FACl as a Bifunctional Additive to Enhance the Performance of Lead-Free Antimony-Based Perovskite Solar Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. In This Experiment, All Reagents Were Used Directly Without Additional Purification
2.2. Preparation of the Electron Transport Layer
2.3. FACl–Cs3Sb2I9 Synthesis
2.4. Solar Cells Fabrication
2.5. Characterization
3. Results and Discussion
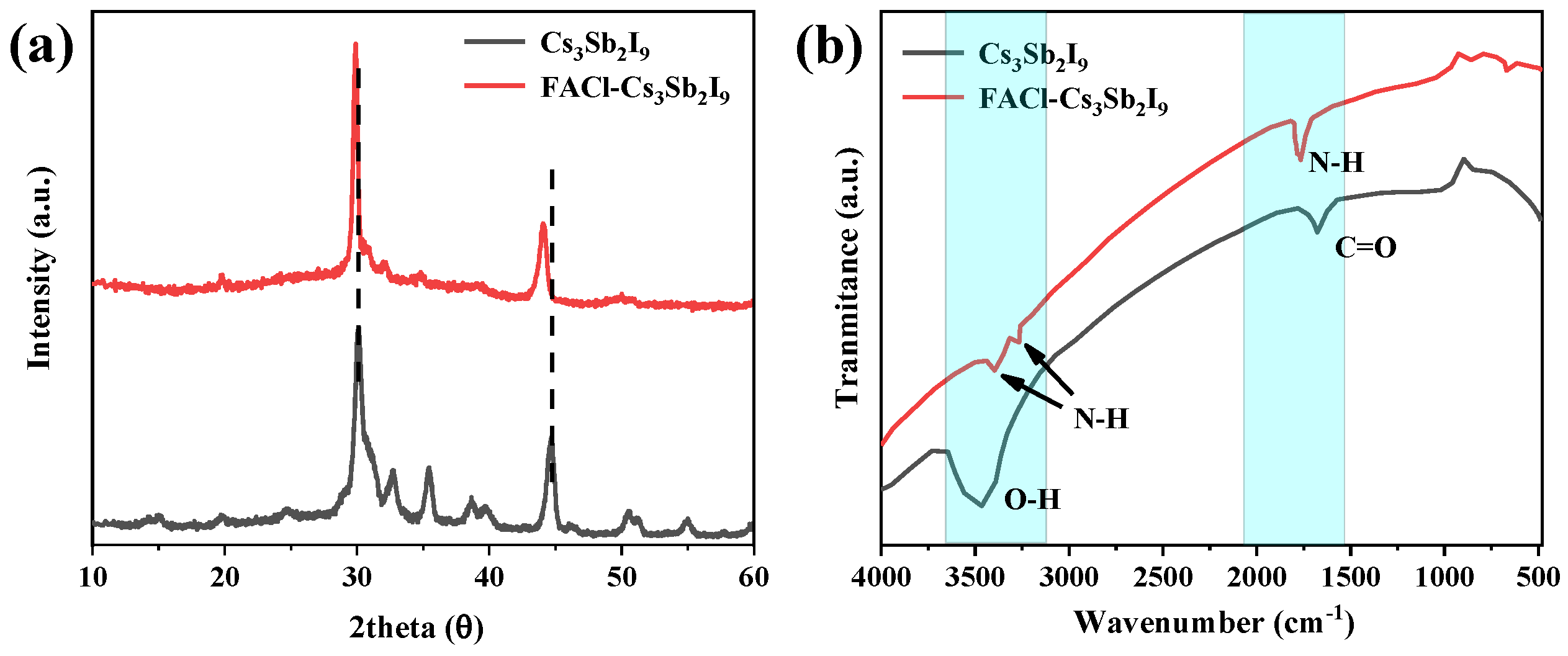
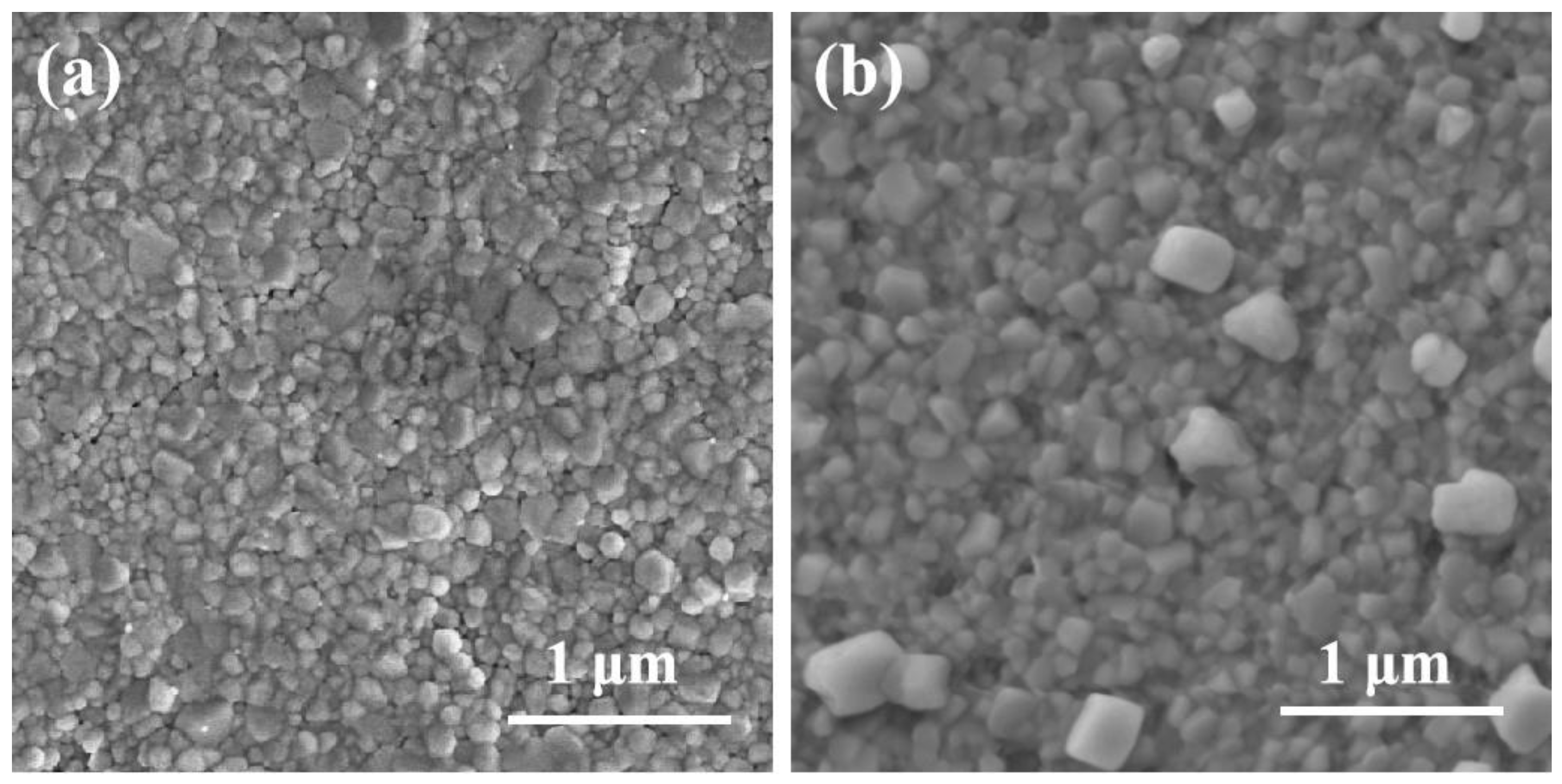
3.1. Structure Formation and Layered Phase Deposition
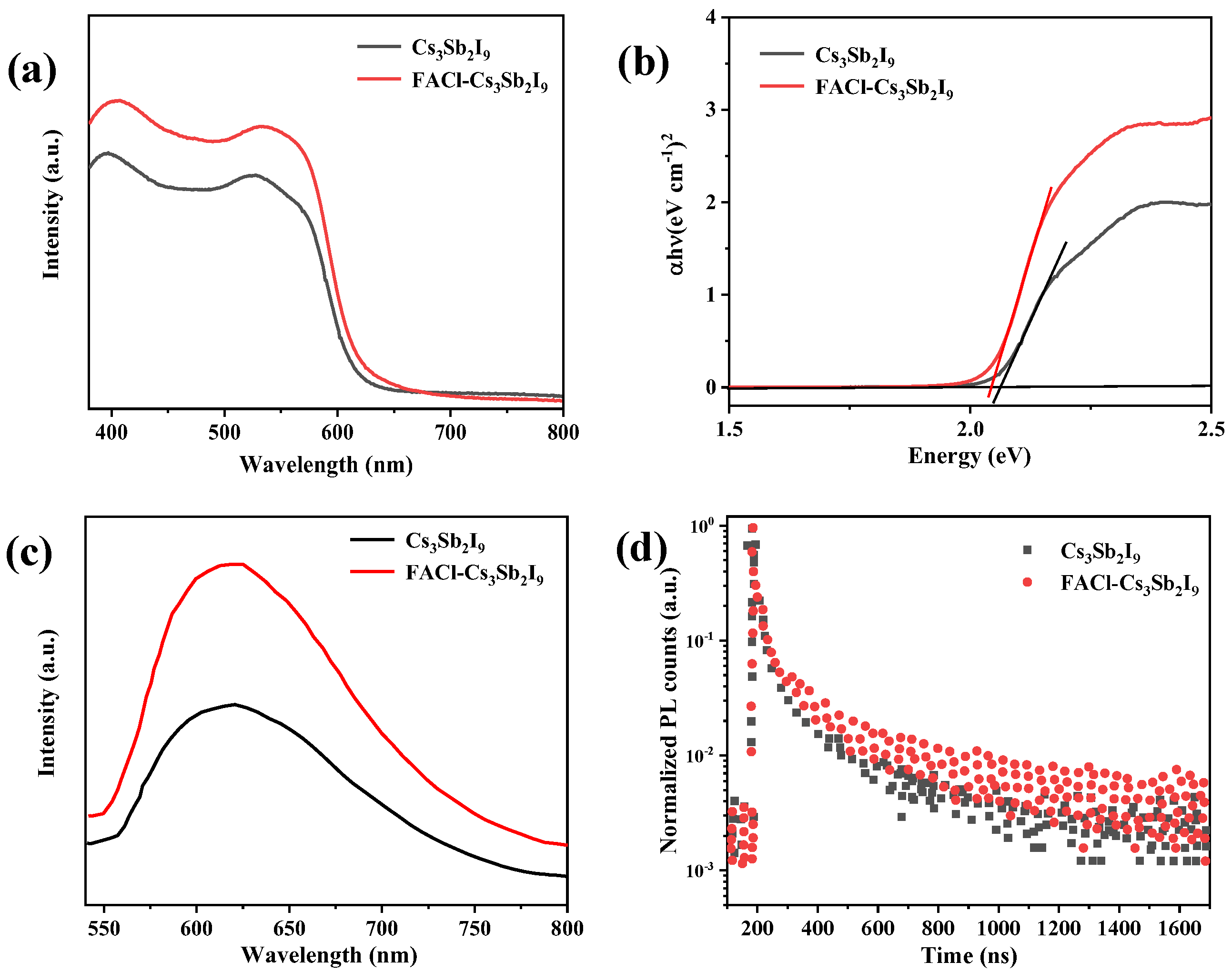
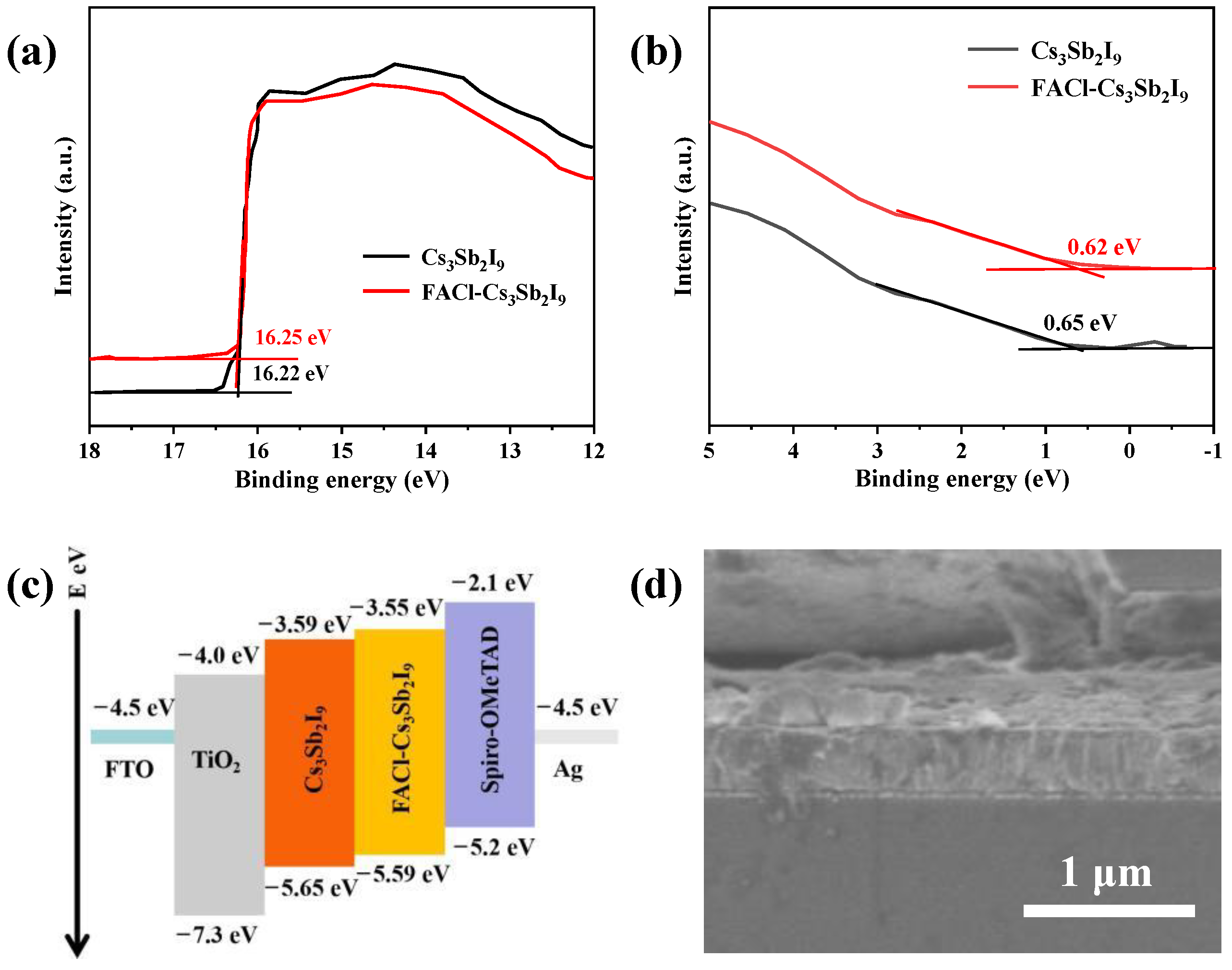
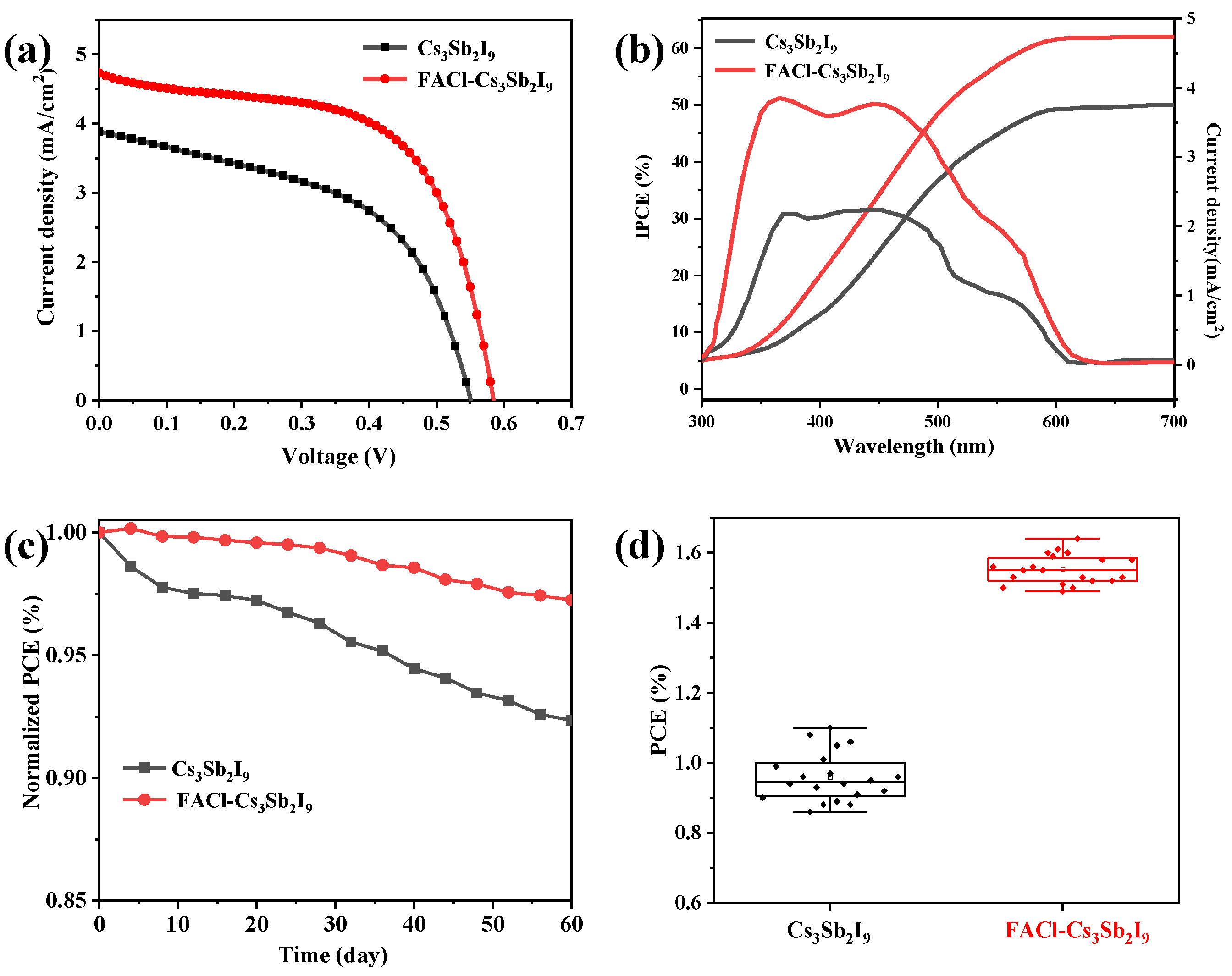
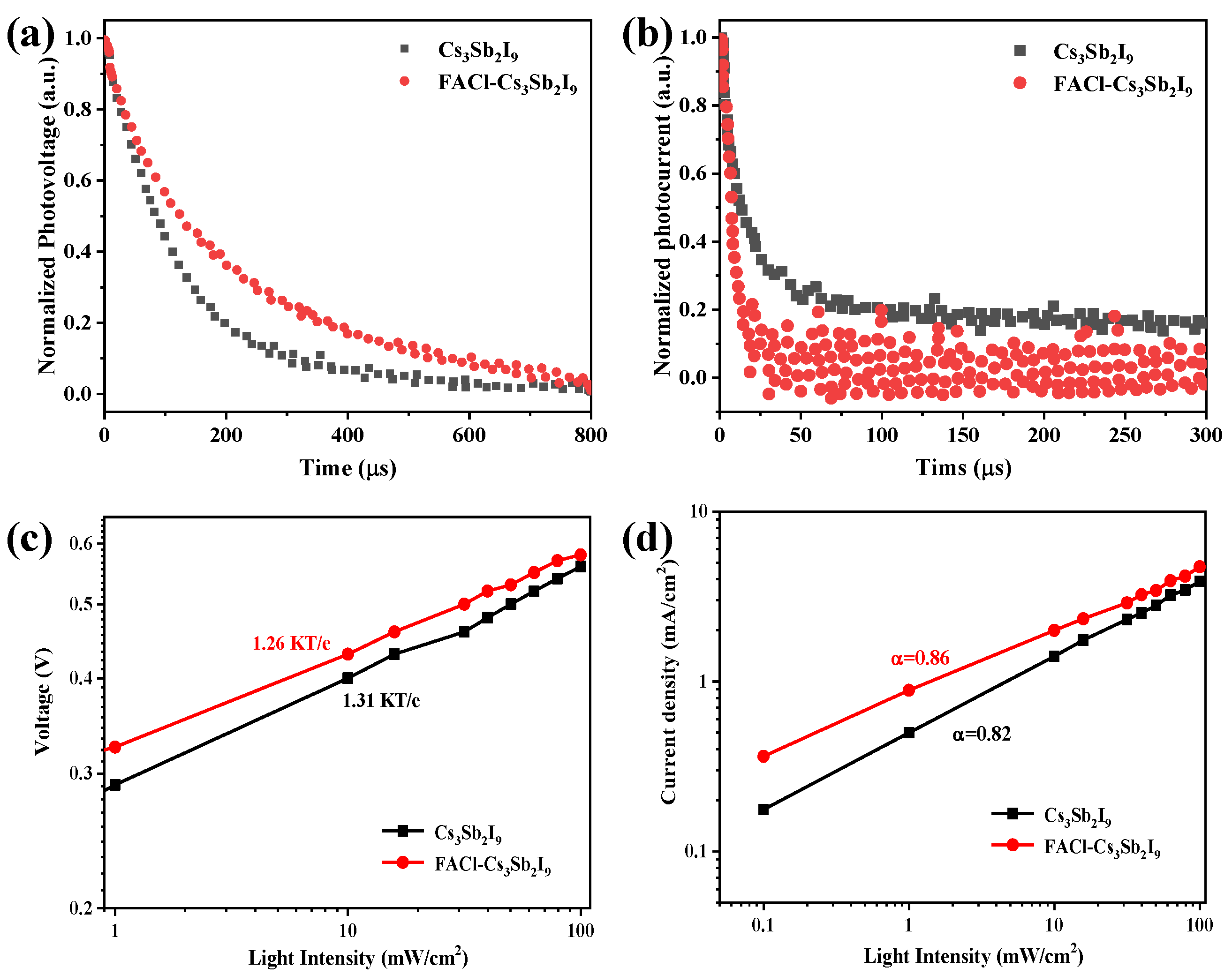
3.2. The Performance of Device and Physical Characterization
| Jsc (mA/cm2) | Voc (V) | FF (%) | η (%) | Ref. | |
|---|---|---|---|---|---|
| Cs3Sb2I9 | 0.13 | 0.40 | 58.0 | 0.03 | [39] |
| MA3Sb2I9 | 1.0 | 0.89 | 55 | 0.49 | [10] |
| MA3Sb2I9 | 1.48 | 0.74 | 52 | 0.57 | [23] |
| Cs3Sb2I9 | 2.34 | 0.62 | 46.18 | 0.67 | [2] |
| Cs3Sb2I9 | 3.55 | 0.61 | 55.8 | 1.21 | [13] |
| Cs3Sb2I9 | 5.31 | 0.72 | 38.97 | 1.49 | [8] |
| FAI-Cs3Sb2I9 | 5.57 | 0.62 | 51.4 | 1.76 | [12] |
| Cs3Sb2I9 | 5.40 | 0.80 | 54.9 | 2.48 | [14] |
| MA3Sb2I9 | 6.64 | 0.70 | 59.6 | 2.77 | [29] |
| FACl–Cs3Sb2I9 | 4.73 | 0.58 | 60.5 | 1.66 | This work |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zheng, Y.; Li, Y.; Zhuang, R. Towards 26% efficiency in inverted perovskite solar cells via interfacial flipped band bending and suppressed deep-level traps. Energy Environ. Sci. 2024, 17, 1153–1162. [Google Scholar]
- Boopathi, K.M.; Karuppuswamy, P.; Singh, A.; Hanmandlu, C.; Lin, L.; Abbas, S.A.; Chang, C.C.; Wang, P.C.; Li, G.; Chu, C.W. Solution-processable antimony-based light-absorbing materials beyond lead halide perovskites. J. Mater. Chem. A 2017, 5, 20843–20850. [Google Scholar]
- Jin, Z.; Zhang, Z.; Xiu, J.; Song, H.; Gatti, T.; He, Z. A critical review on bismuth and antimony halide based perovskites and their derivatives for photovoltaic applications: Recent advances and challenges. J. Mater. Chem. A 2020, 8, 16166–16188. [Google Scholar]
- Saparov, B.; Hong, F.; Sun, J.-P.; Duan, H.-S.; Meng, W.; Cameron, S.; Hill, I.G.; Yan, Y.; Mitzi, D.B. Thin-film preparation and characterization of Cs3Sb2I9: A lead-free layered perovskite semiconductor. Chem. Mater. 2015, 27, 5622–5632. [Google Scholar]
- Cariou, R.; Benick, J.; Feldmann, F.; Höhn, O.; Hauser, H.; Beutel, P.; Razek, N.; Wimplinger, M.; Bläsi, B.; Lackner, D.; et al. III–V-on-silicon solar cells reaching 33% photoconversion efficiency in two-terminal configuration. Nat. Energy 2018, 3, 326–333. [Google Scholar]
- Jiang, X.; Li, H.; Zhou, Q.; Wei, Q.; Wei, M.; Jiang, L.; Wang, Z.; Peng, Z.; Wang, F.; Zang, Z.; et al. One-Step Synthesis of SnI2·(DMSO)x Adducts for High-Performance Tin Perovskite Solar Cells. J. Am. Chem. Soc. 2021, 143, 10970–10976. [Google Scholar]
- Schileo, G.; Grancini, G. Lead or no lead? Availability, toxicity, sustainability and environmental impact of lead-free perovskite solar cells. J. Mater. Chem. C 2021, 9, 67–76. [Google Scholar]
- Singh, A.; Boopathi, K.M.; Mohapatra, A.; Chen, Y.F.; Li, G.; Chu, C.W. Photovoltaic Performance of Vapor-Assisted Solution-Processed Layer Polymorph of Cs3Sb2I9. ACS Appl. Mater. Interfaces 2018, 10, 2566–2573. [Google Scholar]
- Castriotta, L.A.; Zendehdel, M.; Yaghoobi Nia, N.; Leonardi, E.; Löffler, M.; Paci, B.; Generosi, A.; Rellinghaus, B.; Di Carlo, A. Reducing Losses in Perovskite Large Area Solar Technology: Laser Design Optimization for Highly Efficient Modules and Minipanels. Adv. Energy Mater. 2022, 12, 2103420. [Google Scholar]
- Hebig, J.-C.; Kühn, I.; Flohre, J.; Kirchartz, T. Optoelectronic Properties of (CH3NH3)3Sb2I9 Thin Films for Photovoltaic Applications. ACS Energy Lett. 2016, 1, 309–314. [Google Scholar]
- Harikesh, P.C.; Mulmudi, H.K.; Ghosh, B.; Goh, T.W.; Teng, Y.T.; Thirumal, K.; Lockrey, M.; Weber, K.; Koh, T.M.; Li, S.; et al. Rb as an Alternative Cation for Templating Inorganic Lead-Free Perovskites for Solution Processed Photovoltaics. Chem. Mater. 2016, 28, 7496–7504. [Google Scholar] [CrossRef]
- Farooq, U.; Zhang, M.; Chi, D.; Wang, J.; Idris, A.M.; Huang, S.; Pan, Z.; Li, Z. Surface defects passivation with organic salt for highly stable and efficient lead-free Cs3Sb2I9 perovskite solar cells. ACS Appl. Energy Mater. 2023, 6, 10294–10302. [Google Scholar] [CrossRef]
- Yang, A.S.; Cai, T.Y.; Su, L.; Li, Y.S.; He, F.F.; Zhang, Q.P.; Zhou, Y.L.; He, R.; Zhang, K.; Yang, W.B. Review on organic phase change materials for sustainable energy storage. Sustain. Energy Fuels 2022, 6, 5045–5071. [Google Scholar] [CrossRef]
- Umar, F.; Zhang, J.; Jin, Z.; Muhammad, I.; Yang, X.; Deng, H.; Jahangeer, K.; Hu, Q.; Song, H.; Tang, J. Dimensionality Controlling of Cs3Sb2I9 for Efficient All-Inorganic Planar Thin Film Solar Cells by HCl-Assisted Solution Method. Adv. Opt. Mater. 2019, 7, 1801368. [Google Scholar] [CrossRef]
- Johansson, M.B.; Zhu, H.; Johansson, E.M.J. Extended Photo-Conversion Spectrum in Low-Toxic Bismuth Halide Perovskite Solar Cells. J. Phys. Chem. Lett. 2016, 7, 3467–3471. [Google Scholar] [CrossRef]
- Saliba, M.; Matsui, T.; Seo, J.Y.; Domanski, K.; Correa-Baena, J.P.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Tress, W.; Abate, A.; Hagfeldt, A.; et al. Cesium-containing triple cation perovskite solar cells: Improved stability, reproducibility and high efficiency. Energy Environ. Sci. 2016, 9, 1989–1997. [Google Scholar] [CrossRef] [PubMed]
- Turren-Cruz, S.-H.; Hagfeldt, A.; Saliba, M. Methylammonium-free, high-performance, and stable perovskite solar cells on a planar architecture. Science 2018, 362, 449. [Google Scholar] [CrossRef]
- Liu, X.; Guo, Y.; Cheng, Y.; Lu, S.; Li, R.; Chen, J. Advances in chloride additives for high-efficiency perovskite solar cells: Multiple points of view. Chem. Commun. 2023, 59, 13394–13405. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Zhao, Y.; Zhang, X.; Yang, X.; Chen, Y.; Chu, Z.; Ye, Q.; Li, X.; Yin, Z.; You, J. Surface passivation of perovskite film for efficient solar cells. Nat. Photonics 2019, 13, 460–466. [Google Scholar] [CrossRef]
- Jiang, F.; Yang, D.; Jiang, Y.; Liu, T.; Zhao, X.; Ming, Y.; Luo, B.; Qin, F.; Fan, J.; Han, H.; et al. Chlorine-Incorporation-Induced Formation of the Layered Phase for Antimony-Based Lead-Free Perovskite Solar Cells. J Am. Chem. Soc. 2018, 140, 1019–1027. [Google Scholar] [CrossRef]
- Stolterfoht, M.; Caprioglio, P.; Wolff, C.M.; Márquez, J.A.; Nordmann, J.; Zhang, S.; Rothhardt, D.; Hörmann, U.; Redinger, A.; Kegelmann, L. The perovskite/ transport layer interfaces dominate non radiative recombination in efficient perovskite solar cells. Energy Environ. Sci. 2019, 12, 2778–2788. [Google Scholar]
- Matteocci, F.; Razza, S.; Di Giacomo, F.; Casaluci, S.; Mincuzzi, G.; Brown, T.M.; D’Epifanio, A.; Licoccia, S.; Di Carlo, A. Solid-state solar modules based on mesoscopic organometal halide perovskite: A route towards the up-scaling process. Phys. Chem. Chem. Phys. 2014, 16, 3918–3923. [Google Scholar]
- Abdi-Jalebi, M.; Andaji-Garmaroudi, Z.; Pearson, A.J.; Divitini, G.; Cacovich, S.; Philippe, B.; Rensmo, H.; Ducati, C.; Friend, R.H.; Stranks, S.D. Potassium- and Rubidium-Passivated Alloyed Perovskite Films: Optoelectronic Properties and Moisture Stability. ACS Energy Lett. 2018, 3, 2671–2678. [Google Scholar]
- Abdi-Jalebi, M.; Andaji-Garmaroudi, Z.; Cacovich, S.; Stavrakas, C.; Philippe, B.; Richter, J.M.; Alsari, M.; Booker, E.P.; Hutter, E.M.; Pearson, A.J.; et al. Maximizing and stabilizing luminescence from halide perovskites with potassium passivation. Nature 2018, 555, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Noel, N.K.; Stranks, S.D.; Abate, A.; Wehrenfennig, C.; Guarnera, S.; Haghighirad, A.-A.; Sadhanala, A.; Eperon, G.E.; Pathak, S.K.; Johnston, M.B.; et al. Lead-free organic−inorganic tin halide perovskites for photovoltaic applications. Energy Environ. Sci. 2014, 7, 3061–3068. [Google Scholar]
- Fu, H. Review of lead-free halide perovskites as light-absorbers for photovoltaic applications: From materials to solar cells. Sol. Energy Mater. Sol. Cells 2019, 193, 107–132. [Google Scholar]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Solanki, A.; Yadav, P.; Turren-Cruz, S.-H.; Lim, S.S.; Saliba, M.; Sum, T.C. Cation influence on carrier dynamics in perovskite solar cells. Nano Energy 2019, 58, 604–611. [Google Scholar]
- Karuppuswamy, P.; Boopathi, K.M.; Mohapatra, A.; Chen, H.-C.; Wong, K.-T.; Wang, P.-C.; Chu, C.-W. Role of a hydrophobic scaffold in controlling the crystallization of methylammonium antimony iodide for efficient lead-free perovskite solar cells. Nano Energy 2018, 45, 330–336. [Google Scholar]
- McMeekin, D.P.; Sadoughi, G.; Rehman, W.; Eperon, G.E.; Saliba, M.; Hörantner, M.T.; Haghighirad, A.; Sakai, N.; Korte, L.; Rech, B.; et al. A mixed-cation lead mixed-halide perovskite absorber for tandem solar cells. Science 2016, 351, 151–155. [Google Scholar]
- Li, B.; Zhang, Y.; Fu, L.; Yu, T.; Zhou, S.; Zhang, L.; Yin, L. Surface passivation engineering strategy to fully-inorganic cubic CsPbI3 perovskites for high-performance solar cells. Nat. Commun. 2018, 9, 1076. [Google Scholar] [CrossRef]
- Yang, Z.; Fan, J.Z.; Proppe, A.H.; Arquer, F.P.G.d.; Rossouw, D.; Voznyy, O.; Lan, X.; Liu, M.; Walters, G.; Quintero-Bermudez, R.; et al. Mixed-quantum-dot solar cells. Nat. Commun. 2017, 8, 1325. [Google Scholar] [PubMed]
- Sanehira, E.M.; Marshall, A.R.; Christians, J.A.; Harvey, S.P.; Ciesielski, P.N.; Wheeler, L.M.; Schulz, P.; Lin, L.Y.; Beard, M.C.; Luther, J.M. Enhanced mobility CsPbI3 quantum dot arrays for record-efficiency, high-voltage photovoltaic cells. Sci. Adv. 2017, 3, eaao4204. [Google Scholar] [CrossRef] [PubMed]
- Ball, J.M.; Petrozza, A. Defects in perovskite-halides and their effects in solar cells. Nat. Energy 2016, 1, 16149. [Google Scholar]
- Wang, F.; Bai, S.; Tress, W.; Hagfeldt, A.; Gao, F. Defects engineering for high-performance perovskite solar cells. npj Flexi. Electron. 2018, 2, 22. [Google Scholar] [CrossRef]
- Meng, L.; You, J.; Yang, Y. Addressing the stability issue of perovskite solar cells for commercial applications. Nat. Commun. 2018, 9, 5265. [Google Scholar]
- Wu, T.; Liu, X.; Luo, X.; Lin, X.; Cui, D.; Wang, Y.; Segawa, H.; Zhang, Y.; Han, L. Lead-free tin perovskite solar cells. Joule 2021, 5, 863–886. [Google Scholar]
- Wang, R.; Xue, J.; Meng, L.; Lee, J.-W.; Zhao, Z.; Sun, P.; Cai, L.; Huang, T.; Wang, Z.; Wang, Z.-K.; et al. Caffeine Improves the Performance and Thermal Stability of Perovskite Solar Cells. Joule 2019, 3, 1464–1477. [Google Scholar]
- Correa-Baena, J.-P.; Nienhaus, L.; Kurchin, R.C.; Shin, S.S.; Wieghold, S.; Putri Hartono, N.T.; Layurova, M.; Klein, N.D.; Poindexter, J.R.; Polizzotti, A.; et al. A-Site Cation in Inorganic A3Sb2I9 Perovskite Influences Structural Dimensionality; Exciton Binding Energy; and Solar Cell Performance. Chem. Mater. 2018, 30, 3734–3742. [Google Scholar]
- Zuraw, W.; Vinocour Pacheco, F.A.; Sánchez-Diaz, J.; Przypis, Ł.; Mejia Escobar, M.A.; Almosni, S.; Vescio, G.; Martínez-Pastor, J.P.; Garrido, B.; Kudrawiec, R.; et al. Large-Area; Flexible; Lead-Free Sn-Perovskite Solar Modules. ACS Energy Lett. 2023, 8, 4885–4887. [Google Scholar]
- Meng, F.; Liu, X.; Cai, X.; Gong, Z.; Li, B.; Xie, W.; Li, M.; Chen, D.; Yip, H.L.; Su, S.J. Incorporation of rubidium cations into blue perovskite quantum dot light-emitting diodes via FABr-modified multi-cation hot-injection method. Nanoscale 2019, 11, 1295–1303. [Google Scholar] [PubMed]
- Zhang, Z.; Sun, Z.; Song, P.; Guo, D.; Jin, Z. PEDOT:PSS doping strategy of improving both interface energy level matching and photocarrier transport in FASnI3 perovsktie solar cells. Thin Solid Film 2023, 784, 140066. [Google Scholar]
- Chen, B.; Wang, S.; Zhang, X.; Zhu, W.; Cao, Z.; Hao, F. Reducing the Interfacial Voltage Loss in Tin Halides Perovskite Solar Cells. Chem. Eng. J. 2022, 445, 136769. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Gao, Z.; Sun, Z.; Song, P.; Feng, X.; Jin, Z. FACl as a Bifunctional Additive to Enhance the Performance of Lead-Free Antimony-Based Perovskite Solar Cells. Micromachines 2025, 16, 379. https://doi.org/10.3390/mi16040379
Gao X, Gao Z, Sun Z, Song P, Feng X, Jin Z. FACl as a Bifunctional Additive to Enhance the Performance of Lead-Free Antimony-Based Perovskite Solar Cells. Micromachines. 2025; 16(4):379. https://doi.org/10.3390/mi16040379
Chicago/Turabian StyleGao, Xinyu, Zihao Gao, Zhen Sun, Ping Song, Xiyuan Feng, and Zhixin Jin. 2025. "FACl as a Bifunctional Additive to Enhance the Performance of Lead-Free Antimony-Based Perovskite Solar Cells" Micromachines 16, no. 4: 379. https://doi.org/10.3390/mi16040379
APA StyleGao, X., Gao, Z., Sun, Z., Song, P., Feng, X., & Jin, Z. (2025). FACl as a Bifunctional Additive to Enhance the Performance of Lead-Free Antimony-Based Perovskite Solar Cells. Micromachines, 16(4), 379. https://doi.org/10.3390/mi16040379






