Integrated Device for Cancer Nucleic Acid Biomarker Detection at Body Temperature
Abstract
1. Introduction
2. Materials and Methods
2.1. Primer Design
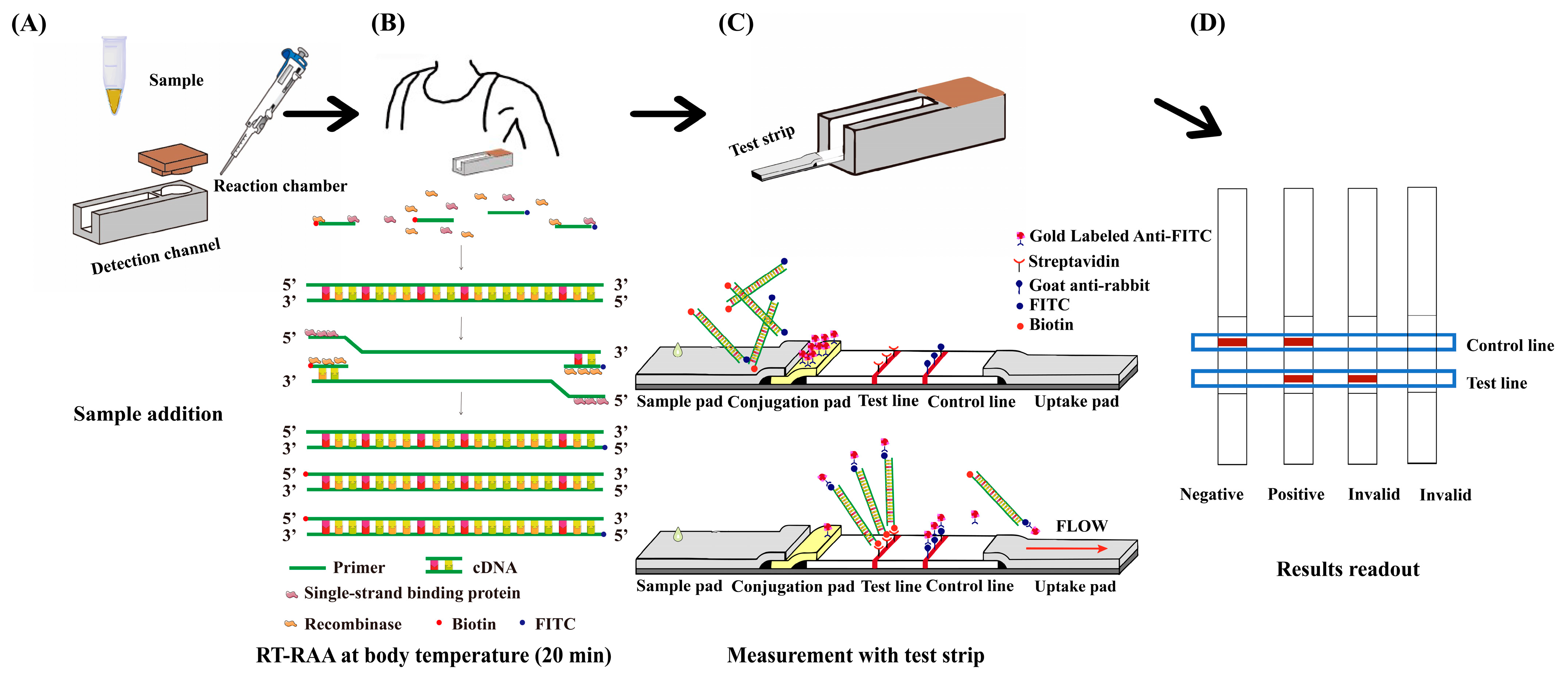
2.2. Integrated Detection Platform Design and Fabrication
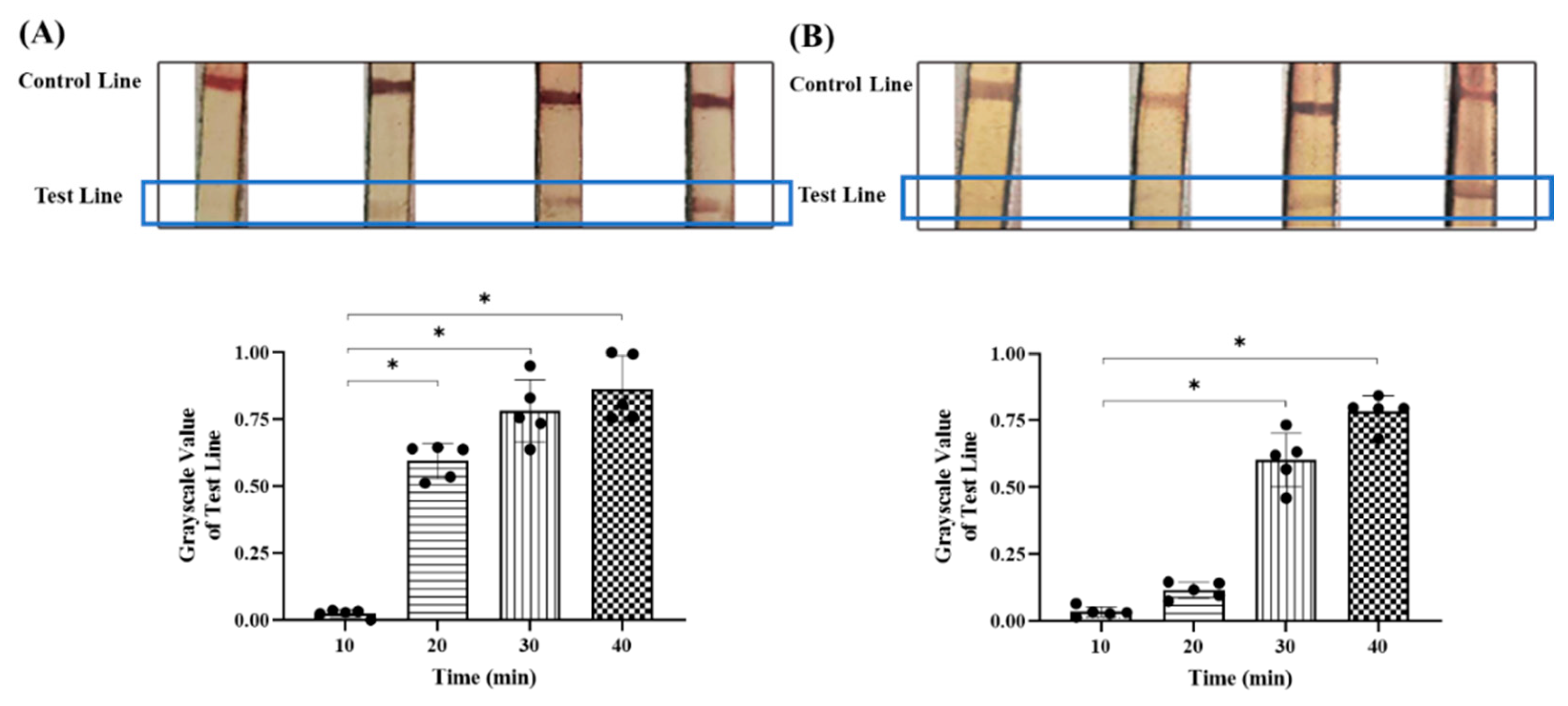
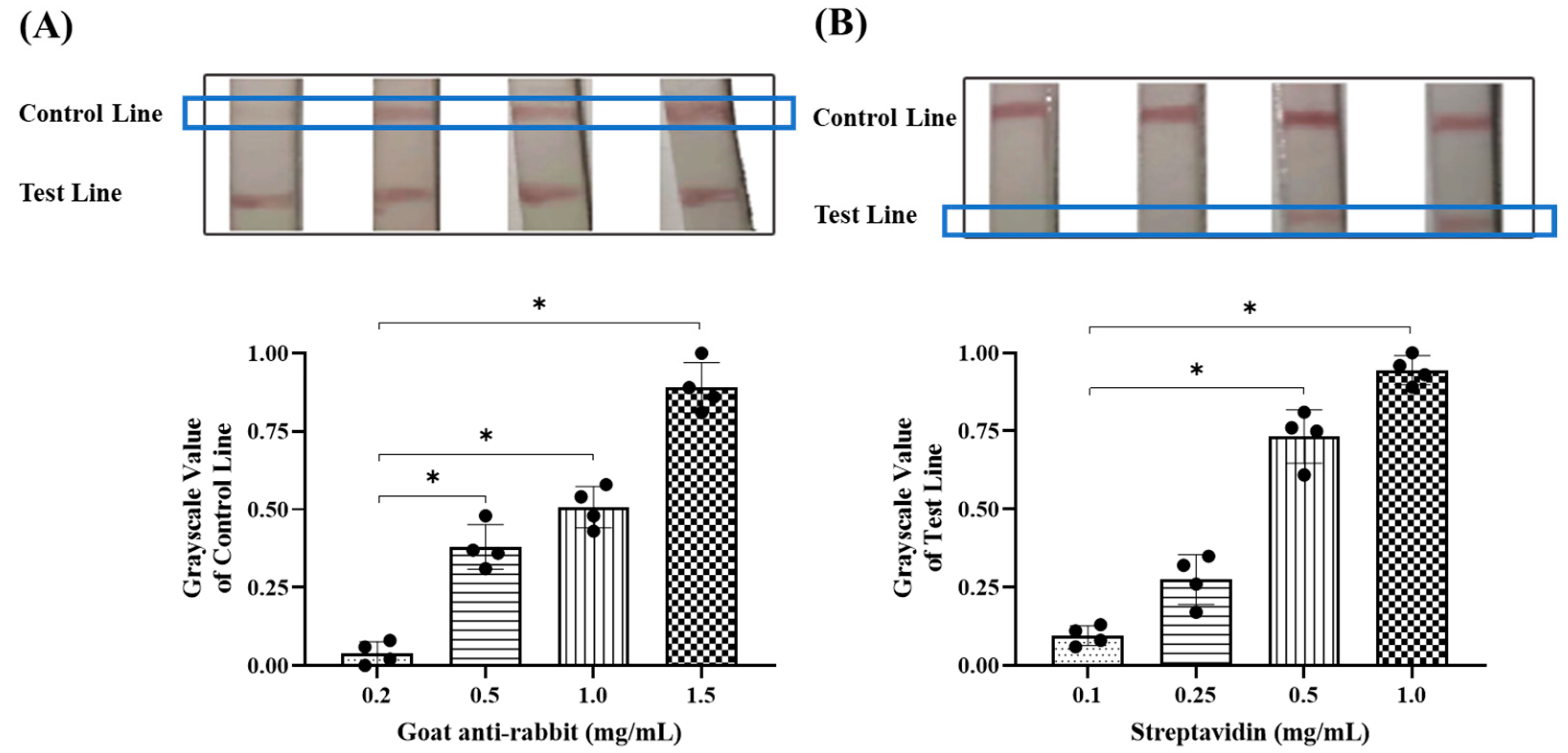
2.3. Optimization of Reaction Conditions
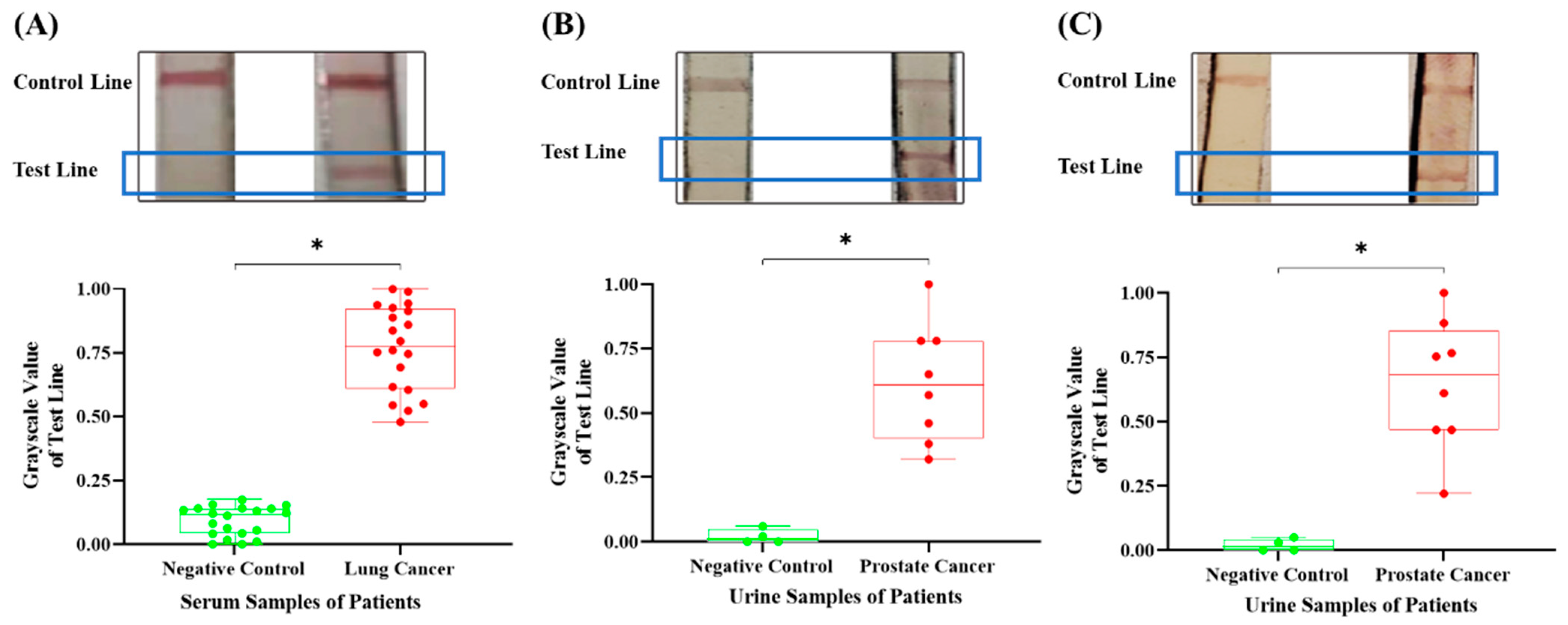
2.4. Detection of Clinical Samples
2.5. Statistical Analysis
3. Results and Discussion
3.1. Optimal Conditions for Colloidal Gold Labeling
3.2. Optimal Detection Conditions for Colloidal Gold Test Strips
3.3. The Detection Results of Different Cancer Nucleic Markers in Clinical Samples Through the Integrated Device
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, S.; Ge, K.; Huo, X.; Yang, K.; Wang, X.; Yang, Y. 2D Fe/Co-MOF/SOX cascade reactors for fast noninvasive detection of sarcosine level in prostate cancer urine. J. Colloid Interface Sci. 2025, 679, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Pateel, G.P.; Senapati, K.; Pandey, A.K. Improving the performance of multi-stage HER2 breast cancer detection in hematoxylin-eosin images based on ensemble deep learning. Biomed. Signal Process. Control 2025, 100, 107016. [Google Scholar]
- Cheng, H.P.; Yang, T.H.; Wang, J.C.; Chuang, H.S. Recent Trends and Innovations in Bead-Based Biosensors for Cancer Detection. Sensors 2024, 24, 2904. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Li, J.; Li, L.; Wang, C.; Liu, X.; Shi, M.; Liu, Z.; Li, K. Construction of test strips for lung cancer detection based on aptamers. J. Pharm. Biomed. Anal. 2024, 242, 115976. [Google Scholar] [CrossRef] [PubMed]
- Nie, Q.; Xu, P.; Liu, J.; Wang, Y.; Huang, Q.; You, R.; Liu, Y.; Li, L.; Lu, Y. Enhanced SERS detection of the colorectal cancer biomarker utilizing a two-dimensional silver substrate. Colloids Surf. B Biointerfaces 2025, 245, 114317. [Google Scholar] [CrossRef]
- Ma, L.H.; Wang, H.B.; Zhang, T.; Xuan, Y.; Li, C.; Chen, W.; Liu, B.; Zhao, Y.D. Visual simultaneous detection of single nucleotide polymorphism of tumor susceptibility gene and marker alpha-fetoprotein based on double-labeled colloidal gold probe with lateral flow strip biosensor. Sens. Actuators B Chem. 2019, 298, 126819. [Google Scholar] [CrossRef]
- Hansen, L.; Nagdeve, S.N.; Suganthan, B.; Ramasamy, R.P. An Electrochemical Nucleic Acid Biosensor for Triple-Negative Breast Cancer Biomarker Detection. Sensors 2024, 24, 5747. [Google Scholar] [CrossRef]
- Xu, S.; Wang, X.; Wu, C.; Zhu, X.; Deng, X.; Wu, Y.; Liu, M.; Huang, X.; Wu, L.; Huang, H. MscI restriction enzyme cooperating recombinase-aided isothermal amplification for the ultrasensitive and rapid detection of low-abundance EGFR mutations on microfluidic chip. Biosens. Bioelectron. 2024, 247, 115925. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Chen, X.D.; Wang, P.; Tu, F.M.; Yu, Y.; Chen, Q.M.; Liu, Z.M. A visual detection assay for Helicobacter pylori in saliva based on recombinase-aided amplification and peptide nucleic acid-assisted split DNAzyme probes. Sens. Actuators B Chem. 2023, 396, 134582. [Google Scholar] [CrossRef]
- Yao, Y.; Zou, M.; Wu, H.; Ma, S.; Gu, X.; Zhou, M.; Zhao, F.; Abudushalamua, G.; Xiao, F.; Chen, Y.; et al. A colloidal gold test strip based on catalytic hairpin assembly for the clinical detection of influenza a virus nucleic acid. Talanta 2023, 265, 124855. [Google Scholar] [CrossRef]
- Wang, X.; Wang, G.; Wang, Y.; Quan, S.; Qi, H.; Sun, L.; Shen, C.; Huang, H.; Jiao, W.; Shen, A. Development and Preliminary Application of Multiplex Loop-Mediated Isothermal Amplification Coupled with Lateral Flow Biosensor for Detection of Mycobacterium tuberculosis Complex. Front. Cell. Infect. Microbiol. 2021, 11, 666492. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Song, G.; Cao, Z.; Pan, J.; Li, X.; Gao, Y.; Qi, J.; Chen, Z.; Fan, G.; et al. Internally controlled recombinase-aided amplification (IC-RAA) assays for the detection of human papillomavirus genotypes 16 and 18 using extracted DNA and samples treated with nucleic acid releasing agent. Arch. Virol. 2020, 165, 2241–2247. [Google Scholar] [CrossRef]
- Fan, X.; Li, L.; Zhao, Y.; Liu, Y.; Liu, C.; Wang, Q.; Dong, Y.; Wang, S.; Chi, T.; Song, F.; et al. Clinical Validation of Two Recombinase-Based Isothermal Amplification Assays (RPA/RAA) for the Rapid Detection of African Swine Fever Virus. Front. Microbiol. 2020, 11, 1696. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Fan, J.; Li, Z.; Zhang, Y.; Qin, Y.; Wu, K.; Li, X.; Li, Y.; Fan, S.; Zhao, M. Development of Recombinase Aided Amplification Combined with Disposable Nucleic Acid Test Strip for Rapid Detection of Porcine Circovirus Type 2. Front. Vet. Sci. 2021, 8, 676294. [Google Scholar] [CrossRef] [PubMed]
- Vindeirinho, J.M.; Pinho, E.; Gomes, L.; Guiomar, R.; Kobialka, R.M.; El Wahed, A.A.; Azevedo, N.F.; Almeida, C. RT-RAA with a lateral flow assay readout based on ssDNA hybridization for detection of RNA viruses—The case of SARS-CoV-2. Sens. Actuators B Chem. 2025, 426, 136864. [Google Scholar] [CrossRef]
- Li, X.; Zhu, S.; Zhang, X.; Ren, Y.; He, J.; Zhou, J.; Yin, L.; Wang, G.; Zhong, T.; Wang, L.; et al. Advances in the application of recombinase-aided amplification combined with CRISPR-Cas technology in quick detection of pathogenic microbes. Front. Bioeng. Biotechnol. 2023, 11, 1215466. [Google Scholar] [CrossRef]
- Tian, Y.; Fan, Z.; Xu, L.; Cao, Y.; Chen, S.; Pan, Z.; Gao, Y.; Li, H.; Zheng, S.; Ma, Y.; et al. CRISPR/Cas13a-assisted rapid and portable HBV DNA detection for low-level viremia patients. Emerg. Microbes Infect. 2023, 12, e2177088. [Google Scholar] [CrossRef]
- Zhu, X.; Zhao, Y.; Zhu, C.; Wang, Y.; Liu, Y.; Su, J. Rapid detection of cagA-positive Helicobacter pylori based on duplex recombinase aided amplification combined with lateral flow dipstick assay. Diagn. Microbiol. Infect. Dis. 2022, 103, 115661. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Wang, L.; Lu, Y.; Chen, C.; Wang, K.; Zhang, L. Recombinase Polymerase Amplification Assay with Lateral Flow Strips for Rapid Detection of Candidiasis Due to Candida parapsilosis. Curr. Microbiol. 2023, 80, 217. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Liu, Y.; Yang, X.; Lubanga, N.; Tai, P.; Xiong, M.; Fan, B.; Yang, X.; Nie, Z.; Zhang, Q.; et al. Rapid visual detection of Helicobacter pylori and vacA subtypes by Dual-Target RAA-LFD assay. Clin. Chim. Acta 2025, 564, 119927. [Google Scholar] [CrossRef]
- Sun, P.; Yang, X.; Liu, J.; Bao, Y.; Qi, J.; Han, X.; Liu, G.; Wang, S.; Tian, M. Development and Application of Colloidal Gold Test Strips for the Rapid Detection of Canine Brucellosis. Biosensors 2024, 14, 388. [Google Scholar] [CrossRef]
- Hu, W.; Yan, Z.; Li, H.; Qiu, J.; Zhang, D.; Li, P.; Pan, Y.; Guo, H. Development of a new colloidal gold immunochromatographic strip for rapid detecting subgroup A of avian leukosis virus using colloidal gold nanoparticles. Biochem. Eng. J. 2019, 148, 16–23. [Google Scholar] [CrossRef]
- Bae, U.; Shim, J.Y.; Lee, H.R.; Shin, J.Y. Serum carcinoembryonic antigen level is associated with arterial stiffness in healthy Korean adult. Clin. Chim. Acta 2013, 415, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Gan, L.; Tian, Z.; Han, J.; Du, B.; Xue, G.; Feng, Y.; Zhao, H.; Cui, J.; Yan, C.; et al. Rapid detection of Burkholderia cepacia complex carrying the 16S rRNA gene in clinical specimens by recombinase-aided amplification. Front. Cell. Infect. Microbiol. 2022, 12, 984140. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Xie, Q.; Li, J.; Pei, Y.; Bai, Y.; Liu, F.; Cui, H.; Shao, G.; Feng, Z. Rapid and sensitive detection of African swine fever virus in pork using recombinase aided amplification combined with QDMs-based test strip. Anal. Bioanal. Chem. 2022, 414, 3885–3894. [Google Scholar] [CrossRef] [PubMed]
- Nie, M.; Zhou, Y.; Li, F.; Deng, H.; Zhao, M.; Huang, Y.; Jiang, C.; Sun, X.; Xu, Z.; Zhu, L. Epidemiological investigation of swine Japanese encephalitis virus based on RT-RAA detection method. Sci. Rep. 2022, 12, 9392. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liang, Q.; Lv, R.; Ahmad, S.; Bano, M.; Weng, G.; Wen, R. Optimization and Validation of Reverse Transcription Recombinase-Aided Amplification (RT-RAA) for Sorghum Mosaic Virus Detection in Sugarcane. Pathogens 2023, 12, 1055. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Z.; Jia, B.; Tu, Z.; Zeng, J.; Pang, J.; Ren, W.; Huang, Z.; He, B.; Wang, Z. Detection of AFB(1) by Immunochromatographic Test Strips Based on Double-Probe Signal Amplification with Nanobody and Biotin-Streptavidin System. Foods 2024, 13, 3396. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Zhang, D.; Wang, Q.; Mao, S.; Kim, E.S.; Kim, N.Y.; Zhou, Q.; Li, Y.; Yao, Z. Detection of carcinoembryonic antigen specificity using microwave biosensor with machine learning. Biosens. Bioelectron. 2025, 269, 116908. [Google Scholar] [CrossRef]
- Gao, Z.; Chen, Z.; Deng, J.; Li, X.; Qu, Y.; Xu, L.; Luo, Y.; Lu, Y.; Liu, T.; Zhao, W.; et al. Measurement of Carcinoembryonic Antigen in Clinical Serum Samples Using a Centrifugal Microfluidic Device. Micromachines 2018, 9, 470. [Google Scholar] [CrossRef]
- Nakayama, T.; Zhao, J.; Takeuchi, D.; Kerdsin, A.; Chiranairadul, P.; Areeratana, P.; Loetthong, P.; Pienpringam, A.; Akeda, Y.; Oishi, K. Colloidal gold-based immunochromatographic strip test compromising optimised combinations of anti-S. suis capsular polysaccharide polyclonal antibodies for detection of Streptococcus suis. Biosens. Bioelectron. 2014, 60, 175–179. [Google Scholar] [CrossRef]
- Chen, W.; Wu, S.; Li, G.; Duan, X.; Sun, X.; Li, S.; Zhao, Y.; Gu, D.; Zeng, G.; Liu, H. Accurate diagnosis of prostate cancer with CRISPR-based nucleic acid test strip by simultaneously identifying PCA3 and KLK3 genes. Biosens. Bioelectron. 2023, 220, 114854. [Google Scholar] [CrossRef]
- Toro, A.U.; Shukla, S.K.; Bansal, P. Micronome Revealed miR-205-5p as Key Regulator of VEGFA During Cancer Related Angiogenesis in Hepatocellular Carcinoma. Mol. Biotechnol. 2023, 65, 1178–1186. [Google Scholar] [CrossRef] [PubMed]

| Gene Primers | Sequence (5′→3′) |
|---|---|
| CEA-F | Biotin-CAAACCGCAGTGACCCAGT |
| CEA-R | FITC-ACTCCAATCATGATGCCGACAG |
| PSA-F | Biotin-TTTCCTTATCATCCTCGCTCCTC |
| PSA-R | FITC-CATGACCTTCACAGCATCCGT |
| PCA3-F | Biotin-GAAGCACCTCGCATTTGTGG |
| PCA3-R | FITC-GGCCAGAAGCTAGCATCCAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Wu, B.; Li, X.; Jin, Y.; Zhang, H.; Liu, B.; Zhang, Z.; Li, N. Integrated Device for Cancer Nucleic Acid Biomarker Detection at Body Temperature. Micromachines 2025, 16, 192. https://doi.org/10.3390/mi16020192
Chen C, Wu B, Li X, Jin Y, Zhang H, Liu B, Zhang Z, Li N. Integrated Device for Cancer Nucleic Acid Biomarker Detection at Body Temperature. Micromachines. 2025; 16(2):192. https://doi.org/10.3390/mi16020192
Chicago/Turabian StyleChen, Chang, Bin Wu, Xuesong Li, Yuhang Jin, Hangyu Zhang, Bo Liu, Zhengyao Zhang, and Na Li. 2025. "Integrated Device for Cancer Nucleic Acid Biomarker Detection at Body Temperature" Micromachines 16, no. 2: 192. https://doi.org/10.3390/mi16020192
APA StyleChen, C., Wu, B., Li, X., Jin, Y., Zhang, H., Liu, B., Zhang, Z., & Li, N. (2025). Integrated Device for Cancer Nucleic Acid Biomarker Detection at Body Temperature. Micromachines, 16(2), 192. https://doi.org/10.3390/mi16020192






