Cell Ratio-Dependent Osteoblast–Endothelial Cell Crosstalk Promoting Osteogenesis–Angiogenesis Coupling via Regulation of Microfluidic Perfusion and Paracrine Signaling
Abstract
1. Introduction
2. Materials and Methods
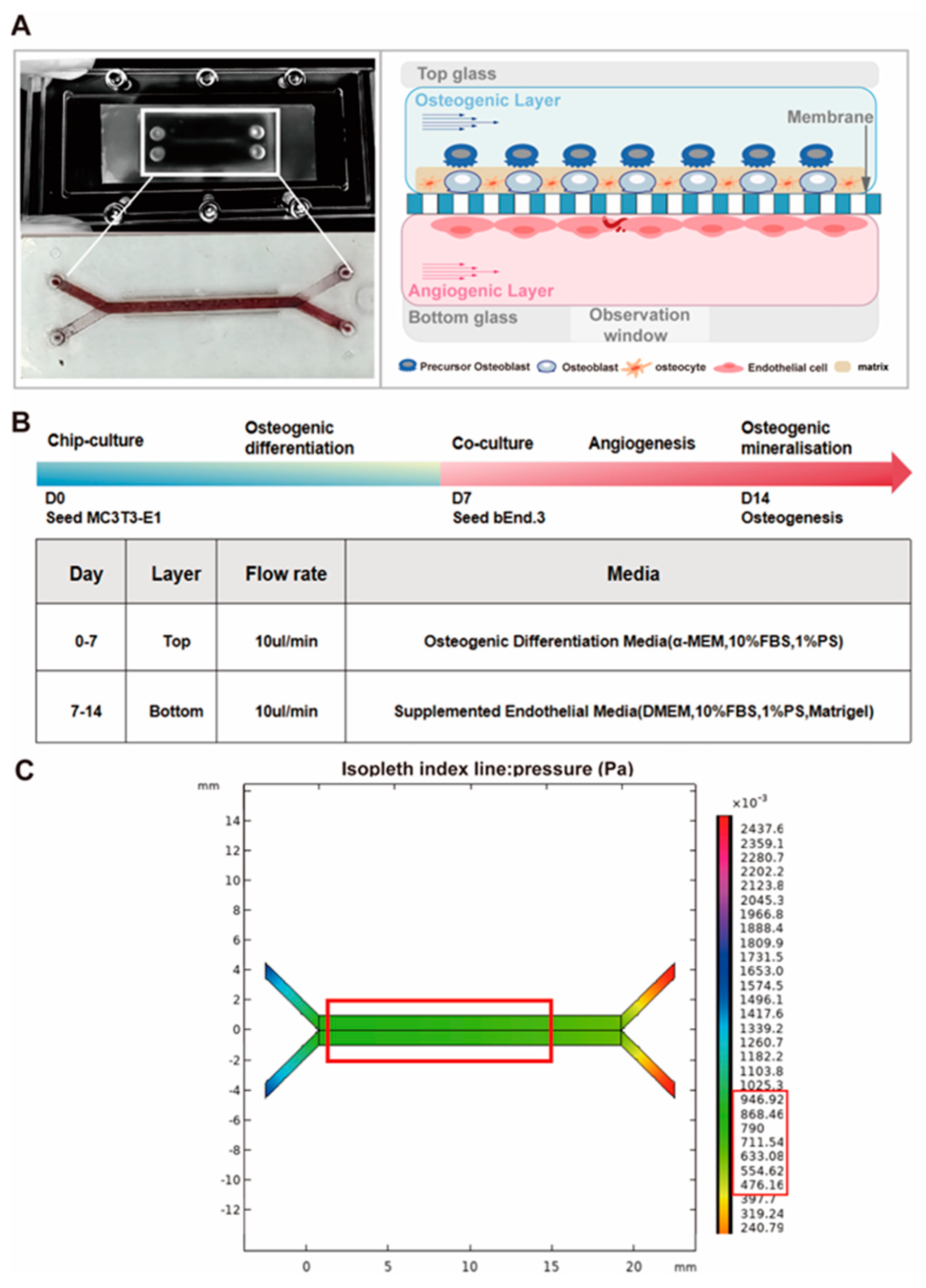
2.1. Development of Chip Platforms
2.2. Flow Rate Analysis
2.3. Cell Culture
2.4. Co-Culture
2.5. Cell Culture on Chip
2.6. Cell Proliferation Assay
2.7. Osteogenic Differentiation Assay
2.8. In Vitro Angiogenesis-Related Assays
2.9. Enzyme-Linked Immunosorbent Assay
2.10. Quantitative Real-Time Polymerase Chain Reaction Analysis
2.11. Immunofluorescence Staining
2.12. Statistical Analysis
3. Results
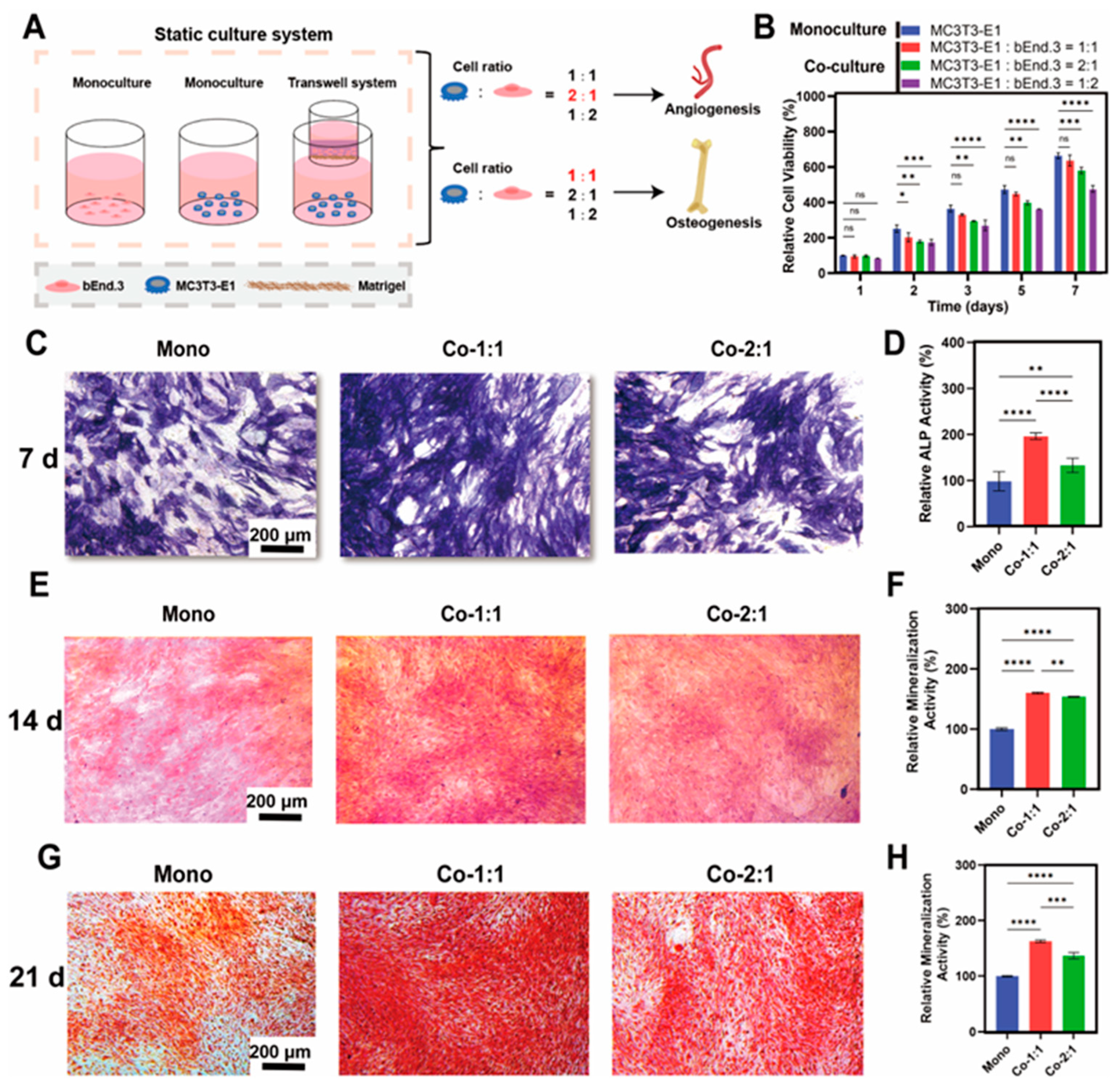
3.1. Osteogenic Benefits of MC3T3-E1 and bEnd.3 Cells Co-Cultured at Different Cell Ratios
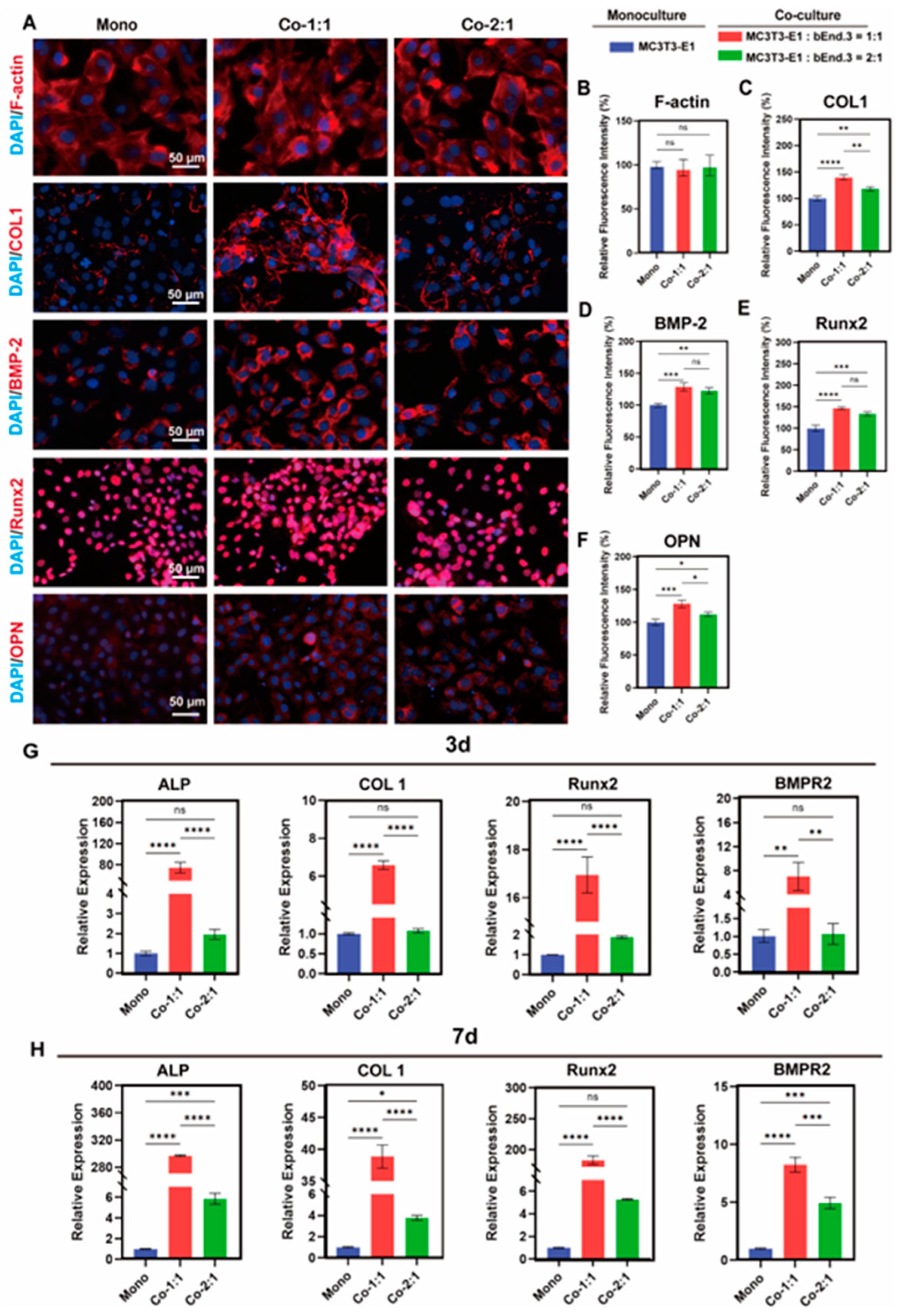
3.2. Expression of Osteogenic Markers in MC3T3-E1 and bEnd.3 Cells Co-Cultured at Different Cell Ratios
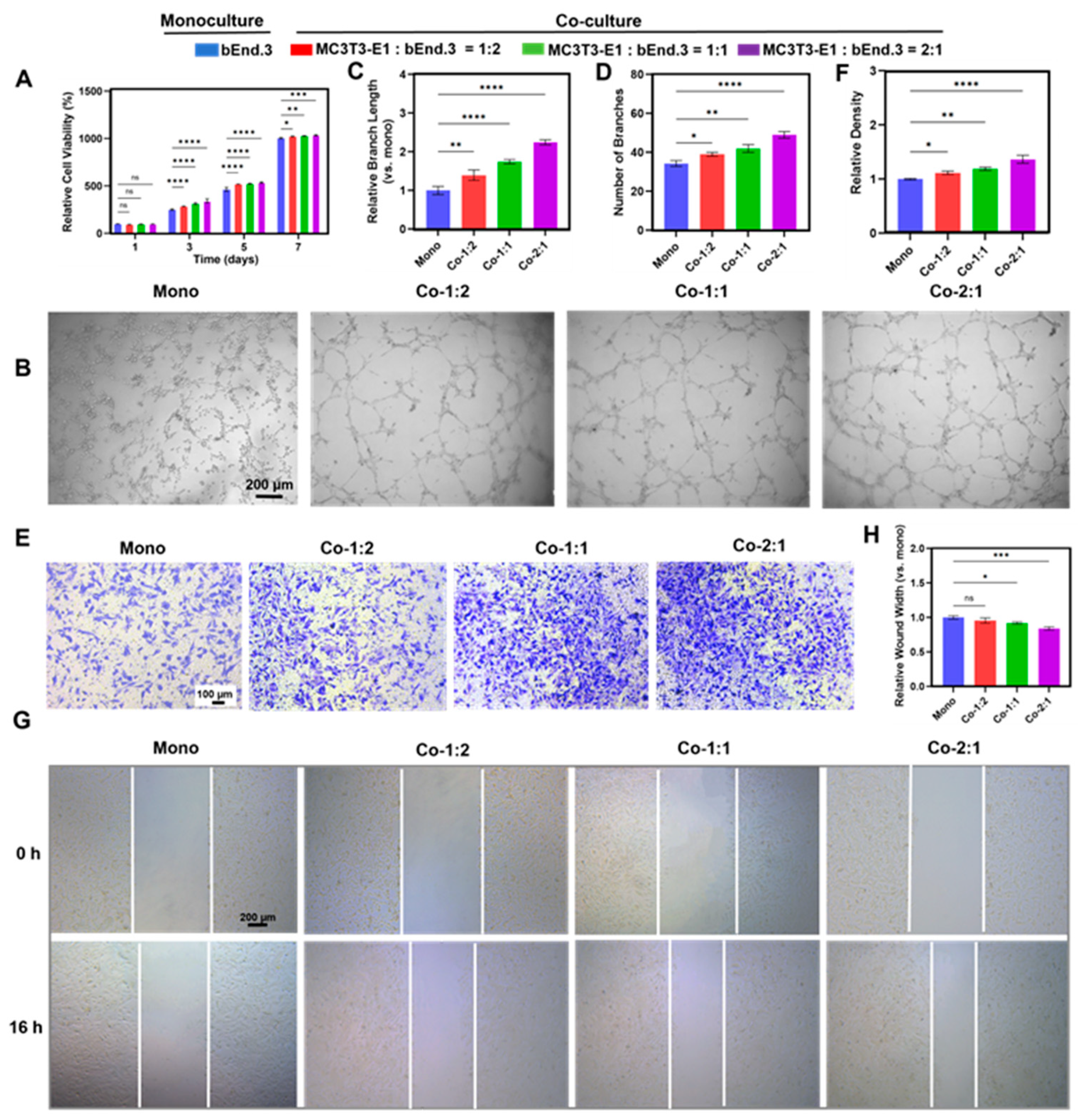
3.3. Angiogenic Benefits of MC3T3-E1 and bEnd.3 Cells Co-Cultured at Different Cell Ratios
3.4. Expression of Angiogenic Markers in MC3T3-E1 and bEnd.3 Cells Co-Cultured at Different Cell Ratios
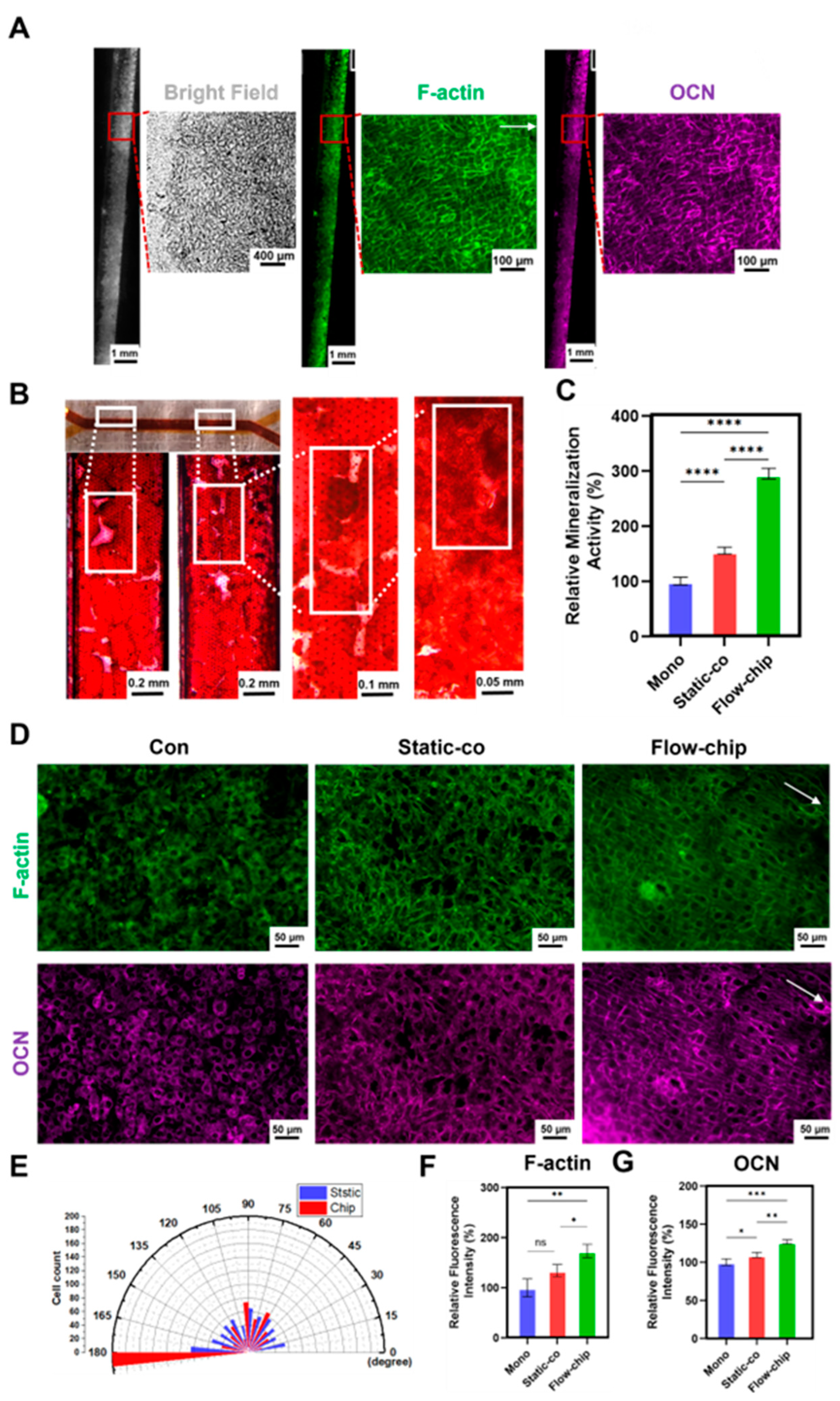
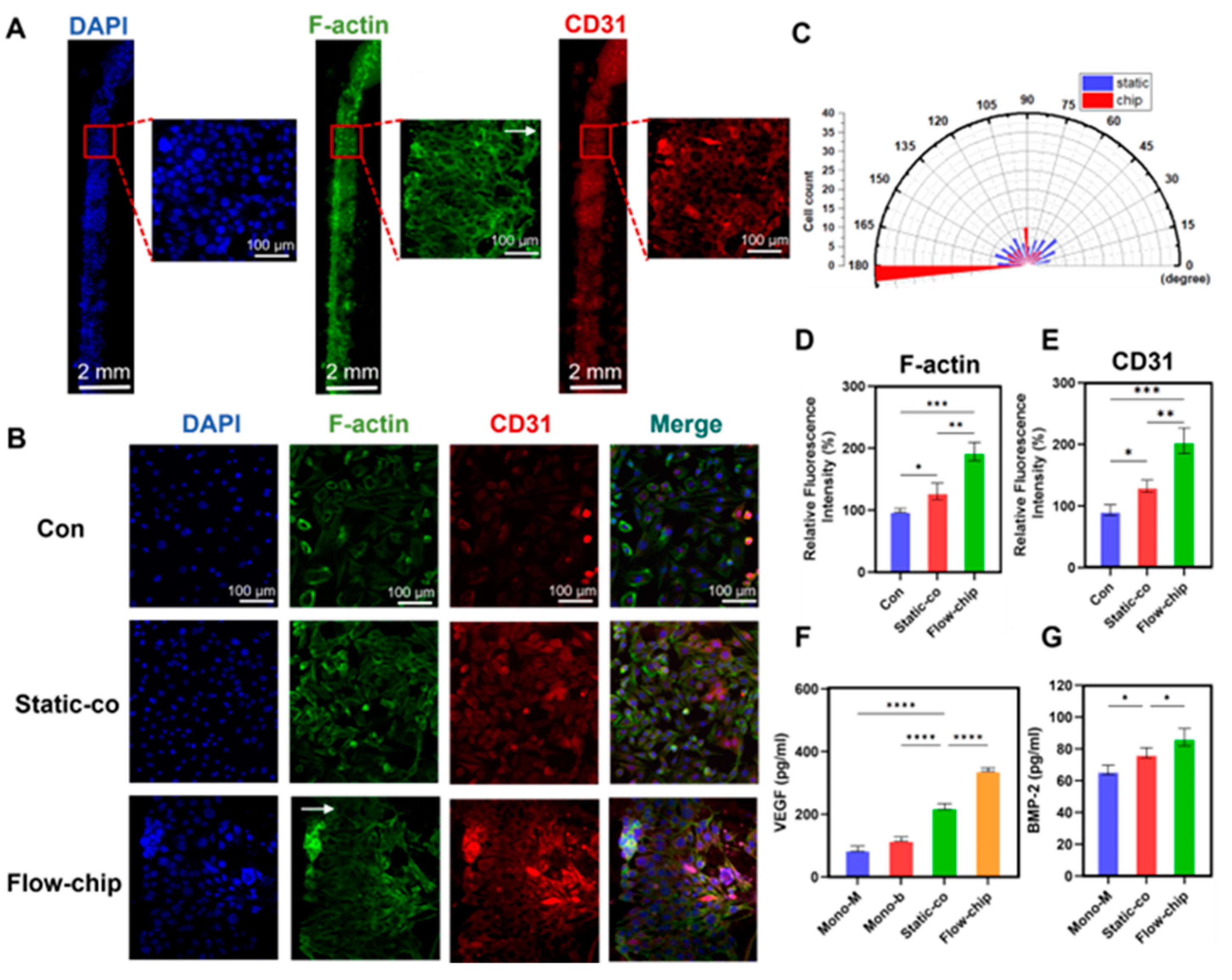
3.5. Osteogenesis–Angiogenesis Coupling on a Chip
3.6. The Osteogenic Effect of Osteogenesis–Angiogenesis Coupling in a Microfluidic Chip
3.7. The Angiogenic Effect of Osteogenesis–Angiogenesis Coupling in a Microfluidic Chip
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VEGF | Vascular endothelial growth factor |
| BMP-2 | Bone morphogenetic protein-2 |
| HIF-1α | Hypoxia-inducible factor-1α |
| ECs | Endothelial cells |
| Elisa | Enzyme-linked immunosorbent assay |
| ARS | Alizarin Red S |
| MSCs | Mesenchymal stem cells |
| PDMS | Polydimethylsiloxane |
| PET | Polyester |
| PMMA | Polymethyl methacrylate |
| CCK-8 | Cell Counting Kit-8 |
| ALP | Alkaline phosphatase |
| VEGF-A | Vascular endothelial growth factor A |
| VEGFR1 | Vascular endothelial growth factor receptor 1 |
| VEGFR2 | Vascular endothelial growth factor receptor 2 |
| COL1 | Collagen type I |
| RT-qPCR | Quantitative reverse transcription polymerase chain reaction |
| BMPR2 | Bone morphogenetic protein receptor type 2 |
| Runx2 | Runt-related transcription factor 2 |
| CD31 | Platelet endothelial cell adhesion molecule-1 |
| FITC | Fluorescein isothiocyanate |
| DAPI | 4′,6-diamidino-2′-phenylindole |
| OPN | Osteopontin |
| F-actin | Filamentous actin |
| OCN | Osteocalcin |
| TGF-β | Transforming growth factor-beta |
References
- Peng, Y.; Wu, S.; Li, Y.; Crane, J.L. Type H blood vessels in bone modeling and remodeling. Theranostics 2020, 10, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Hankenson, K.D.; Zimmerman, G.; Marcucio, R. Biological perspectives of delayed fracture healing. Injury 2014, 45, S8–S15. [Google Scholar] [CrossRef]
- Kusumbe, A.P.; Ramasamy, S.K.; Adams, R.H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 2014, 507, 323–328. [Google Scholar] [CrossRef]
- Saran, U.; Gemini Piperni, S.; Chatterjee, S. Role of angiogenesis in bone repair. Arch. Biochem. Biophys. 2014, 561, 109–117. [Google Scholar] [CrossRef]
- Schlundt, C.; Saß, R.A.; Bucher, C.H.; Bartosch, S.; Hauser, A.E.; Volk, H.-D.; Duda, G.N.; Schmidt-Bleek, K. Complex Spatio-Temporal Interplay of Distinct Immune and Bone Cell Subsets during Bone Fracture Healing. Cells 2024, 13, 40. [Google Scholar] [CrossRef]
- Stegen, S.; van Gastel, N.; Carmeliet, G. Bringing new life to damaged bone: The importance of angiogenesis in bone repair and regeneration. Bone 2015, 70, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Grosso, A.; Burger, M.G.; Lunger, A.; Schaefer, D.J.; Banfi, A.; Di Maggio, N. It Takes Two to Tango: Coupling of Angiogenesis and Osteogenesis for Bone Regeneration. Front. Bioeng. Biotechnol. 2017, 5, 68. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, T.A. The cell and molecular biology of fracture healing. Clin. Orthop. Rel. Res. 1998, 355, S7–S21. [Google Scholar] [CrossRef]
- Romeo, S.G.; Alawi, K.M.; Rodrigues, J.; Singh, A.; Kusumbe, A.P.; Ramasamy, S.K. Endothelial proteolytic activity and interaction with non-resorbing osteoclasts mediate bone elongation. Nat. Cell Biol. 2019, 21, 430–441. [Google Scholar] [CrossRef]
- Cui, Z.; Crane, J.; Xie, H.; Jin, X.; Zhen, G.; Li, C.; Xie, L.; Wang, L.; Bian, Q.; Qiu, T.; et al. Halofuginone attenuates osteoarthritis by inhibition of TGF-β activity and H-type vessel formation in subchondral bone. Ann. Rheum. Dis. 2016, 75, 1714–1721. [Google Scholar] [CrossRef]
- Hu, K.; Olsen, B.R. Osteoblast-derived VEGF regulates osteoblast differentiation and bone formation during bone repair. J. Clin. Investig. 2016, 126, 509–526. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Olsen, B.R. Vascular endothelial growth factor control mechanisms in skeletal growth and repair. Dev. Dyn. 2017, 246, 227–234. [Google Scholar] [CrossRef]
- Schipani, E.; Maes, C.; Carmeliet, G.; Semenza, G.L. Regulation of Osteogenesis-Angiogenesis Coupling by HIFs and VEGF. J. Bone Miner. Res. 2009, 24, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Yallowitz, A.; Qin, A.; Wu, Z.; Shin, D.Y.; Kim, J.-M.; Debnath, S.; Ji, G.; Bostrom, M.P.; Yang, X.; et al. Targeting skeletal endothelium to ameliorate bone loss. Nat. Med. 2018, 24, 823–833. [Google Scholar] [CrossRef]
- Riddle, R.C.; Khatri, R.; Schipani, E.; Clemens, T.L. Role of hypoxia-inducible factor-1α in angiogenic–osteogenic coupling. J. Mol. Med. 2009, 87, 583–590. [Google Scholar] [CrossRef]
- Wang, Y.; Wan, C.; Deng, L.; Liu, X.; Cao, X.; Gilbert, S.R.; Bouxsein, M.L.; Faugere, M.-C.; Guldberg, R.E.; Gerstenfeld, L.C.; et al. The hypoxia-inducible factor α pathway couples angiogenesis to osteogenesis during skeletal development. J. Clin. Investig. 2007, 117, 1616–1626. [Google Scholar] [CrossRef]
- Goers, L.; Freemont, P.; Polizzi, K.M. Co-culture systems and technologies: Taking synthetic biology to the next level. J. R. Soc. Interface 2014, 11, 20140065. [Google Scholar] [CrossRef] [PubMed]
- Bok, J.-S.; Byun, S.-H.; Park, B.-W.; Kang, Y.-H.; Lee, S.-L.; Rho, G.-J.; Hwang, S.-C.; Woo, D.K.; Lee, H.-J.; Byun, J.-H. The Role of Human Umbilical Vein Endothelial Cells in Osteogenic Differentiation of Dental Follicle-Derived Stem Cells in In Vitro Co-cultures. Int. J. Med. Sci. 2018, 15, 1160–1170. [Google Scholar] [CrossRef]
- Bogdanowicz, D.R.; Lu, H.H. Studying cell-cell communication in co-culture. Biotechnol. J. 2013, 8, 395–396. [Google Scholar] [CrossRef]
- Vis, M.A.M.; Ito, K.; Hofmann, S. Impact of Culture Medium on Cellular Interactions in in vitro Co-culture Systems. Front. Bioeng. Biotechnol. 2020, 8, 911. [Google Scholar] [CrossRef]
- Xu, C.; Liu, H.; He, Y.; Li, Y.; He, X. Endothelial progenitor cells promote osteogenic differentiation in co-cultured with mesenchymal stem cells via the MAPK-dependent pathway. Stem Cell Res. Ther. 2020, 11, 537. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.; Zhang, H.; Hou, J.; Wan, L.; Cheng, W.; Wang, X.; Dong, D.; Chen, C.; Xia, J.; Guo, J.; et al. VEGF secreted by mesenchymal stem cells mediates the differentiation of endothelial progenitor cells into endothelial cells via paracrine mechanisms. Mol. Med. Rep. 2017, 17, 1667–1675. [Google Scholar] [CrossRef]
- Wu, W.; Zhao, Z.; Wang, Y.; Zhu, G.; Tan, K.; Liu, M.; Li, L. Biomechanical Effects of Mechanical Stress on Cells Involved in Fracture Healing. Orthop. Surg. 2024, 16, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.F. Flow-mediated endothelial mechanotransduction. Physiol. Rev. 1995, 75, 519–560. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Geng, B.; Wang, H.; Wang, s.; Zhao, D.; He, J.; Lu, F.; An, J.; Wang, C.; Xia, Y. Fluid shear stress-induced down-regulation of microRNA-140-5p promotes osteoblast proliferation by targeting VEGFA via the ERK5 pathway. Connect. Tissue Res. 2022, 63, 156–168. [Google Scholar] [CrossRef]
- Zhao, Y.; Richardson, K.; Yang, R.; Bousraou, Z.; Lee, Y.K.; Fasciano, S.; Wang, S. Notch signaling and fluid shear stress in regulating osteogenic differentiation. Front. Bioeng. Biotechnol. 2022, 10, 1007430. [Google Scholar] [CrossRef]
- Ingber, D.E. Is it Time for Reviewer 3 to Request Human Organ Chip Experiments Instead of Animal Validation Studies? Adv. Sci. 2020, 7, 2002030. [Google Scholar] [CrossRef]
- Wang, Y.I.; Abaci, H.E.; Shuler, M.L. Microfluidic blood-brain barrier model provides in vivo-like barrier properties for drug permeability screening. Biotechnol. Bioeng. 2017, 114, 184–194. [Google Scholar] [CrossRef]
- Kimura, H.; Sakai, Y.; Fujii, T. Organ/body-on-a-chip based on microfluidic technology for drug discovery. Drug Metab. Pharmacokinet. 2018, 33, 43–48. [Google Scholar] [CrossRef]
- Ma, C.; Peng, Y.; Li, H.; Chen, W. Organ-on-a-Chip: A New Paradigm for Drug Development. Trends Pharmacol. Sci. 2021, 42, 119–133. [Google Scholar] [CrossRef]
- Zhang, B.; Korolj, A.; Lai, B.F.L.; Radisic, M. Advances in organ-on-a-chip engineering. Nat. Rev. Mater. 2018, 3, 257–278. [Google Scholar] [CrossRef]
- Ingber, D.E. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat. Rev. Genet. 2022, 23, 467–491. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.N.; Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef]
- Mou, X.; Shah, J.; Roye, Y.; Du, C.; Musah, S. An ultrathin membrane mediates tissue-specific morphogenesis and barrier function in a human kidney chip. Sci. Adv. 2024, 10, eadn2689. [Google Scholar] [CrossRef]
- Vormann, M.K.; Gijzen, L.; Hutter, S.; Boot, L.; Nicolas, A.; van den Heuvel, A.; Vriend, J.; Ng, C.P.; Nieskens, T.T.G.; van Duinen, V.; et al. Nephrotoxicity and Kidney Transport Assessment on 3D Perfused Proximal Tubules. AAPS J. 2018, 20, 90. [Google Scholar] [CrossRef]
- Weinbaum, S.; Cowin, S.C.; Zeng, Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J. Biomech. 1994, 27, 339–360. [Google Scholar] [CrossRef]
- Van der Meer, A.D.; Vermeul, K.; Poot, A.A.; Feijen, J.; Vermes, I. A microfluidic wound-healing assay for quantifying endothelial cell migration. Am. J. Physiol. Heart Circul. Physiol. 2010, 298, H719–H725. [Google Scholar] [CrossRef]
- Wittkowske, C.; Reilly, G.C.; Lacroix, D.; Perrault, C.M. In Vitro Bone Cell Models: Impact of Fluid Shear Stress on Bone Formation. Front. Bioeng. Biotechnol. 2016, 4, 87. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.L.; Turner, C.H. Mechanotransduction and the functional response of bone to mechanical strain. Calcif. Tissue Int. 1995, 57, 344–358. [Google Scholar] [CrossRef]
- Rubin, J.; Rubin, C.; Jacobs, C.R. Molecular pathways mediating mechanical signaling in bone. Gene 2006, 367, 1–16. [Google Scholar] [CrossRef]
- Park, J.S.; Burckhardt, C.J.; Lazcano, R.; Solis, L.M.; Isogai, T.; Li, L.; Chen, C.S.; Gao, B.; Minna, J.D.; Bachoo, R.; et al. Mechanical regulation of glycolysis via cytoskeleton architecture. Nature 2020, 578, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.; Ma, X.; Li, S.; Li, T.; Li, Z.; Qian, Y.; Shafiq, M.; Wang, J.; Zhou, X.; He, C. Bone Microenvironment-Mimetic Scaffolds with Hierarchical Microstructure for Enhanced Vascularization and Bone Regeneration. Adv. Funct. Mater. 2022, 32, 2200011. [Google Scholar] [CrossRef]
- Duda, G.N.N.; Geissler, S.; Checa, S.; Tsitsilonis, S.; Petersen, A.; Schmidt-Bleek, K. The decisive early phase of bone regeneration. Nat. Rev. Rheumatol. 2023, 19, 78–95. [Google Scholar] [CrossRef] [PubMed]
- Bahney, C.S.; Zondervan, R.L.; Allison, P.; Theologis, A.; Ashley, J.W.; Ahn, J.; Miclau, T.; Marcucio, R.S.; Hankenson, K.D. Cellular biology of fracture healing. J. Orthop. Res. 2019, 37, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Hankenson, K.D.; Gagne, K.; Shaughnessy, M. Extracellular signaling molecules to promote fracture healing and bone regeneration. Adv. Drug Deliv. Rev. 2015, 94, 3–12. [Google Scholar] [CrossRef]
- Yang, C.; Tibbitt, M.W.; Basta, L.; Anseth, K.S. Mechanical memory and dosing influence stem cell fate. Nat. Mater. 2014, 13, 645–652. [Google Scholar] [CrossRef]
- Martino, F.; Perestrelo, A.R.; Vinarský, V.; Pagliari, S.; Forte, G. Cellular Mechanotransduction: From Tension to Function. Front. Physiol. 2018, 9, 824. [Google Scholar] [CrossRef]
- Lin, H.; Sohn, J.; Shen, H.; Langhans, M.T.; Tuan, R.S. Bone marrow mesenchymal stem cells: Aging and tissue engineering applications to enhance bone healing. Biomaterials 2019, 203, 96–110. [Google Scholar] [CrossRef]
- Loi, F.; Córdova, L.A.; Pajarinen, J.; Lin, T.-H.; Yao, Z.; Goodman, S.B. Inflammation, fracture and bone repair. Bone 2016, 86, 119–130. [Google Scholar] [CrossRef]
- Pajarinen, J.; Lin, T.; Gibon, E.; Kohno, Y.; Maruyama, M.; Nathan, K.; Lu, L.; Yao, Z.; Goodman, S.B. Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials 2019, 196, 80–89. [Google Scholar] [CrossRef]
- Wan, Q.Q.; Qin, W.P.; Ma, Y.X.; Shen, M.J.; Li, J.; Zhang, Z.B.; Chen, J.H.; Tay, F.R.; Niu, L.N.; Jiao, K. Crosstalk between Bone and Nerves within Bone. Adv. Sci. 2021, 8, 2003390. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Yague, M.A.; Abbah, S.A.; McNamara, L.; Zeugolis, D.I.; Pandit, A.; Biggs, M.J. Biomimetic approaches in bone tissue engineering: Integrating biological and physicomechanical strategies. Adv. Drug Deliv. Rev. 2015, 84, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yu, L.; Liu, F.; Wan, L.; Deng, Z. The effect of cytokines on osteoblasts and osteoclasts in bone remodeling in osteoporosis: A review. Front. Immunol. 2023, 14, 1222129. [Google Scholar] [CrossRef]
- Zha, Y.; Li, Y.; Lin, T.; Chen, J.; Zhang, S.; Wang, J. Progenitor cell-derived exosomes endowed with VEGF plasmids enhance osteogenic induction and vascular remodeling in large segmental bone defects. Theranostics 2021, 11, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Olsen, B.R. The roles of vascular endothelial growth factor in bone repair and regeneration. Bone 2016, 91, 30–38. [Google Scholar] [CrossRef]
- Kang, M.; Lee, S.; Seo, J.-P.; Lee, E.-B.; Ahn, D.; Shin, J.; Paik, Y.-K.; Jo, D. Cell-permeable bone morphogenetic protein 2 facilitates bone regeneration by promoting osteogenesis. Mater. Today Bio 2024, 25, 100983. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Collins, J.M.; Lang, A.; Parisi, C.; Moharrer, Y.; Nijsure, M.P.; Kim, J.H.; Ahmed, S.; Szeto, G.L.; Qin, L.; Gottardi, R.; et al. YAP and TAZ couple osteoblast precursor mobilization to angiogenesis and mechanoregulation in murine bone development. Dev. Cell 2024, 59, 211–227. [Google Scholar] [CrossRef]
- Lim, X.R.; Harraz, O.F. Mechanosensing by Vascular Endothelium. Annu. Rev. Physiol. 2024, 86, 71–97. [Google Scholar] [CrossRef]
- Nerem, R.M. Hemodynamics and the vascular endothelium. J. Biomech. Eng. Trans. ASME 1993, 115, 510–514. [Google Scholar] [CrossRef]
- Kasper, G.; Dankert, N.; Tuischer, J.; Hoeft, M.; Gaber, T.; Glaeser, J.D.; Zander, D.; Tschirschmann, M.; Thompson, M.; Matziolis, G.; et al. Mesenchymal Stem Cells Regulate Angiogenesis According to Their Mechanical Environment. Stem Cells 2007, 25, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Gardner, O.F.W.; Fahy, N.; Alini, M.; Stoddart, M.J. Differences in human mesenchymal stem cell secretomes during chondrogenic induction. Eur. Cells Mater. 2016, 31, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Thi, M.M.; Suadicani, S.O.; Spray, D.C. Fluid Flow-induced Soluble Vascular Endothelial Growth Factor Isoforms Regulate Actin Adaptation in Osteoblasts. J. Biol. Chem. 2010, 285, 30931–30941. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Chen, S.; Fan, W.; Zhang, S.; Chen, X. Cell Ratio-Dependent Osteoblast–Endothelial Cell Crosstalk Promoting Osteogenesis–Angiogenesis Coupling via Regulation of Microfluidic Perfusion and Paracrine Signaling. Micromachines 2025, 16, 539. https://doi.org/10.3390/mi16050539
Wang Y, Chen S, Fan W, Zhang S, Chen X. Cell Ratio-Dependent Osteoblast–Endothelial Cell Crosstalk Promoting Osteogenesis–Angiogenesis Coupling via Regulation of Microfluidic Perfusion and Paracrine Signaling. Micromachines. 2025; 16(5):539. https://doi.org/10.3390/mi16050539
Chicago/Turabian StyleWang, Yuexin, Shu Chen, Wenwen Fan, Sixian Zhang, and Xi Chen. 2025. "Cell Ratio-Dependent Osteoblast–Endothelial Cell Crosstalk Promoting Osteogenesis–Angiogenesis Coupling via Regulation of Microfluidic Perfusion and Paracrine Signaling" Micromachines 16, no. 5: 539. https://doi.org/10.3390/mi16050539
APA StyleWang, Y., Chen, S., Fan, W., Zhang, S., & Chen, X. (2025). Cell Ratio-Dependent Osteoblast–Endothelial Cell Crosstalk Promoting Osteogenesis–Angiogenesis Coupling via Regulation of Microfluidic Perfusion and Paracrine Signaling. Micromachines, 16(5), 539. https://doi.org/10.3390/mi16050539




