A Novel Variable Volume Capillary Microgripper for Micromanipulation in Aqueous Media
Abstract
1. Introduction
2. Materials and Methods
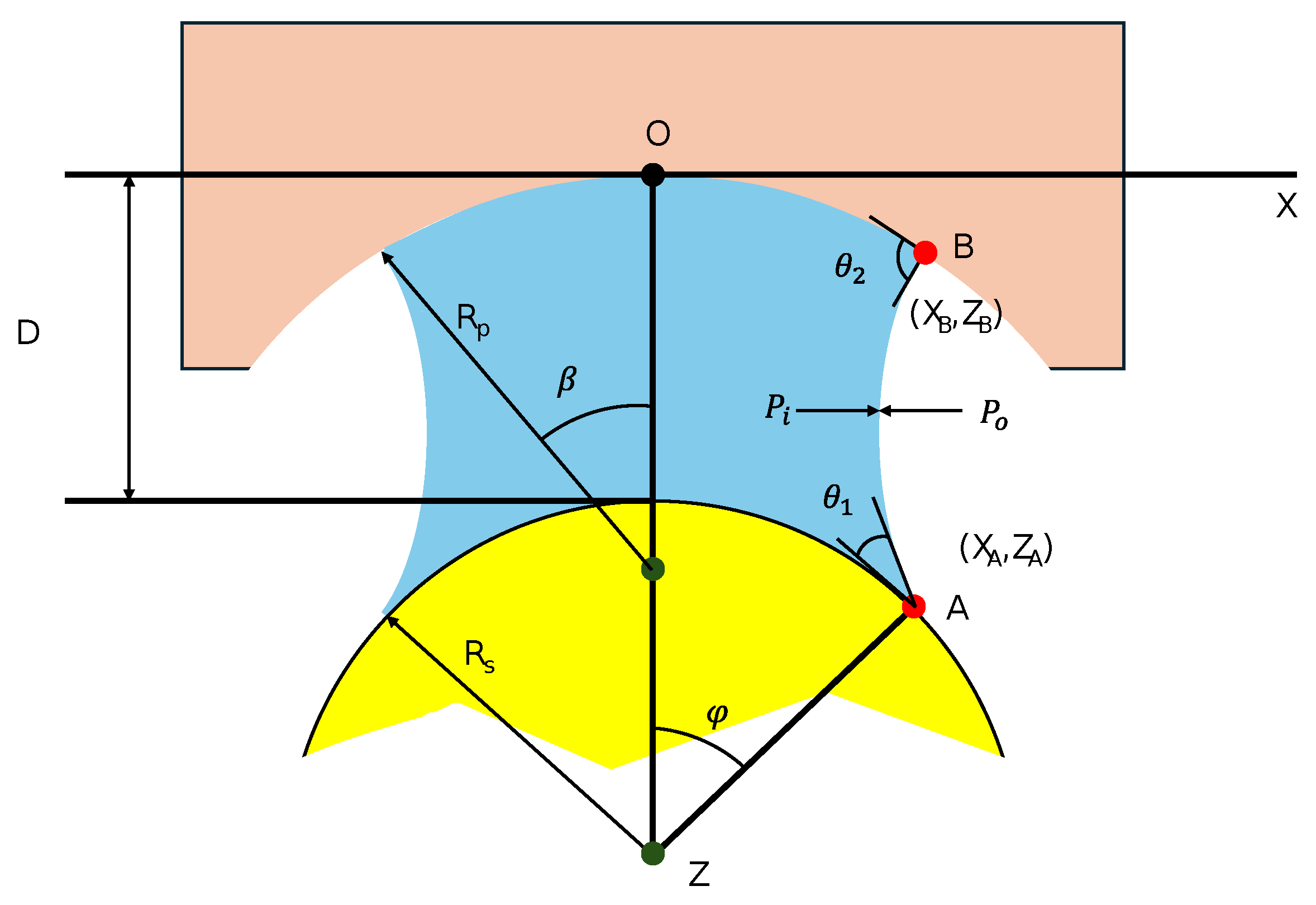
2.1. Theoretical Background of Capillary Bridging
2.2. Simulations
2.2.1. Governing Equations
2.2.2. Initial and Boundary Conditions
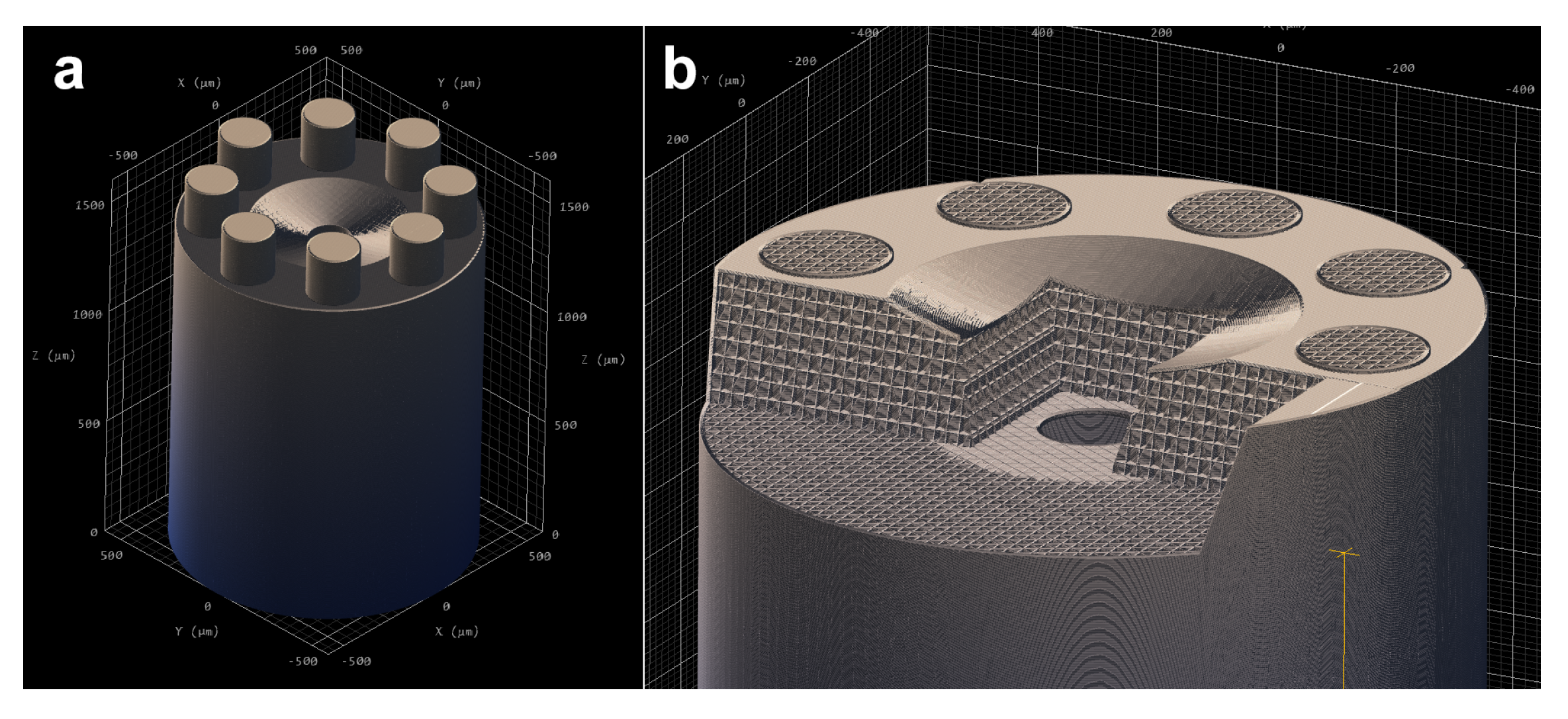
2.2.3. Mesh and Simulation
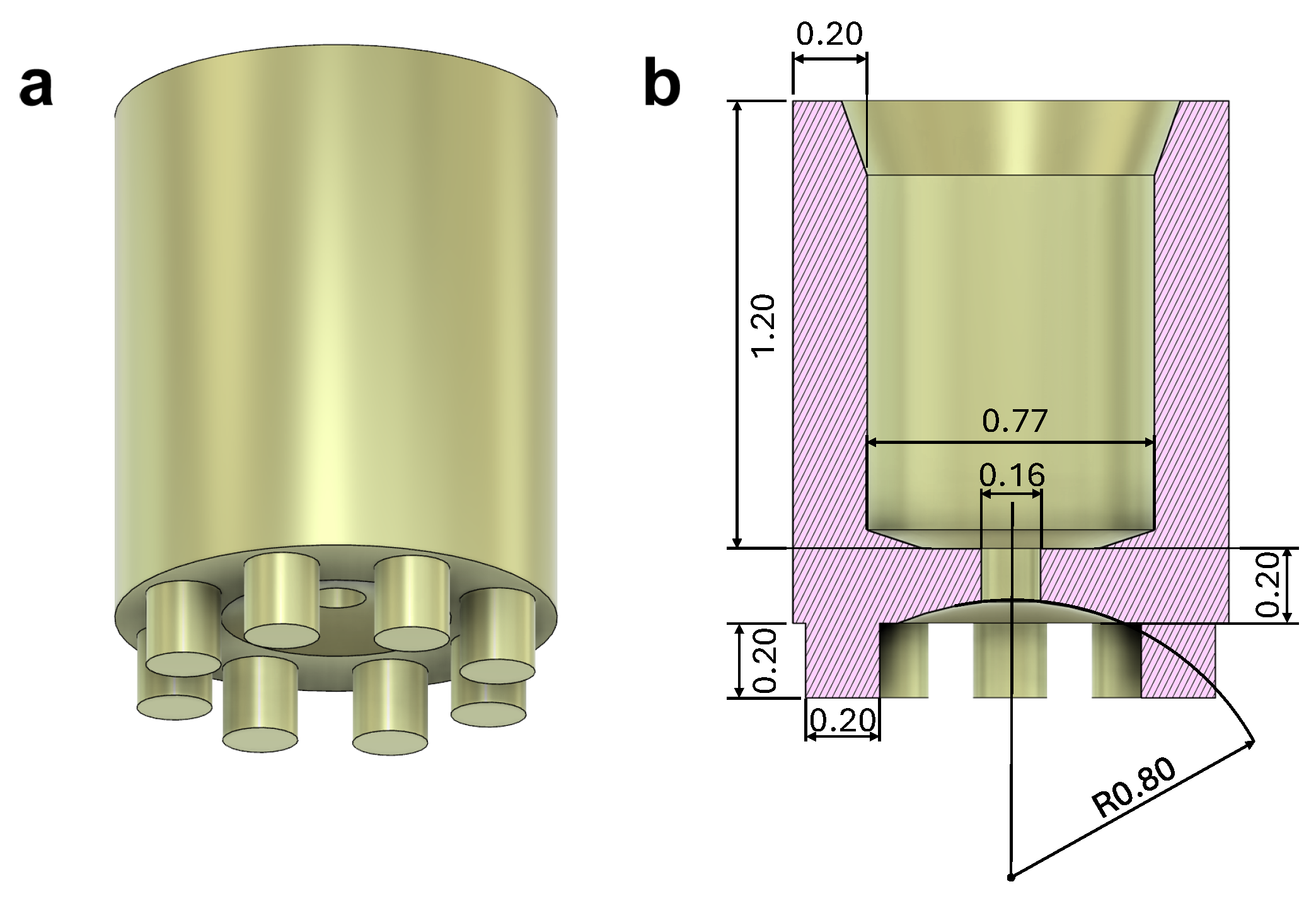
2.3. Microgripper Fabrication
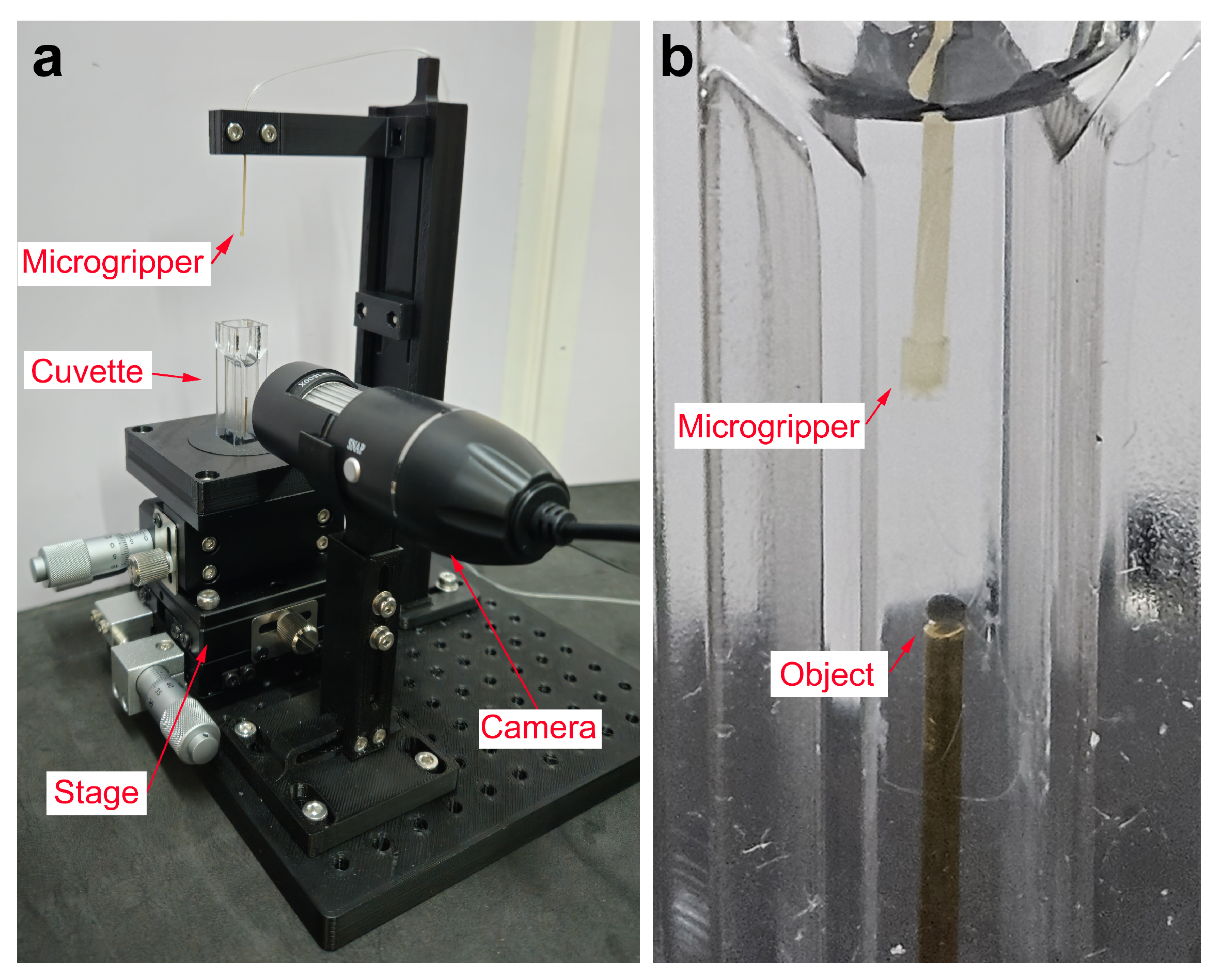
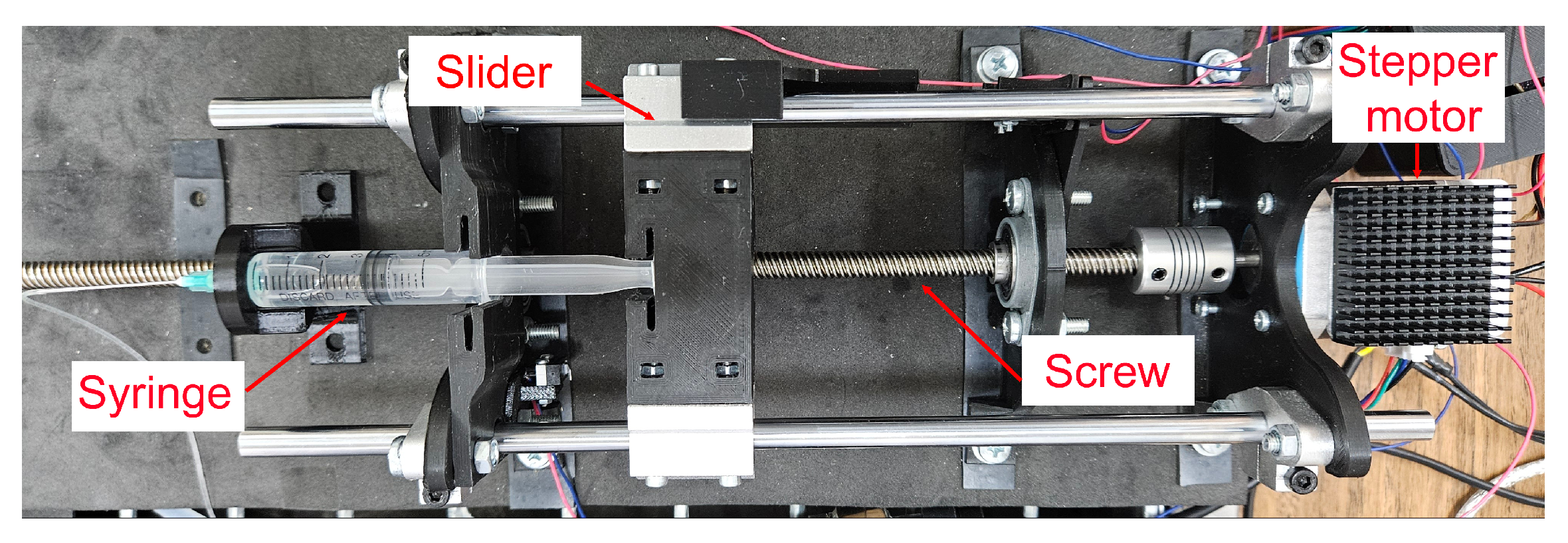
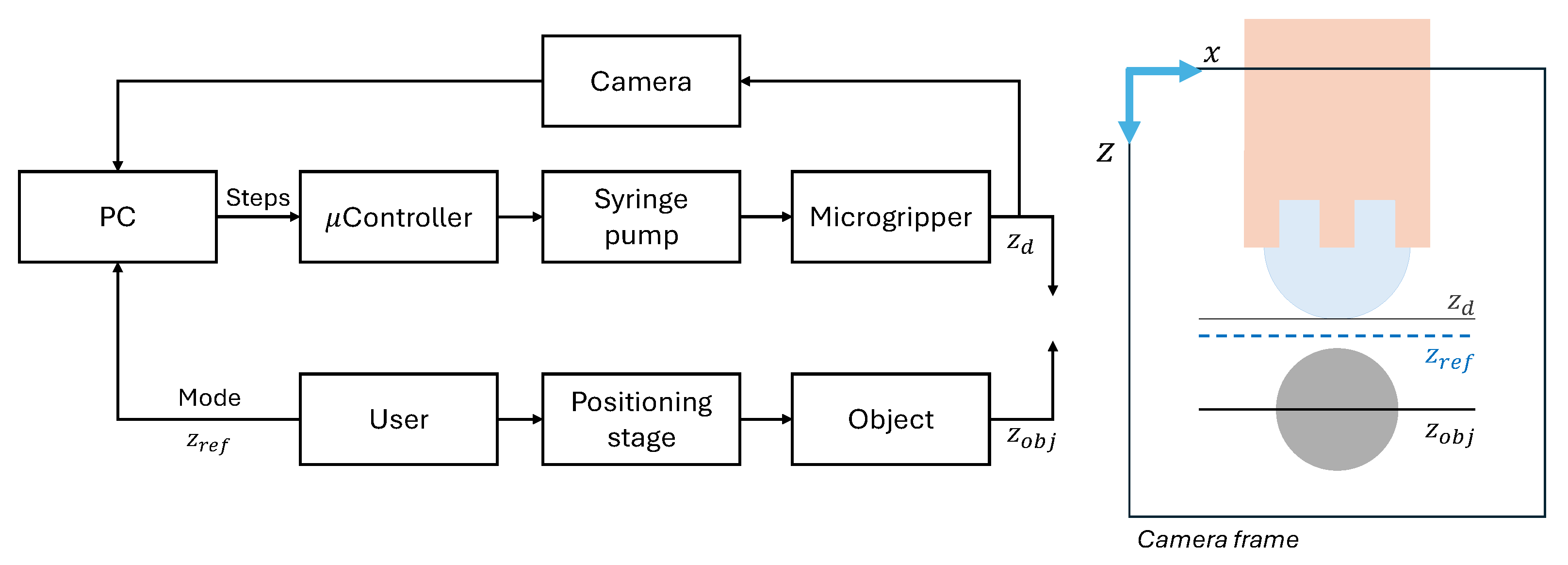
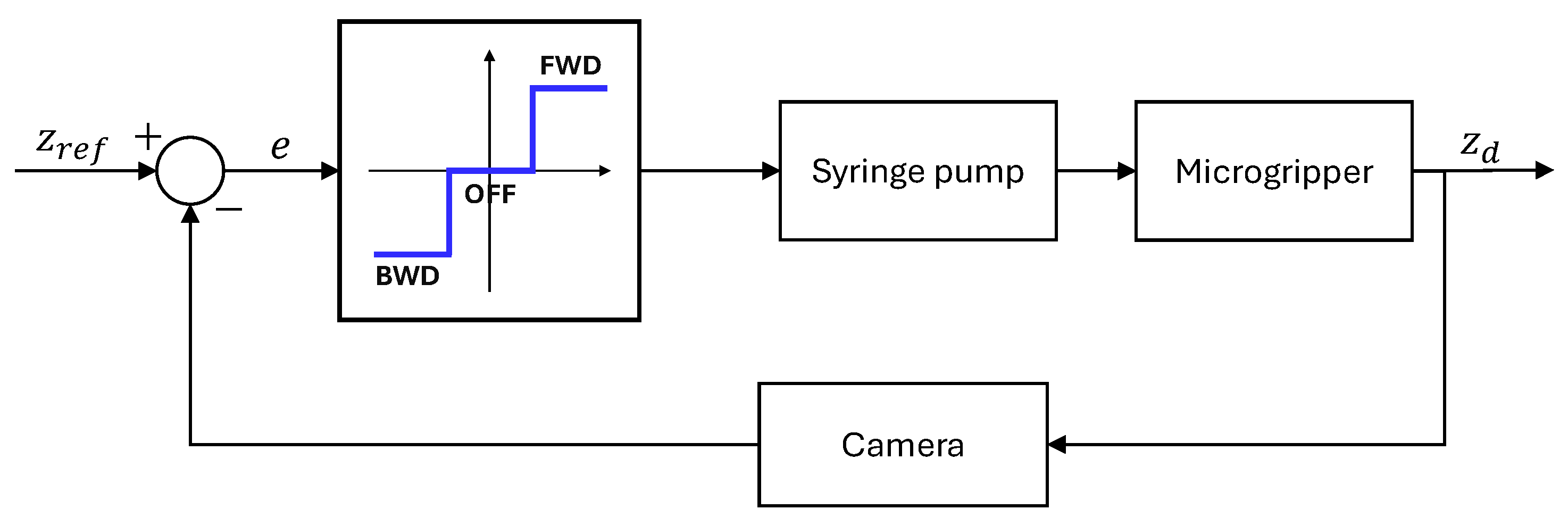
2.4. Experimental Setup
- Actuation mechanism:A syringe pump injects oil into the microgripper via a connecting tube. It utilizes a threaded rod to drive the syringe plunger, allowing for controlled fluid injection or retraction based on the rod rotational direction. The syringe barrel is fixed, and the plunger is attached to a sliding carriage moving along support rods. Limit switches define the travel range.
- Motor and feedback: A stepper motor drives the threaded rod. An AS5600 (ams OSRAM AG, Austria) magnetic encoder provides rotational feedback for precise control.
- Control unit: An Arduino DUE serves as the main controller, processing inputs from the limit switches, magnetic encoder, and managing the DRV8825 (Texas Instrument, Dallas, TX, USA) motor driver (mounted on a custom PCB). An Arduino UNO is used solely to power the motor encoder.
- Fluidic components: A 5 mL syringe with a Luer Lock connection is employed. A 21G needle connects the syringe to a flexible tube with inner diameter (ID) of mm, outer diameter (OD) of 1 mm, and a length of approximately m. This specific choice of long, thin, and flexible tubing is dictated by the requirements of the primary envisioned application: enabling the microgripper to be guided through the lumen of a long medical catheter to reach internal target sites within the body. A rigid tube (ID: mm, OD: mm) is press-fit into the microgripper inlet and connected to the flexible tube.
- Control algorithm:
- -
- The Arduino DUE program controls the stepper motor steps based on feedback from the magnetic encoder (via I2C protocol) and incorporates limit switch sensor logic. It receives high-level commands (steps, direction, and speed) via serial communication.
- -
- A Python 3.10 program running on a PC manages the overall process, including visualization, real-time vision system analysis (using an attached camera), and sending commands to the Arduino for motor control. Its primary objective in the control loop is to adjust the syringe piston movement precisely to maintain the droplet lower apex aligned with a defined reference line in the captured image.
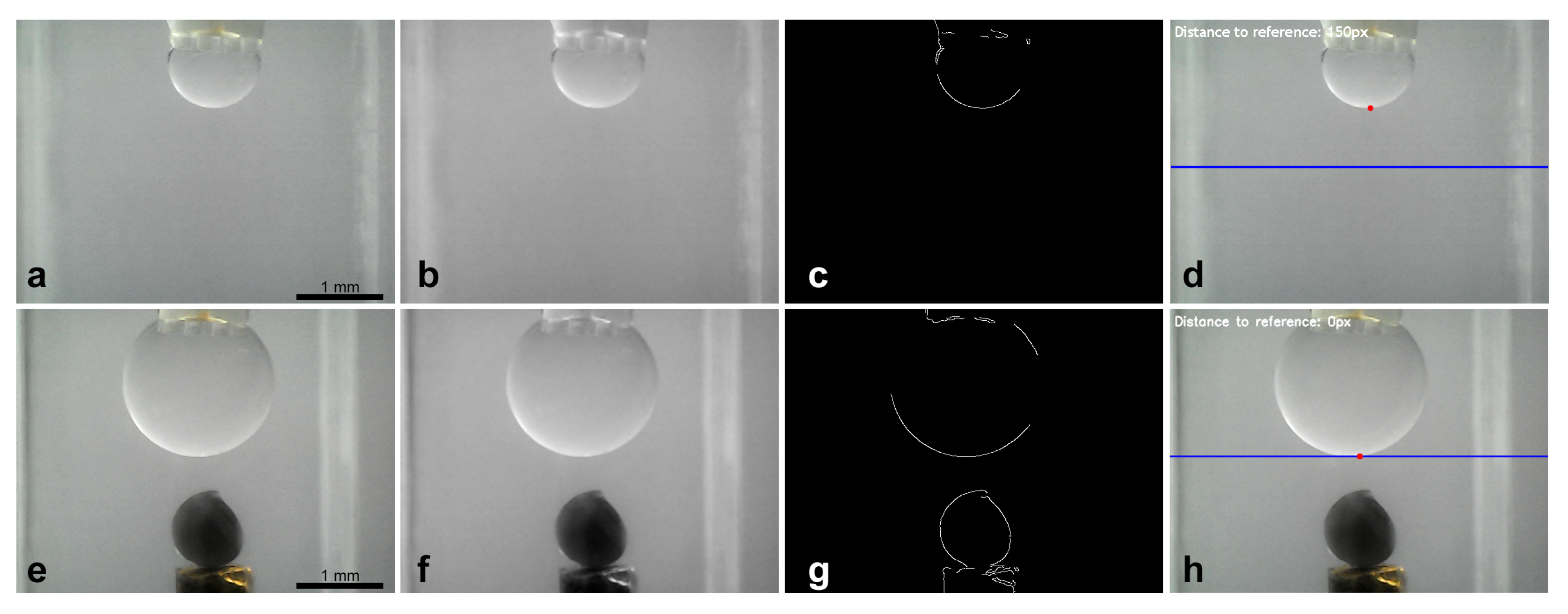
2.5. Image Processing
3. Results and Discussion
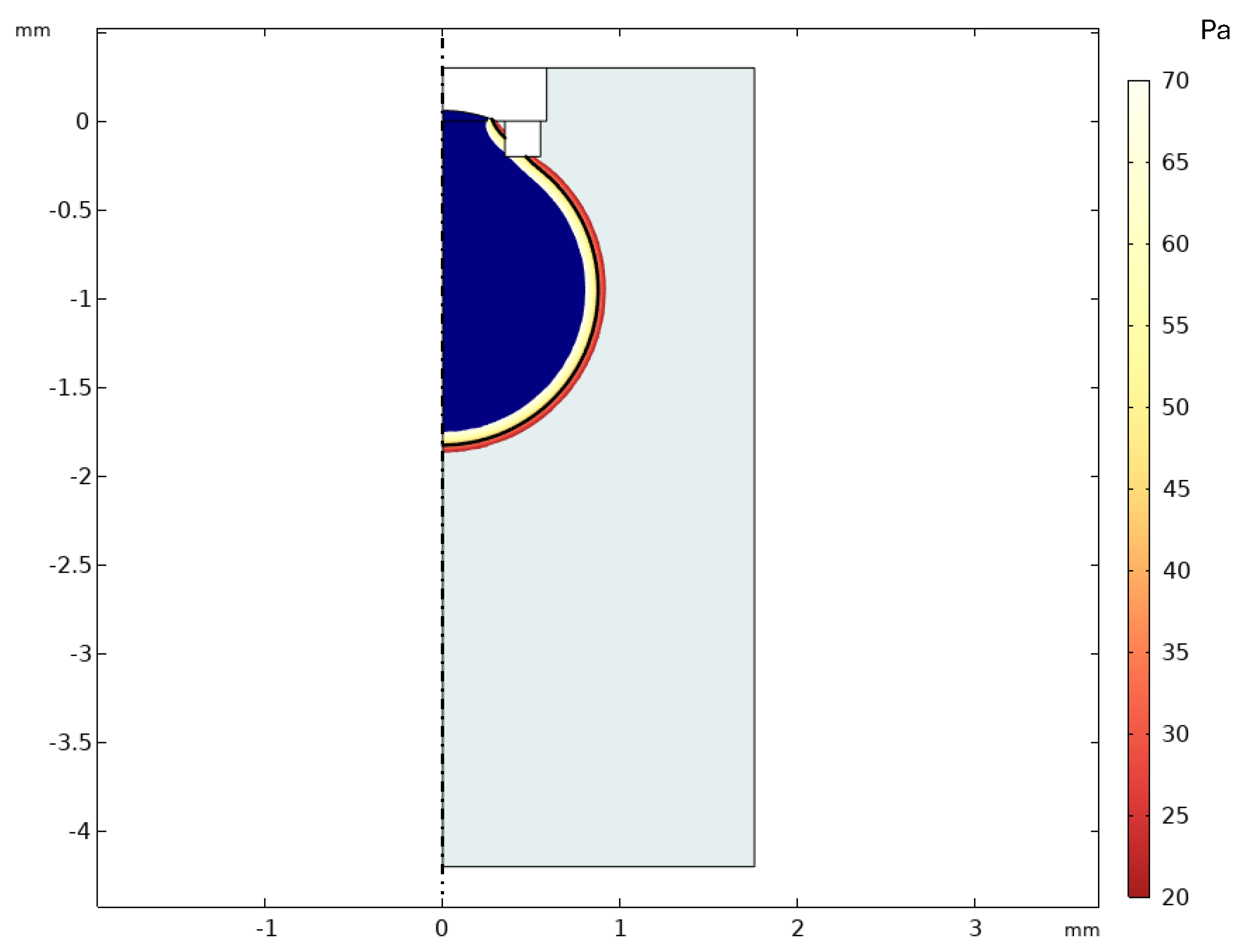
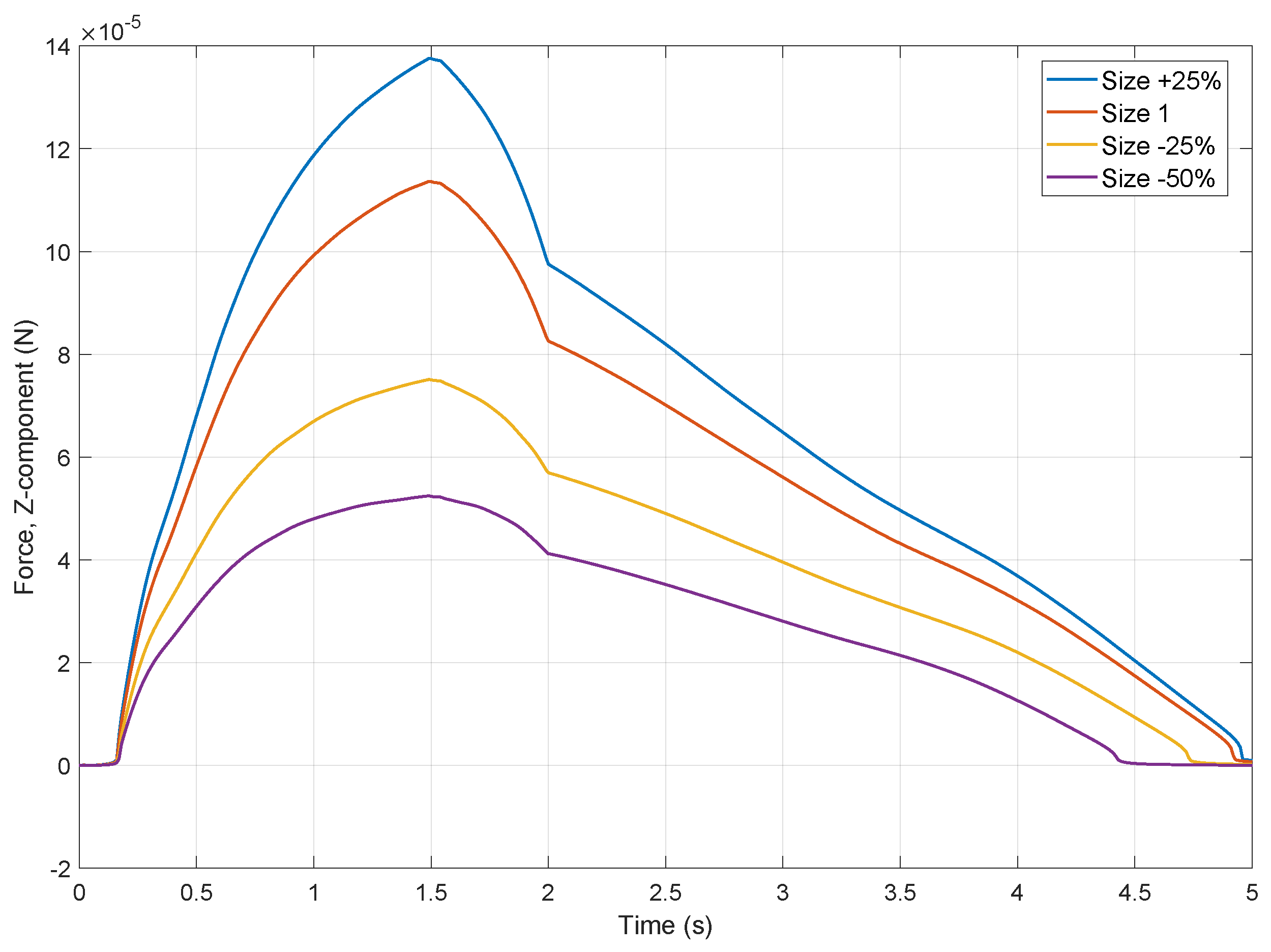
3.1. Simulation
3.2. Droplet Control
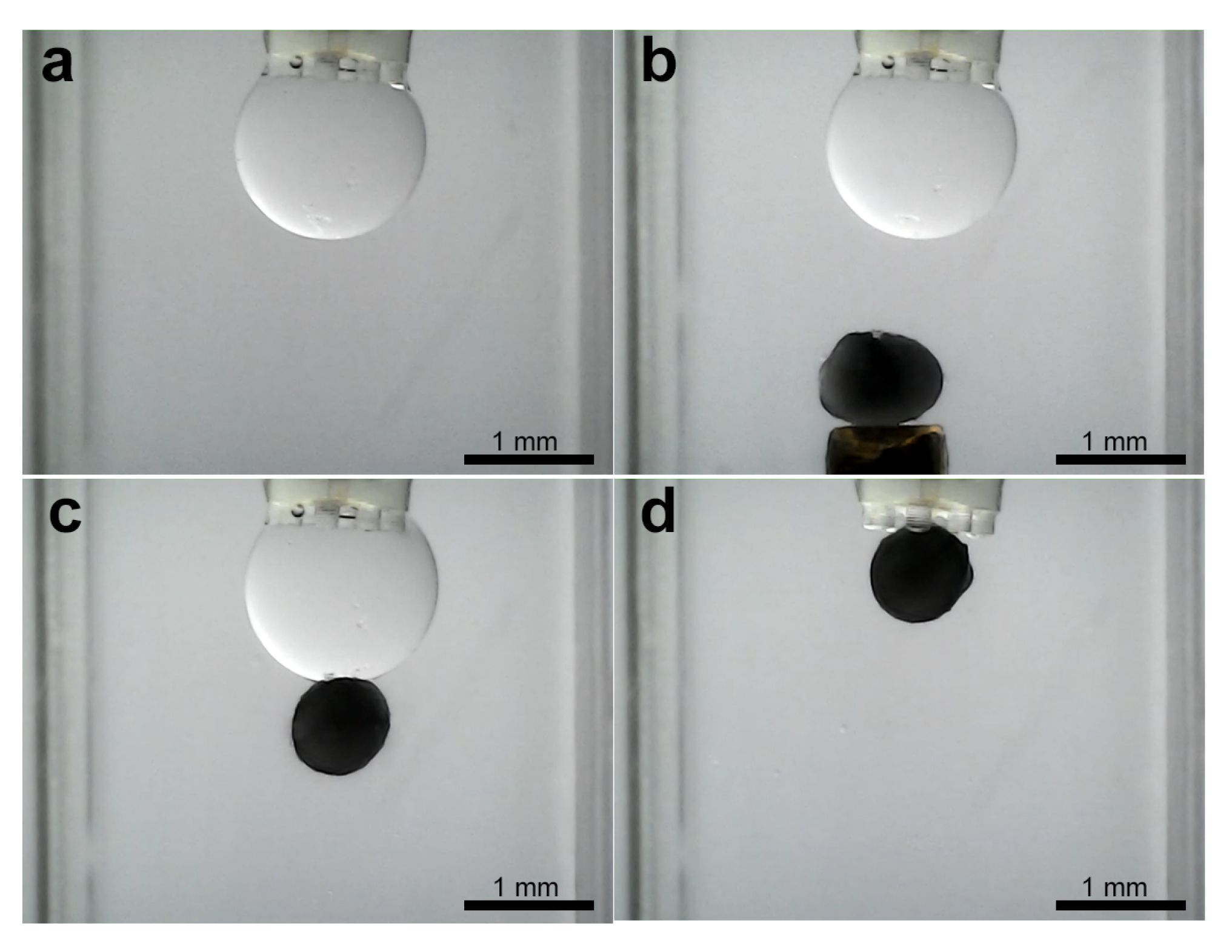
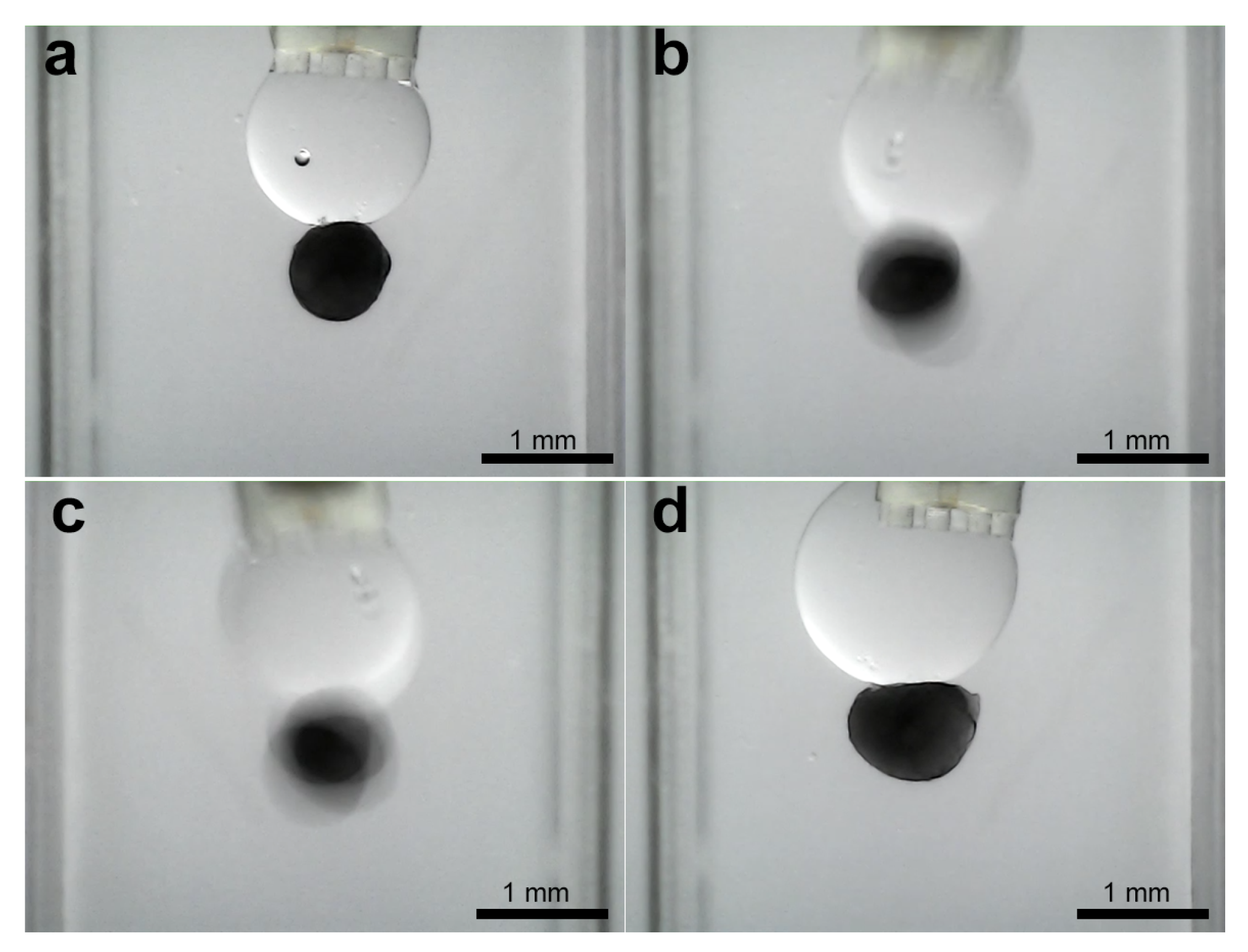
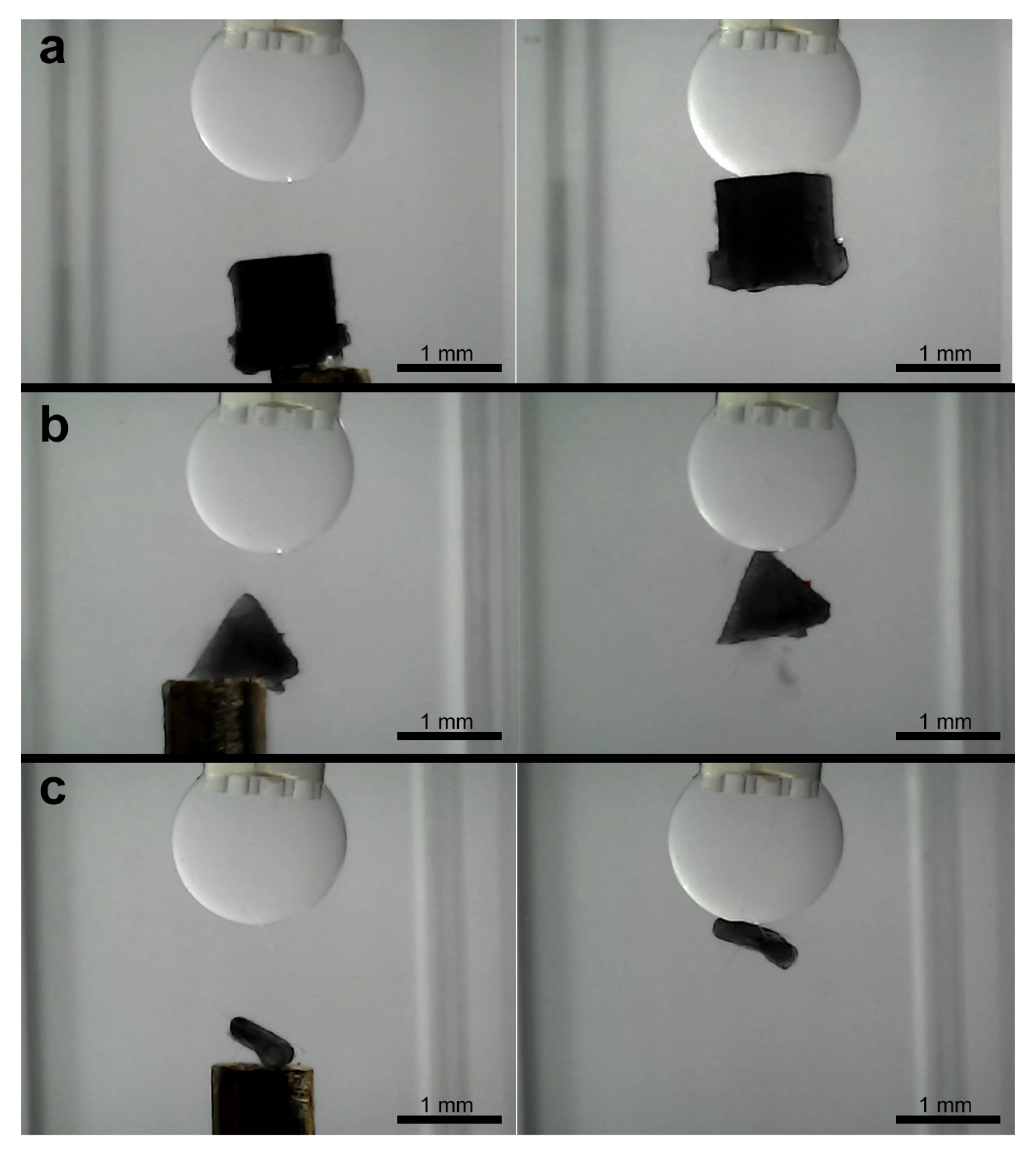
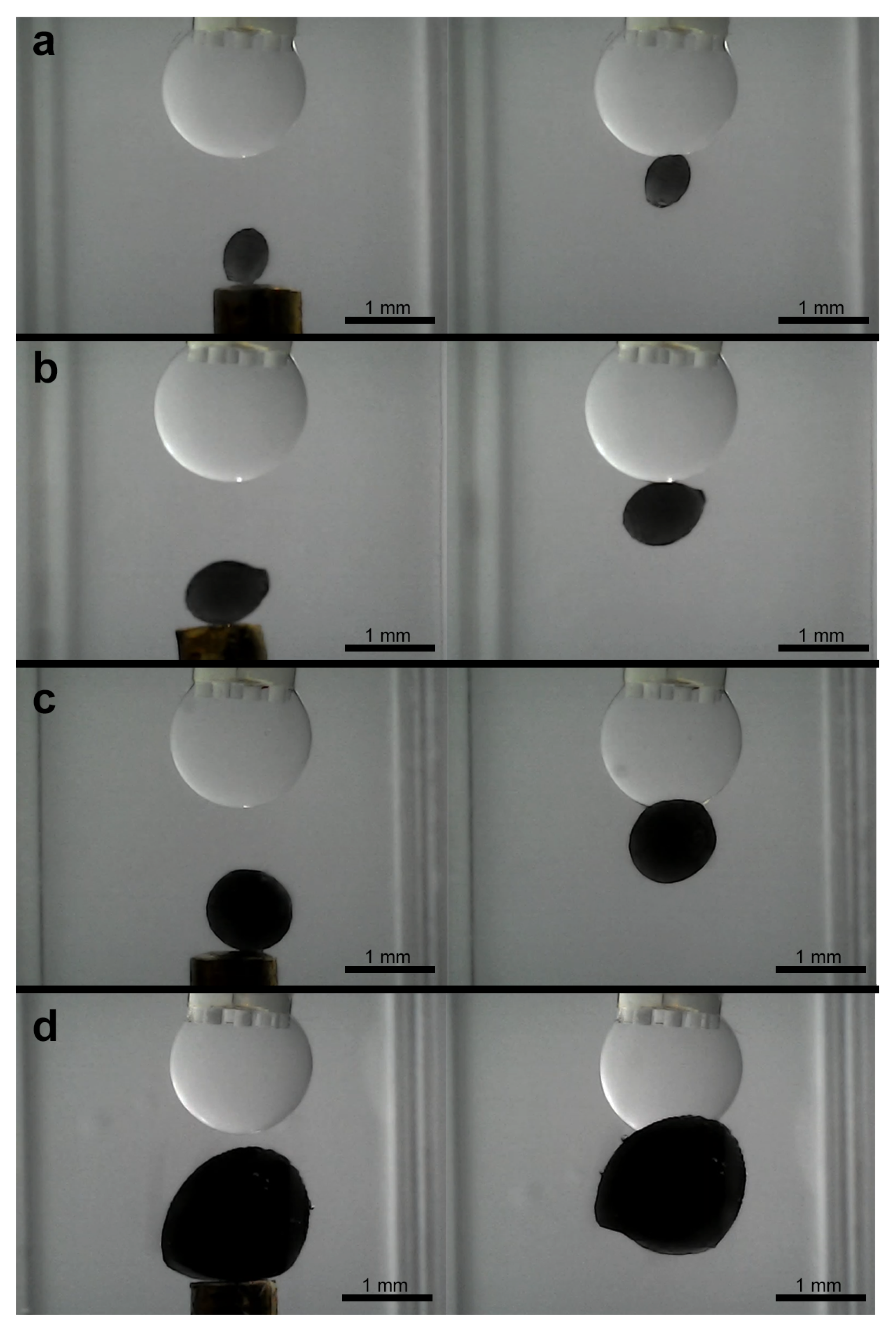
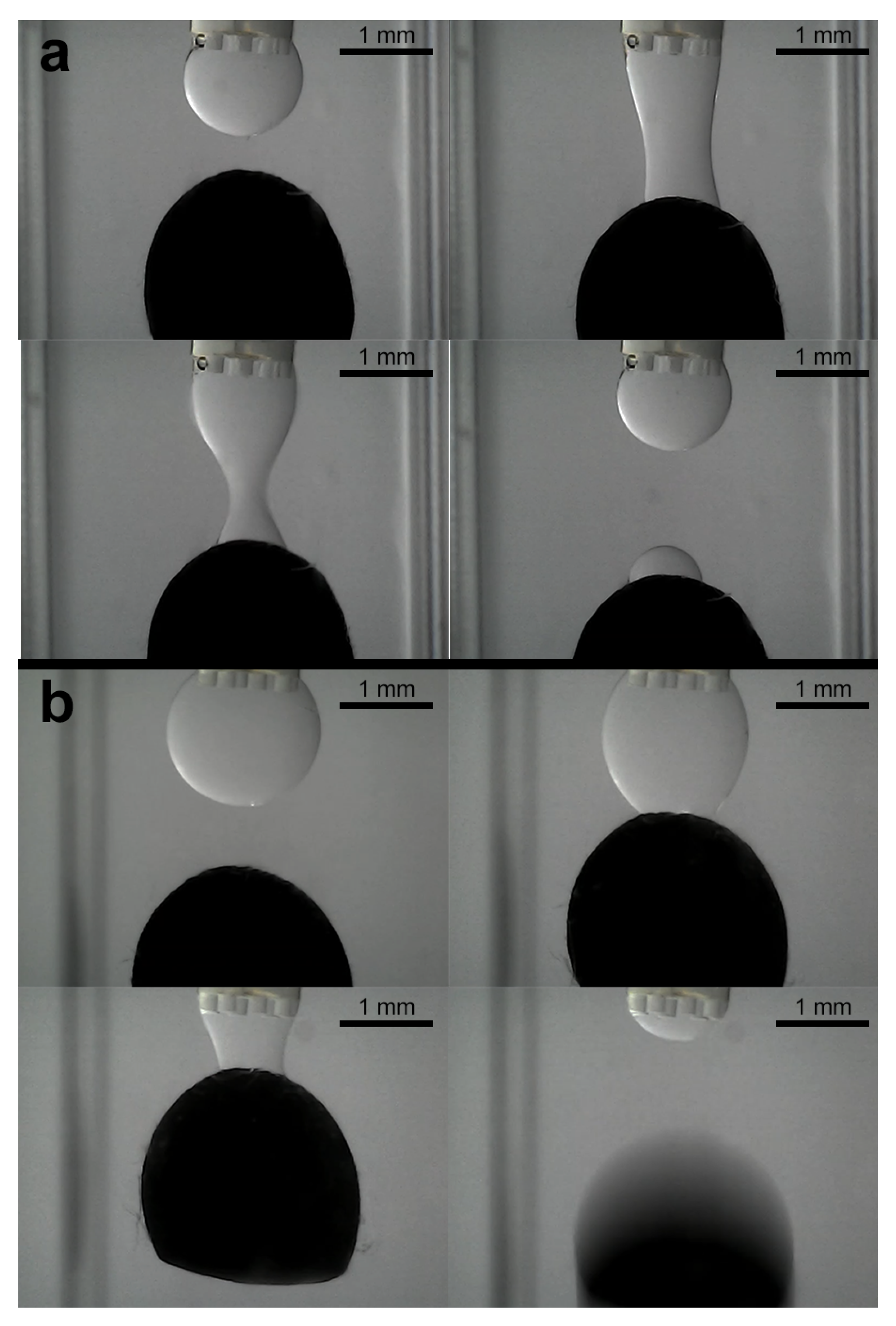
3.3. Experiments for Capturing and Releasing
- Expanded operational domain: The most significant advantage is the ability to operate directly in aqueous media. This opens up applications in biological sample handling, culture media, underwater microassembly, or manipulation in other liquid environments where air-based capillary grippers are inherently unsuitable due to the lack of a stable liquid–gas interface and the high surface tension of the surrounding medium.
- Stability and elimination of evaporation: Conventional grippers using volatile fluids like water in air face limitations due to evaporation, which alters droplet volume, changes capillary forces unpredictably, and limits the operational time [13]. Our use of non-volatile silicone oil completely circumvents this issue, enabling stable, long-term operation and facilitating the precise, feedback-controlled volume adjustments demonstrated in our experiments.
- Interfacial tension versus surface tension: The driving force in our system is the interfacial tension between silicone oil and water, which is notably lower than the surface tension of water in air [21]. While this might suggest inherently lower maximum gripping forces, the net force required to hold an object in an aqueous environment is also influenced by buoyancy, which counteracts gravitational forces. Furthermore, our results show that the combination of this interfacial tension and the ability to dynamically control the bridge volume, and thus the contact area/angle, provides sufficient adhesion for securely capturing and manipulating the target microobjects as evidenced by the resistance to shearing forces during capture. Dynamic volume control thus becomes a crucial tool to compensate for or tune forces derived from lower interfacial tension compared to air-based systems.
- Release mechanism: The non-volatility, while beneficial for stability, introduces a distinct release challenge compared to systems where controlled evaporation can be used as a passive release mechanism [5,6]. The residual oil film necessitates active detachment strategies (like shearing or flushing) after droplet retraction. This contrasts with air-based systems where simple bridge breaking or evaporation might suffice, representing a key design trade-off for achieving aqueous operation. An exception to this was observed during the manipulation of the largest ellipsoid. This suggests that for objects near the upper size limit of the gripper capability, the force balance can become more sensitive to volume changes, potentially offering a passive release pathway that is less prevalent with smaller objects where residual film adhesion dominates more strongly.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fantoni, G.; Santochi, M.; Dini, G.; Tracht, K.; Scholz-Reiter, B.; Fleischer, J.; Kristoffer Lien, T.; Seliger, G.; Reinhart, G.; Franke, J.; et al. Grasping devices and methods in automated production processes. CIRP Ann.-Manuf. Technol. 2014, 63, 679–701. [Google Scholar] [CrossRef]
- Urbano, D.G.; Noventa, G.; Ghidoni, A.; Lezzi, A.M. A semi-empirical fluid dynamic model of a vacuum microgripper based on cfd analysis. Appl. Sci. 2021, 11, 7482. [Google Scholar] [CrossRef]
- Lambert, P.; Letier, P.; Delchambre, A. Capillary and surface tension forces in the manipulation of small parts. In Proceedings of the IEEE International Symposium on Assembly and Task Planning, Besancon, France, 10–11 July 2003; pp. 54–59. [Google Scholar] [CrossRef]
- de Gennes, P.G.; Brochard-Wyart, F.; Quéré, D. Capillarity and Wetting Phenomena; Springer: New York, NY, USA, 2004; pp. 215–259. [Google Scholar] [CrossRef]
- Chafaï, A.; Ibrahimi, A.; Lambert, P. A Volume-Tuning Capillary Gripper That Enhances Handling Capabilities and Enables Testing of Micro-Components. Micromachines 2022, 13, 1323. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, W.; Ito, T.; Tanaka, K.; Tokui, R.; Fuchiwaki, O. Capillary Force Gripper for Complex-Shaped Micro-Objects with Fast Droplet Forming by On-Off Control of a Piston Slider. IEEE Robot. Autom. Lett. 2019, 4, 3695–3702. [Google Scholar] [CrossRef]
- Tanaka, K.; Ito, T.; Nishiyama, Y.; Fukuchi, E.; Fuchiwaki, O. Double-Nozzle Capillary Force Gripper for Cubical, Triangular Prismatic, and Helical 1-mm-Sized-Objects. IEEE Robot. Autom. Lett. 2022, 7, 1324–1331. [Google Scholar] [CrossRef]
- Butt, H.J.; Kappl, M. Normal capillary forces. Adv. Colloid Interface Sci. 2009, 146, 48–60. [Google Scholar] [CrossRef]
- Jantzen, S.; Stein, M.; Kniel, K.; Dietzel, A. Microclamping principles from the perspective of micrometrology—A review. Precis. Eng. 2017, 50, 538–550. [Google Scholar] [CrossRef]
- Arutinov, G.; Mastrangeli, M.; Van Heck, G.; Lambert, P.; Den Toonder, J.M.; Dietzel, A.; Smits, E.C. Capillary Gripping and Self-Alignment: A Route Toward Autonomous Heterogeneous Assembly. IEEE Trans. Robot. 2015, 31, 1033–1043. [Google Scholar] [CrossRef]
- Sariola, V.; Liimatainen, V.; Tolonen, T.; Udd, R.; Zhou, Q. Silicon capillary gripper with self-alignment capability. In Proceedings of the IEEE International Conference on Robotics and Automation, Shanghai, China, 9–13 May 2011; pp. 4098–4103. [Google Scholar] [CrossRef]
- Song, L.; Chang, B.; Feng, Y.; Jin, J.; Zhou, Q. Self-Alignment Capillary Gripper for Microfiber Manipulation. IEEE/ASME Trans. Mechatron. 2023, 28, 1957–1965. [Google Scholar] [CrossRef]
- Tadrist, L.; Motte, L.; Rahli, O.; Tadrist, L. Characterization of interface properties of fluids by evaporation of a capillary bridge. R. Soc. Open Sci. 2019, 6, 191608. [Google Scholar] [CrossRef]
- Fantoni, G.; Hansen, H.N.; Santochi, M. A new capillary gripper for mini and micro parts. CIRP Ann.-Manuf. Technol. 2013, 62, 17–20. [Google Scholar] [CrossRef]
- Yang, S.; Mielniczuk, B.; Saïd El Youssoufi, M.; Hueckel, T. A note on evolution of pressure and flow within an evaporating capillary bridge. Eur. Phys. J. E 2018, 41, 140. [Google Scholar] [CrossRef] [PubMed]
- Dehaeck, S.; Cavaiani, M.; Chafai, A.; Tourtit, Y.; Vitry, Y.; Lambert, P. Hybrid two-scale fabrication of sub-millimetric capillary grippers. Micromachines 2019, 10, 224. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.N.G.; Millet, O.; Gagneux, G. On the capillary bridge between spherical particles of unequal size: Analytical and experimental approaches. Contin. Mech. Thermodyn. 2019, 31, 225–237. [Google Scholar] [CrossRef]
- Fan, Z.; Liu, Z.; Huang, C.; Zhang, W.; Lv, Z.; Wang, L. Capillary forces between concave gripper and spherical particle for micro-objects gripping. Micromachines 2021, 12, 285. [Google Scholar] [CrossRef]
- Alias, A.B.; Huang, H.Y.; Yao, D.J. A Review on Microfluidics: An Aid to Assisted Reproductive Technology. Molecules 2021, 26, 4354. [Google Scholar] [CrossRef]
- Iacovacci, V.; Diller, E.; Ahmed, D.; Menciassi, A. Medical Microrobots. Annu. Rev. Biomed. Eng. 2024, 26, 561–591. [Google Scholar] [CrossRef]
- Peters, F.; Arabali, D. Interfacial tension between oil and water measured with a modified contour method. Colloids Surf. Physicochem. Eng. Asp. 2013, 426, 1–5. [Google Scholar] [CrossRef]
- Mendichi, R.; Schieroni, A.G.; Piovani, D.; Allegrini, D.; Ferrara, M.; Romano, M.R. Comparative study of chemical composition, molecular and rheological properties of silicone oil medical devices. Transl. Vis. Sci. Technol. 2019, 8, 1–8. [Google Scholar] [CrossRef]
- Seborg, D.E.; Edgar, T.F.; Mellichamp, D.A.; Doyle, F.J., III. Process Dynamics and Control; John Wiley & Sons: Hoboken, NJ, USA, 2017; Volume 53, p. 136. [Google Scholar]
- Ito, T.; Fukuchi, E.; Tanaka, K.; Nishiyama, Y.; Watanabe, N.; Fuchiwaki, O. Vision Feedback Control for the Automation of the Pick-and-Place of a Capillary Force Gripper. Micromachines 2022, 13, 1270. [Google Scholar] [CrossRef]
- Iazzolino, A.; Tourtit, Y.; Chafaï, A.; Gilet, T.; Lambert, P.; Tadrist, L. Pick up and release of micro-objects: A motion-free method to change the conformity of a capillary contact. Soft Matter 2020, 16, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Z.; Xu, Q. Recent design and development of piezoelectric-actuated compliant microgrippers: A review. Sensors Actuators A Phys. 2021, 331, 113002. [Google Scholar] [CrossRef]
- Barbot, A.; Ortiz, F.; Bolopion, A.; Gauthier, M.; Lambert, P. Exploiting Liquid Surface Tension in Microrobotics. Annu. Rev. Control Robot. Auton. Syst. 2023, 6, 313–334. [Google Scholar] [CrossRef]



| Reference Position (px) | Initial Position (px) | Steady-State Error (px) | Oscillation Amplitude (px) | Oscillation Period (s) | Reference-Normalized ISE |
|---|---|---|---|---|---|
| 250 | 108 | 0.0500 | 7.1841 | 32.5364 | 15.3340 |
| 230 | 181 | −1.0000 | 3.1587 | 20.2985 | 1.1491 |
| 150 | 227 | 0.0500 | 28.2167 | 27.6115 | 13.6832 |
| 150 | 202 | −0.3000 | 19.6545 | 23.8660 | 5.1483 |
| 115 | 148 | −0.4500 | 7.5858 | 20.3094 | 1.8236 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancha-Sánchez, E.; Serrano-Balbontín, A.J.; Tejado, I.; Vinagre, B.M. A Novel Variable Volume Capillary Microgripper for Micromanipulation in Aqueous Media. Micromachines 2025, 16, 633. https://doi.org/10.3390/mi16060633
Mancha-Sánchez E, Serrano-Balbontín AJ, Tejado I, Vinagre BM. A Novel Variable Volume Capillary Microgripper for Micromanipulation in Aqueous Media. Micromachines. 2025; 16(6):633. https://doi.org/10.3390/mi16060633
Chicago/Turabian StyleMancha-Sánchez, Enrique, Andrés J. Serrano-Balbontín, Inés Tejado, and Blas M. Vinagre. 2025. "A Novel Variable Volume Capillary Microgripper for Micromanipulation in Aqueous Media" Micromachines 16, no. 6: 633. https://doi.org/10.3390/mi16060633
APA StyleMancha-Sánchez, E., Serrano-Balbontín, A. J., Tejado, I., & Vinagre, B. M. (2025). A Novel Variable Volume Capillary Microgripper for Micromanipulation in Aqueous Media. Micromachines, 16(6), 633. https://doi.org/10.3390/mi16060633








