Lead-Free Potassium Sodium Niobate-Based Wearable Ultrasonic Patches for Blood Pressure Detection
Abstract
1. Introduction
2. Materials and Methods
3. Results
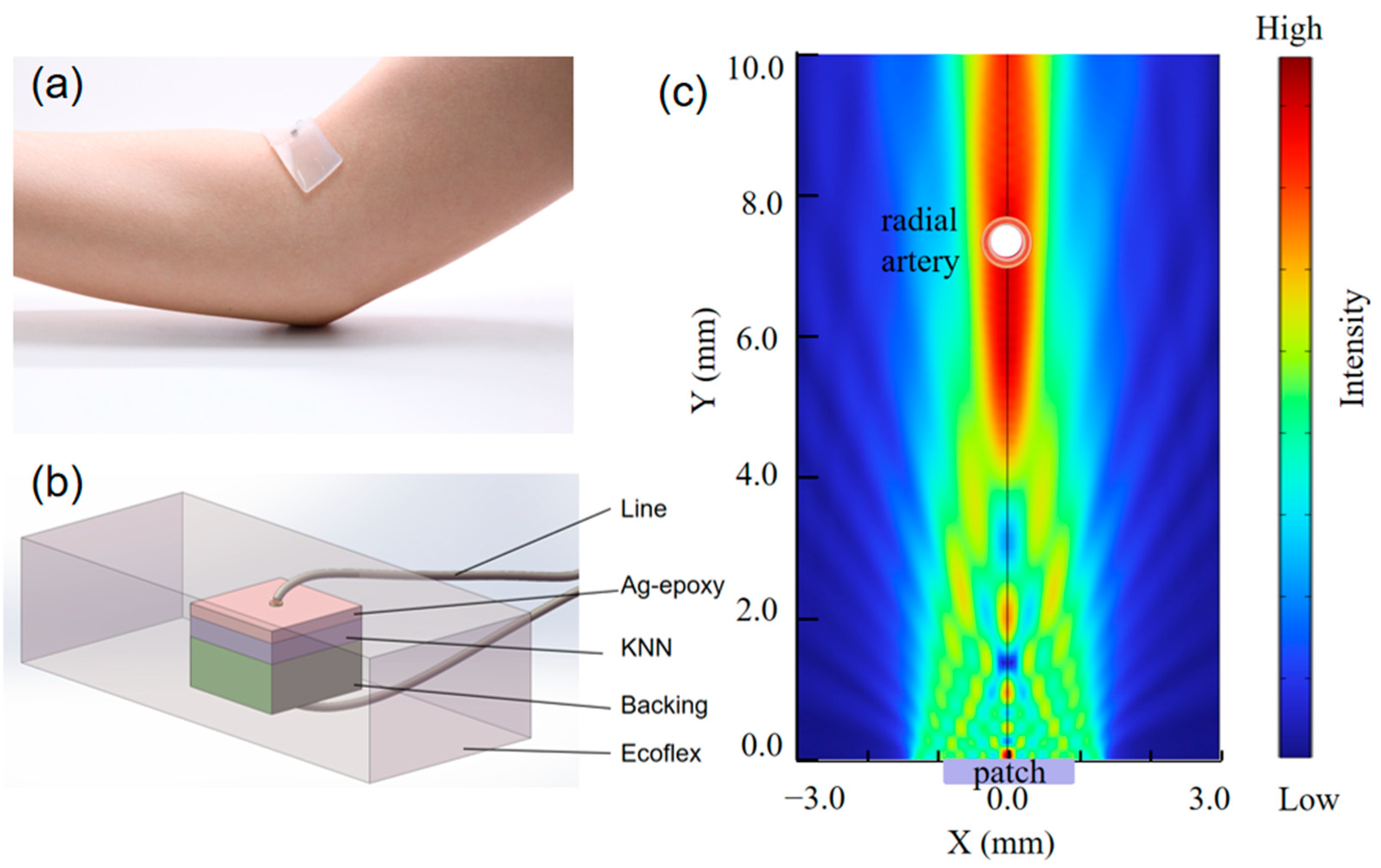
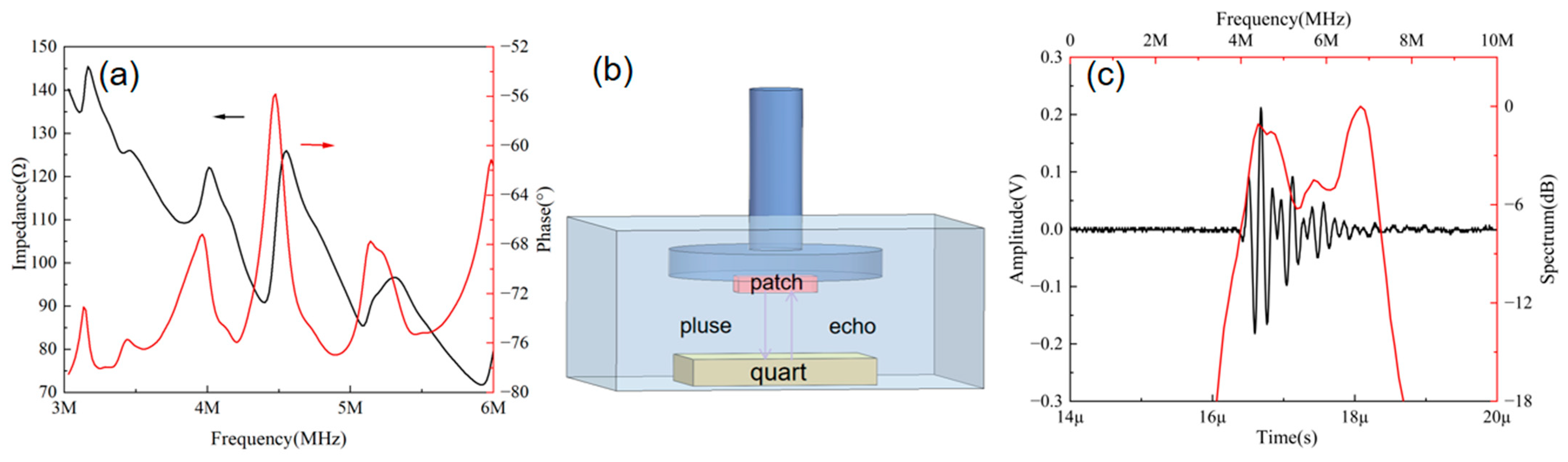
3.1. Characterization of Ultrasonic Patches
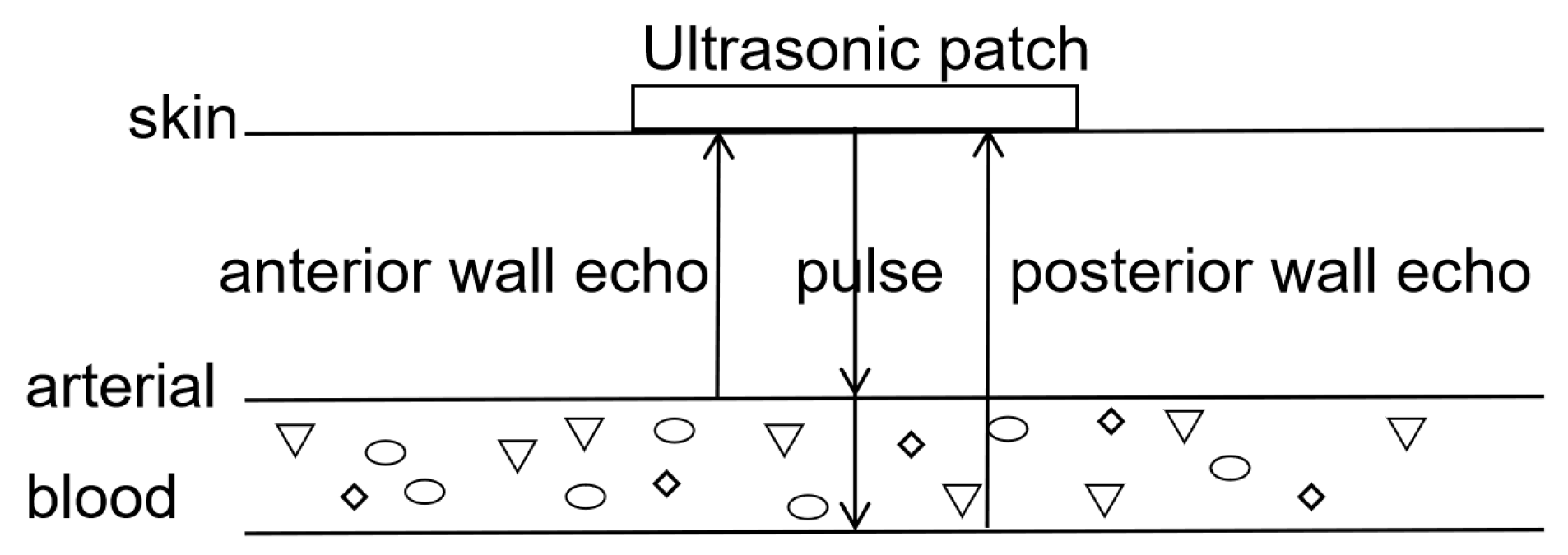
3.2. Principle of Blood Pressure Monitoring
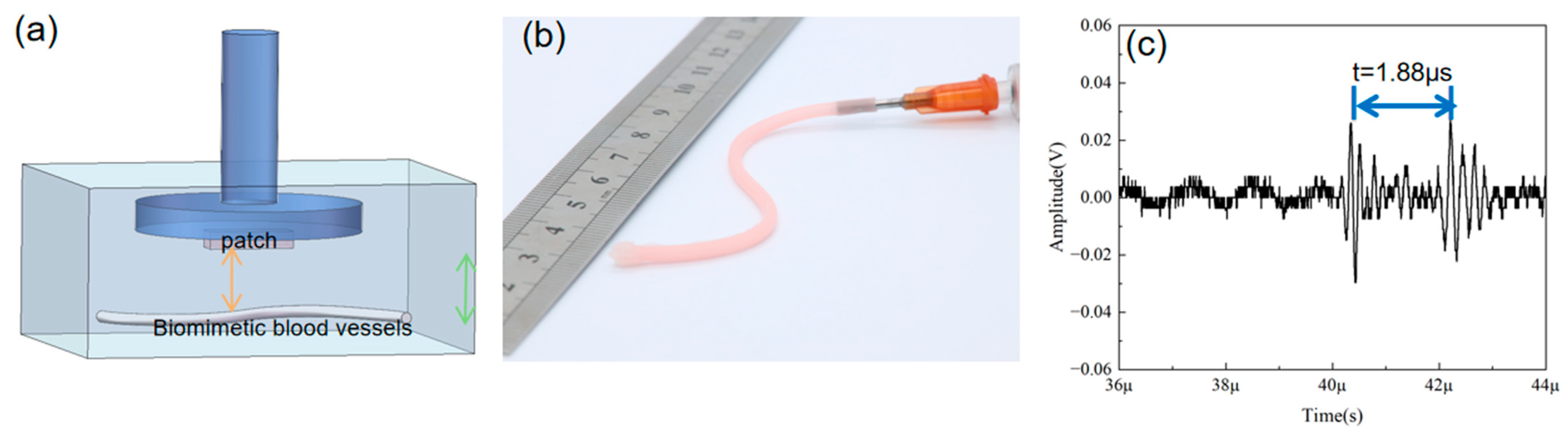
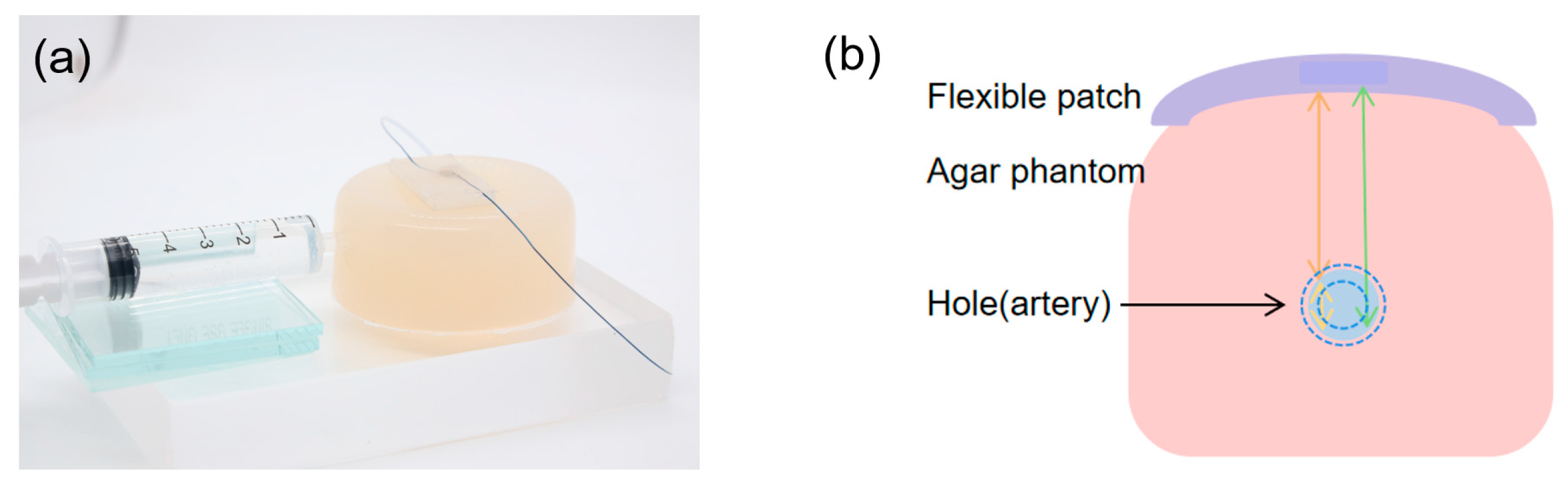
3.3. Biomimetic Vascular Testing Experiment
3.4. Construction and Testing of Bionic Vascular System
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Report on Hypertension: The Race Against a Silent Killer; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Li, W.; Gnanenthiran, S.R.; Schutte, A.E.; Tan, I. Blood pressure time at target and its prognostic value for cardiovascular outcomes: A scoping review. Hypertens. Res. 2024, 47, 2337–2350. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, S.; Ricci, Z.; Quattrone, D.; Tofani, L.; Tujjar, O.; Villa, G.; Romano, S.M.; De Gaudio, A.R. Accuracy of invasive arterial pressure monitoring in cardiovascular patients: An observational study. Crit. Care 2014, 18, 644. [Google Scholar] [PubMed]
- Kaur, G.; Morton, T.; Khairy, M.; Foy, M.; Gardner-Gray, J. Invasive Arterial BP Measurements in the Emergency Department—When, if Ever, is it Indicated? Curr. Hypertens. Rep. 2024, 27, 3. [Google Scholar] [PubMed]
- Peng, C.; Chen, M.; Sim, J.K.; Zhu, Y.; Jiang, X. Noninvasive and Nonocclusive Blood Pressure Monitoring via a Flexible Piezo-Composite Ultrasonic Sensor. IEEE Sens. J. 2021, 21, 2642–2650. [Google Scholar]
- Zhao, L.; Liang, C.; Huang, Y.; Zhou, G.; Xiao, Y.; Ji, N.; Zhang, Y.-T.; Zhao, N. Emerging sensing and modeling technologies for wearable and cuffless blood pressure monitoring. NPJ Digit. Med. 2023, 6, 93. [Google Scholar] [CrossRef]
- Samartkit, P.; Pullteap, S. Non-invasive continuous blood pressure sensors in biomedical engineering research: A review. Sens. Actuators A Phys. 2024, 367, 115084. [Google Scholar] [CrossRef]
- Perera, Y.; Raitt, J.; Poole, K.; Metcalfe, D.; Lewinsohn, A. Non-invasive versus arterial pressure monitoring in the pre-hospital critical care environment: A paired comparison of concurrently recorded measurements. Scand. J. Trauma Resusc. Emerg. Med. 2024, 32, 77. [Google Scholar]
- Quan, X.; Liu, J.; Roxlo, T.; Siddharth, S.; Leong, W.; Muir, A.; Cheong, S.-M.; Rao, A. Advances in Non-Invasive Blood Pressure Monitoring. Sensors 2021, 21, 427. [Google Scholar] [CrossRef]
- Seo, J.; Kim, Y. Ultrasound imaging and beyond: Recent advances in medical ultrasound. Biomed. Eng. Lett. 2017, 7, 57–58. [Google Scholar]
- Wang, F.; Jin, P.; Feng, Y.; Fu, J.; Wang, P.; Liu, X.; Zhang, Y.; Ma, Y.; Yang, Y.; Yang, A.; et al. Flexible Doppler ultrasound device for the monitoring of blood flow velocity. Sci. Adv. 2021, 7, eabi9283. [Google Scholar]
- Wang, C.; Li, X.; Hu, H.; Zhang, L.; Huang, Z.; Lin, M.; Zhang, Z.; Yin, Z.; Huang, B.; Gong, H.; et al. Monitoring of the central blood pressure waveform via a conformal ultrasonic device. Nat. Biomed. Eng. 2018, 2, 687–695. [Google Scholar]
- Yan, J.; Li, Y.; Peng, Y.; Yao, S. Acoustic metasurface embedded with thin-walled plate based on phase modulation for multi-angle broadband sound absorption. Thin-Walled Struct. 2024, 199, 111839. [Google Scholar]
- European Parliament and Council. Directive 2011/65/EU on the restriction of the use of certain hazardous substances in electrical and electronic equipment (recast). Off. J. Eur. Union 2011, 23, 88–110. [Google Scholar]
- ISO 10993-17:2023; Biological Evaluation of Medical Devices—Part 17: Establishment of Allowable Limits for Leachable Substances. International Organization for Standardization: Geneva, Switzerland, 2023.
- GB/T 16886:2022; Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing within a Risk Management Process. General Administration of Quality Supervision, Inspection and Quarantine of China: Beijing, China, 2022.
- Xing, J.; Mo, M.; Chen, Y.; Tan, Z.; Zhu, J. Decoding the piezo-contributions of potassium sodium niobate ceramics. J. Phys. Chem. C 2024, 128, 658–666. [Google Scholar]
- Thomas, B.; Sumam, K.S. Blood Flow in Human Arterial System—A Review. Procedia Technol. 2016, 24, 339–346. [Google Scholar]
- Hu, H.; Hu, C.; Guo, W.; Zhu, B.; Wang, S. Wearable ultrasound devices: An emerging era for biomedicine and clinical translation. Ultrasonics 2024, 142, 107401. [Google Scholar]
- Chen, J.; Liu, J.; Chen, W.; Shang, D.; Zhang, Q.; Li, Y.; Zheng, H.; Gu, D.; Wu, D.; Ma, T. Skin-Conformable Flexible and Stretchable Ultrasound Transducer for Wearable Imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2024, 71, 811–820. [Google Scholar]
- Chen, W.; Liu, J.; Lei, S.; Yang, Z.; Zhang, Q.; Li, Y.; Huang, J.; Dong, Y.; Zheng, H.; Wu, D.; et al. Flexible Ultrasound Transducer with Embedded Optical Shape Sensing Fiber for Biomedical Imaging Applications. IEEE Trans. Biomed. Eng. 2023, 70, 2841–2851. [Google Scholar]
- Xue, H.; Jin, J.; Tan, Z.; Chen, K.; Lu, G.; Zeng, Y.; Hu, X.; Peng, X.; Jiang, L.; Wu, J. Flexible, biodegradable ultrasonic wireless electrotherapy device based on highly self-aligned piezoelectric biofilms. Sci. Adv. 2024, 10, eadn0260. [Google Scholar]
- Zeng, Y.; Sun, X.; Zhang, J.; Chang, C.-F.; Liu, B.; Gong, C.; Ji, J.; Zhang, B.Z.; Wang, Y.; Ren, M.X.; et al. High-frequency wearable ultrasound array belt for small animal echocardiography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2024, 71, 3492197. [Google Scholar]
- Mayet, J.; Hughes, A. Cardiac and vascular pathophysiology in hypertension. Heart 2003, 89, 1104–1109. [Google Scholar] [PubMed]
- Soleimani, E.; Dizaji, M.M.; Fatouraee, N.; Saberi, H. Assessing the blood pressure waveform of the carotid artery using an ultrasound image processing method. Ultrasonography 2017, 36, 144–152. [Google Scholar] [PubMed]
- Song, C.; Carlson, S. Radial Artery Access for Peripheral Vascular Interventions: A Review of the Literature. Ann. Vasc. Surg. 2024, 107, 55–59. [Google Scholar] [PubMed]
- Athaya, T.; Choi, S. A Review of Noninvasive Methodologies to Estimate the Blood Pressure Waveform. Sensors 2022, 22, 3953. [Google Scholar] [CrossRef]
- Pries, A.R.; Reglin, B.; Secomb, T.W. Remodeling of blood vessels: Responses of diameter and wall thickness to hemodynamic and metabolic stimuli. Hypertension 2005, 46, 725–731. [Google Scholar]

| KNN-Cr | PZT-5H | |
|---|---|---|
| Velocity (m/s) | 5283 | 2890 |
| Density (kg/m3) | 4353 | 7900 |
| Dielectric constant | 3639 | 1390 |
| kt | 0.41 | 0.52 |
| Z (MRayl) | 23 | 23 |
| d33 (pC/N) | 465 | 680 |
| KNN-Cr | |
|---|---|
| Thickness of piezoelectric layer (mm) | 0.47 |
| Thickness of matching layer (mm) | 0.096 |
| Thickness of backing layer (mm) | 1.00 |
| Size of KNN-Cr (mm2) | 2.8 × 2.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Quan, Y.; Xing, J.; Tan, Z.; Sun, X.; Lou, L.; Fei, C.; Zhu, J.; Yang, Y. Lead-Free Potassium Sodium Niobate-Based Wearable Ultrasonic Patches for Blood Pressure Detection. Micromachines 2025, 16, 392. https://doi.org/10.3390/mi16040392
Sun Y, Quan Y, Xing J, Tan Z, Sun X, Lou L, Fei C, Zhu J, Yang Y. Lead-Free Potassium Sodium Niobate-Based Wearable Ultrasonic Patches for Blood Pressure Detection. Micromachines. 2025; 16(4):392. https://doi.org/10.3390/mi16040392
Chicago/Turabian StyleSun, Yajun, Yi Quan, Jie Xing, Zhi Tan, Xinhao Sun, Lifei Lou, Chunlong Fei, Jianguo Zhu, and Yintang Yang. 2025. "Lead-Free Potassium Sodium Niobate-Based Wearable Ultrasonic Patches for Blood Pressure Detection" Micromachines 16, no. 4: 392. https://doi.org/10.3390/mi16040392
APA StyleSun, Y., Quan, Y., Xing, J., Tan, Z., Sun, X., Lou, L., Fei, C., Zhu, J., & Yang, Y. (2025). Lead-Free Potassium Sodium Niobate-Based Wearable Ultrasonic Patches for Blood Pressure Detection. Micromachines, 16(4), 392. https://doi.org/10.3390/mi16040392








