A Versatile and Modular Microfluidic System for Dynamic Cell Culture and Cellular Interactions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
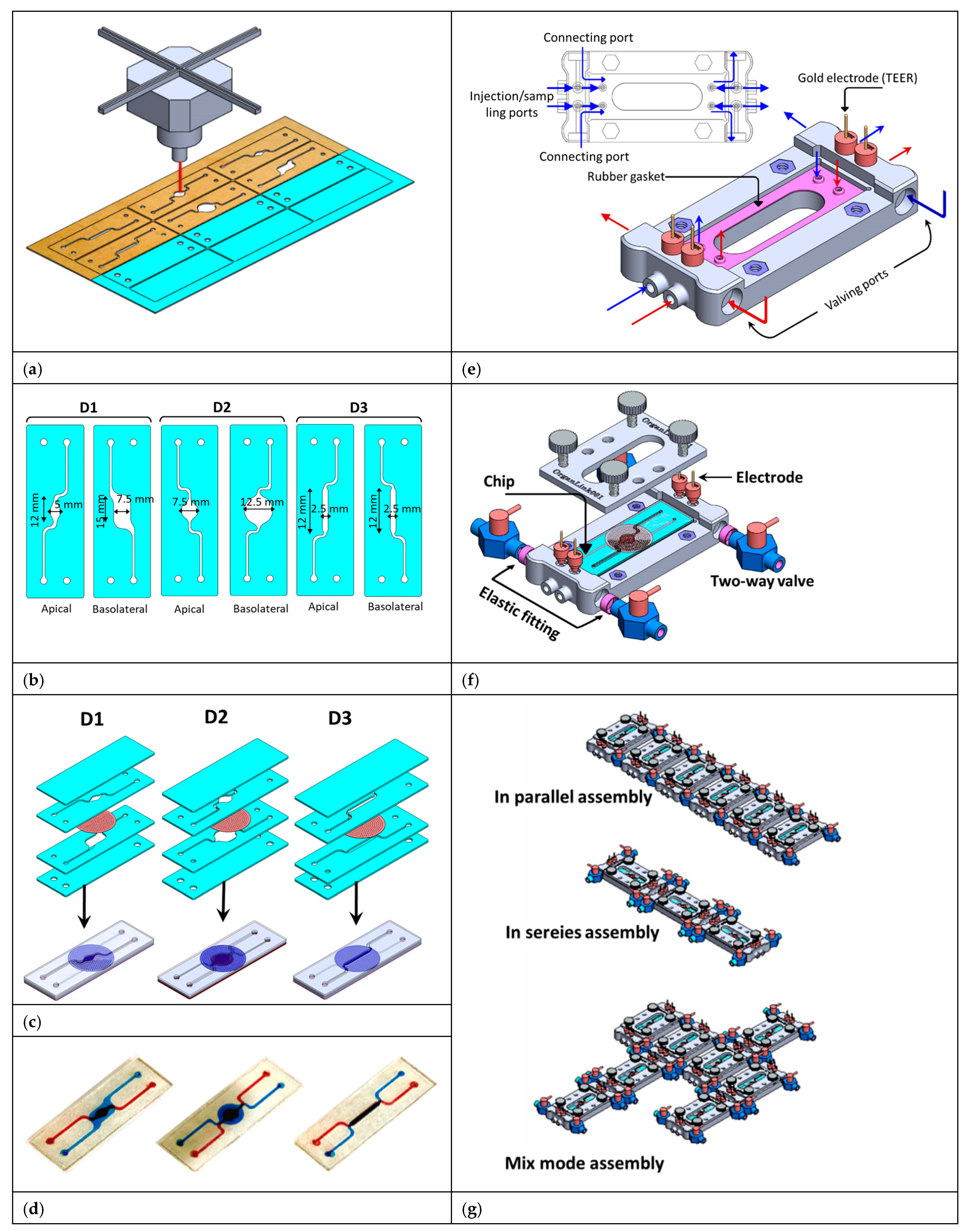
2.2. Chip Fabrication
2.3. Fabrication of the Fluid Routing Unit
2.4. Chip Assembly and Inter-Chip Fluid Routing
2.5. Characterization of the Chip-to-Chip Crosstalk
2.6. System Characterization and Cell Culture
2.7. Immunofluorescence Staining of Intercellular Tight Junctions
2.8. TEER Measurements
2.9. Trans-Epithelial Permeability
2.10. Immune Cell Culture
3. Results and Discussions
3.1. Characterization of the Fluidic Routing Through the Chip Assemblies
3.2. Multi-Intestinal Epithelium System
3.3. EC–Immune Cell Co-Culture and Cytokine Expression Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Curry, S.H. Why have so many drugs with stellar results in laboratory stroke models failed in clinical trials? A theory based on allometric relationships. Ann. N. Y. Acad. Sci. 2003, 993, 69–74. [Google Scholar] [CrossRef]
- Knight, J.B.; Balcombe, J. Animal carcinogenicity studies: 1. Poor human predictivity. Altern. Lab. Anim. 2006, 34, 19–27. [Google Scholar] [CrossRef]
- Bhatia, N.S.; Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Perestrelo, A.R.; Águas, A.C.; Rainer, A.; Forte, G. Microfluidic organ/body-on-a-chip devices at the convergence of biology and microengineering. Sensors 2015, 15, 31142–31170. [Google Scholar] [CrossRef] [PubMed]
- Skardal, A.; Shupe, T.; Atala, A. Organoid-on-a-chip and body-on-a-chip systems for drug screening and disease modeling. Drug Discov. Today 2016, 21, 1399–1411. [Google Scholar] [CrossRef] [PubMed]
- Mencattini, A.; Mattei, F.; Schiavoni, G.; Gerardino, A.; Businaro, L.; Di Natale, C.; Martinelli, E. From petri dishes to organ on chip platform: The increasing importance of machine learning and image analysis. Front. Pharmacol. 2019, 10, 100. [Google Scholar] [CrossRef]
- Ramadan, Q.; Zourob, M. Organ-on-a-chip engineering: Toward bridging the gap between lab and industry. Biomicrofluidics 2020, 14, 041501. [Google Scholar] [CrossRef] [PubMed]
- Abu-Dawas, S.; Alawami, H.; Zourob, M.; Ramadan, Q. Design and Fabrication of Low-Cost Microfluidic Chips and Microfluidic Routing System for Reconfigurable Multi-(Organ-on-a-Chip) Assembly. Micromachines 2021, 12, 1542. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kongsuphol, P.; Gourikutty, S.B.N.; Ramadan, Q. Human adipocyte differentiation and characterization in a perfusion-based cell culture device. Biomed. Microdevices 2017, 19, 18. [Google Scholar] [CrossRef] [PubMed]
- Ronaldson-Bouchard, K.; Teles, D.; Yeager, K.; Tavakol, D.N.; Zhao, Y.; Chramiec, A.; Tagore, S.; Summers, M.; Stylianos, S.; Tamargo, M.; et al. A multi-organ chip with matured tissue niches linked by vascular flow. Nat. Biomed. Eng. 2022, 6, 351–371. [Google Scholar] [CrossRef]
- Esch, M.B.; Ueno, H.; Applegate, D.R.; Shuler, M.L. Modular, pumpless body-on-a-chip platform for the co-culture of GI tract epithelium and 3D primary liver tissue. Lab Chip 2016, 16, 2719–2729. [Google Scholar] [CrossRef]
- Bhise, N.S.; Manoharan, V.; Massa, S.; Tamayol, A.; Ghaderi, M.; Miscuglio, M.; Lang, Q.; Zhang, Y.S.; Shin, S.R.; Calzone, G.; et al. A liver-on-a-chip platform with bioprinted hepatic spheroids. Biofabrication 2016, 8, 014101. [Google Scholar] [CrossRef]
- Fatehullah, A.; Tan, S.H.; Barker, N. Organoids as an in vitro model of human development and disease. Nat. Cell Biol. 2016, 18, 246–254. [Google Scholar] [CrossRef]
- Bazzoun, D.; Lelièvre, S.; Talhouk, R. Polarity proteins as regulators of cell junction complexes: Implications for breast cancer. Pharmacol. Ther. 2013, 138, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Bryant, D.M.; Mostov, K.E. From cells to organs: Building polarized tissue. Nat. Rev. Mol. Cell. Biol. 2008, 9, 887–901. [Google Scholar] [CrossRef]
- Ramadan, Q.; Fardous, R.S.; Hazaymeh, R.; Alshmmari, S.; Zourob, M. Pharmacokinetics-On-a-Chip: In Vitro Microphysiological Models for Emulating of Drugs ADME. Adv. Biol. 2021, 5, 2100775. [Google Scholar] [CrossRef] [PubMed]
- Otani, T.; Furuse, M. Tight junction structure and function revisited. Trends Cell Biol. 2020, 30, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D.E. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat. Rev. Genet. 2022, 23, 467–491. [Google Scholar] [CrossRef]
- Leung, C.M.; de Haan, P.; Ronaldson-Bouchard, K.; Kim, G.-A.; Ko, J.; Rho, H.S.; Chen, Z.; Habibovic, P.; Jeon, N.L.; Takayama, S.; et al. A guide to the organ-on-a-chip. Nat. Rev. Methods Primers 2022, 2, 33. [Google Scholar] [CrossRef]
- Vogt, N. Modeling multi-organ systems on a chip. Nat. Methods 2022, 19, 641. [Google Scholar] [CrossRef]
- Sung, J.H. Pharmacokinetic-based multi-organ chip for recapitulating organ interactions. Methods Cell Biol. 2018, 146, 183–197. [Google Scholar] [CrossRef]
- Lee, S.H.; Sung, J.H. Organ-on-a-Chip Technology for Reproducing Multiorgan Physiology. Adv. Healthcare Mater. 2018, 7, 1700419. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, R.; Togo, S.; Kimura, M.; Shinozawa, T.; Koido, M.; Koike, H.; Thompson, W.; Karns, R.A.; Mayhew, C.N.; McGrath, P.S.; et al. Modeling steatohepatitis in humans with pluripotent stem cell-derived organoids. Cell Metab. 2019, 30, 374–384.e6. [Google Scholar] [CrossRef] [PubMed]
- Vatine, G.D.; Barrile, R.; Workman, M.J.; Sances, S.; Barriga, B.K.; Rahnama, M.; Barthakur, S.; Kasendra, M.; Lucchesi, C.; Kerns, J.; et al. Human iPSC-derived blood-brain barrier chips enable disease modeling and personalized medicine applications. Cell Stem Cell 2019, 24, 995–1005.e6. [Google Scholar] [CrossRef]
- Ramadan, Q.; Hazaymeh, R.; Zourob, M. Immunity-on-a-Chip: Integration of Immune Components into the Scheme of Organ-on-a-Chip Systems. Adv. Biol. 2023, 7, e2200312. [Google Scholar] [CrossRef]
- Wikman-Larhed, A.; Artursson, P. Co-cultures of human intestinal goblet (HT29-H) and absorptive (Caco-2) cells for studies of drug and peptide absorption. Eur. J. Pharm. Sci. 1995, 3, 171–183. [Google Scholar] [CrossRef]
- Maurer, M.; Gresnigt, M.S.; Last, A.; Wollny, T.; Berlinghof, F.; Pospich, R.; Cseresnyes, Z.; Medyukhina, A.; Graf, K.; Groeger, M.; et al. A three-dimensional immunocompetent intestine-on-chip model as in vitro platform for functional and microbial interaction studies. Biomaterials 2019, 220, 119396. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.; Hinojosa, C.D.; Ingber, D.E.; Kim, H.J. Human intestinal morphogenesis controlled by transepithelial morphogen gradient and flow-dependent physical cues in a microengineered gut-on-a-chip. iScience 2019, 15, 391. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, Q.; Jafarpoorchekab, H.; Huang, C.; Silacci, P.; Carrara, S.; Koklü, G.; Ghaye, J.; Ramsden, J.; Ruffert, C.; Vergeres, G.; et al. NutriChip: Nutrition analysis meets microfluidics. Lab Chip 2013, 13, 196–203. [Google Scholar] [CrossRef]
- Vergères, G.; Bogicevic, B.; Buri, C.; Carrara, S.; Chollet, M.; Corbino-Giunta, L.; Egger, L.; Gille, D.; Kopf-Bolanz, K.; Laederach, K.; et al. The NutriChip project–translating technology into nutritional knowledge. Br. J. Nutr. 2012, 108, 762–768. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kus, M.; Ibragimow, I.; Piotrowska-Kempisty, H. Caco-2 Cell Line Standardization with Pharmaceutical Requirements and In Vitro Model Suitability for Permeability Assays. Pharmaceutics 2023, 15, 2523. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.-S.L.; Thakker, D.R. Applications of the Caco-2 model in the design and development of orally active drugs: Elucidation of biochemical and physical barriers posed by the intestinal epithelium. Adv. Drug Deliv. Rev. 1997, 23, 77–98. [Google Scholar] [CrossRef]
- Lopez-Escalera, S.; Wellejus, A. Evaluation of Caco-2 and human intestinal epithelial cells as in vitro models of colonic and small intestinal integrity. Biochem. Biophys. Rep. 2022, 31, 101314. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran Sulfate Sodium (DSS)-Induced Colitis in Mice. Curr. Protoc. Immunol. 2014, 104, 1–14. [Google Scholar] [CrossRef]
- Eichele, D.D.; Kharbanda, K.K. Dextran sodium sulfate colitis murine model: An indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J. Gastroenterol. 2017, 23, 6016–6029. [Google Scholar] [CrossRef]
- Kiesler, P.; Fuss, I.J.; Strober, W. Experimental Models of Inflammatory Bowel Diseases. Med. Hyg. 2001, 59, 241–248. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Rajasekaran, N.; Choi, J.-S.; Kim, J.-H.; Jang, M.H.; Shin, Y.K.; Suh, P.-G.; Ryu, S.H. Intestinal Epithelial Cell-Specific Deletion of PLD2 Alleviates DSS-Induced Colitis by Regulating Occludin. Sci. Rep. 2017, 7, 1573. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.W.; Choi, S.; Kim, S.Y.; Yoon, Y.S.; Kang, J.-H.; Oh, S.H. Allyl Isothiocyanate Ameliorates Dextran Sodium Sulfate-Induced Colitis in Mouse by Enhancing Tight Junction and Mucin Expression. Int. J. Mol. Sci. 2018, 19, 2025. [Google Scholar] [CrossRef]
- Bednarek, R. In Vitro Methods for Measuring the Permeability of Cell Monolayers. Methods Protoc. 2022, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Co, J.Y.; Margalef-Català, M.; Monack, D.M.; Amieva, M.R. Controlling the polarity of human gastrointestinal organoids to investigate epithelial biology and infectious diseases. Nat. Protoc. 2021, 16, 5171–5192. [Google Scholar] [CrossRef] [PubMed]
- Kongsuphol, P.; Gupta, S.; Liu, Y.; Bhuvanendran Nair Gourikutty, S.; Biswas, S.K.; Ramadan, Q. In vitro micro-physiological model of the inflamed human adipose tissue for immune-metabolic analysis in type II diabetes. Sci. Rep. 2019, 9, 4887. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Guo, J.; Yan, W.; Xu, L. Macrophage polarization in inflammatory bowel disease. Cell Commun. Signal. 2023, 21, 367. [Google Scholar] [CrossRef] [PubMed]
- Orliaguet, L.; Dalmas, E.; Drareni, K.; Venteclef, N.; Alzaid, F. Mechanisms of Macrophage Polarization in Insulin Signaling and Sensitivity. Front. Endocrinol. 2020, 11, 516860. [Google Scholar] [CrossRef] [PubMed]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramadan, Q.; Hazaymeh, R.; Zourob, M. A Versatile and Modular Microfluidic System for Dynamic Cell Culture and Cellular Interactions. Micromachines 2025, 16, 237. https://doi.org/10.3390/mi16020237
Ramadan Q, Hazaymeh R, Zourob M. A Versatile and Modular Microfluidic System for Dynamic Cell Culture and Cellular Interactions. Micromachines. 2025; 16(2):237. https://doi.org/10.3390/mi16020237
Chicago/Turabian StyleRamadan, Qasem, Rana Hazaymeh, and Mohammed Zourob. 2025. "A Versatile and Modular Microfluidic System for Dynamic Cell Culture and Cellular Interactions" Micromachines 16, no. 2: 237. https://doi.org/10.3390/mi16020237
APA StyleRamadan, Q., Hazaymeh, R., & Zourob, M. (2025). A Versatile and Modular Microfluidic System for Dynamic Cell Culture and Cellular Interactions. Micromachines, 16(2), 237. https://doi.org/10.3390/mi16020237







