Abstract
Porous carbon particles (PCPs) prepared from sucrose via the hydrothermal method and its modified forms with polyethyleneimine (PEI) as PCP-PEI were used as templates as in situ metal nanoparticles as M@PCP and M@PCP-PEI (M:Co, Ni, or Cu), respectively. The prepared M@PCP and M@PCP-PEI composites were used as catalysts in the hydrolysis of NaBH4 and NH3BH3 to produce hydrogen (H2). The amount of Co nanoparticles within the Co@PCP-PEI structure was steadily increased via multiple loading/reducing cycles, e.g., from 29.8 ± 1.1 mg/g at the first loading/reducing cycles to 44.3 ± 4.9 mg/g after the third loading/reducing cycles. The Co@PCP-PEI catalyzed the hydrolysis of NaBH4 within 120 min with 251 ± 1 mL H2 production and a 100% conversion ratio with a 3.8 ± 0.3 mol H2/(mmol cat·min) turn-over frequency (TOF) and a lower activation energy (Ea), 29.3 kJ/mol. In addition, the Co@PCP-PEI-catalyzed hydrolysis of NH3BH3 was completed in 28 min with 181 ± 1 mL H2 production at 100% conversion with a 4.8 ± 0.3 mol H2/(mmol cat·min) TOF value and an Ea value of 32.5 kJ/mol. Moreover, Co@PCP-PEI composite catalysts were afforded 100% activity up to 7 and 5 consecutive uses in NaBH4 and NH3B3 hydrolysis reactions, respectively, with all displaying 100% conversions for both hydrolysis reactions in the 10 successive uses of the catalyst.
1. Introduction
Improvements in human welfare and health resulting from industrial progress has led to increased energy consumption, resulting in lateral effects on climate change and therefore increased demand for more sustainable and greener energy sources to counteract fossil fuel-associated problems [1,2,3,4]. Currently, the world’s primary source of hydrogen (H2) is mostly from fossil fuels. For example, at the end of 2021, it was found that 47% of hydrogen comes from natural gas, 27% from coal, 22% from oil (as a by-product), and only 4% from water via electrolysis [5,6]; however, there is considerable potential for H2 to evolve into a sustainable energy resource through the adoption of new and alternative sources. Current advancements have identified non-fossil fuel-based hydrides as viable candidates for H2 production, addressing the challenges associated with traditional fossil fuel reliance [7,8,9]. Consequently, the development of versatile catalysts that can facilitate H2 production from these hydrides has become increasingly significant. The generation of efficient catalysts not only enhances the viability of H2 as a green energy source but also contributes to the overarching objective of reducing dependence on fossil fuels and mitigating environmental impacts [10,11,12]. The release of H2 from hydrogen-rich inorganic hydrides, e.g., sodium borohydride (NaBH4) [13,14], ammonium borane (NH3BH3) [14,15], hydrazine hydrate (N2H4H2O) [16,17], magnesium hydrides (MgH2) [18,19], and tetrahydroxy boron (B2(OH)4) [20,21], has been considered as a feasible, inexpensive, and effective solution to energy and environmental problems. However, fast and controlled H2 production from these inorganic hydrides necessitates catalysts that are also non-toxic. Amongst the potential H2 carriers, substantial efforts have been made in the design of low-cost and non-noble metal catalysts with a focus on the hydrolysis of NaBH4 (Equation (1)) and NH3BH3 (Equation (2)) [22,23,24,25]. As given in Equations (1) and (2), H2 generation from NaBH4 and NH3BH3 only generates non-toxic metaborates.
NaBH4 (aq) + 2H2O → 4H2 (g) + NaBO2 (aq) + heat
NH3BH3 (aq) + 2H2O → 3H2 (g) + (NH4)BO2 (aq) + heat
NaBH4 has practical advantages such as high gravimetric hydrogen storage capacity (10.8% by weight), chemical stability, room temperature inflammability, and recyclability of hydrolysis by-products [26]. NH3BH3, on the other hand, is non-toxic, fully soluble, and extremely stable at room temperature with a high hydrogen concentration (19.6% by weight) [27]. Consequently, NaBH4 and NH3BH3 are regarded as the most feasible amongst the chemical hydrogen storage compounds for a range of applications [28,29,30,31]. The non-precious metals such as Co, Ni, and Cu can be readily employed in NaBH4 and NH3BH3 hydrolysis reactions to provide significant cost-saving alternatives in hydrogen generation research for commercial applications in addition to their non-toxic nature [31].
This research complements the findings of Glavee et al., who examined the synthesis of nanoscale particles via the reaction of sodium borohydride (NaBH4) with a range of metal salts, including those of cobalt, nickel, iron, and copper [32,33,34,35]. Metal nanoparticles are frequently used as catalysts to enhance/control the reaction rates for many different catalytic reactions. However, the high surface energy of metal nanoparticles tends to cause agglomerates and bigger particles to form, and their ease of oxidation resulting in changes to the surface properties of metal nanoparticles causes an eventual decline in activity. As a result, various materials such as polymeric hydrogels [10], carbon materials [36,37], mesoporous materials [38,39], clay [40,41], and zeolite [42,43] have been employed to stabilize and coat nanoparticles to prevent aggregation and oxidation and/or deactivation.
Therefore, in this investigation, porous carbon particles (PCPs) and their polyethyleneimine (PEI)-modified PCP-PEI forms were used as a template to prepare Co, Ni, and Cu metal nanoparticles in situ as M@PCP and M@PCP-PEI (M:Co, Ni, or Cu), respectively. The prepared M@PCP and M@PCP-PEI composites then were tested as catalysts for the hydrolysis of NaBH4 and NH3BH3 to produce H2. The amount of metal nanoparticles was increased via multiple loading/reducing cycles within PCP-based materials. The effects of template, metal species, the amount of metal particle, and temperature on the catalytic activity of the metal catalysts in H2 generation reactions from the hydrolysis of NaBH4 and NH3BH3 reaction were studied. The turn-over frequency (TOF, mol H2/(mmol cat·min)) and hydrogen generation rate (HGR, mL H2/(g cat·min)) values of M@PCP and M@PCP-PEI (M:Co, Ni, or Cu) composite catalysts for the reactions were calculated and compared. Activation energy (Ea), enthalpy (ΔH), and entropy (ΔS) were determined for the Co@PCP-PEI-catalyzed hydrolysis of both NaBH4 and NH3BH3. Moreover, the reuse of Co@PCP-PEI composite catalysts in the hydrolysis of both NaBH4 and NH3BH3 reaction was examined.
2. Materials and Methods
2.1. Materials
Sucrose (Carlo Erba, Val-de-Reuil, France), tetraethoxysilane (TEOS, 98%, Sigma Aldrich, Milwaukee, WI, USA), ammonium hydroxide (NH4OH, 25%, Sigma Aldrich, Milwaukee, WI, USA), and ethanol (ethanol absolute anhydrous, ≥99.9%, Carlo Erba, Cornaredo, Italy) were used for the preparation of porous carbon particles (PCPs). Sulfuric acid (H2SO4, 95–97%, Merck, Darmstadt, Germany), nitric acid (HNO3, ≥65%, Sigma-Aldrich, Milwaukee, WI, USA), dimethylformamide (DMF, 99%, Sigma Aldrich Milwaukee, WI, USA), epichlorohydrin (ECH, 99%, Sigma-Aldrich, Milwaukee, WI, USA), and polyethyleneimine (PEI, 50% in water, Mw:1800, Sigma-Aldrich, Milwaukee, WI, USA) were used in the modification of PCPs. Cobalt chloride hexahydrate (CoCl2.6H2O, 98%, Acros, Geel, Belgium), nickel chloride hexahydrate (NiCl2.6H2O, 98%, Acros, Geel, Belgium), and copper chloride (CuCl2 anhydrous, 98%, Acros, Geel, Belgium) were used as corresponding metal ion sources. Sodium borohydride (NaBH4, 98%, Merck, Darmstadt, Germany) was used as a reducing agent and for the preparation of metal nanoparticles. Also, both sodium borohydride (NaBH4, 98%, Merck) and ammonia–borane (NH3BH3, 97%, Aldrich, Milwaukee, WI, USA) were used for the production of hydrogen from hydrolysis reactions. Double distilled water was used for washing the prepared particles.
2.2. Synthesis and Modification of PCPs
All details about the synthesis of PCPs and modification of PCPs with PEI (PCP-PEI) were reported in the literature in our earlier study [44] and performed accordingly.
2.3. In Situ Metal Particle Synthesis Within PCP-PEI
Chloride salts of related metal ions were used in the preparation of Co, Ni, and Cu metal nanoparticles within PCP and PCP-PEI structures. Accordingly, 1.0 g of PCP and PCP-PEI was placed in 250 mL of 1000 ppm aqueous Co(II), Ni(II), and Cu(II) solutions separately, which were mixed at a mixing speed of 500 rpm for 4 h to load the related metal ions into the PCP or PCP-PEI structures. Then, the metal ion-loaded PCP-M(II) and PCP-PEI-M(II) (M:Co, Ni, or Cu) structures were placed separately in a freshly prepared 0.1 M 50 mL aqueous NaBH4 solution under a constant mixing speed of 500 rpm. The metal ions were converted to the relevant metal nanoparticles as the reaction was completed, upon which no more gas evolution was observed. Then, these prepared M@PCP and M@PCP-PEI (M:Co, Ni, or Cu) composites were used as catalysts to produce H2 from NaBH4 and NH3BH3 hydrolysis reactions.
The amounts of in situ synthesized metal nanoparticles within PCP and PCP-PEI were determined by atomic absorption spectroscopy (Thermo, ICA 3500 AA SPECTRO, Bedford, MA, USA) from the metal ion solution obtained by treating M@PCP and M@PCP-PEI composites with 5 M 20 mL HCl three times for 8 h at a 500 rpm mixing rate to dissolve the metal nanoparticles from the M@PCP and M@PCP-PEI composites.
High-contrast transmission electron microscopy (CTEM, FEI 120 kV, Hillsboro, OR, USA) was utilized to evaluate the morphology and dimensions of in situ synthesized metal nanoparticles within PCP-PEIs. For all transmission electron microscopy (TEM) analyses, M@PCP-PEI particles were initially dispersed in ethanol and subjected to ultrasonic cleaning for a duration of 1.45 min. Subsequently, a drop of the resulting suspension was placed onto a formvar-coated TEM grid and then allowed to dry overnight, and the corresponding images were acquired.
2.4. Catalytic Activity of M@PCP and M@PCP-PEI (M:Co, Ni, or Cu) Composites
2.4.1. Hydrolysis of NaBH4
After adding certain quantities of M@PCP and M@PCP-PEI (M:Co, Ni, or Cu) composites, which contained the same amount (mmol) of metal particles, 0.0476 mmol M within M@PCP, and 0.0788 mmol M within M@PCP-PEI), were placed in a reaction flask containing 50 mM (0.0965 g) NaBH4 in 50 mL distilled water for the hydrolysis of NaBH4. The reaction parameters for the hydrolysis reactions were 50 mL 50 mM NaBH4 and a mixing rate of 1000 rpm at 30 °C. According to the NaBH4 hydrolysis reaction (Equation (1)), the produced H2 was recorded as a function of time via a water-filled inverted graded cylinder based on replaced water volume with generated H2 gas.
2.4.2. Hydrolysis of NH3BH3
After adding certain quantities of M@PCP-PEI (M:Co, Ni, or Cu) composites, 0.0788 mmol M) in a reaction flask containing 50 mM (0.0795 g) NH3BH3 in 50 mL distilled water, the hydrolysis of NH3BH3 was carried out. The reaction parameters in the hydrolysis reactions were 50 mL 50 mM NH3BH3 and a mixing rate of 1000 rpm at 30 °C. According to the hydrolysis reaction of NH3BH3 (Equation (2)), the produced H2 was also recorded as a function of time.
2.5. Activation Parameters for NaBH4 and NH3BH3 Hydrolysis Catalyzed by Co@PCP-PEI Composites
Activation parameters such as activation energy (Ea), enthalpy (ΔH), and entropy (ΔS) were calculated for the Co@PCP-PEI composite-catalyzed hydrolysis of both NaBH4 and NH3BH3 according to Arrhenius (Equation (3)) and Eyring (Equation (4)) equations.
where k is the reaction rate constant, which was calculated according to a zero-order kinetic expression, Ea is the activation energy, T is the absolute temperature (K), kB is the Boltzmann constant (1.381 × 10−23 J K−1), h is Planck’s constant (6.626 × 10−34 J·s), ΔH is the activation enthalpy, ΔS is the entropy, and R is the gas constant (8.314 JK−1 mol−1).
k = A × e [Ea/RT]
ln (k/T) = −(ΔH/R)(1/T) + ln(kB/h) + ΔS/R
2.6. Reuse of Catalyst in Hydrolysis of NaBH4 and NH3BH3
The reusabilities of Co@PCP-PEI composite catalysts in the hydrolysis of NaBH4 and NH3BH3 were investigated following the literature [12,38]. The reusability parameters, such as conversion% and activity%, of catalysts were compared. The conversion% was defined as the produced amount of hydrogen via the catalyzed reaction according to the stoichiometry of both the hydrolysis of NaBH4 and NH3BH3 as given in Equation (1) and Equation (2), respectively. The activity% was defined as the ratio of the initial H2 production rate for each consecutive use based on half the amount of H2 that is produced stoichiometrically as the measure of catalyzing efficiency or potency of Co@PCP-PEI composite catalysts for the hydrolysis of NaBH4 and NH3BH3. For the investigation of the reusability of catalysts, after the initial hydrolysis of NaBH4 and NH3BH3, fresh NaBH4 and NH3BH3 at the same quantities as before (0.0965 g for NaBH4 and 0.0795 g for NH3BH3) were added individually nine more times, and the change in the conversion% and activity% of the catalysts were calculated for each use. All the reusability tests of Co@PCP-PEI composite catalysts in the H2 production reaction for the hydrolysis of NaBH4 and NH3BH3 were performed in triplicate, and the results of the conversion% and activity% of catalysts were presented as their averages with standard deviations.
3. Results and Discussion
3.1. Synthesis and Characterization M@PCP and M@PCP-PEI Composite Catalysts
The details for the synthesis and characterization of PCP and PCP-PEI structures, which were used here as templates for in situ metal particle preparations, were reported in our previous study [44]. The modification of PCPs with PEI was confirmed with the appearance of −NH2 peaks at 1604 cm−1 in the FT-IR as well as the change in the surface charge of PCP that was −12.5 ± 2.7 mV and increased to +13.4 ± 3.1 mV after PEI modification [38]. The particle size of PCP particles was reported as 967 ± 61 nm and increased to 1123 ± 92 nm after PEI modification, and the surface area of PCPs decreased from 723 ± 57 m2/g to 611 ± 75 m2/g upon PEI modification. Moreover, these prepared PCP-PEI structures were used as catalysts in the methanolysis of NaBH4 [44]. Here, PCP and PCP-PEI structures were used as a template in the synthesis of metal nanoparticles such as Co, Ni, and Cu nanoparticles in situ as the schematic presentation of the employed process is illustrated in Figure 1.
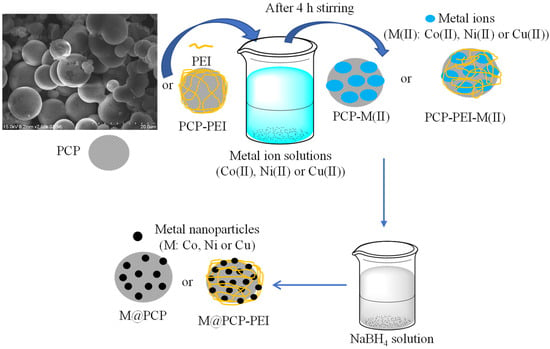
Figure 1.
Schematic presentation of Co, Ni, or Cu metal nanoparticle synthesis within PCP and PCP-PEI structures.
The PCP and PCP-PEI particles were placed into 1000 ppm 250 mL of Co(II), Ni(II), and Cu(II) metal ion solutions and stirred for 4h at 500 rpm to load the corresponding metal ions into PCP and PCP-PEI structures. Finally, the metal ion-loaded PCP and PCP-PEI structures were placed into 0.1 M 50 mL aqueous NaBH4 solutions separately and stirred at 500 rpm until the gas evolution stopped. Then, the obtained M@PCP and M@PCP-PEI (M:Co, Ni, or Cu) composites were used as catalysts for the hydrolysis of both NaBH4 and NH3BH3 to produce H2. A comparative analysis of the powder X-ray diffraction (X-RD, Panalytical X’Pert Pro MPD X-Ray Diffractometer, AE Almelo, The Netherlands) patterns for PCP and M@PCP composites is presented in Figure S1. The X-RD data revealed two prominent diffraction peaks at 2θ values of 23.1° (002) and 43.24° (100), which are associated with carbon, as indicated by the PCP X-RD pattern shown in Figure S1 [45,46]. In contrast, the X-RD patterns of the Co@PCP composites exhibited no significant alterations. Conversely, the Ni@PCP composites displayed additional peaks at 2θ = 35.2° (111) and 61.3° (200), which correspond to nickel species within the PCP matrix [47,48]. Furthermore, the X-RD pattern for the Cu@PCP composites indicated several peaks characteristic of copper structures, specifically at 2θ = 36.6°, 42.6°, and 61.8°, corresponding to the (111), (200), and (220) planes of Cu2O, respectively [49,50]. Additionally, the peaks at 2θ = 43.7° and 74.1° associated with the (111) and (220) planes were attributed to copper nanoparticles [49,50]. The X-RD patterns for PCP-PEI and the related M@PCP-PEI composites have been documented in previous studies conducted by our research group [51]. To further validate and quantify the in situ synthesis of Co, Ni, and Cu particles within the PCPs, atomic absorption spectroscopy (AAS) analyses were performed, and the quantity of metal nanoparticles were determined. The corresponding results are given in Table 1.

Table 1.
The amounts of Co, Ni, and Cu metal nanoparticles within PCP-based structures.
The amount of metal nanoparticles within PCP and PCP-PEI was determined by atomic absorption spectroscopy (AAS) analysis. For this purpose, the metal particle containing the carbon particle composite weighing 100 mg was treated with 5 M 20 mL HCl at room temperature at 500 rpm for 8 h three times. Then, the eluted M(II) ions in the solution were analyzed with AAS. The amounts of M(II) ions were determined with AAS as summarized in Table 1. It was observed that the metal ion contents of M@PCP composites were lower than those of M@PCP-PEI composites, as expected. The presence of amine groups in PCP-PEI led to greater M(II) binding ability due to amine–M(II) complex formation.
The amount of Co metal particles in PCP and PCP-PEI were determined as 19.2 ± 0.9 and 29.8 ± 1.1 mg/g, respectively. On the other hand, Ni contents of Ni@PCP and Ni@PCP-PEI structures were calculated as 13.9 ± 1.0 and 48.2 ± 2.4 mg/g, respectively. Similarly, Cu content in Cu@PCP-PEI was almost 3-fold higher than in Cu@PCP at 31.3 ± 1.9 compared to 90.4 ± 3.2 mg/g.
3.2. Catalytic Activity of M@PCP and M@PCP-PEI Composites in Hydrogen Production Reaction from Hydrolysis of NaBH4 and NH3BH3
The catalytic activity of metal-free PCP-PEI structures in the methanolysis of NaBH4 was reported earlier in our previous study [44]. Here, the catalytic activity of M@PCP and M@PCP-PEI (M:Co, Ni, or Cu) composite particles for H2 production reactions from the hydrolysis of NaBH4 and NH3BH3 were examined. The experimental setup used to determine the catalytic activity of M@PCP and M@PCP-PEI composites was a 50 mL water-filled round bottom flask containing catalysts, and 50 mM NaBH4/NH3BH3. This reaction flask was connected with a trap containing concentrated sulfuric acid that was also connected to the inverted volumetric cylinder filled with water. In this set up, the H2 generated in the flask was transferred from the trap to collect any water moisture and then to the water-filled volumetric cylinder. Then, the produced H2 was replaced with water in the volumetric cylinder, enabling the easy reading of the volume of produced H2.
3.2.1. Hydrogen Production from Hydrolysis of NaBH4
The catalytic activities of M@PCP and M@PCP-PEI (M:Co, Ni, or Cu) composites in the hydrolysis of NaBH4 were compared and the related graphs are given in Figure 2. To compare the catalytic activities of M@PCP composites in the NaBH4 hydrolysis reaction, 156 mg of Co@PCP, 200 mg of Ni@PCP, and 96 mg of Cu@PCP composites, with all having around 0.048 mmol metal nanoparticles, were used. It can be clearly seen in Figure 2a that the Co@PCP composites catalyzed the hydrolysis of NaBH4 completely in 300 min with 251 ± 1 mL of H2 production. On the other hand, Ni@PCP composites catalyzed the same reaction completely in 540 min with 251 ± 1 mL of H2 produced. However, Cu@PCP composites did not exhibit any catalytic activity for NaBH4 hydrolysis as only 100 mL of H2 was produced in 150 min, which is equal to the amount of produced H2 from the self-hydrolysis reaction of NaBH4 (without catalyst) in 150 min. Moreover, the catalytic activities of M@PCP-PEI (M:Co, Ni, or Cu) for NaBH4 hydrolysis were also compared and the results are presented in Figure 2b. For this objective, 156 mg of Co@PCP-PEI, which equates to 0.0788 mmol metal particles, and equal mmol metal particles containing 96 mg Ni@PCP-PEI and 55 mg Cu@PCP-PEI composites were used for the catalytic hydrolysis of NaBH4. As clearly seen, both Co@PCP-PEI and Ni@PCP-PEI composites catalyzed the reactions much faster than Co@PCP and Ni@PCP composites. The hydrolysis of NaBH4 catalyzed by Co@PCP-PEI and Ni@PCP-PEI composites were completed in 120 and 210 min, respectively, with both producing 251 ± 1 mL of H2. As the amounts of metal nanoparticles are higher within Co@PCP-PEI and Ni@PCP-PEI composites compared to Co@PCP and Ni@PCP composites, these results are reasonable.
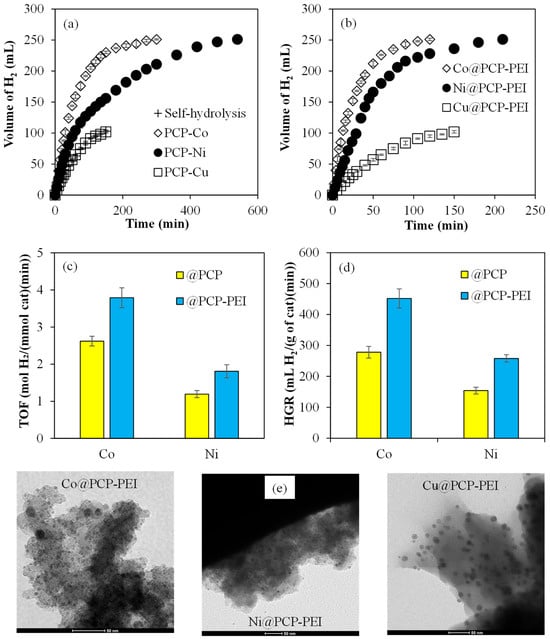
Figure 2.
The catalytic activity of (a) M@PCP and (b) M@PCP-PEI composites on the hydrolysis of NaBH4 to produce H2; comparison of (c) TOF and (d) HGR values of M@PCP and M@PCP-PEI composite-catalyzed NaBH4 hydrolysis reactions, and (e) TEM images of M@PCP-PEI composites [reaction conditions: M:Co, Ni, or Cu, 0.0476 mmol M for M@PCP, 0.0788 mmol M for M@PCP-PEI, 50 mL water, 0.0965 g NaBH4, 30 °C, 1000 rpm].
For the comparison of the catalytic activity of M@PCP and M@PCP-PEI composite catalysts, important parameters such as turn-over frequency (TOF, mol H2/(mmol cat·min)) and hydrogen generation rate (HGR, mL H2/(g cat·min)) for M@PCP and M@PCP-PEI composites were calculated and are illustrated in Figure 2c,d, respectively. For both TOF and HGR calculations, the number of catalysts (moles) was taken into consideration as the particles have an approximate 10 nm size range, assuming most metal nanoparticles possess many active sites in the composite systems and 100% are active. In Figure 2c, the calculated TOF values for both Co@PCP-PEI and Ni@PCP-PEI are 3.8 ± 0.3 and 1.8 ± 0.2 mol H2/(mmol cat·min), respectively. These are almost 1.5-fold higher than the values for Co@PCP and Ni@PCP, which are 1.6 ± 0.1 and 1.2 ± 0.1 (mol H2/(mmol cat·min)), respectively. The calculated HGR values for the M@PCP and M@PCP-PEI composite-catalyzed hydrolysis of NaBH4 are shown in Figure 2d. The HGR values of 452 ± 31 for Co@PCP-PEI and 258 ± 12 mL H2/(g cat·min) for Ni@PCP-PEI are higher than the values for Co@PCP and Ni@PCP which are 278 ± 19 and 154 ± 11 mL H2/(g cat·min), respectively. The effects of the amount of metal particle and temperature on the catalytic activity of the Co@PCP-PEI catalyst in the hydrolysis of NaBH4 were also investigated because of the higher TOF and HGR values among the prepared catalysts used in the hydrolysis of NaBH4. Moreover, to confirm the presence of in situ synthesized metal nanoparticles within PCP-based materials and their sizes, TEM images of M@PCP-PEI composites with higher metal nanoparticle content and catalytic activity were taken and are given in Figure 2e. The dimensions of in situ synthesized Co and Ni nanoparticles within PCP-PEI are about 5 and 10 nm, whereas Cu nanoparticles exhibited slightly bigger particles sizes varying from 10 to 20 nm.
The amounts of Co metal particles within Co@PCP-PEI composites were increased by multiple loading and reducing cycles. For example, after Co metal nanoparticles were synthesized within PCP-PEI as Co@PCP-PEI, these composites were then placed in 250 mL 1000 ppm aqueous Co(II) ion solutions and stirred for 4 h for the second loading of Co(II) ions into Co@PCP-PEI composites. Then, these two-time Co(II) ion-loaded Co@PCP-PEI composites were washed with water to remove unbound Co(II) ions on the surfaces, then placed into a freshly prepared 50 mL 0.1 M NaBH4 solution to reduce for a second time the loaded Co(II) ions to Co metal nanoparticles, and stirred at 500 rpm until the evolution of the gas stopped as an indication of the reduction of Co(II) to the corresponding Co metal nanoparticles. This reloading/reducing cycle was repeated one more time for the preparation Co@PCP-PEI composites. The amounts of Co metal particles in Co@PCP-PEI composites after the first, second, and third loading/reducing process were determined as 29.8 ± 1.1, 35.6 ± 2.2, and 44.3 ± 4.9 mg/g, respectively, and are given in Table 1. It is obvious that the amount of metal nanoparticles within PCP-PEI can easily increase via multiple loading/reducing cycles. The effects of the amount of Co metal particles within the Co@PCP-PEI composite on its catalytic activity were also investigated and the results are given in Figure 3a. The catalytic activity of one-time loaded/reduced Co@PCP-PEI composite-catalyzed hydrolysis of NaBH4 was completed in 120 min with 251 ± 1 mL of H2 production, whereas the same amount of H2 was produced in 80 and 50 min after the second and third Co(II) ion-loaded/reduced cycles. The comparison of TOF and HGR values for multiple loaded/reduced Co@PCP-PEI composite-catalyzed reactions is shown in Figure 3b. The calculated TOF values for Co@PCP-PEI composite-catalyzed reactions increased from 3.8 ± 0.3 mol H2/(mmol cat·min) to 5.5 ± 0.5 mol H2/(mmol cat·min) with increasing loading/reducing cycles of Co@PCP-PEI catalysts. In addition, the calculated HGR values also increased from 452 ± 31 mL H2/(g cat·min) to 729 ± 49 mL H2/(g cat·min) for the Co@PCP-PEI-catalyzed reaction from one cycle to three. The increase in the amount of Co metal nanoparticles within the Co@PCP-PEI composite catalysts also led to an increase in catalytic activity for the hydrolysis of NaBH4, which exhibited higher TOF and HGR values.
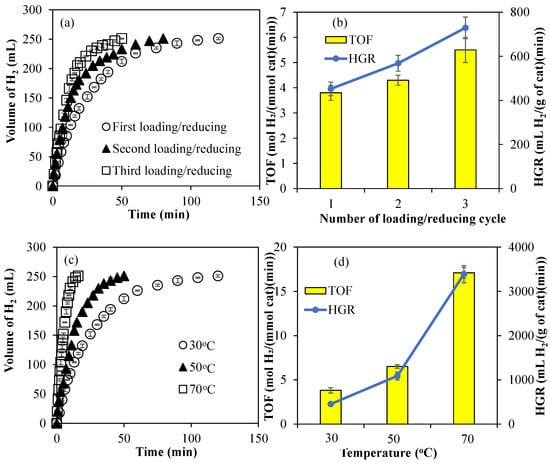
Figure 3.
(a) The effect of the amount of Co metal nanoparticles on the catalytic activity of Co@PCP-PEI composites in the hydrolysis of NaBH4; (b) comparison of TOF and HGR values of multiple-Co(II)-loaded/reduced Co@PCP-PEI composite catalyst; (c) effect of temperature on the hydrolysis of NaBH4 catalyzed by Co@PCP-PEI composite catalysts (Co: 29.8 ± 1.1 mg/g); (d) comparison of TOF and HGR values of Co@PCP-PEI composite catalysts for the hydrolysis of NaBH4 carried out at different temperatures (Co: 29.8 ± 1.1 mg/g) [reaction conditions: 50 mL water, 0.0965 g NaBH4, mixing rate: 1000 rpm].
The effect of temperature on the catalytic activity of the Co@PCP-PEI-catalyzed hydrolysis of NaBH4 was also investigated by carrying the catalyzed reactions at 30, 50, and 70 °C. In Figure 3c, the Co@PCP-PEI-catalyzed hydrolysis of NaBH4 at 30, 50, and 70 °C was completed in 120, 50, and 16 min, respectively each with the same amount of H2 produced, 251 ± 1 mL. The reaction rates for the Co@PCP-PEI-catalyzed hydrolysis of NaBH4 was increased with the increase in the temperature, as expected. Additionally, as demonstrated in Figure 3d, the TOF and HGR values of the Co@PCP-PEI-catalyzed hydrolysis of NaBH4 also increased with the increase in reaction temperature from 30 to 70 °C. The TOF value for the Co@PCP-PEI-catalyzed reaction, 3.8 ± 0.3 mol H2/(mmol cat·min) at 30 °C, was increased almost 5-fold by increasing the temperature to 70 °C with a 17.1 ± 0.6 mol H2/(mmol cat·min) TOF value. Similarly, the HGR values of the Co@PCP-PEI-catalyzed hydrolysis of NaBH4 at 30 °C was increased by almost 8-fold at 70 °C, i.e., from 452 ± 31 to 3390 ± 193 mL H2/(g cat·min). The HGR values obtained at 30 °C for NaBH4 hydrolysis reactions utilizing Co@PCP-PEI composites are comparatively lower than those reported for other catalysts containing Co metal nanoparticles reported in the literature, e.g., the NaBH4 hydrolysis reaction catalyzed by B-doped Co3O4 nanowires with 7055 mL H2/(g cat·min) [52], nitrogen-doped mesoporous graphitic carbon-encapsulated cobalt nanoparticles (Co@NMGC) with 3575 mL H2/(g cat·min) [53], bacterial cellulose/Co-B (BC/Co-B) nanocomposites with 3887 mL H2/(g cat·min) [54], Co nanoparticles supported on carbon nanospheres (CNSs) (CNSs@Co) with 7447 mL H2/(g cat·min) [55], Co-CeOx/nitrogen-doped carbon nanosheet (NCNS) with 28,410 mL H2/(g cat·min) [56], CoB/TiO2−x catalyst with 3070 mL H2/(g cat·min) [57], Co6FeAl-LDH catalyst with 4955 mL H2/(g cat·min) [58], Co(30%)/Fe3O4@GO with 6005 mL H2/(g cat·min) [59], and g-C3N4/Co–Mo–B/Ni foam with 9958 mL H2/(g cat·min) values [60]. Nevertheless, these composites revealed promising potential to perform competitively at elevated temperatures. It is important to acknowledge that the necessity for high operational temperatures may pose economic and energy-related challenges for the synthesized catalyst. Furthermore, an increase in the concentration of Co nanoparticles incorporated into the PCP-PEI matrix is associated with an improvement in the HGR value. Therefore, this limitation can be mitigated by increasing the concentration of Co nanoparticles within the PCP-PEI composites.
3.2.2. H2 Production for Hydrolysis of NH3BH3
Another H2 carrier, NH3BH3, can also be catalyzed by M@PCP-PEI (M:Co, Ni, or Cu) composites to produce H2. Therefore, M@PCP-PEI (M:Co, Ni, or Cu) composites were used as a catalyst in the hydrolysis of NH3BH3. As illustrated in Figure 4a, the catalytic performance of the M@PCP-PEI (M:Co, Ni, or Cu) composite catalyst was compared using 156 mg of Co@PCP-PEI, 96 mg of Ni@PCP-PEI, and 55 mg of Cu@PCP-PEI composites, which refers to 0.0788 mmol metal nanoparticles in the hydrolysis of NH3BH3. Co@PCP-PEI composites catalyzed the complete hydrolysis of NH3BH3 in 28 min with 181 ± 1 mL H2 production, which was faster than Ni@PCP-PEI and Cu@PCP-PEI composite-catalyzed reactions, which were completed in 70 and 130 min, respectively, with 181 ± 1 mL H2 production.
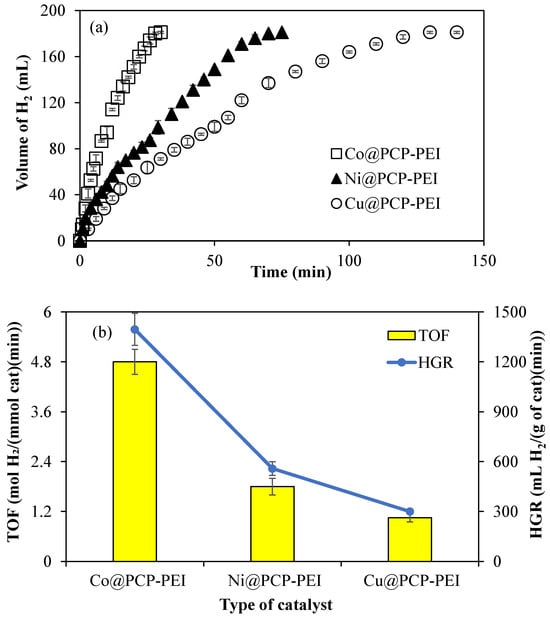
Figure 4.
(a) The catalytic activity of M@PCP-PEI composite catalysts in the hydrolysis of NH3BH3 to produce H2, and (b) comparison of TOF and HGR values of the M@PCP-PEI composite catalyst [reaction condition: M:Co, Ni, or Cu, 0.0788 mmol M, 50 mL water, 0.0795 g NH3BH3, 30 °C, 1000 rpm].
The comparison of TOF and HGR values for the M@PCP-PEI (M:Co, Ni, or Cu)-catalyzed hydrolysis of NH3BH3 is given in Figure 4b. It is obvious that Co@PCP-PEI composites exhibited higher TOF and HGR values, at 4.8 ± 0.3 mol H2/(mmol cat·min) and 1395 ± 96 mL H2/(g cat·min), respectively, than the other two composite catalysts. These TOF and HGR values calculated for Co@PCP-PEI catalyzed reactions are almost 2- and 4-fold higher than the calculated TOF and HGR values for Ni@PCP-PEI and Cu@PCP-PEI composite-catalyzed reactions, respectively.
The effects of the amount of metal nanoparticles and the reaction temperature on the hydrolysis of NH3BH3 were investigated for Co@PCP-PEI composite catalysts due to their higher TOF and HGR values. As presented in Figure 5a, the effect of the amounts of Co nanoparticles within PCP-PEI is increased with multiple loading/reducing cycles, as mentioned previously. The catalytic activity of Co@PCP-PEI composites in the hydrolysis of NH3BH3 was increased with the increase in the amount of Co metal nanoparticles. The Co@PCP-PEI composite-catalyzed hydrolysis of NH3BH3 was completed in 28 min with 181 ± 1 mL H2 production, whereas the same reaction was completed in 21 and 9.5 min, respectively, for two- and three-time Co(II)-loaded/reduced Co@PCP-PEI composites as the catalyst, with each producing 181 ± 1 mL H2. The comparison of TOF and HGR values of the one-, two-, and three-time Co(II) ion-loaded/reduced composite-catalyzed hydrolysis of NH3BH3 also revealed that these values increased with the increase in amount of Co metal nanoparticles (or increased number of loaded/reduced cycles), as shown in Figure 5b. The TOF value of the one-time Co(II) ion-loaded/reduced CP-PEI, Co@PCP-PEI composite-catalyzed hydrolysis of NH3BH3 is 4.8 ± 0.3 mol H2/(mmol cat·min) and increased to 7.9 ± 0.3 mol H2/(mmol cat·min) upon using three-time Co(II) ion-loaded/reduced Co@PCP-PEI composite catalysts. Similarly, the HGR values were calculated for first- and third-time Co(II) ion-loaded Co@PCP-PEI composites that were used in the hydrolysis of NH3BH3 and were calculated as 1395 ± 96 and 2766 ± 162 mL H2/(g cat·min), respectively. The calculated TOF and HGR values for the first-time Co(II) ion-loaded/reduced composite catalyst were increased almost 2-fold upon three-time Co(II) ion-loaded/reduced cycles for Co@PCP-PEI composite-catalyzed reactions. This is reasonable as the increased amount of Co metal particles affords higher catalytic performance than lesser amounts of Co metal particle-containing Co@PCP-PEI composite catalysts. On the other hand, it can be clearly seen from Figure 5c that the increase in the reaction temperature of the Co@PCP-PEI-catalyzed hydrolysis of NH3BH3 increased the reaction rates as anticipated. The hydrolysis of NH3BH3 was completed in 28, 12, and 6 min in the presence of the Co@PCP-PEI catalyst at 30, 50, and 70 °C, respectively, with all producing 181 ± 1 mL H2.
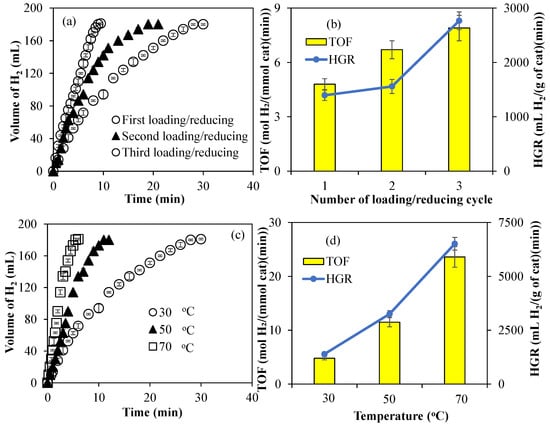
Figure 5.
(a) The effect of the amounts of Co metal nanoparticles on the catalytic activity of Co@PCP-PEI composites in the hydrolysis of NH3BH3; (b) comparison of TOF and HGR values; (c) the effect of temperature on the Co@PCP-PEI composite-catalyzed hydrolysis of NH3BH3; (d) comparison of TOF and HGR values at different temperatures [reaction conditions: 50 mL water, 0.07955 g NH3BH3, 1000 rpm].
It is also evident from the comparison of TOF and HGR values as illustrated in Figure 5d for the Co@PCP-PEI-catalyzed hydrolysis of NH3BH3 at 30, 50, and 70 °C that the increase in the reaction temperature increases the values of TOF and HGR. The TOF value of 4.8 ± 0.3 mol H2/(mmol cat·min) and the HGR value of 1395 ± 96 mL H2/(g cat·min) for the Co@PCP-PEI-catalyzed hydrolysis of NH3BH3 at 30 °C were increased almost 5-fold and calculated as 23.6 ± 0.3 mol H2/(mmol cat·min) (TOF value) and 6514 ± 293 mL H2/(g cat·min) (HGR value), respectively at 70 °C. The calculated HGR values for the Co@PCP-PEI-catalyzed NH3BH3 hydrolysis reaction are already competitive with those of similar studies reported in the literature such as those of the Co–P/Ni foam-catalyzed hydrolysis of NH3BH3 with 1248 mL H2/(g cat·min) [61] and CoB nanowire-catalyzed hydrolysis of NH3BH3 with 2667 mL H2/(g cat·min) [62].
3.3. Activation Parameters for Co@PCP-PEI-Catalyzed Hydrolysis of Both NaBH4 and NH3BH3
The activation energy (Ea), enthalpy (ΔH) and entropy (ΔS) for the Co@PCP-PEI-catalyzed hydrolysis of NaBH4 and NH3BH3 were calculated using Arrhenius and Eyring equations from the half H2 production curves with time at 30, 50, and 70 °C. The corresponding Arrhenius and Eyring plots of the Co@PCP-PEI-catalyzed hydrolysis of both NaBH4 and NH3BH3 are given in Figure S2. From this figure, the calculated Ea, ΔH, and ΔS are summarized in Table 2.

Table 2.
The Ea, ΔH, and ΔS values for the Co@PCP-PEI composite-catalyzed hydrolysis of NaBH4 and NH3BH3 and their comparison with some similar studies reported in the literature.
The Ea values for the Co@PCP-PEI=catalyzed hydrolysis of both NaBH4 and NH3BH3 were calculated as 29.5 and 32.3 kJ/mol, respectively. The Ea value of the Co@PCP-PEI-catalyzed hydrolysis of NaBH4 was compared with those of similar studies reported in the literature; these varied, with most being higher. For example, NaBH4 hydrolysis reaction were catalyzed by B-doped Co3O4 nanowires with Ea = 29.7 kJ/mol [52], nitrogen-doped mesoporous graphitic carbon-encapsulated cobalt nanoparticles (Co@NMGC) with Ea = 35.2 kJ/mol [53], bacterial cellulose/Co-B (BC/Co-B) nanocomposites with Ea = 56.4 kJ/mol [54], Co nanoparticles supported on carbon nanospheres (CNSs) (CNSs@Co) with Ea = 40.8 kJ/mol [55], Co-CeOx/nitrogen-doped carbon nanosheet (NCNS) with Ea = 44.2 kJ/mol [56], CoB/TiO2-x catalyst with Ea = 57.0 kJ/mol [57], Co6FeAl-LDH catalyst with Ea = 35.5 kJ/mol [58], Co(30%)/Fe3O4@GO with Ea = 44.4 kJ/mol [59], and g-C3N4/Co–Mo–B/Ni foam with Ea = 52.6 kJ/mol [60]. In this study, the Ea value (29.5 kJ/mol) is lower than the reported energy activation values of the last two years of the Co-based catalysts, as presented in Table 2. On the other hand, the determined Ea value for the Co@PCP-PEI-catalyzed hydrolysis of NH3BH3, 32.3 kJ/mol, is also competitive with reported activation energy values for the same reaction reported in the literature, such as the Co–Mo–B/Ni foam-catalyzed hydrolysis of NH3BH3 with Ea = 44.3 kJ/mol [61], Co–P/Ni foam-catalyzed hydrolysis of NH3BH3 with Ea = 48.0 kJ/mol [62], CoB nanowire-catalyzed hydrolysis of NH3BH3 with Ea = 16.2 kJ/mol [63], Ag@Pd composite-catalyzed hydrolysis of NH3BH3 with Ea = 50.1 kJ/mol [64], and Ru1Ni1.90/nitrogen-doped carbon skeleton (NCS)-catalyzed hydrolysis of NH3BH3 with Ea = 26.5 kJ/mol [65]. Therefore, it is apparent that Co@PCP-PEI composite catalysts are more favorable materials in terms of H2 generation using either of the H2 sources, NaBH4 and NH3BH3.
3.4. Reusability of Co@PCP-PEI Composite Catalyst
The cost consideration of catalysts in industrial applications is one of the most important constraints. The reusability of catalysts to reduce costs in industrial applications is of paramount significance. Therefore, the reusability of Co@PCP-PEI composites in both hydrolysis reactions of NaBH4 and NH3BH3 were tested, and the corresponding graphs are given in Figure 6. In Figure 6a, the reuse of Co@PCP-PEI composite catalysts in the hydrolysis of NaBH4 is given and 100% conversions were attained for all hydrolyses of NaBH4 up to 10 consecutive uses. On the other hand, the activity% of the Co@PCP-PEI composite catalyst for the hydrolysis of NaBH4 in 10 consecutive usages revealed that the activity remains at 100% for up to 7 consecutive uses, and after the 10th use, approximately 85% of its activity is preserved.
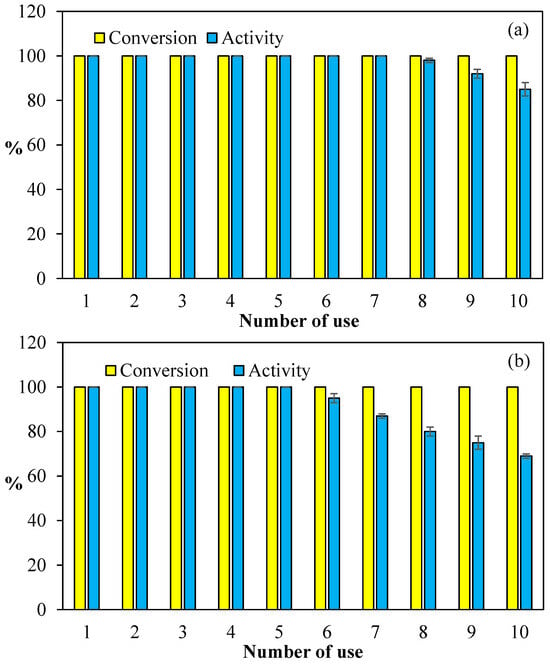
Figure 6.
The reusability of Co@PCP-PEI composite catalysts in the hydrolysis of (a) NaBH4 and (b) NH3BH3 [reaction conditions: 0.0788 mmol Co, 50 mL water, 0.0965 g NaBH4, 0.0795 g NH3BH3, 30 °C, 1000 rpm].
Additionally, the reusability of Co@PCP-PEI composite catalysts in the hydrolysis of NH3BH3 was also compared and the results are shown in Figure 6b. As presented, the Co@PCP-PEI composite-catalyzed hydrolysis of NH3BH3 afford 100% conversion even at the 10th consecutive use. On the other hand, the activity of this catalyst maintained its activity% up to the 5th use at 100%, and slowly decreased, e.g., between the 6th-10th use, the activity% was reduced from 95 ± 2 to 69 ± 1%. Nevertheless, the Co@PCP-PEI composite catalysts exhibited almost 70% activity at the 10th repetitive use.
Overall, the seven-time and 5-time successive uses of Co@PCP-PEI composite catalysts afford 100% activity in the hydrolysis of NaBH4 and NH3BH3, standing out as the most important feature of these catalysts along with their 100% conversion capability with up to 10 repeated uses, making this catalyst system a promising material for industrial applications. The observed reduction in the catalytic activity% of Co@PCP-PEI during the hydrolysis reactions of NaBH4 and NH3BH3 is due to the accumulation of reaction by-products on the catalyst surface. This phenomenon has been reported in the existing literature and was confirmed with XRD and FT-IR analyses [66,67].
4. Conclusions
PCP and PCP-PEI structures were successfully used as templates to prepare metal nanoparticles such as Co, Ni, and Cu, in situ. The prepared M@PCP and M@PCP-PEI (M:Co, Ni, or Cu) composites were used as catalysts for the hydrolysis of both NaBH4 and NH3BH3 to produce H2. The hydrolysis of NaBH4 and NH3BH3 catalyzed by Co@PCP-PEI resulted in higher TOF and HGR values than the M@PCP and M@PCP-PEI (M:Ni or Cu) composite catalysts. The TOF values for the Co@PCP-PEI composite-catalyzed hydrolysis of NaBH4 and hydrolysis of NH3BH3 were calculated as 3.8 ± 0.3 and 4.8 ± 0.3 mol H2/(mmol cat·min), respectively; the HGR values were calculated as 452 ± 31 and 1395 ± 96 mL H2/(g cat·min), in the same order. Moreover, the determined Ea value for the Co@PCP-PEI composite-catalyzed hydrolysis of NaBH4 was 29.3 kJ/mol, which is lower than the Ea value of the Co@PCP-PEI composite-catalyzed hydrolysis of NH3BH3, which was 32.5 kJ/mol. However, these Ea values are competitive with those of similar reported studies in literature. It was further demonstrated that the Co@PCP-PEI composite possesses high reuse capability, with 100% conversions up to 10 successive uses in the hydrolysis of NaBH4 and NH3BH3. After seven and five repetitive deployments, 100% of the activities were obtained and there was a slight reduction afterwards. As a result, M@PCP-PEI (M:Co, Ni, and Cu) catalyst systems with transition metal nanoparticles can be presumed economically viable and may be employed in sophisticated H2-driven devices for clean and environmentally benign applications. A significant finding of the relevant research is its contribution for the development of multifunctional materials that can also be used in other applications including the catalytic reduction of even CO2 while simultaneously adsorbing it, e.g., M@PCP or M@PCP-PEI were reported for these purposes [51,68]. These materials provide multiple advantages to address many issues beyond renewable energy sources contributing to mitigating global warming.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/mi16020172/s1, Figure S1: The XRD patterns of M@PCP (M:Co, Ni, or Cu) composites.; Figure S2: The Arrhenius graphs of the hydrolysis of (a) NaBH4 and (b) NH3BH3, and Eyring graphs of the hydrolysis of (c) NaBH4 and (d) NH3BH3 catalyzed by Co@PCP-PEI composites.
Author Contributions
Conceptualization, N.S.; methodology, S.D. and N.S.; validation, S.D., O.P.; formal analysis, S.D., O.P. and N.S.; investigation, S.D., O.P. and N.S.; resources, N.S.; writing—original draft preparation, S.D. and O.P.; writing—review and editing, N.S.; visualization, N.S.; supervision, N.S.; project administration, N.S.; funding acquisition, N.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All the data generated in this research is retained within the manuscript and in Supplementary Materials.
Acknowledgments
Support from Çanakkale Onsekiz Mart University, The Scientific Research Commission (COMU-BAP: FBA-2018-1291) is greatly acknowledged.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Clarke, L.; Eom, J.; Marten, E.H.; Horowitz, R.; Kyle, P.; Link, R.; Mignone, B.K.; Mundra, A.; Zhou, Y. Effects of long-term climate change on global building energy expenditures. Energy Econ. 2018, 72, 667–677. [Google Scholar] [CrossRef]
- Cronin, J.; Anandarajah, G.; Dessens, O. Climate change impacts on the energy system: A review of trends and gaps. Clim. Change 2018, 151, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, S.; Osman, A.I.; Doran, J.; Rooney, D.W. Strategies for mitigation of climate change: A review. Environ. Chem. Lett. 2020, 18, 2069–2094. [Google Scholar] [CrossRef]
- Wilbanks, T.; Bilello, D.; Bull, S. Effects of Climate Change on Energy Production and Use in the United States; DigitalCommons @ University of Nebraska: Lincoln, NE, USA, 2008. [Google Scholar]
- Megía, P.J.; Vizcaíno, A.J.; Calles, J.A.; Carrero, A. Hydrogen Production Technologies: From Fossil Fuels toward Renewable Sources. A Mini Review. Energy Fuels 2021, 35, 16403–16415. [Google Scholar] [CrossRef]
- Amin, M.; Shah, H.H.; Fareed, A.G.; Khan, W.U.; Chung, E.; Zia, A.; Rahman Farooqi, Z.U.; Lee, C. Hydrogen production through renewable and non-renewable energy processes and their impact on climate change. Int. J. Hydrogen Energy 2022, 47, 33112–33134. [Google Scholar] [CrossRef]
- Ishaq, H.; Dincer, I. Comparative assessment of renewable energy-based hydrogen production methods. Renew. Sustain. Energy Rev. 2021, 135, 110192. [Google Scholar] [CrossRef]
- Wang, M.; Wang, G.; Sun, Z.; Zhang, Y.; Xu, D. Review of renewable energy-based hydrogen production processes for sustainable energy innovation. Glob. Energy Interconnect. 2019, 2, 436–443. [Google Scholar] [CrossRef]
- Chi, J.; Yu, H. Water electrolysis based on renewable energy for hydrogen production. Chin. J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- Sahiner, N. Soft and flexible hydrogel templates of different sizes and various functionalities for metal nanoparticle preparation and their use in catalysis. Prog. Polym. Sci. 2013, 38, 1329–1356. [Google Scholar] [CrossRef]
- Dincer, I.; Acar, C. Review and evaluation of hydrogen production methods for better sustainability. Int. J. Hydrogen Energy 2015, 40, 11094–11111. [Google Scholar] [CrossRef]
- Li, Q.; Kim, H. Hydrogen production from NaBH4 hydrolysis via Co-ZIF-9 catalyst. Fuel Process. Technol. 2012, 100, 43–48. [Google Scholar] [CrossRef]
- Li, R.; Zhang, F.; Zhang, J.; Dong, H. Catalytic hydrolysis of NaBH4 over titanate nanotube supported Co for hydrogen production. Int. J. Hydrogen Energy 2022, 47, 5260–5268. [Google Scholar] [CrossRef]
- Demirci, S.; Sahiner, N. Superior reusability of metal catalysts prepared within poly(ethylene imine) microgels for H2 production from NaBH4 hydrolysis. Fuel Process. Technol. 2014, 127, 88–96. [Google Scholar] [CrossRef]
- Chen, W.; Lv, G.; Fu, J.; Ren, H.; Shen, J.; Cao, J.; Liu, X. Demonstration of Controlled Hydrogen Release Using Rh@GQDs during Hydrolysis of NH3BH3. ACS Appl. Mater. Interfaces 2021, 13, 50017–50026. [Google Scholar] [CrossRef]
- Jicsinszky, L.; Iványi, R. Catalytic transfer hydrogenation of sugar derivatives. Carbohydr. Polym. 2001, 45, 139–145. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, X.-Y.; Wu, H.; Lei, Y.-Z.; Li, J.-H. Highly efficient reduction of nitro compounds: Recyclable Pd/C-catalyzed transfer hydrogenation with ammonium formate or hydrazine hydrate as hydrogen source. Synth. Commun. 2018, 48, 2475–2484. [Google Scholar] [CrossRef]
- Zhang, J.; Hou, Q.; Guo, X.; Yang, X. Modified MgH2 Hydrogen Storage Properties Based on Grapefruit Peel-Derived Biochar. Catalysts 2022, 12, 517. [Google Scholar] [CrossRef]
- Pukazhselvan, D.; Shaula, A.L.; Mikhalev, S.M.; Bdikin, I.; Fagg, D.P. Elucidating Evidence for the In Situ Reduction of Graphene Oxide by Magnesium Hydride and the Consequence of Reduction on Hydrogen Storage. Catalysts 2022, 12, 735. [Google Scholar] [CrossRef]
- Chen, W.; Shen, J.; Huang, Y.; Liu, X.; Astruc, D. Catalyzed Hydrolysis of Tetrahydroxydiboron by Graphene Quantum Dot-Stabilized Transition-Metal Nanoparticles for Hydrogen Evolution. ACS Sustain. Chem. Eng. 2020, 8, 7513–7522. [Google Scholar] [CrossRef]
- Yang, K.; Wang, P.; Sun, Z.-Y.; Guo, M.; Zhao, W.; Tang, X.; Wang, G. Hydrogen-Bonding Controlled Nickel-Catalyzed Regioselective Cyclotrimerization of Terminal Alkynes. Org. Lett. 2021, 23, 3933–3938. [Google Scholar] [CrossRef] [PubMed]
- Dragan, M. Hydrogen Storage in Complex Metal Hydrides NaBH4: Hydrolysis Reaction and Experimental Strategies. Catalysts 2022, 12, 356. [Google Scholar] [CrossRef]
- Liao, J.; Wu, Y.; Feng, Y.; Hu, H.; Zhang, L.; Qiu, J.; Li, J.; Liu, Q.; Li, H. Boosted Catalytic Activity toward the Hydrolysis of Ammonia Borane by Mixing Co- and Cu-Based Catalysts. Catalysts 2022, 12, 426. [Google Scholar] [CrossRef]
- İzgi, M.S.; Şahin, Ö.; Onat, E.; Saka, C. Epoxy-activated acrylic particulate polymer-supported Co–Fe–Ru–B catalyst to produce H2 from hydrolysis of NH3BH3. Int. J. Hydrogen Energy 2020, 45, 22638–22648. [Google Scholar] [CrossRef]
- Kytsya, A.; Berezovets, V.; Verbovytskyy, Y.; Bazylyak, L.; Kordan, V.; Zavaliy, I.; Yartys, V.A. Bimetallic Ni-Co nanoparticles as an efficient catalyst of hydrogen generation via hydrolysis of NaBH4. J. Alloys Compd. 2022, 908, 164484. [Google Scholar] [CrossRef]
- Kojima, Y.; Kawai, Y.; Nakanishi, H.; Matsumoto, S. Compressed hydrogen generation using chemical hydride. J. Power Sources 2004, 135, 36–41. [Google Scholar] [CrossRef]
- Zhao, J.; Shi, J.; Zhang, X.; Cheng, F.; Liang, J.; Tao, Z.; Chen, J. A Soft Hydrogen Storage Material: Poly(Methyl Acrylate)-Confined Ammonia Borane with Controllable Dehydrogenation. Adv. Mater. 2010, 22, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, H.N. A review on hydrogen generation from the hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2021, 46, 726–765. [Google Scholar] [CrossRef]
- Zhu, Q.-L.; Xu, Q. Immobilization of Ultrafine Metal Nanoparticles to High-Surface-Area Materials and Their Catalytic Applications. Chem 2016, 1, 220–245. [Google Scholar] [CrossRef]
- Khalily, M.A.; Eren, H.; Akbayrak, S.; Susapto, H.H.; Biyikli, N.; Özkar, S.; Guler, M.O. Facile Synthesis of Three-Dimensional Pt-TiO 2 Nano-networks: A Highly Active Catalyst for the Hydrolytic Dehydrogenation of Ammonia-Borane. Angew. Chem. 2016, 128, 12445–12449. [Google Scholar] [CrossRef]
- Balčiūnaitė, A.; Sukackienė, Z.; Antanavičiūtė, K.; Vaičiūnienė, J.; Naujokaitis, A.; Tamašauskaitė-Tamašiūnaitė, L.; Norkus, E. Investigation of hydrogen generation from sodium borohydride using different cobalt catalysts. Int. J. Hydrogen Energy 2021, 46, 1989–1996. [Google Scholar] [CrossRef]
- Glavee, G.N.; Klabunde, K.J.; Sorensen, C.M.; Hadjipanayis, G.C. Sodium borohydride reduction of cobalt ions in nonaqueous media. Formation of ultrafine particles (nanoscale) of cobalt metal. Inorg. Chem. 1993, 32, 474–477. [Google Scholar] [CrossRef]
- Glavee, G.N.; Klabunde, K.J.; Sorensen, C.M.; Hadjipanayis, G.C. Borohydride Reduction of Nickel and Copper Ions in Aqueous and Nonaqueous Media. Controllable Chemistry Leading to Nanoscale Metal and Metal Boride Particles. Langmuir 1994, 10, 4726–4730. [Google Scholar] [CrossRef]
- Glavee, G.N.; Klabunde, K.J.; Sorensen, C.M.; Hadjipanayis, G.C. Borohydride reduction of cobalt ions in water. Chemistry leading to nanoscale metal, boride, or borate particles. Langmuir 1993, 9, 162–169. [Google Scholar] [CrossRef]
- Glavee, G.N.; Klabunde, K.J.; Sorensen, C.M.; Hadjipanayis, G.C. Chemistry of Borohydride Reduction of Iron(II) and Iron(III) Ions in Aqueous and Nonaqueous Media. Formation of Nanoscale Fe, FeB, and Fe2B Powders. Inorg. Chem. 1995, 34, 28–35. [Google Scholar] [CrossRef]
- He, L.; Weniger, F.; Neumann, H.; Beller, M. Synthesis, Characterization, and Application of Metal Nanoparticles Supported on Nitrogen-Doped Carbon: Catalysis beyond Electrochemistry. Angew. Chem. Int. Ed. 2016, 55, 12582–12594. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, N.; Wang, Y.; Xuan, X.; Yang, X.; Zhou, J. Nitrogen-doped microporous carbon material decorated with metal nanoparticles derived from solid Zn/Co zeolitic imidazolate framework with high selectivity for CO2 separation. Fuel 2020, 265, 116972. [Google Scholar] [CrossRef]
- Fukuoka, A.; Araki, H.; Sakamoto, Y.; Sugimoto, N.; Tsukada, H.; Kumai, Y.; Akimoto, Y.; Ichikawa, M. Template Synthesis of Nanoparticle Arrays of Gold and Platinum in Mesoporous Silica Films. Nano Lett. 2002, 2, 793–795. [Google Scholar] [CrossRef]
- Davidson, M.; Ji, Y.; Leong, G.J.; Kovach, N.C.; Trewyn, B.G.; Richards, R.M. Hybrid Mesoporous Silica/Noble-Metal Nanoparticle Materials—Synthesis and Catalytic Applications. ACS Appl. Nano Mater. 2018, 1, 4386–4400. [Google Scholar] [CrossRef]
- Glotov, A.; Vutolkina, A.; Pimerzin, A.; Vinokurov, V.; Lvov, Y. Clay nanotube-metal core/shell catalysts for hydroprocesses. Chem. Soc. Rev. 2021, 50, 9240–9277. [Google Scholar] [CrossRef]
- Zhou, C.H. An overview on strategies towards clay-based designer catalysts for green and sustainable catalysis. Appl. Clay Sci. 2011, 53, 87–96. [Google Scholar] [CrossRef]
- Peron, D.V.; Zholobenko, V.L.; de la Rocha, M.R.; Oberson de Souza, M.; Feris, L.A.; Marcilio, N.R.; Ordomsky, V.V.; Khodakov, A.Y. Nickel–zeolite composite catalysts with metal nanoparticles selectively encapsulated in the zeolite micropores. J. Mater. Sci. 2019, 54, 5399–5411. [Google Scholar] [CrossRef]
- Juneau, M.; Liu, R.; Peng, Y.; Malge, A.; Ma, Z.; Porosoff, M.D. Characterization of Metal-zeolite Composite Catalysts: Determining the Environment of the Active Phase. ChemCatChem 2020, 12, 1826–1852. [Google Scholar] [CrossRef]
- Demirci, S.; Yildiz, M.; Inger, E.; Sahiner, N. Porous carbon particles as metal-free superior catalyst for hydrogen release from methanolysis of sodium borohydride. Renew. Energy 2020, 147, 69–76. [Google Scholar] [CrossRef]
- Rajan, A.S.; Sampath, S.; Shukla, A.K. An in situ carbon-grafted alkaline iron electrode for iron-based accumulators. Energy Environ. Sci. 2014, 7, 1110. [Google Scholar] [CrossRef]
- Liu, F.; Li, H.; Liao, D.; Xu, Y.; Yu, M.; Deng, S.; Zhang, G.; Xiao, T.; Long, J.; Zhang, H.; et al. Carbon quantum dots derived from the extracellular polymeric substance of anaerobic ammonium oxidation granular sludge for detection of trace Mn(vii) and Cr(vi). RSC Adv. 2020, 10, 32249–32258. [Google Scholar] [CrossRef]
- Feng, J.; Zong, Y.; Sun, Y.; Zhang, Y.; Yang, X.; Long, G.; Wang, Y.; Li, X.; Zheng, X. Optimization of porous FeNi3/N-GN composites with superior microwave absorption performance. Chem. Eng. J. 2018, 345, 441–451. [Google Scholar] [CrossRef]
- Shen, Z.; Zu, Y.; Chen, Y.; Gong, J.; Sun, C. Microwave absorption performance of porous carbon particles modified by nickel with different morphologies. J. Mater. Sci. Technol. 2023, 137, 79–90. [Google Scholar] [CrossRef]
- Cheirmadurai, K.; Biswas, S.; Murali, R.; Thanikaivelan, P. Green synthesis of copper nanoparticles and conducting nanobiocomposites using plant and animal sources. RSC Adv. 2014, 4, 19507. [Google Scholar] [CrossRef]
- Khan, A.; Rashid, A.; Younas, R.; Chong, R. A chemical reduction approach to the synthesis of copper nanoparticles. Int. Nano Lett. 2016, 6, 21–26. [Google Scholar] [CrossRef]
- Ari, B.; Inger, E.; Sunol, A.K.; Sahiner, N. Optimized Porous Carbon Particles from Sucrose and Their Polyethyleneimine Modifications for Enhanced CO2 Capture. J. Compos. Sci. 2024, 8, 338. [Google Scholar] [CrossRef]
- Yuan, H.; Wang, S.; Ma, Z.; Kundu, M.; Tang, B.; Li, J.; Wang, X. Oxygen vacancies engineered self-supported B doped Co3O4 nanowires as an efficient multifunctional catalyst for electrochemical water splitting and hydrolysis of sodium borohydride. Chem. Eng. J. 2021, 404, 126474. [Google Scholar] [CrossRef]
- Li, J.; Hong, X.; Wang, Y.; Luo, Y.; Huang, P.; Li, B.; Zhang, K.; Zou, Y.; Sun, L.; Xu, F.; et al. Encapsulated cobalt nanoparticles as a recoverable catalyst for the hydrolysis of sodium borohydride. Energy Storage Mater. 2020, 27, 187–197. [Google Scholar] [CrossRef]
- Peng, C.; Li, T.; Zou, Y.; Xiang, C.; Xu, F.; Zhang, J.; Sun, L. Bacterial cellulose derived carbon as a support for catalytically active Co–B alloy for hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2021, 46, 666–675. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, G.; Zhang, L.; Wang, W.; Miao, W.; Chen, K.; Cheng, L.; Li, Y.; Han, S. Ultrafine cobalt nanoparticles supported on carbon nanospheres for hydrolysis of sodium borohydride. Renew. Energy 2020, 162, 345–354. [Google Scholar] [CrossRef]
- Yao, L.; Li, X.; Peng, W.; Yao, Q.; Xia, J.; Lu, Z.H. Co-CeO: Xnanoparticles anchored on a nitrogen-doped carbon nanosheet: A synergistic effect for highly efficient hydrolysis of sodium borohydride. Inorg. Chem. Front. 2021, 8, 1056–1065. [Google Scholar] [CrossRef]
- Shen, J.; Xu, D.; Ji, J.; Zhang, Q.; Fan, X. In situ evolved defective TiO2 as robust support for CoB-catalyzed hydrolysis of NaBH4. Int. J. Hydrogen Energy 2023, 48, 1001–1010. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, G.; Yin, J.; Chen, J.; Tang, C.; Liu, C.; Li, Q.; Wang, T.; Li, F.; Yao, C.; et al. Photo-thermal synergic enhancement of Co FeAl-LDHs for hydrogen generation from hydrolysis of NaBH4. Appl. Surf. Sci. 2023, 610, 155325. [Google Scholar] [CrossRef]
- Mirshafiee, F.; Rezaei, M. Co/Fe3O4@GO catalyst for one-step hydrogen generation from hydrolysis of NaBH4: Optimization and kinetic study. Int. J. Hydrogen Energy 2023, 48, 32356–32370. [Google Scholar] [CrossRef]
- Ren, J.; Ma, J.; Xu, F.; Zhang, D.; Zhang, K.; Cao, Z.; Wu, S.; Sun, Q.; Wang, Y.; Li, G. Hydrogen generation from hydrolysis of NaBH4 solution with efficient g-C3N4/Co–Mo–B/Ni foam catalyst. Int. J. Hydrogen Energy 2024, 50, 1213–1222. [Google Scholar] [CrossRef]
- Eom, K.S.; Kim, M.J.; Kim, R.H.; Nam, D.H.; Kwon, H.S. Characterization of hydrogen generation for fuel cells via borane hydrolysis using an electroless-deposited Co-P/Ni foam catalyst. J. Power Sources 2010, 195, 2830–2834. [Google Scholar] [CrossRef]
- Yan, J.; Liao, J.; Li, H.; Wang, H.; Wang, R. Magnetic field induced synthesis of amorphous CoB alloy nanowires as a highly active catalyst for hydrogen generation from ammonia borane. Catal. Commun. 2016, 84, 124–128. [Google Scholar] [CrossRef]
- Dai, H.B.; Gao, L.L.; Liang, Y.; Kang, X.D.; Wang, P. Promoted hydrogen generation from ammonia borane aqueous solution using cobalt-molybdenum-boron/nickel foam catalyst. J. Power Sources 2010, 195, 307–312. [Google Scholar] [CrossRef]
- Xu, P.; Lu, W.; Zhang, J.; Zhang, L. Efficient Hydrolysis of Ammonia Borane for Hydrogen Evolution Catalyzed by Plasmonic Ag@Pd Core-Shell Nanocubes. ACS Sustain. Chem. Eng. 2020, 8, 12366–12377. [Google Scholar] [CrossRef]
- He, Y.; Peng, Y.; Wang, Y.; Long, Y.; Fan, G. Air-engaged fabrication of nitrogen-doped carbon skeleton as an excellent platform for ultrafine well-dispersed RuNi alloy nanoparticles toward efficient hydrolysis of ammonia borane. Fuel 2021, 297, 120750. [Google Scholar] [CrossRef]
- Demirci, S.; Yildiz, M.; Sahiner, N. Phosphazene-based covalent organic polymers as metal-free catalysts with improved H2 generation from NaBH4 in methanol with superior catalytic activity and re-generation ability. J. Environ. Chem. Eng. 2024, 12, 112066. [Google Scholar] [CrossRef]
- Demirci, S.; Sunol, A.K.; Sahiner, N. Catalytic activity of amine functionalized titanium dioxide nanoparticles in methanolysis of sodium borohydride for hydrogen generation. Appl. Catal. B Environ. 2020, 261, 118242. [Google Scholar] [CrossRef]
- Ari, B.; Sunol, A.K.; Sahiner, N. Highly re-usable porous carbon-based particles as adsorbents for the development of CO2 capture technologies. J. CO2 Util. 2024, 82, 102767. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).