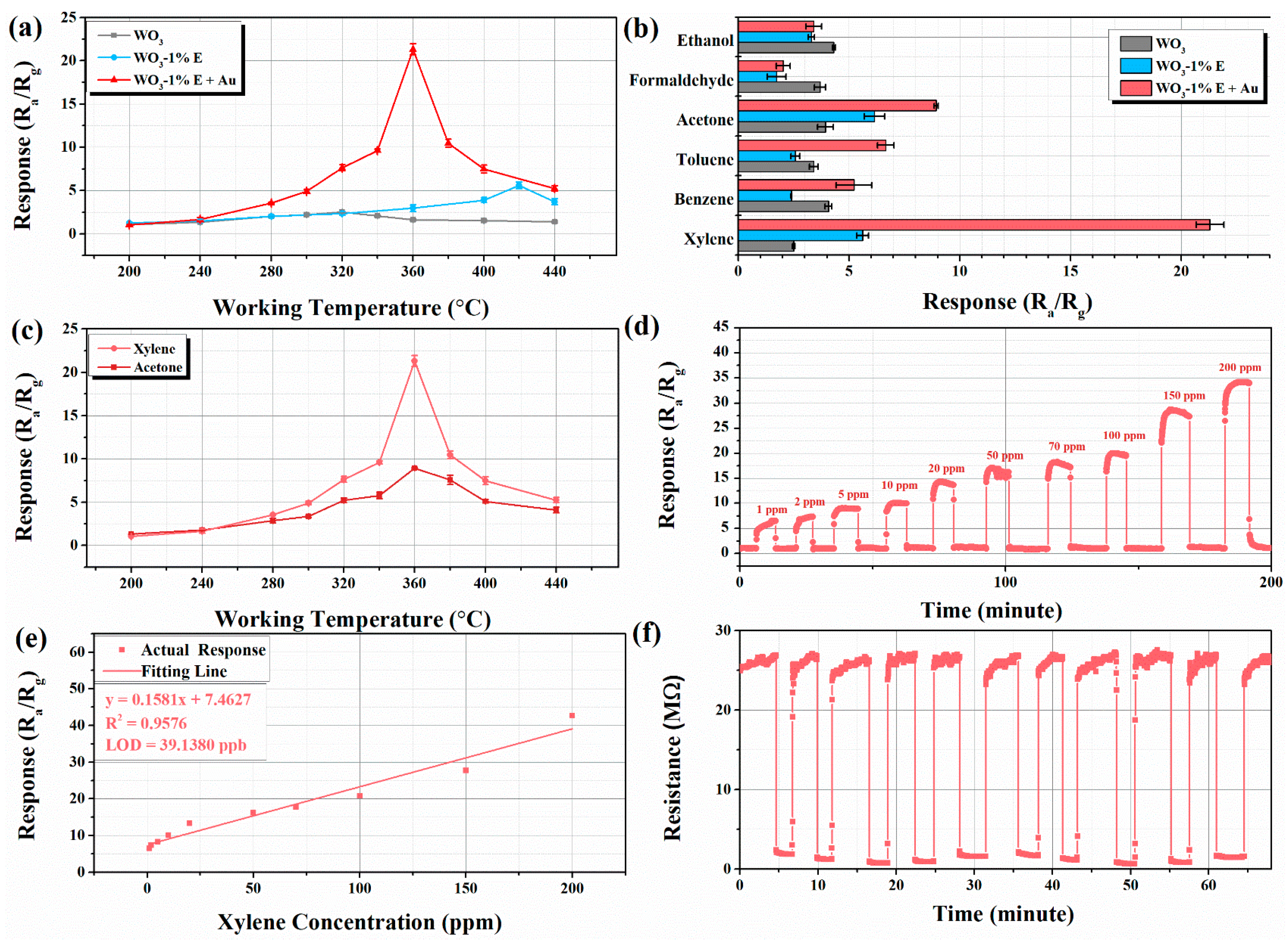
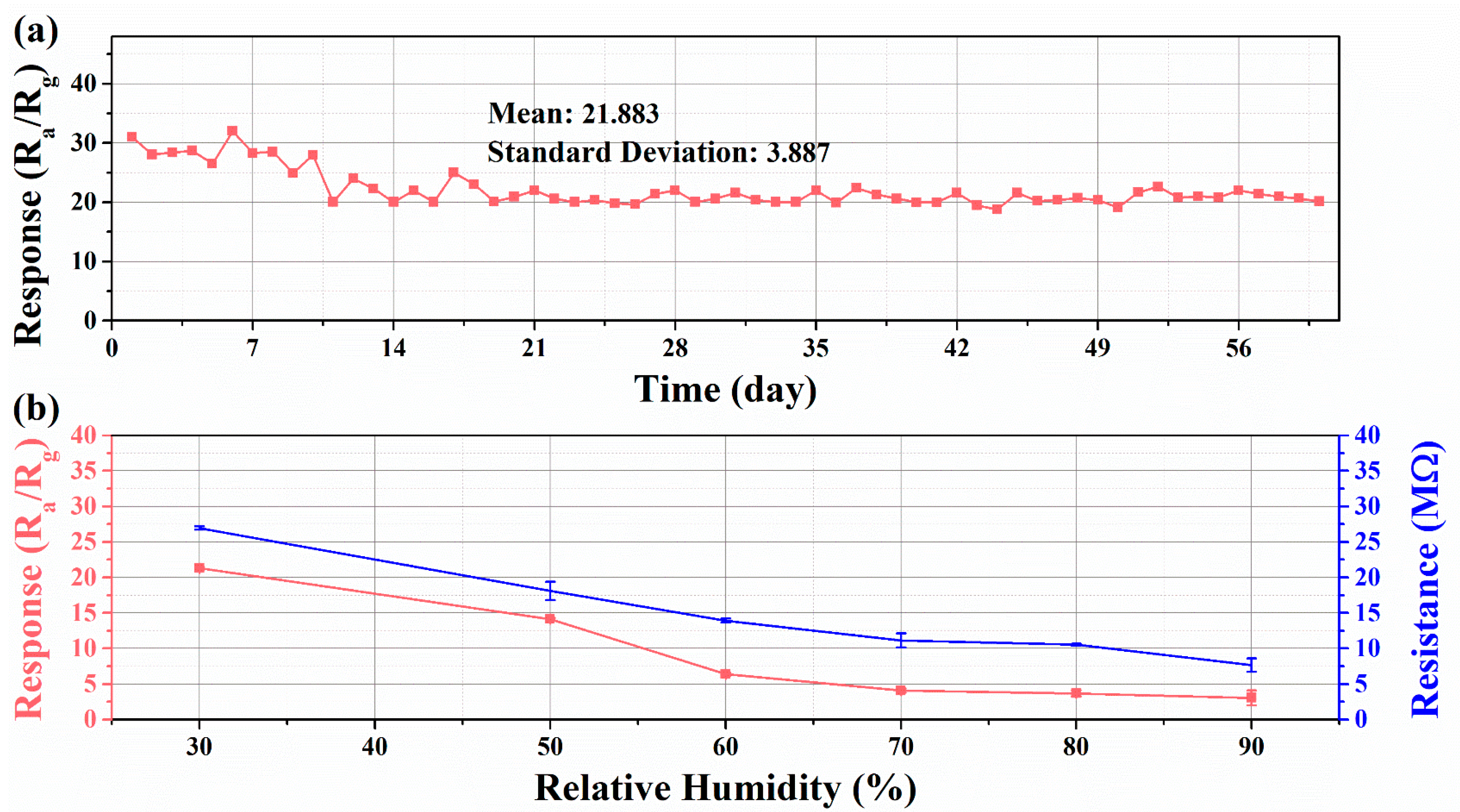
3.2. Gas Sensing Performance
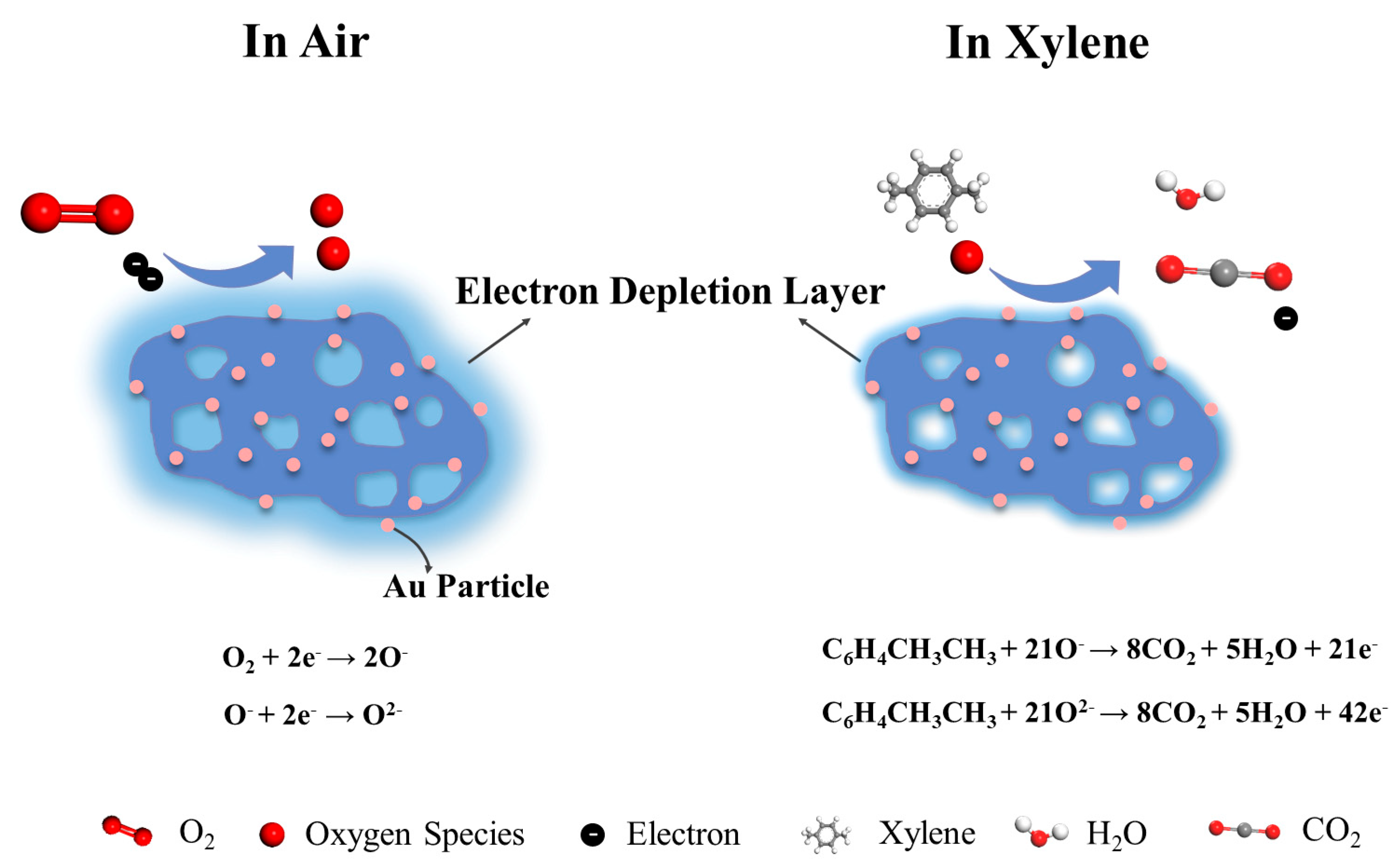
3.3. Sensing Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lu, Y.; Liu, J.; Lu, B.; Jiang, A.; Wan, C. Study on the removal of indoor VOCs using biotechnology. J. Hazard. Mater. 2010, 182, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Woellner, M.; Hausdorf, S.; Klein, N.; Mueller, P.; Smith, M.W.; Kaskel, S. Adsorption and Detection of Hazardous Trace Gases by Metal-Organic Frameworks. Adv. Mater. 2018, 30, 1704679. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, A.; Kim, J.-H.; Kim, H.W.; Kim, S.S. Resistive-based gas sensors for detection of benzene, toluene and xylene (BTX) gases: A review. J. Mater. Chem. C 2018, 6, 4342–4370. [Google Scholar] [CrossRef]
- Kim, H.J.; Yoon, J.W.; Choi, K.I.; Jang, H.W.; Umar, A.; Lee, J.H. Ultraselective and sensitive detection of xylene and toluene for monitoring indoor air pollution using Cr-doped NiO hierarchical nanostructures. Nanoscale 2013, 5, 7066–7073. [Google Scholar] [CrossRef]
- Gao, H.; Wei, D.; Lin, P.; Liu, C.; Sun, P.; Shimanoe, K.; Yamazoe, N.; Lu, G. The design of excellent xylene gas sensor using Sn-doped NiO hierarchical nanostructure. Sens. Actuators B-Chem. 2017, 253, 1152–1162. [Google Scholar] [CrossRef]
- Zhao, Q.; Du, Q.; Yang, Y.; Zhao, Z.; Cheng, J.; Bi, F.; Shi, X.; Xu, J.; Zhang, X. Effects of regulator ratio and guest molecule diffusion on VOCs adsorption by defective UiO-67: Experimental and theoretical insights. Chem. Eng. J. 2022, 433, 134510. [Google Scholar] [CrossRef]
- Yang, X.; Deng, Y.; Yang, H.; Liao, Y.; Cheng, X.; Zou, Y.; Wu, L.; Deng, Y. Functionalization of Mesoporous Semiconductor Metal Oxides for Gas Sensing: Recent Advances and Emerging Challenges. Adv. Sci. 2023, 10, 2204810. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, M.; Liang, K.; Turak, A.; Zhang, B.; Meng, D.; Wang, C.; Qu, F.; Cheng, W.; Yang, M. An acetone gas sensor based on nanosized Pt-loaded Fe2O3 nanocubes. Sens. Actuators B-Chem. 2019, 290, 59–67. [Google Scholar] [CrossRef]
- Xie, S.; Zhao, C.; Shen, J.; Wei, J.; Liu, H.; Pan, Y.; Zhao, Y.; Zhu, Y. Hierarchical Flower-like WO3 Nanospheres Decorated with Bimetallic Au and Pd for Highly Sensitive and Selective Detection of 3-Hydroxy-2-butanone Biomarker. ACS Sens. 2023, 8, 728–738. [Google Scholar] [CrossRef]
- Van Toan, N.; Hung, C.M.; Hoa, N.D.; Van Duy, N.; Le, D.T.T.; Hoa, N.T.T.; Viet, N.N.; Phuoc, P.H.; Van Hieu, N. Enhanced NH3 and H2 gas sensing with H2S gas interference using multilayer SnO2/Pt/WO3 nanofilms. J. Hazard. Mater. 2021, 412, 125181. [Google Scholar] [CrossRef]
- Hu, J.; Xiong, X.; Guan, W.; Long, H.; Zhang, L.; Wang, H. Self-templated flower-like WO3-In2O3 hollow microspheres for conductometric acetone sensors. Sens. Actuators B-Chem. 2022, 361, 131705. [Google Scholar] [CrossRef]
- Dong, C.; Zhao, R.; Yao, L.; Ran, Y.; Zhang, X.; Wang, Y. A review on WO3 based gas sensors: Morphology control and enhanced sensing properties. J. Alloys Compd. 2020, 820, 153194. [Google Scholar] [CrossRef]
- Kang, M.; Cho, I.; Park, J.; Jeong, J.; Lee, K.; Lee, B.; Del Orbe Henriquez, D.; Yoon, K.; Park, I. High Accuracy Real-Time Multi-Gas Identification by a Batch-Uniform Gas Sensor Array and Deep Learning Algorithm. ACS Sens. 2022, 7, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.S.; Na, H.G.; Mirzaei, A.; Bang, J.H.; Oum, W.; Han, S.; Choi, S.-W.; Kim, M.; Jin, C.; Kim, S.S.; et al. Room-temperature NO2 sensor based on electrochemically etched porous silicon. J. Alloys Compd. 2019, 811, 151975. [Google Scholar] [CrossRef]
- Yuan, T.; Ma, Z.; Nekouei, F.; Zhang, W.; Xu, J. Zeolitic imidazolate framework-derived n-ZnO/p-Co3O4 heterojunction by ion-etching method for superior CO toxic gas sensor. Sens. Actuators B-Chem. 2023, 374, 132717. [Google Scholar] [CrossRef]
- Xuan, J.; Wang, L.; Zou, Y.; Li, Y.; Zhang, H.; Lu, Q.; Sun, M.; Yin, G.; Zhou, A. Room-temperature gas sensor based on in situ grown, etched and W-doped ZnO nanotubes functionalized with Pt nanoparticles for the detection of low-concentration H2S. J. Alloys Compd. 2022, 922, 166158. [Google Scholar] [CrossRef]
- Gruber, D.; Kraus, F.; Müller, J. A novel gas sensor design based on CH4/H2/H2O plasma etched ZnO thin films. Sens. Actuators B-Chem. 2003, 92, 81–89. [Google Scholar] [CrossRef]
- Lee, J.S.; Park, J.W.; Shin, S.M. Fabrication of a micro catalytic gas sensor using thin film process and Si anisotropic etching techniques. Sens. Actuators B-Chem. 1997, 45, 265–269. [Google Scholar] [CrossRef]
- Hazra, S.K.; Basu, S. High sensitivity and fast response hydrogen sensors based on electrochemically etched porous titania thin films. Sens. Actuators B-Chem. 2006, 115, 403–411. [Google Scholar] [CrossRef]
- Radzali, R.; Hassan, Z.; Zainal, N.; Yam, F.K. Preparation of porous InAlGaN/Si(111) by photoelectrochemical etching for high performance hydrogen gas sensors at room temperature. Sens. Actuators B-Chem. 2015, 213, 276–284. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, Y.; Liu, Y.; Zhang, X. KOH post-etching-induced rough silicon nanowire array for H2 gas sensing application. Nanotechnology 2016, 27, 465502. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jung, Y.; Sung, S.H.; Lee, G.; Kim, J.; Seong, J.; Shim, Y.-S.; Jun, S.C.; Jeon, S. High-performance gas sensor array for indoor air quality monitoring: The role of Au nanoparticles on WO3, SnO2, and NiO-based gas sensors. J. Mater. Chem. A 2021, 9, 1159–1167. [Google Scholar] [CrossRef]
- van den Broek, J.; Abegg, S.; Pratsinis, S.E.; Güntner, A.T. Highly selective detection of methanol over ethanol by a handheld gas sensor. Nat. Commun. 2019, 10, 4420. [Google Scholar] [CrossRef]
- Kumar, S.; Lawaniya, S.D.; Agarwal, S.; Yu, Y.T.; Nelamarri, S.R.; Kumar, M.; Mishra, Y.K.; Awasthi, K. Optimization of Pt nanoparticles loading in ZnO for highly selective and stable hydrogen gas sensor at reduced working temperature. Sens. Actuators B-Chem. 2023, 375, 132943. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhang, H.; Li, J.; Meng, F.; Yang, Z.; Zhang, H. Ag Nanoparticle-Modified ZnO-In2O3 Nanocomposites for Low-Temperature Rapid Detection Hydrogen Gas Sensors. IEEE Trans. Instrum. Meas. 2023, 72, 1–12. [Google Scholar] [CrossRef]
- Zhu, L.Y.; Ou, L.X.; Mao, L.W.; Wu, X.Y.; Liu, Y.P.; Lu, H.L. Advances in Noble Metal-Decorated Metal Oxide Nanomaterials for Chemiresistive Gas Sensors: Overview. Nano-Micro Lett. 2023, 15, 89. [Google Scholar] [CrossRef]
- Park, S.; Lim, Y.; Oh, D.; Ahn, J.; Park, C.; Kim, M.; Jung, W.; Kim, J.; Kim, I.-D. Steering selectivity in the detection of exhaled biomarkers over oxide nanofibers dispersed with noble metals. J. Mater. Chem. A 2023, 11, 3535–3545. [Google Scholar] [CrossRef]
- Barbosa, M.S.; Suman, P.H.; Kim, J.J.; Tuller, H.L.; Varela, J.A.; Orlandi, M.O. Gas sensor properties of Ag- and Pd-decorated SnO micro-disks to NO2, H2 and CO: Catalyst enhanced sensor response and selectivity. Sens. Actuators B-Chem. 2017, 239, 253–261. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Wang, Y.; Li, X.; Cheng, P.; Zhao, Y.; Dang, F.; Zhang, Y. Hydrothermal synthesis of Au@SnO2 hierarchical hollow microspheres for ethanol detection. Sens. Actuators B-Chem. 2020, 319, 128299. [Google Scholar] [CrossRef]
- Zhang, X.; Lan, W.; Xu, J.; Luo, Y.; Pan, J.; Liao, C.; Yang, L.; Tan, W.; Huang, X. ZIF-8 derived hierarchical hollow ZnO nanocages with quantum dots for sensitive ethanol gas detection. Sens. Actuators B-Chem. 2019, 289, 144–152. [Google Scholar] [CrossRef]
- Liu, L.; Li, X.; Dutta, P.K.; Wang, J. Room temperature impedance spectroscopy-based sensing of formaldehyde with porous TiO2 under UV illumination. Sens. Actuators B-Chem. 2013, 185, 1–9. [Google Scholar] [CrossRef]
- Wei, D.; Huang, Z.; Wang, L.; Chuai, X.; Zhang, S.; Lu, G. Hydrothermal synthesis of Ce-doped hierarchical flower-like In2O3 microspheres and their excellent gas-sensing properties. Sens. Actuators B-Chem. 2018, 255, 1211–1219. [Google Scholar] [CrossRef]
- Li, H.; Wu, C.-H.; Liu, Y.-C.; Yuan, S.-H.; Chiang, Z.-X.; Zhang, S.; Wu, R.-J. Mesoporous WO3-TiO2 heterojunction for a hydrogen gas sensor. Sens. Actuators B-Chem. 2021, 341, 130035. [Google Scholar] [CrossRef]
- Hu, J.; Zhuang, Z.; Liu, F.; Liu, X.; Liu, Z. Investigation of grain boundary and orientation effects in polycrystalline metals by a dislocation-based crystal plasticity model. Comput. Mater. Sci. 2019, 159, 86–94. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Han, S.D.; Cho, B.K.; Brinzari, V. Grain Size Effects in Sensor Response of Nanostructured SnO2-and In2O3-Based Conductometric Thin Film Gas Sensor. Crit. Rev. Solid State Mater. Sci. 2009, 34, 1–17. [Google Scholar] [CrossRef]
- Solis, J.L.; Saukko, S.; Kish, L.; Granqvist, C.G.; Lantto, V. Semiconductor gas sensors based on nanostructured tungsten oxide. Thin Solid Film. 2001, 391, 255–260. [Google Scholar] [CrossRef]
- Gurlo, A. Nanosensors: Towards morphological control of gas sensing activity. SnO2, In2O3, ZnO and WO3 case studies. Nanoscale 2011, 3, 154–165. [Google Scholar] [CrossRef]
- Verma, A.; Yadav, B.C. 2D/2D Nanostructured System Based on WO3/WS2 for Acetone Sensor and Breath Analyzer. ACS Appl. Nano Mater. 2023, 6, 5493–5507. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, T.; Fan, Q.Y.; Liu, Y.; Zhang, A.A.; Li, Z.Q.; Hao, Y.H.; Chen, L.; Liu, F.R.; Gu, X.J.; et al. Discovering Surface Structure and the Mechanism of Graphene Oxide-Triggered CeO2-WO3/TiO2 Catalysts for NO Abatement with NH3. ACS Catal. 2022, 12, 8386–8403. [Google Scholar] [CrossRef]
- Zhang, X.L.; Huang, W.; Xia, Z.X.; Xian, M.; Bu, F.; Liang, F.B.; Feng, D.X. One-pot synthesis of S-scheme WO3/BiOBr heterojunction nanoflowers enriched with oxygen vacancies for enhanced tetracycline photodegradation. Sep. Purif. Technol. 2022, 290, 120897. [Google Scholar] [CrossRef]
- Nayak, A.K.; Das, A.K.; Pradhan, D. High Performance Solid-State Asymmetric Supercapacitor using Green Synthesized Graphene-WO3 Nanowires Nanocomposite. ACS Sustain. Chem. Eng. 2017, 5, 10128–10138. [Google Scholar] [CrossRef]
- Yang, D.J.; Kamienchick, I.; Youn, D.Y.; Rothschild, A.; Kim, I.D. Ultrasensitive and Highly Selective Gas Sensors Based on Electrospun SnO2 Nanofibers Modified by Pd Loading. Adv. Funct. Mater. 2010, 20, 4258–4264. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Kou, J.; Sun, C.B. Highly efficient electroextraction of gold and simultaneous iodine regeneration using ionic liquid 1-ethyl-3-methylimidazolium dicyanamide. Sep. Purif. Technol. 2023, 318, 123961. [Google Scholar] [CrossRef]
- Qiu, Y.H.; Wang, Y.P. Controllable synthesis of W18O49 nanoneedles for high-performance NO2gas sensors. J. Alloys Compd. 2023, 944, 169199. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, J.F.; Wang, W.; Fan, Y.Z.; Liu, C.X.; Zhou, J.R.; Liu, D.L.; Ruan, S.P. Au-Pd modified SnS2 nanosheets for conductometric detection of xylene gas. Sens. Actuators B-Chem. 2022, 351, 130907. [Google Scholar] [CrossRef]
- Li, X.; Tan, T.; Ji, W.; Zhou, W.L.; Bao, Y.W.; Xia, X.H.; Zeng, Z.F.; Gao, Y. Remarkably Enhanced Methane Sensing Performance at Room Temperature via Constructing a Self-Assembled Mulberry-Like ZnO/SnO2 Hierarchical Structure. Energy Environ. Mater. 2023, 7, e12624. [Google Scholar] [CrossRef]
- Wang, X.; Wang, T.K.; Si, G.K.; Li, Y.; Zhang, S.W.; Deng, X.L.; Xu, X.J. Oxygen vacancy defects engineering on Ce-doped alpha-Fe2O3 gas sensor for reducing gases. Sens. Actuators B-Chem. 2020, 302, 127165. [Google Scholar] [CrossRef]
- Tai, H.L.; Duan, Z.H.; Wang, Y.; Wang, S.; Jiang, Y.D. Paper-Based Sensors for Gas, Humidity, and Strain Detections: A Review. ACS Appl. Mater. Interfaces 2020, 12, 31037–31053. [Google Scholar] [CrossRef]
- Liu, S.; Wang, M.; Ge, C.; Zhang, X.; Lei, S.; Hussain, S.; Wang, M.; Qiao, G.; Liu, G. ZnO/Ti3C2 composite with oxygen vacancies and Schottky barrier for effective detection of ppb-level NO2 at room temperature. Appl. Surf. Sci. 2023, 610, 155440. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, C.; Zhang, R.; Zhang, Y.; Wang, Y.; Zhang, Z.; Cao, J. Construction of hierarchical In2O3/In2S3 microsphere heterostructures for TEA detection. J. Mater. 2022, 8, 1113–1121. [Google Scholar] [CrossRef]
- Ueda, T.; Boehme, I.; Hyodo, T.; Shimizu, Y.; Weimar, U.; Barsan, N. Effects of Gas Adsorption Properties of an Au-Loaded Porous In2O3 Sensor on NO2-Sensing Properties. ACS Sens. 2021, 6, 4019–4028. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Choi, P.G.; Masuda, Y. Large-lateral-area SnO2 nanosheets with a loose structure for high-performance acetone sensor at the ppt level. J. Hazard. Mater. 2023, 455, 131592. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Ruan, S.; Zhang, N.; Yin, Y.; Guo, S.; Chen, Y.; Zhang, H.; Li, C. Synthesis and characterization of Cr-doped WO3 nanofibers for conductometric sensors with high xylene sensitivity. Sens. Actuators B-Chem. 2018, 265, 355–364. [Google Scholar] [CrossRef]
- Han, X.; Niu, Y.; Yu, C.; Liu, Z.; Huang, H.; Huang, H.; Li, S.; Guo, W.; Tan, X.; Qiu, J. Ultrafast construction of interfacial sites by wet chemical etching to enhance electrocatalytic oxygen evolution. Nano Energy 2020, 69, 104367. [Google Scholar] [CrossRef]
- Cui, X.X.; Tian, X.; Xiao, X.C.; Chen, T.; Wang, Y.D. Au Modified Hollow Cube Sn-MOF Derivatives for Highly Sensitive, Great Selective, and Stable Detection of n-Butanol at Room Temperature. Adv. Mater. Technol. 2023, 8, 2300572. [Google Scholar] [CrossRef]
- Wang, X.H.; Meng, X.N.; Gao, W. Ultrahigh-response sensor based on hierarchical Pd-WO3 nanoflowers for rapid hydrogen detection. Sens. Actuators B-Chem. 2023, 387, 133790. [Google Scholar] [CrossRef]
- Horprathum, M.; Srichaiyaperk, T.; Samransuksamer, B.; Wisitsoraat, A.; Eiamchai, P.; Limwichean, S.; Chananonnawathorn, C.; Aiempanakit, K.; Nuntawong, N.; Patthanasettakul, V.; et al. Ultrasensitive Hydrogen Sensor Based on Pt-Decorated WO3 Nanorods Prepared by Glancing-Angle dc Magnetron Sputtering. ACS Appl. Mater. Interfaces 2014, 6, 22051–22060. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, J.; Zhu, M.; Ju, D.X.; Xu, H.Y.; Cao, B.Q. High-performance gas sensor based on ZnO nanowires functionalized by Au nanoparticles. Sens. Actuators B-Chem. 2014, 199, 339–345. [Google Scholar] [CrossRef]
- Hjiri, M. Strategies to detect xylene gas using chemoresistive gas sensors: An overview. J. Ind. Eng. Chem. 2024, 139, 56–73. [Google Scholar] [CrossRef]
- Li, F.; Guo, S.J.; Shen, J.L.; Shen, L.; Sun, D.M.; Wang, B.; Chen, Y.; Ruan, S.P. Xylene gas sensor based on Au-loaded WO3· H2O nanocubes with enhanced sensing performance. Sens. Actuators B-Chem. 2017, 238, 364–373. [Google Scholar] [CrossRef]
- Xie, S.H.; Liu, Y.X.; Deng, J.G.; Zhao, X.T.; Yang, J.; Zhang, K.F.; Han, Z.; Arandiyan, H.; Dai, H.X. Effect of transition metal doping on the catalytic performance of Au-Pd/3DOM Mn2O3 for the oxidation of methane and o-xylene. Appl. Catal. B-Environ. 2017, 206, 221–232. [Google Scholar] [CrossRef]
- Park, S.W.; Jeong, S.Y.; Yoon, J.W.; Lee, J.H. General Strategy for Designing Highly Selective Gas-Sensing Nanoreactors: Morphological Control of SnO2 Hollow Spheres and Configurational Tuning of Au Catalysts. ACS Appl. Mater. Interfaces 2020, 12, 51607–51615. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Lei, Z.; Li, X.; Meng, Y.; Cui, W.; Xu, Y.; Hao, X.; Sun, S.; Cheng, P. Etched Tungsten Oxide Modified with Au for Quick Xylene Detection. Micromachines 2025, 16, 646. https://doi.org/10.3390/mi16060646
Wang Y, Lei Z, Li X, Meng Y, Cui W, Xu Y, Hao X, Sun S, Cheng P. Etched Tungsten Oxide Modified with Au for Quick Xylene Detection. Micromachines. 2025; 16(6):646. https://doi.org/10.3390/mi16060646
Chicago/Turabian StyleWang, Yinglin, Zhaohui Lei, Xu Li, Yantong Meng, Wanting Cui, Yiyang Xu, Xidong Hao, Shanfu Sun, and Pengfei Cheng. 2025. "Etched Tungsten Oxide Modified with Au for Quick Xylene Detection" Micromachines 16, no. 6: 646. https://doi.org/10.3390/mi16060646
APA StyleWang, Y., Lei, Z., Li, X., Meng, Y., Cui, W., Xu, Y., Hao, X., Sun, S., & Cheng, P. (2025). Etched Tungsten Oxide Modified with Au for Quick Xylene Detection. Micromachines, 16(6), 646. https://doi.org/10.3390/mi16060646







