Metal Oxide Nanocatalysts for the Electrochemical Detection of Propofol
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus
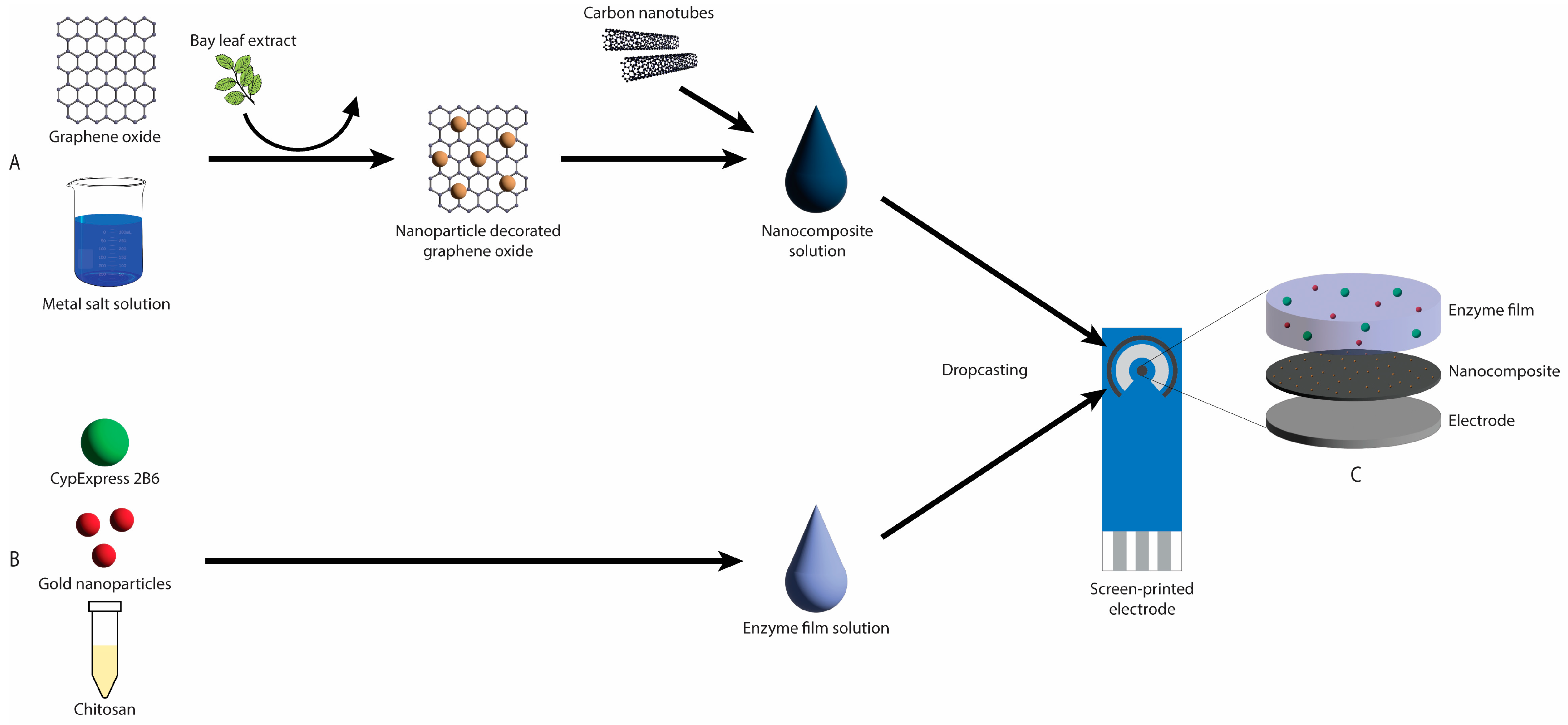
2.3. Nanocomposite Synthesis
2.4. Nanoparticle Characterisation
2.4.1. Ultraviolet–Visible Spectroscopy
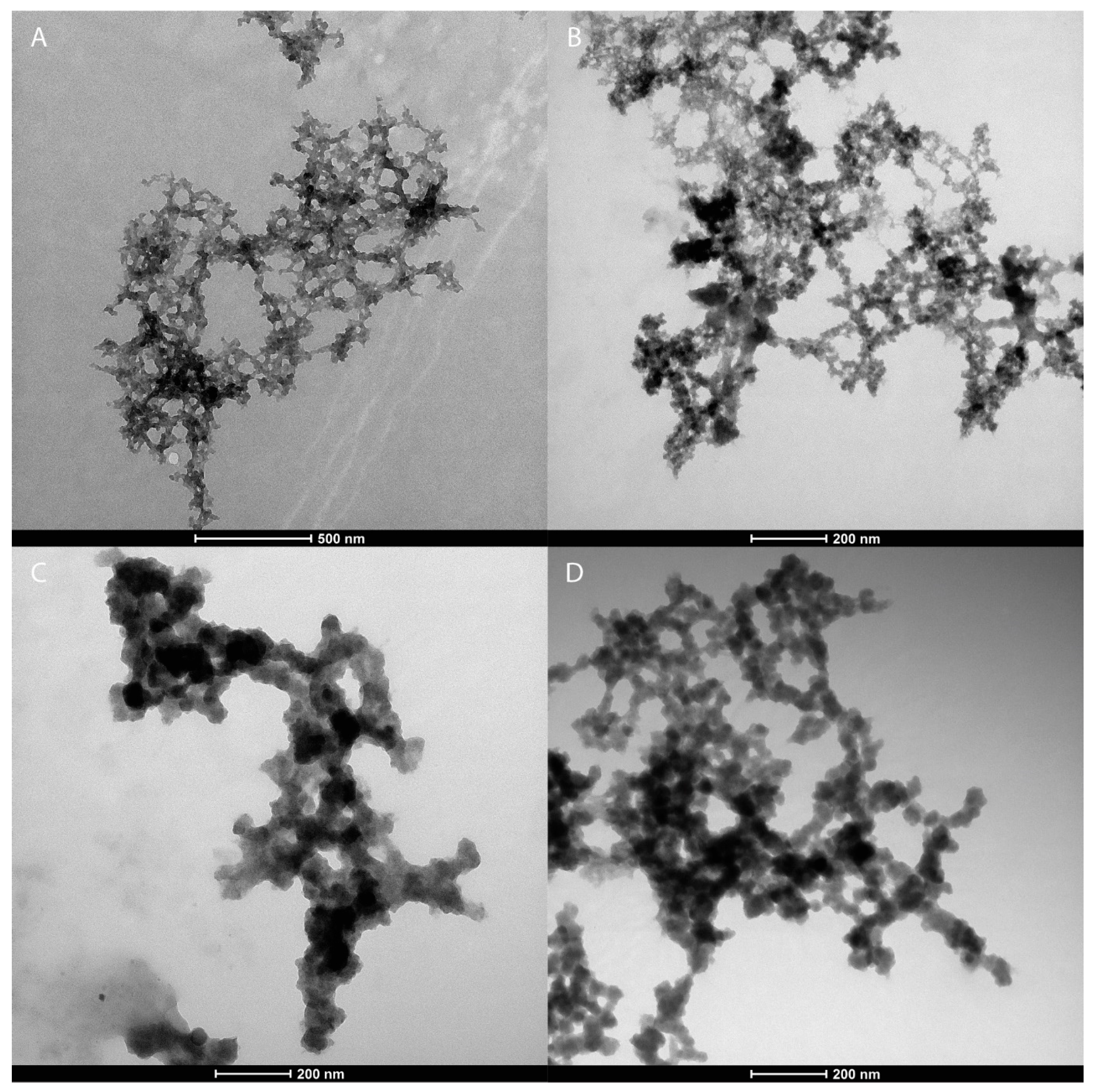
2.4.2. Electron Microscopy
2.5. Electrode Functionalisation
2.6. Electrochemical Measurement
2.7. Baseline Correction
3. Results and Discussion
3.1. Nanoparticle Characterisation
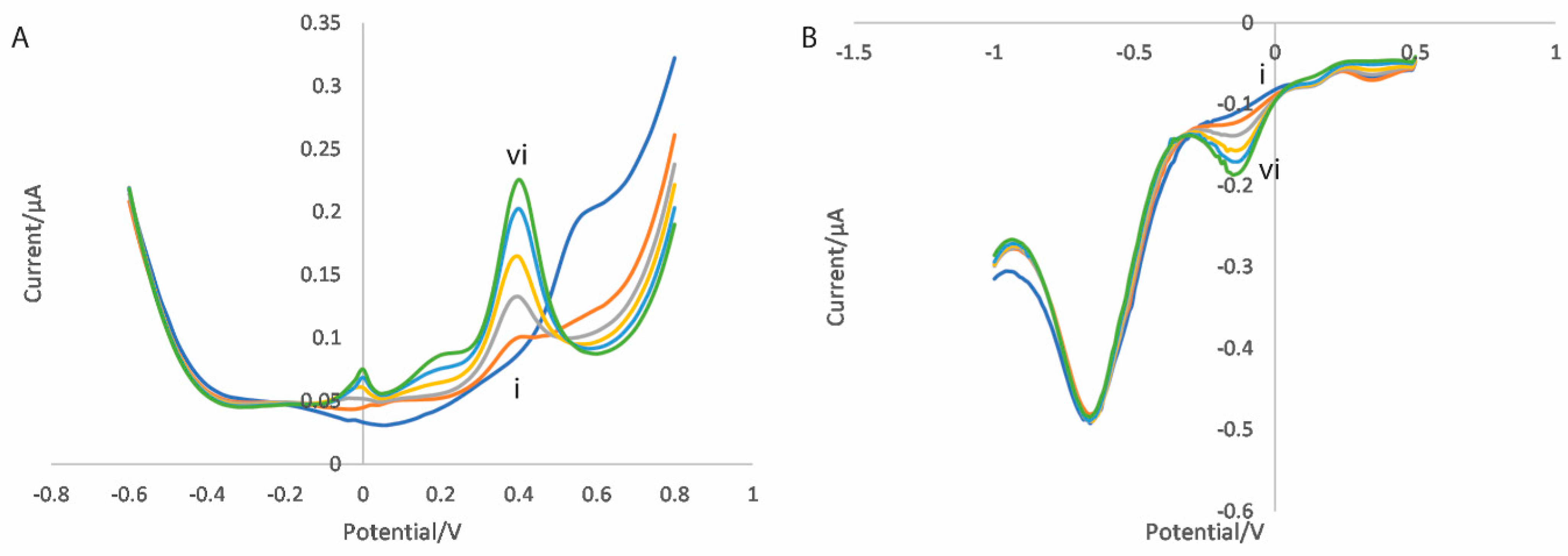
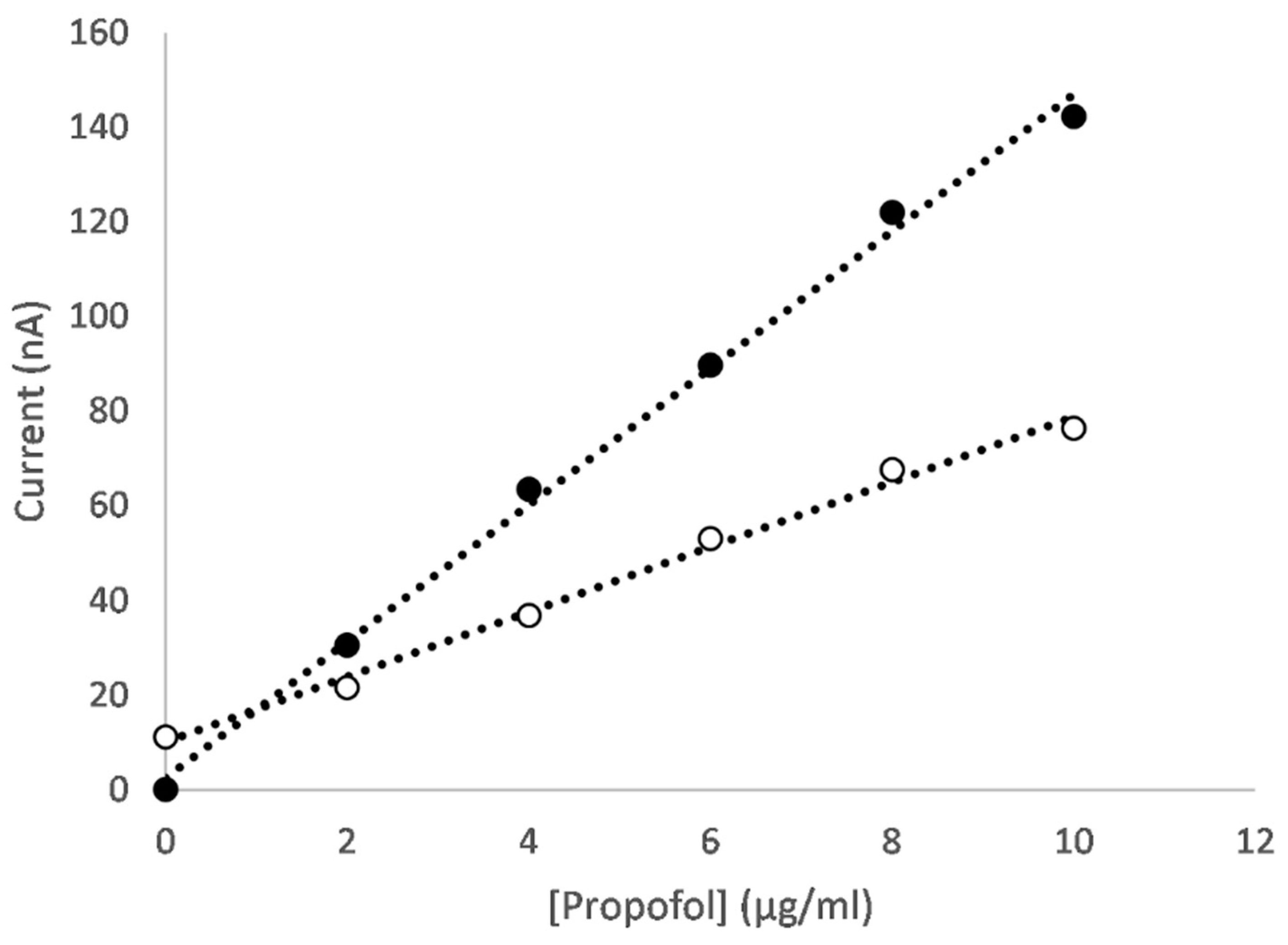
3.2. Electrochemical Measurement
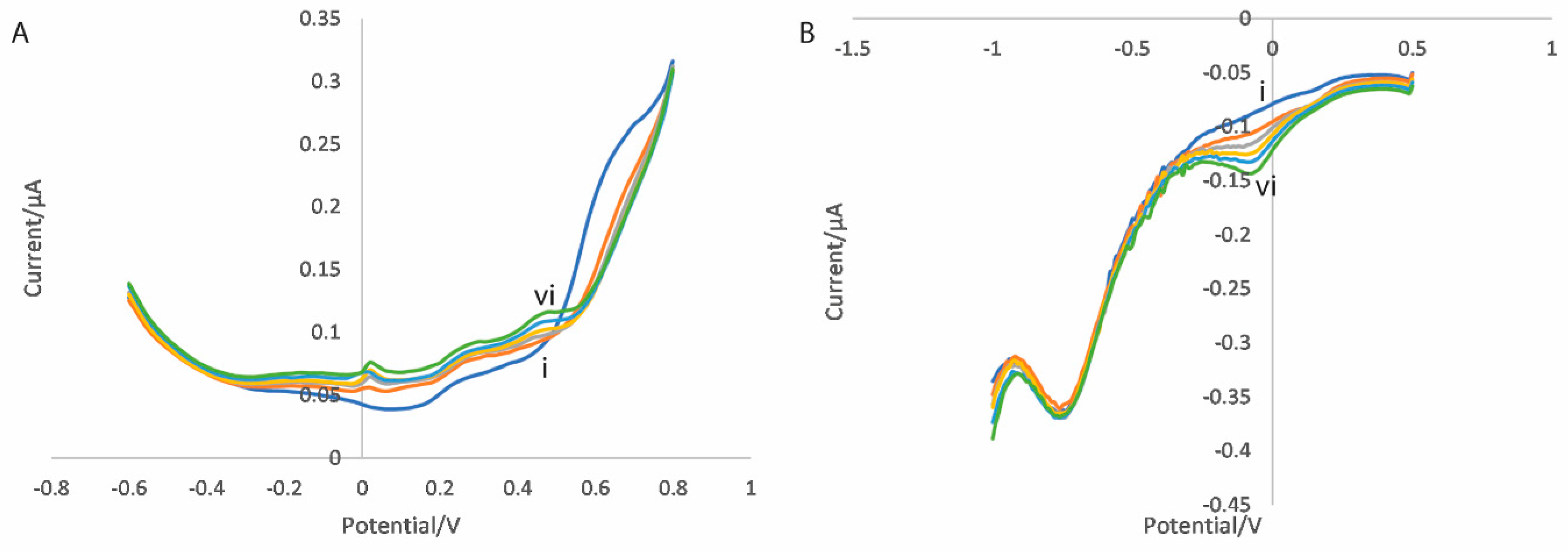
3.2.1. Dual Scan Optimisation
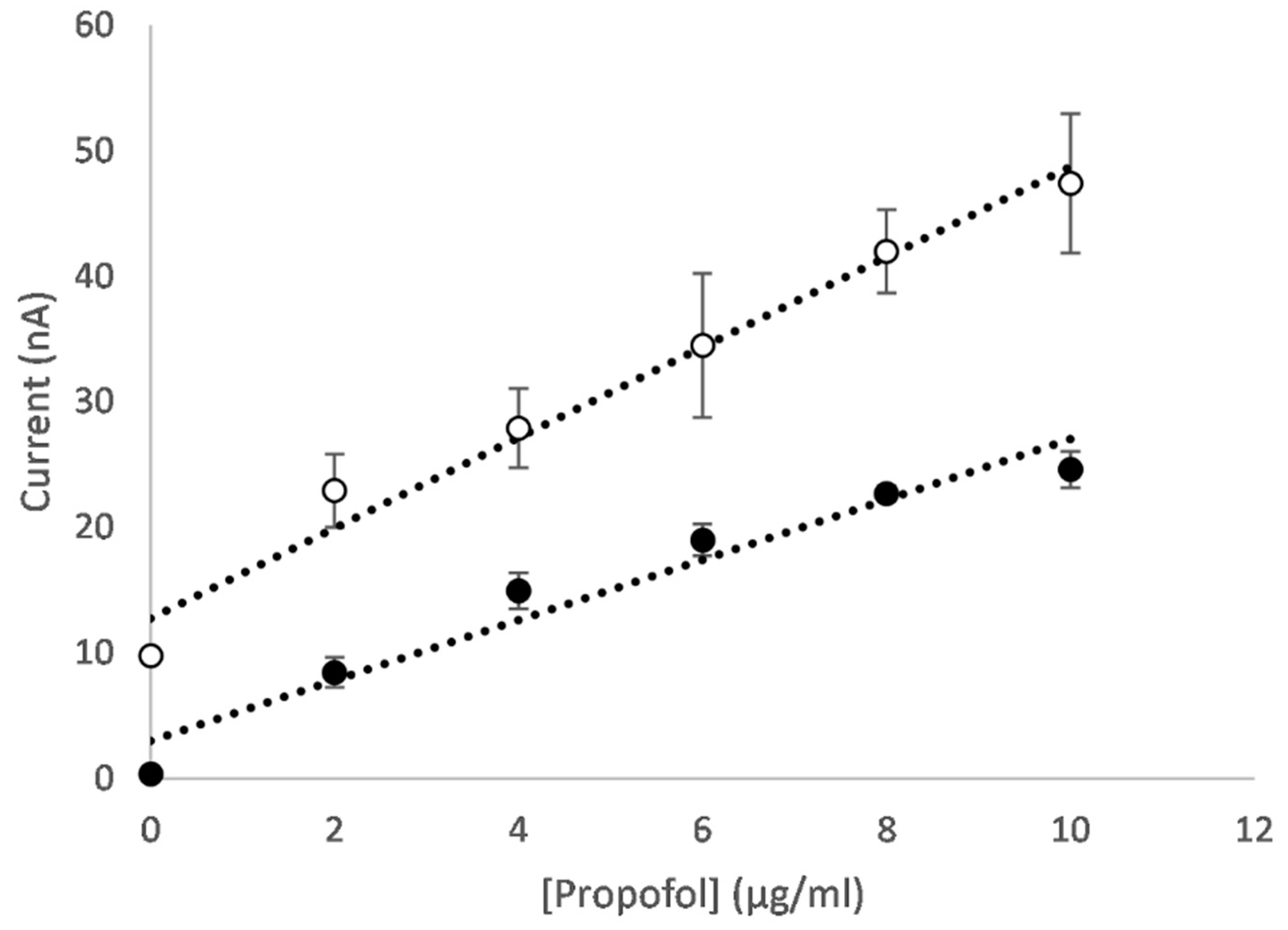
3.2.2. Metal Oxide Nanocomposites
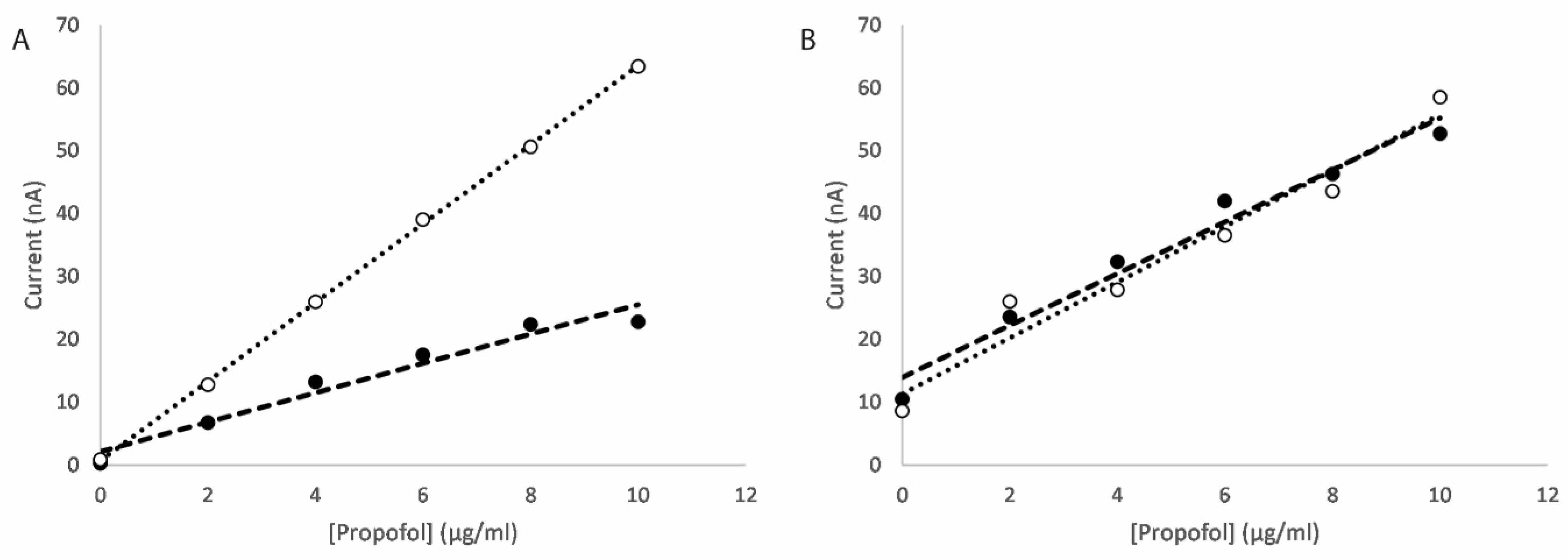
3.2.3. Mixed Metal Oxide Nanocomposites
3.2.4. Interference
4. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferrag, C.; Kerman, K. Grand challenges in nanomaterial-based electrochemical sensors. Front. Sens. 2020, 1, 583822. [Google Scholar] [CrossRef]
- Islam, T.; Hasan, M.M.; Awal, A.; Nurunnabi, M.; Ahammad, A.J.S. Metal nanoparticles for electrochemical sensing: Progress and challenges in the clinical transition of point-of-care testing. Molecules 2020, 25, 5787. [Google Scholar] [CrossRef] [PubMed]
- Gawande, M.B.; Pandey, R.K.; Jayaram, R.V. Role of mixed metaloxides in catalysis science—Versatile applications in organic synthesis. Catal. Sci. Technol. 2012, 2, 1113–1125. [Google Scholar] [CrossRef]
- Lim, W.Q.; Gao, Z. Metal oxide nanoparticles in electroanalysis. Electroanalysis 2015, 27, 2074–2090. [Google Scholar] [CrossRef]
- George, J.M.; Antony, A.; Mathew, B. Metal oxide nanoparticles in electrochemical sensing and biosensing: A review. Microchim. Acta 2018, 185, 358. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, J. Sensors and biosensors based on metal oxide nanomaterials. Trends Anal. Chem. 2019, 121, 115690. [Google Scholar] [CrossRef]
- Vedrine, J.C. Importance, features and uses of metal oxide catalysts in heterogeneous catalysis. Chin. J. Catal. 2019, 40, 1627–1636. [Google Scholar] [CrossRef]
- Hwa, K.-Y.; Subramani, B. Synthesis of zinc oxide nanoparticles on graphene-carbon nanotube hybrid for glucose biosensor applications. Biosens. Bioelectron. 2014, 62, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Tashkhourian, J.; Hemmateenejad, B.; Beigizadeh, H.; Hosseini-Sarvari, M.; Razmi, Z. ZnO nanoparticles and multiwalled carbon nanotubes modified carbon paste electrode for determination of naproxen using electrochemical techniques. J. Electroanal. Chem. 2014, 714–715, 103–108. [Google Scholar] [CrossRef]
- Alam, M.K.; Rahman, M.M.; Abbas, M.; Torati, S.R.; Asiri, A.M.; Kim, D.; Kim, C. Ultra-sensitive 2-nitrophenol detection based on reduced graphene oxide/ZnO nanopcomposites. J. Electroanal. Chem. 2017, 788, 66–73. [Google Scholar] [CrossRef]
- Siddegowda, K.S.; Mahesh, B.; Chamaraja, N.A.; Roopashree, B.; Kumara Swamy, N.; Nanjundaswamy, G.S. Zinc oxide nanoparticles supported on multi-walled carbon nanotube modified electrode for electrochemical sensing of a fluoroquinolone drug. Electroanalysis 2020, 32, 1–11. [Google Scholar] [CrossRef]
- Gu, W.; Wang, M.; Mao, X.; Wang, Y.; Li, L.; Xia, W. A facile sensitive L-tyrosine electrochemical sensor based on a coupled CuO/Cu2O nanoparticles and multi-walled carbon nanotubes nanocomposite film. Anal. Methods 2015, 7, 1313. [Google Scholar] [CrossRef]
- Mei, L.-P.; Feng, J.-J.; Wu, L.; Chen, J.-R.; Shen, L.; Xie, Y.; Wang, A.-J. A glassy carbon electrode modified with porous Cu2O nanospheres on reduced graphene oxide support for simultaneous sensing of uric acid and dopamine with high selectivity over ascorbic acid. Microchim. Acta 2016, 183, 2039–2046. [Google Scholar] [CrossRef]
- Erogul, S.; Bas, S.Z.; Ozmen, M.; Yildiz, S. A new electrochemical sensor based on Fe3O4 functionalized graphene oxide-gold nanoparticle composite film for simultaneous determination of catechol and hyroquinone. Electrochim. Acta 2015, 186, 302–313. [Google Scholar] [CrossRef]
- Aryal, K.P.; Jeong, H.K. Hematite-thermally reduced graphite oxide composite for electrochemical sensing of dopamine. Chem. Phys. Lett. 2019, 723, 133–138. [Google Scholar] [CrossRef]
- Sato, N.; Haruta, M.; Ohta, Y.; Sasagawa, K.; Ohta, J.; Pewnim, N.; Jongprateep, O. Fe2O3/MWCNTs modified microdialysis electrode for dopamine detection. Mater. Res. Express 2020, 7, 015701. [Google Scholar] [CrossRef]
- Uwaya, G.E.; Fayemi, O.E. Electrochemical detection of choline at f-MWCNT/Fe3O4 nanocomposite modified glassy carbon electrode. Mater. Res. Express 2021, 8, 055403. [Google Scholar] [CrossRef]
- Elhag, S.; Ibupoto, Z.H.; Liu, X.; Nur, O.; Willander, M. Dopamine wide range detection sensor based on modified Co3O4 nanowires electrode. Sens. Actuators B 2014, 203, 543–549. [Google Scholar] [CrossRef]
- Shahid, M.; Zhan, Y.; Ahmend, W.; Sagadevan, S. Cobalt oxide-based nanomaterial for electrochemical sensor applications. Malays. Nano Int. J. 2021, 1, 47–63. [Google Scholar] [CrossRef]
- Deroco, P.B.; Vicentini, F.C.; Fatibello-Filho, O. An electrochemical sensor for the simultaneous determination of paracetamol and codeine using a glassy carbon electrode modified with nickel oxide nanoparticles and carbon black. Electroanalysis 2015, 27, 2214–2220. [Google Scholar] [CrossRef]
- Hussain, M.M.; Rahman, M.M.; Asiri, A.M. Ultrasensitive and selective 4-aminophenol chemical sensor development based on nickel oxide nanoparticles decorated carbon nanotube nanocomposites for green environment. J. Environ. Sci. 2017, 53, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Muthuchamy, N.; Gopalan, A.; Lee, K.-P. Highly selective non-enzymatic electrochemical sensor based on a titanium dioxide nanowire-poly(3-aminophenyl boronic acid)-gold nanoparticle ternary nanocomposite. RSC Adv. 2018, 8, 2138–2147. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Wu, L.; Miao, J.; Wei, W.; Liu, A.; Liu, S. Titanium dioxide and polypyrrole molecularly imprinted polymer nanocomposites based electrochemical sensor for highly selective detection of p-nonylphenol. Anal. Chmica Acta 2019, 1080, 84–94. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, B.; Chen, Z.; Zuo, X. Innovative electrochemical sensor using TiO2 nanomaterials to detect phosphopeptides. Anal. Chem. 2021, 93, 10635–10643. [Google Scholar] [CrossRef] [PubMed]
- Gurban, A.-M.; Burtan, D.; Rotariu, L.; Bala, C. Manganese oxide based screen-printed sensor for xenoestrogens. Sens. Actuators B 2015, 210, 273–280. [Google Scholar] [CrossRef]
- Rao, D.; Zhang, X.; Sheng, Q.; Zheng, J. Highly improved sensing of dopamine by using glassy carbon electrode modfied with MnO2, graphene, carbon nanotubes and gold nanoparticles. Microchim. Acta 2016, 183, 2597–2604. [Google Scholar] [CrossRef]
- Yao, Z.; Yang, X.; Niu, Y.; Wu, F.; Hu, Y.; Yang, Y. Voltammetric dopaine sensor based on a gold electrode modified with reduced graphene oxide and Mn3O4 on gold nanoparticles. Microchim. Acta 2017, 184, 2081–2088. [Google Scholar] [CrossRef]
- Reddy, S.; Swamy, B.E.K.; Chandra, U.; Mahathesha, K.R.; Sathisha, T.V.; Jayadevappa, H. Synthesis of MgFe2O4 nanoparticles and MgFe2O4 nanoparticles/CPE for electrochemical investigation of dopamine. Anal. Methods 2011, 3, 2792–2796. [Google Scholar] [CrossRef]
- Reddy, S.; Swamy, B.E.K.; Aruna, S.; Kumar, M.; Shashanka, R.; Jayadevappa, H. Preparation of NiO/ZnO hybrid nanoparticles for electrochemical sensing of dopamine and uric acid. Chem. Sens. 2012, 2, 7. [Google Scholar]
- Qin, Z.; Cheng, Q.; Lu, Y.; Li, J. Facile synthesis of hierarchically mesoporous NiCo2O4 nanowires for sensitive nonenzymatic glucose detection. Appl. Phys. A 2017, 123, 492. [Google Scholar] [CrossRef]
- Tian, X.; Liu, L.; Li, Y.; Yang, C.; Zhou, Z.; Nie, Y.; Wang, Y. Nonenzymatic electrochemical sensor based on CuO-TiO2 for sensitive and selective detecion of methyl parathion pesticide in ground water. Sens. Actuators B 2018, 256, 135–142. [Google Scholar] [CrossRef]
- Kumar, Y.; Pramanik, P.; Das, D.K. Electrochemical detection of paracetamol and dopamine molecules using nano-particles of cobalt ferrite and manganese ferrite modified with graphite. Heliyon 2019, 5, e02031. [Google Scholar] [CrossRef] [PubMed]
- Chavali, M.S.; Nikolova, M.P. Metal oxide nanoparticles and their applications in nanotechnoloy. SN Appl. Sci. 2019, 1, 607. [Google Scholar] [CrossRef]
- Yoon, Y.; Truong, P.L.; Lee, D.; Ko, S.H. Metal-oxide nanomaterials synthesis and applications in flexible and wearable sensors. ACS Nanosci. Au 2022, 2, 64–92. [Google Scholar] [CrossRef] [PubMed]
- Makarov, V.V.; Love, A.J.; Sinitsyna, O.V.; Makarova, S.S.; Yaminsky, I.V.; Taliansky, M.E.; Kalinina, N.O. “Green” nanotechnologies: Synthesis of metal nanoparticles using plants. Acta Naturae 2014, 6, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Zikalala, N.; Matshetshe, K.; Parani, S.; Oluwafemi, O.S. Biosynthesis protocols for colloidal metal oxide nanoparticles. Nano-Struct. Nano-Objects 2018, 16, 288–299. [Google Scholar] [CrossRef]
- Barr, J.; Egan, T.D.; Sandoval, N.F.; Zomorodi, K.; Cohane, C.; Gambus, P.L.; Shafer, S.L. Propofol dosing regimens for ICU sedation based upon an integrated pharmacokinetic-pharmackodynamic model. Anesthesiology 2001, 95, 324–333. [Google Scholar] [CrossRef]
- Barr, J.; Fraser, G.L.; Puntillo, K.; Ely, E.W.; Gélinas, C.; Dasta, J.F.; Davidson, J.E.; Devlin, J.W.; Kress, J.P.; Joffe, A.M.; et al. Clinical practice guidelines for the management of pain. Crit. Care Med. 2013, 41, 263–306. [Google Scholar] [CrossRef] [PubMed]
- Devlin, J.W.; Skrobik, Y.; Gélinas, C.; Needham, D.M.; Slooter, A.J.; Pandharipande, P.P.; Watson, P.L.; Weinhouse, G.L.; Nunnally, M.E.; Rochwerg, B.; et al. “Clinical practice guidlines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit. Care Med. 2018, 46, e825–e873. [Google Scholar] [CrossRef]
- Rowe, K.; Fletcher, S. Sedation in the intensive care unit. Contin. Educ. Anaesth. Crit. Care Pain 2008, 8, 50–55. [Google Scholar] [CrossRef]
- Hughes, C.G.; McGrane, S.; Pandharipande, P.P. Sedation in the intensive care setting. Clin. Pharmacol. Adv. Appl. 2012, 4, 53–63. [Google Scholar]
- Nies, R.J.; Muller, C.; Pfister, R.; Binder, P.S.; Nosseir, N.; Nettersheim, F.S.; Kuhr, K.; Wiesen, M.H.J.; Kochanek, M.; Michels, G. Monitoring of sedation depth in intensive care unit by therapeutic drug monitoring? A prospective observation study of medical intensive care patients. J. Intensive Care 2018, 6, 62. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.L.; Proudfoot, C.W.; Cann, K.F.; Walsh, T.S. The incidence of sub-optimal sedation in the ICU: A systematic review. Crit. Care 2009, 13, R204. [Google Scholar] [CrossRef] [PubMed]
- Mazoit, J.X.; Samii, K. Binding of propofol to blood components: Implications for pharmacokinetics and for pharmacodynamics. Br. J. Clin. Pharmacol. 1999, 47, 35–42. [Google Scholar] [CrossRef]
- Ferreira, M.; Varela, H.; Torresi, R.M.; Treiliosi-Filho, G. Electrode passivation caused by polymerization of different phenolic compounds. Electrochim. Acta 2006, 52, 434–442. [Google Scholar] [CrossRef]
- Choukairi, M.; Bouchta, D.; Bounab, L.; Ben Atyah, M.; Elkhamlichi, R.; Chaouket, F.; Raissouni, I.; Rodriguez, I.N. Electrochemical detection of uric acid and ascorbic acid: Application in serum. J. Electroanal. Chem. 2015, 758, 117–124. [Google Scholar] [CrossRef]
- Nikpanje, E.; Bahmaei, M.; Sharif, A.M. Determination of ascorbic acid, acetaminophen, and caffeine in urine, blood serum by electrochemical sensor based on ZnO-Zn2SnO4-SnO2 nanocomposite and graphene. J. Electrochem. Sci. Technol. 2021, 12, 173–187. [Google Scholar] [CrossRef]
- Ferrier, D.C.; Kiely, J.; Luxton, R. Cytochrome P450 2B6 amperometric biosensor for continuous monitoring of propofol. IEEE Sens. J. 2021, 21, 23730–23736. [Google Scholar] [CrossRef]
- Ferrier, D.C.; Kiely, J.; Luxton, R. Metal oxide decorated carbon nanocomposite electrodes for propofol monitoring. Biosens. Bioelectron. X 2022, 12, 100286. [Google Scholar] [CrossRef]
- Ferrier, D.C.; Kiely, J.; Luxton, R. Molecular displacement approach for the electrochemical detection of protein-bound propofol. Sens. Bio-Sens. Res. 2024, 46, 100710. [Google Scholar] [CrossRef]
- Viezbicke, B.D.; Patel, S.; Davis, B.E.; Birnie, D.P. Evaluation of the Tauc method for optical absorption edge determination: ZnO thin films as a model system. Phys. Status Solidi B 2015, 252, 1700–1710. [Google Scholar] [CrossRef]
- Lassoued, A.; Lassoued, M.S.; Dkhil, B.; Ammar, S.; Gari, A. Synthesis, struyctural, morphological, optical and magnetic characterization of iron oxide nanoparticles by precipitation method: Effect of varying nature of precursor. Phys. E Low-Dimens. Syst. Nanostruct. 2018, 97, 328–334. [Google Scholar] [CrossRef]
- Thielsch, R.; Bohme, T.; Reiche, R.; Schlafer, D.; Bauer, H.-D.; Bottcher, H. Quantum-size effects of PbS nanocrystallites in evaporated composite films. Nanostruct. Mater. 1998, 10, 131–149. [Google Scholar] [CrossRef]
- Regenthal, R.; Krueger, M.; Koeppel, C.; Preiss, R. Drug levels: Therapeutic and toxic serum/plasma concentrations of common drugs. J. Clin. Monit. 1999, 15, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.R.; Akbar, S.A.; Morris, P.A. Nanoscale metal oxide-nased heterojunctions for gas sensing: A review. Sens. Actuators B Chem. 2014, 204, 250–272. [Google Scholar] [CrossRef]
- Fomekong, R.L.; Tsobnang, P.K.; Magnin, D.; Hermans, S.; Delcorte, A.; Ngolui, J.L. Coprecipitation of nickel malonate: A facile and reproducible synthesis route for Ni1-xZnxO nanoparticles and Ni1-xZnxO/ZnO nanocomposites via pyrolysis. J. Solid State Chem. 2015, 230, 381–389. [Google Scholar] [CrossRef]
- Etape, E.P.; Ngolui, L.J.; Foba-Tendo, J.; Yufanyi, D.M.; Namondo, B.V. Synthesis and characterization of CuO, TiO2, and CuO-TiO2 mixed oxide by a modified oxalate route. Hindawi 2017, 2017, 4518654. [Google Scholar]
- Sramkova, I.; Amorim, C.G.; Sklenarova, H.; Montenegro, M.C.B.M.; Horstkotte, B.; Araujo, A.N.; Solich, P. Fully automated analytical procedure for propofol determination by sequential injection technique with spectrophotometric and fluorimetric detections. Talanta 2014, 118, 104–110. [Google Scholar] [CrossRef]
- Diao, J.; Wang, T.; Li, L. Graphene quantum dots as nanoprobes for fluorescent detection of propofol in emulsions. R. Soc. Open Sci. 2019, 6, 181753. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Pettigrew, D.M.; Bates, S.; Laitenberger, P.G.; Troughton, G. Performance evaluation of a whole blood propofol analyser. J. Clin. Monit. Comput. 2012, 26, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Gad-Kariem, E.A.; Abounassif, M.A. Colorimetric determination of propofol in bulk form, dosage form and biological fluids. Anal. Lett. 2000, 33, 2515–2531. [Google Scholar] [CrossRef]
- Hong, C.-C.; Lin, C.-C.; Hong, C.-L.; Lin, Z.-X.; Chung, M.-H.; Hsieh, P.-W. Handheld analyzer with on-chip molecularly-imprinted biosensors for electrochemical detection of propofol in plasman samples. Biosens. Bioelectron. 2016, 86, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Bosch, M.E.; Sanchez, A.J.R.; Rojas, F.S.; Ojeda, C.B. Determination of paracetamol: Historical evolution. J. Pharm. Biomed. Anal. 2006, 42, 291–321. [Google Scholar] [CrossRef] [PubMed]
- Hanks, F.; McKenzie, C. Paracetamol in intensive care—Intravenous, oral or not at all? Anaesthesia 2016, 71, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Eastwood, G.M.; Bailey, M.; Gattas, D.; Kruger, P.; Saxena, M.; Santamaria, J.D.; Bellomo, R. Paracetamol therapy and outcome of critically ill patients: A multicenter retrosepctive observational study. Crit. Care 2015, 19, 162–172. [Google Scholar] [CrossRef]
- Narayana, P.V.; Reddy, T.M.; Gopal, P.; Naidu, G.R. Electrochemical sensing of paracetamol and its simultaneous resolution in the presence of dopamine and folic acid at a multi-walled carbon nanotubes/poly(glycine) composite modified electrode. Anal. Meth. 2014, 6, 9459–9468. [Google Scholar] [CrossRef]
- Morris, M.E.; Levy, G. Renal clearance and serum protein binding of acetaminophen and its major conjugates in humans. J. Pharm. Sci. 1984, 73, 1038–1041. [Google Scholar] [CrossRef]
- Milligan, T.P.; Morris, H.C.; Hammond, P.M.; Price, C.P. Studies on paracetamol binding to serum proteins. Ann. Clin. Biochem. 1994, 31, 492–496. [Google Scholar] [CrossRef]
- Brahim, S.; Narinesingh, D.; Guiseppi-Elie, A. Bio-smart hydrogels: Co-joined molecular recognition and signal transduction in biosensor fabrication and drug delivery. Biosens. Bioelectron. 2002, 17, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Patella, B.; Sortino, A.; Mazzara, F.; Aiello, G.; Drago, G.; Torino, C.; Vilasi, A.; O’Riordan, A.; Inguanta, R. Electrochemical detection of dopamine with negligible interference from ascorbic and uric acid by means of reduced graphene oxide and metals-NPs based electrodes. Anal. Chim. Acta 2021, 1187, 339124. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2016, 213, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.H.; Irwin, W.C.; Fesser, J.; Massey, K.L. Interference of ascorbic acid with chemical analytes. Ann. Clin. Biochem. 2005, 42, 475–477. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Serban, A.I.; Fafaneata, C. Electrochemical methods for ascorbic acid determination. Electrochim. Acta 2014, 121, 443–460. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.-H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Kiew, S.F.; Boakye-Ansah, S.; Lau, S.Y.; Barhoum, A.; Danquah, M.K.; Rodrigues, J. Green approaches for the synthesis of metal and metal oxide nanoparticles using microbial and plant extracts. Nanoscale 2022, 14, 2534–2571. [Google Scholar] [CrossRef] [PubMed]
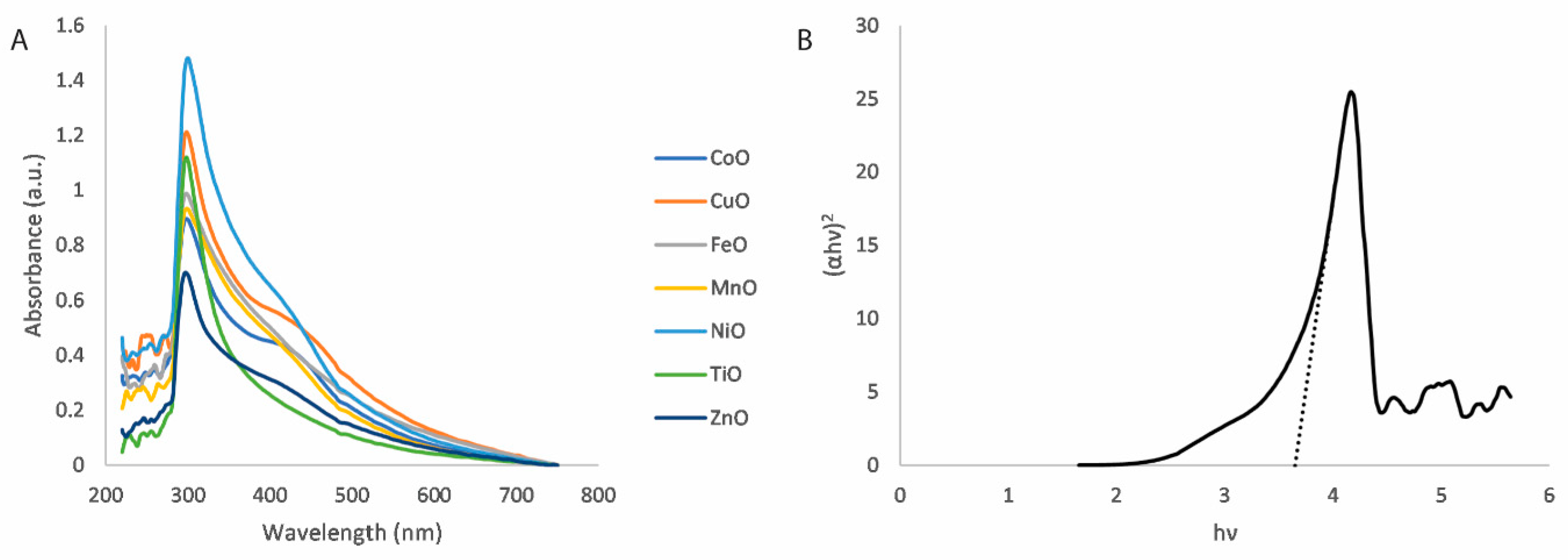



| Metal Oxide Nanoparticle | Bandgap Energy (eV) |
|---|---|
| Cobalt oxide | 3.56 |
| Copper oxide | 3.65 |
| Iron oxide | 3.46 |
| Manganese oxide | 3.39 |
| Nickel oxide | 3.56 |
| Titanium oxide | 3.73 |
| Zinc oxide | 3.75 |
| Scan | Nanocatalyst | Sensitivity (nA/μg/mL/mm2) | Detection Limit (μg/mL) |
|---|---|---|---|
| Oxidation | CuTiO | 3.05 | 0.46 |
| Oxidation | ZnTiO | 3.03 | 3.33 |
| Reduction | CuTiO | 4.58 | 0.47 |
| Reduction | ZnTiO | 4.34 | 6.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrier, D.C.; Kiely, J.; Luxton, R. Metal Oxide Nanocatalysts for the Electrochemical Detection of Propofol. Micromachines 2025, 16, 120. https://doi.org/10.3390/mi16020120
Ferrier DC, Kiely J, Luxton R. Metal Oxide Nanocatalysts for the Electrochemical Detection of Propofol. Micromachines. 2025; 16(2):120. https://doi.org/10.3390/mi16020120
Chicago/Turabian StyleFerrier, David C., Janice Kiely, and Richard Luxton. 2025. "Metal Oxide Nanocatalysts for the Electrochemical Detection of Propofol" Micromachines 16, no. 2: 120. https://doi.org/10.3390/mi16020120
APA StyleFerrier, D. C., Kiely, J., & Luxton, R. (2025). Metal Oxide Nanocatalysts for the Electrochemical Detection of Propofol. Micromachines, 16(2), 120. https://doi.org/10.3390/mi16020120






