Phase Engineering of Nanomaterials: Tailoring Crystal Phases for High-Performance Batteries and Supercapacitors
Abstract
1. Introduction
2. Phase Engineering in Metal Nanomaterials
Direct Synthesis of Unconventional Phase Metal Nanomaterials
3. Phase Transformation in Metal Nanomaterials
3.1. Thermal Activation
3.2. High Pressure
3.3. Surface Modification
3.4. Secondary Growth
3.5. Electron/Ion Beam Irradiation
3.6. Mechanical Deformation
3.7. Other Approaches
4. Phase Engineering in Transition Metal Dichalcogenide (TMD) Nanomaterials
4.1. Colloidal/Hydrothermal Synthesis
4.2. Gas–Solid Reaction
4.3. Salt-Assisted Synthesis
4.4. CVD Synthesis
5. Phase Engineering in Metal-Organic Framework (MOF) Nanomaterials
6. Electrochemical Energy Storage in Phase Engineering Nanomaterials
6.1. Rechargeable Batteries
6.2. Metal-Ion Batteries
6.3. Lithium-Sulfur Batteries
6.4. Supercapacitors
7. Conclusions
Funding
Conflicts of Interest
Nomenclature, Greek Symbols, Subscripts, Superscripts, Acronyms, and Abbreviations
| Symbol | Definition/Description |
| C | Specific capacitance (F g−1 or mF cm−2) |
| E | Energy density (Wh kg−1) |
| P | Power density (W kg−1) |
| V | Voltage (V) |
| I | Current (A or mA) |
| Q | Charge or capacity (C or mAh g−1) |
| t | Time (s or h) |
| R | Resistance (Ω) |
| σ | Electrical conductivity (S cm−1) |
| η | Efficiency (%) |
| d | Thickness or diameter (nm or µm) |
References
- Chen, Y.; Lai, Z.; Zhang, X.; Fan, Z.; He, Q.; Tan, C.; Zhang, H. Phase engineering of nanomaterials. Nat. Rev. Chem. 2020, 4, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Yun, Q.; Ge, Y.; Shi, Z.; Liu, J.; Wang, X.; Zhang, A.; Huang, B.; Yao, Y.; Luo, Q.; Zhai, L.; et al. Recent Progress on Phase Engineering of Nanomaterials. Chem. Rev. 2023, 123, 13489–13692. [Google Scholar] [CrossRef]
- Kim, J.H.; Sung, H.; Lee, G.-H. Phase Engineering of Two-Dimensional Transition Metal Dichalcogenides. Small Sci. 2024, 4, 2300093. [Google Scholar] [CrossRef]
- Leng, K.; Chen, Z.; Zhao, X.; Tang, W.; Tian, B.; Nai, C.T.; Zhou, W.; Loh, K.P. Phase Restructuring in Transition Metal Dichalcogenides for Highly Stable Energy Storage. ACS Nano 2016, 10, 9208–9215. [Google Scholar] [CrossRef]
- Rahmatinejad, J.; Raisi, B.; Liu, X.; Zhang, X.; Sadeghi Chevinli, A.; Yang, L.; Ye, Z. 1T-2H Mixed-Phase MoS2 Stabilized with a Hyperbranched Polyethylene Ionomer for Mg2+/Li+ Co-Intercalation Toward High-Capacity Dual-Salt Batteries. Small 2024, 20, 2304878. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Y.; Wen, G.; Zhang, X.; Huang, X. Intercalation-induced electronic reconstruction: Unlocking stable 1T-MoS(2) with expanded interlayers for lithium-ion batteries. J. Colloid Interface Sci. 2025, 697, 137947. [Google Scholar] [CrossRef]
- Marinov, A.D.; Bravo Priegue, L.; Shah, A.R.; Miller, T.S.; Howard, C.A.; Hinds, G.; Shearing, P.R.; Cullen, P.L.; Brett, D.J.L. Ex Situ Characterization of 1T/2H MoS2 and Their Carbon Composites for Energy Applications, a Review. ACS Nano 2023, 17, 5163–5186. [Google Scholar] [CrossRef]
- Nguyen, T.; Montemor, M.d.F. Metal Oxide and Hydroxide–Based Aqueous Supercapacitors: From Charge Storage Mechanisms and Functional Electrode Engineering to Need-Tailored Devices. Adv. Sci. 2019, 6, 1801797. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Duan, X.; Liu, J.; Ye, Y.; Lv, F.; Liu, T.; Wang, Q.; Zhang, X. Recent progress on transition metal oxides as advanced materials for energy conversion and storage. Energy Storage Mater. 2021, 42, 317–369. [Google Scholar] [CrossRef]
- Shon, J.K.; Lee, H.S.; Park, G.O.; Yoon, J.; Park, E.; Park, G.S.; Kong, S.S.; Jin, M.; Choi, J.-M.; Chang, H.; et al. Discovery of abnormal lithium-storage sites in molybdenum dioxide electrodes. Nat. Commun. 2016, 7, 11049. [Google Scholar] [CrossRef]
- Yang, J.; Ma, M.; Sun, C.; Zhang, Y.; Huang, W.; Dong, X. Hybrid NiCo2S4@MnO2 heterostructures for high-performance supercapacitor electrodes. J. Mater. Chem. A 2015, 3, 1258–1264. [Google Scholar] [CrossRef]
- Wu, H.B.; Lou, X.W. Metal-organic frameworks and their derived materials for electrochemical energy storage and conversion: Promises and challenges. Sci. Adv. 2017, 3, eaap9252. [Google Scholar] [CrossRef]
- Yang, D.; Li, M.; Zheng, X.; Han, X.; Zhang, C.; Jacas Biendicho, J.; Llorca, J.; Wang, J.; Hao, H.; Li, J.; et al. Phase Engineering of Defective Copper Selenide toward Robust Lithium–Sulfur Batteries. ACS Nano 2022, 16, 11102–11114. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Li, Q.; Wang, Z.; Deepak, F.L. Atomic-scale dynamic observation reveals temperature-dependent multistep nucleation pathways in crystallization. Nanoscale Horiz. 2019, 4, 1302–1309. [Google Scholar] [CrossRef]
- Strauss, F.; Kitsche, D.; Ma, Y.; Teo, J.H.; Goonetilleke, D.; Janek, J.; Bianchini, M.; Brezesinski, T. Operando Characterization Techniques for All-Solid-State Lithium-Ion Batteries. Adv. Energy Sustain. Res. 2021, 2, 2100004. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, X.; Chen, Y. Introduction: Phase Engineering of Nanomaterials. Chem. Rev. 2024, 124, 245–247. [Google Scholar] [CrossRef]
- Xie, Y.; Ma, W.; Jiang, D.; Li, W.; Yang, R.; Panchal, S.; Fowler, M.; Zhang, Y. A high-fidelity online monitoring algorithm for multiple physical fields in battery pack. Appl. Energy 2025, 398, 126443. [Google Scholar] [CrossRef]
- Mangiri, R.; Ramachandran, T.; Kumar, Y.A.; Ghosh, A.; Al-Sehemi, A.G.; Yadav, A.K.; Mani, D. Surface engineering of M5X4 MXenes for next-gen energy solutions. Mater. Today Chem. 2025, 48, 102864. [Google Scholar] [CrossRef]
- Mohapatra, J.R.; Moharana, M.K.; Panchal, S. Indirect liquid-cooled lithium-ion battery module with improved circuitous minichannel cold plate design: A numerical study involving the effect of different flow configurations. J. Therm. Anal. Calorim. 2025, 1–28. [Google Scholar] [CrossRef]
- Fan, Y.; Yang, H.; Ye, C.; Yang, W.; Panchal, S.; Fraser, R.; Fowler, M.; Dong, H. State of health estimation of lithium-ion batteries based on the fusion of aging feature extraction and SSA-ELM machine learning algorithms. Ionics 2025, 31, 7897. [Google Scholar] [CrossRef]
- Shabeer, Y.; Madani, S.S.; Panchal, S.; Fowler, M. Performance optimization of high energy density aluminum-air batteries: Effects of operational parameters and electrolyte composition. Future Batter. 2025, 6, 100082. [Google Scholar] [CrossRef]
- Li, W.; Xie, Y.; Yang, R.; Fan, Y.; Zhang, K.; Panchal, S.; Zhang, Y. A Holistic Electrothermal Profiles Online Sensing Method With Sparse Sensor System in Large-Format Battery Pack. IEEE Trans. Ind. Electron. 2025, 72, 10257–10266. [Google Scholar] [CrossRef]
- Madani, S.S.; Allard, F.; Shabeer, Y.; Fowler, M.; Panchal, S.; Ziebert, C.; Mekhilef, S.; Dou, S.X.; See, K.; Wang, Z. Exploring the Aging Dynamics of Lithium-Ion Batteries for Enhanced Lifespan Understanding. J. Phys. Conf. Ser. 2025, 2968, 012017. [Google Scholar] [CrossRef]
- Huang, X.; Li, S.; Huang, Y.; Wu, S.; Zhou, X.; Li, S.; Gan, C.L.; Boey, F.; Mirkin, C.A.; Zhang, H. Synthesis of hexagonal close-packed gold nanostructures. Nat. Commun. 2011, 2, 292. [Google Scholar] [CrossRef]
- Fan, Z.; Bosman, M.; Huang, X.; Huang, D.; Yu, Y.; Ong, K.P.; Akimov, Y.A.; Wu, L.; Li, B.; Wu, J.; et al. Stabilization of 4H hexagonal phase in gold nanoribbons. Nat. Commun. 2015, 6, 7684. [Google Scholar] [CrossRef]
- Fan, Z.; Chen, Y.; Zhu, Y.; Wang, J.; Li, B.; Zong, Y.; Han, Y.; Zhang, H. Epitaxial growth of unusual 4H hexagonal Ir, Rh, Os, Ru and Cu nanostructures on 4H Au nanoribbons. Chem. Sci. 2017, 8, 795–799. [Google Scholar] [CrossRef]
- Liu, C.; Li, T.; Li, G.; Nobusada, K.; Zeng, C.; Pang, G.; Rosi, N.L.; Jin, R. Observation of Body-Centered Cubic Gold Nanocluster. Angew. Chem. Int. Ed. 2015, 54, 9826–9967. [Google Scholar] [CrossRef]
- Zhou, M.; Higaki, T.; Hu, G.; Sfeir, M.Y.; Chen, Y.; Jiang, D.-e.; Jin, R. Three-orders-of-magnitude variation of carrier lifetimes with crystal phase of gold nanoclusters. Science 2019, 364, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.L.; Li, Z.; Duan, H.H.; Cheng, Z.Y.; Li, Y.D.; Zhu, J.; Yu, R. Formation of Hexagonal-Close Packed (HCP) Rhodium as a Size Effect. J. Am. Chem. Soc. 2017, 139, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Bae, J.-H.; Jo, H.; Park, H.-Y.; Lee, S.; Hong, S.J.; Chun, H.; Cho, M.K.; Kim, J.; Kim, J.; et al. Metastable hexagonal close-packed palladium hydride in liquid cell TEM. Nature 2022, 603, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.C.; Chen, C.Y.; Chen, W.M.; Yu, A.B. Role of Citric Acid in the Formation of Silver Nanoplates through a Synergistic Reduction Approach. Langmuir 2010, 26, 4400–4408. [Google Scholar] [CrossRef] [PubMed]
- Zhelev, D.V.; Zheleva, T.S. Silver nanoplates with ground or metastable structures obtained from template-free two-phase aqueous/organic synthesis. J. Appl. Phys. 2014, 115, 044309. [Google Scholar] [CrossRef]
- Taneja, P.; Banerjee, R.; Ayyub, P.; Dey, G.K. Observation of a hexagonal $(4H)$ phase in nanocrystalline silver. Phys. Rev. B 2001, 64, 033405. [Google Scholar] [CrossRef]
- Han, S.; Xia, G.-J.; Cai, C.; Wang, Q.; Wang, Y.-G.; Gu, M.; Li, J. Gas-assisted transformation of gold from fcc to the metastable 4H phase. Nat. Commun. 2020, 11, 552. [Google Scholar] [CrossRef]
- García-Cerda, L.A.; Bernal-Ramos, K.M.; Montemayor, S.M.; Quevedo-López, M.A.; Betancourt-Galindo, R.; Bueno-Báques, D. Preparation of hcp and fcc Ni and Ni/NiO Nanoparticles Using a Citric Acid Assisted Pechini-Type Method. J. Nanomater. 2011, 2011, 162495. [Google Scholar] [CrossRef]
- Sekhar, M.C.; Reddy, B.P.; Kuchi, C.; Basha, C.K.; Al-Zahrani, F.A.; Mangiri, R. Enhanced solar-driven photocatalytic hydrogen production, dye degradation, and supercapacitor functionality using MoS2–TiO2 nanocomposite. Ceram. Int. 2024, 50, 38679–38687. [Google Scholar] [CrossRef]
- Fan, Z.; Luo, Z.; Chen, Y.; Wang, J.; Li, B.; Zong, Y.; Zhang, H. Synthesis of 4H/fcc-Au@M (M = Ir, Os, IrOs) Core-Shell Nanoribbons For Electrocatalytic Oxygen Evolution Reaction. Small 2016, 12, 3908–3913. [Google Scholar] [CrossRef]
- Gaikar, P.S.; Navale, S.T.; Jadhav, V.V.; Shinde, P.V.; Dubal, D.P.; Arjunwadkar, P.R.; Stadler, F.J.; Naushad, M.; Ghfar, A.A.; Mane, R.S. A simple wet-chemical synthesis, reaction mechanism, and charge storage application of cobalt oxide electrodes of different morphologies. Electrochim. Acta 2017, 253, 151–162. [Google Scholar] [CrossRef]
- Tonelli, D.; Scavetta, E.; Gualandi, I. Electrochemical Deposition of Nanomaterials for Electrochemical Sensing. Sensors 2019, 19, 1186. [Google Scholar] [CrossRef]
- Wang, J.; Liu, G.; Yun, Q.; Zhou, X.; Liu, X.; Chen, Y.; Cheng, H.; Ge, Y.; Huang, J.; Hu, Z. Epitaxial Growth of Unconventional 4H-Pd Based Alloy Nanostructures on 4H-Au Nanoribbons towards Highly Efficient Electrocatalytic Methanol Oxidation. Acta Phys.-Chim. Sin. 2023, 39, 2305034. [Google Scholar] [CrossRef]
- Dai, Z.; Zang, X.; Yang, J.; Sun, C.; Si, W.; Huang, W.; Dong, X. Template Synthesis of Shape-Tailorable NiS2 Hollow Prisms as High-Performance Supercapacitor Materials. ACS Appl. Mater. Interfaces 2015, 7, 25396. [Google Scholar] [CrossRef]
- Wang, Y.; Hall, A.S. Pulsed Electrodeposition of Metastable Pd31Bi12 Nanoparticles for Oxygen Reduction Electrocatalysis. ACS Energy Lett. 2020, 5, 17–22. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, M.; Li, T.; Cui, X.; Shi, Y.; Zhu, X.; Wangyang, P.; Yang, D.; Li, J. Effect of current on electrodeposited MnO2 as supercapacitor and lithium-ion battery electrode. Vacuum 2022, 195, 110692. [Google Scholar] [CrossRef]
- Wu, G.; Zheng, X.; Cui, P.; Jiang, H.; Wang, X.; Qu, Y.; Chen, W.; Lin, Y.; Li, H.; Han, X.; et al. A general synthesis approach for amorphous noble metal nanosheets. Nat. Commun. 2019, 10, 4855. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, Z.; Lu, B. Ultrafine Au nanoparticles decorated NiCo2O4 nanotubes as anode material for high-performance supercapacitor and lithium-ion battery applications. Nano Energy 2014, 7, 114–123. [Google Scholar] [CrossRef]
- Xuan, Y.; Tan, L.; Cheng, B.; Zhang, F.; Chen, X.; Ge, M.; Zeng, Q.; Zeng, Z. Pressure-Induced Phase Transitions in Nanostructured Silicon. J. Phys. Chem. C 2020, 124, 27089–27096. [Google Scholar] [CrossRef]
- Chee, S.W.; Arce-Ramos, J.M.; Li, W.; Genest, A.; Mirsaidov, U. Structural changes in noble metal nanoparticles during CO oxidation and their impact on catalyst activity. Nat. Commun. 2020, 11, 2133. [Google Scholar] [CrossRef]
- Li, Q.; Niu, W.; Liu, X.; Chen, Y.; Wu, X.; Wen, X.; Wang, Z.; Zhang, H.; Quan, Z. Pressure-Induced Phase Engineering of Gold Nanostructures. J. Am. Chem. Soc. 2018, 140, 15783–15790. [Google Scholar] [CrossRef]
- Shi, Z.; Wu, Y.; Ruan, X.; Zhai, W.; Li, Z.; Zhai, L.; Zhang, A.; Zhang, H. Perspectives on phase engineering of nanomaterials. Natl. Sci. Rev. 2024, 11, nwae289. [Google Scholar] [CrossRef]
- Cheng, H.; Yang, N.; Liu, X.; Yun, Q.; Goh, M.H.; Chen, B.; Qi, X.; Lu, Q.; Chen, X.; Liu, W.; et al. Aging amorphous/crystalline heterophase PdCu nanosheets for catalytic reactions. Natl. Sci. Rev. 2019, 6, 955–961. [Google Scholar] [CrossRef]
- Liu, S.; Kang, L.; Zhang, J.; Jung, E.; Lee, S.; Jun, S.C. Structural engineering and surface modification of MOF-derived cobalt-based hybrid nanosheets for flexible solid-state supercapacitors. Energy Storage Mater. 2020, 32, 167–177. [Google Scholar] [CrossRef]
- Liu, J.; Niu, W.; Liu, G.; Chen, B.; Huang, J.; Cheng, H.; Hu, D.; Wang, J.; Liu, Q.; Ge, J.; et al. Selective Epitaxial Growth of Rh Nanorods on 2H/fcc Heterophase Au Nanosheets to Form 1D/2D Rh–Au Heterostructures for Highly Efficient Hydrogen Evolution. J. Am. Chem. Soc. 2021, 143, 4387–4396. [Google Scholar] [CrossRef]
- Sánchez-Iglesias, A.; Carbó-Argibay, E.; Glaria, A.; Rodríguez-González, B.; Pérez-Juste, J.; Pastoriza-Santos, I.; Liz-Marzán, L.M. Rapid Epitaxial Growth of Ag on Au Nanoparticles: From Au Nanorods to Core–Shell Au@Ag Octahedrons. Chem.—A Eur. J. 2010, 16, 5558–5563. [Google Scholar] [CrossRef]
- Fei, M.; Zhang, R.; Li, L.; Li, J.; Ma, Z.; Zhang, K.; Li, Z.; Yu, Z.; Xiao, Q.; Yan, D. Epitaxial growth of MnFe2O4 nanosheets arrays for supercapacitor. Electrochim. Acta 2021, 368, 137586. [Google Scholar] [CrossRef]
- Sutter, E.; Huang, Y.; Komsa, H.P.; Ghorbani-Asl, M.; Krasheninnikov, A.V.; Sutter, P. Electron-Beam Induced Transformations of Layered Tin Dichalcogenides. Nano Lett. 2016, 16, 4410–4416. [Google Scholar] [CrossRef]
- Song, Y.; Li, N.; Kang, J.; Li, Z.; Hong, N.; Han, S.; Chen, L.; Zhang, S.; Liu, C.; Song, C.; et al. Heterostructure-anchored 3D CNT-bridged graphene architecture via layer-by-layer structural engineering for thick electrodes of supercapacitors. Chem. Eng. J. 2024, 497, 154557. [Google Scholar] [CrossRef]
- Liu, D.; Liu, J.; Wang, Q.; Du, P.; Wei, W.; Liu, P. PANI coated microporous graphene fiber capable of subjecting to external mechanical deformation for high performance flexible supercapacitors. Carbon 2019, 143, 147–153. [Google Scholar] [CrossRef]
- Nicolaescu, M.; Popescu, M.-I.; Codrean, C.; Hulka, I.; Orha, C.; Lazau, C.; Bandas, C.; Duteanu, N. Fabrication and performance evaluation of the NPC/amorphous-Sn2P2O7 by dealloying of CuNiSnP alloy for supercapacitor application. Sustain. Energy Technol. Assess. 2025, 80, 104383. [Google Scholar] [CrossRef]
- Li, F.; Luo, S.; Qu, F.; Wang, D.; Li, C.; Liu, X. Electrochemical Dealloying Preparation and Morphology Evolution of Nanoporous Au with Enhanced SERS Activity. Coatings 2023, 13, 489. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Z.; Cui, Y.; Niu, X.; Chen, M.; Liang, P.; Liu, J.; Liu, R.; Li, J.; He, X. Dealloying-Derived Nanoporous Cu6Sn5 Alloy as Stable Anode Materials for Lithium-Ion Batteries. Materials 2021, 14, 4348. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, J.; Kan, D.; He, J.; Song, M.; Pang, J.; Wei, S.; Chen, K. The Recent Progress of Two-Dimensional Transition Metal Dichalcogenides and Their Phase Transition. Crystals 2022, 12, 1381. [Google Scholar] [CrossRef]
- Manzeli, S.; Ovchinnikov, D.; Pasquier, D.; Yazyev, O.V.; Kis, A. 2D transition metal dichalcogenides. Nat. Rev. Mater. 2017, 2, 17033. [Google Scholar] [CrossRef]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef]
- Eda, G.; Maier, S.A. Two-Dimensional Crystals: Managing Light for Optoelectronics. ACS Nano 2013, 7, 5660–5665. [Google Scholar] [CrossRef]
- Chhowalla, M.; Jena, D.; Zhang, H. Two-dimensional semiconductors for transistors. Nat. Rev. Mater. 2016, 1, 16052. [Google Scholar] [CrossRef]
- Yu, Y.; Nam, G.-H.; He, Q.; Wu, X.-J.; Zhang, K.; Yang, Z.; Chen, J.; Ma, Q.; Zhao, M.; Liu, Z.; et al. High phase-purity 1T′-MoS2- and 1T′-MoSe2-layered crystals. Nat. Chem. 2018, 10, 638–643. [Google Scholar] [CrossRef]
- Voiry, D.; Mohite, A.; Chhowalla, M. Phase engineering of transition metal dichalcogenides. Chem. Soc. Rev. 2015, 44, 2702–2712. [Google Scholar] [CrossRef]
- Duan, X.; Zhang, H. Introduction: Two-Dimensional Layered Transition Metal Dichalcogenides. Chem. Rev. 2024, 124, 10619–10622. [Google Scholar] [CrossRef]
- Tang, Q.; Jiang, D.-E. Stabilization and Band-Gap Tuning of the 1T-MoS2 Monolayer by Covalent Functionalization. Chem. Mater. 2015, 27, 3743–3748. [Google Scholar] [CrossRef]
- Li, Z.; Zhai, W.; Yang, H.; Zhai, L.; Long, X.; Shi, Z.; Zhang, A.; Lai, Z.; He, Q.; Zhang, H. Phase engineering of two-dimensional transition metal dichalcogenides for surface-enhanced Raman scattering. Matter 2025, 8, 102210. [Google Scholar] [CrossRef]
- Kim, D.; Pandey, J.; Jeong, J.; Cho, W.; Lee, S.; Cho, S.; Yang, H. Phase Engineering of 2D Materials. Chem. Rev. 2023, 123, 11230–11268. [Google Scholar] [CrossRef]
- He, Q.; Sheng, B.; Zhu, K.; Zhou, Y.; Qiao, S.; Wang, Z.; Song, L. Phase Engineering and Synchrotron-Based Study on Two-Dimensional Energy Nanomaterials. Chem. Rev. 2023, 123, 10750–10807. [Google Scholar] [CrossRef]
- Dai, B.; Su, Y.; Guo, Y.; Wu, C.; Xie, Y. Recent Strategies for the Synthesis of Phase-Pure Ultrathin 1T/1T′ Transition Metal Dichalcogenide Nanosheets. Chem. Rev. 2024, 124, 420–454. [Google Scholar] [CrossRef]
- Endres, E.J.; Bairan Espano, J.R.; Koziel, A.; Peng, A.R.; Shults, A.A.; Macdonald, J.E. Controlling Phase in Colloidal Synthesis. ACS Nanosci. Au 2024, 4, 158–175. [Google Scholar] [CrossRef]
- Zhou, P.; Collins, G.; Hens, Z.; Ryan, K.M.; Geaney, H.; Singh, S. Colloidal WSe2 nanocrystals as anodes for lithium-ion batteries. Nanoscale 2020, 12, 22307–22316. [Google Scholar] [CrossRef]
- Kang, S.; Wang, C.; Chen, J.; Meng, T.; E, J. Progress on solvo/hydrothermal synthesis and optimization of the cathode materials of lithium-ion battery. J. Energy Storage 2023, 67, 107515. [Google Scholar] [CrossRef]
- Wei, L.; Sevilla, M.; Fuertes, A.B.; Mokaya, R.; Yushin, G. Hydrothermal Carbonization of Abundant Renewable Natural Organic Chemicals for High-Performance Supercapacitor Electrodes. Adv. Energy Mater. 2011, 1, 356–361. [Google Scholar] [CrossRef]
- Chen, Y.; Yao, Y.; Zhao, W.; Wang, L.; Li, H.; Zhang, J.; Wang, B.; Jia, Y.; Zhang, R.; Yu, Y.; et al. Precise solid-phase synthesis of CoFe@FeOx nanoparticles for efficient polysulfide regulation in lithium/sodium-sulfur batteries. Nat. Commun. 2023, 14, 7487. [Google Scholar] [CrossRef]
- Sung, J.; Shin, C. Recent Studies on Supercapacitors with Next-Generation Structures. Micromachines 2020, 11, 1125. [Google Scholar] [CrossRef]
- Gan, Y.; Wang, C.; Chen, X.; Liang, P.; Wan, H.; Liu, X.; Tan, Q.; Wu, H.; Rao, H.; Wang, H.; et al. High conductivity Ni12P5 nanowires as high-rate electrode material for battery-supercapacitor hybrid devices. Chem. Eng. J. 2020, 392, 123661. [Google Scholar] [CrossRef]
- Zhu, W.; Kamali, A.R. Molten Salt-Assisted Catalytic Preparation of MoS2/α-MoO3/Graphene as High-Performance Anode of Li-Ion Battery. Catalysts 2023, 13, 499. [Google Scholar] [CrossRef]
- Hong, W.; Wang, X.; Zheng, H.; Li, R.; Wu, R.; Chen, J.S. Molten-Salt-Assisted Synthesis of Nitrogen-Doped Carbon Nanosheets Derived from Biomass Waste of Gingko Shells as Efficient Catalyst for Oxygen Reduction Reaction. Processes 2021, 9, 2124. [Google Scholar] [CrossRef]
- Martínez-Morales, C.; Romero-Serrano, A.; López-Rodríguez, J.; Arellanes-Lozada, P. Molten Salt Synthesis and Electrochemical Evaluation of Na/Ag-Containing MnxOy Composites for Pseudocapacitor Applications. Materials 2025, 18, 3869. [Google Scholar] [CrossRef]
- Kim, H.; Jung, Y.; Lee, W.; Jeon, Y.-P.; Hong, J.-Y.; Lee, J.U. Sustainable MXene Synthesis via Molten Salt Method and Nano-Silicon Coating for Enhanced Lithium-Ion Battery Performance. Molecules 2025, 30, 812. [Google Scholar] [CrossRef]
- Saeed, M.; Alshammari, Y.; Majeed, S.A.; Al-Nasrallah, E. Chemical Vapour Deposition of Graphene—Synthesis, Characterisation, and Applications: A Review. Molecules 2020, 25, 3856. [Google Scholar] [CrossRef]
- Banciu, C.A.; Nastase, F.; Istrate, A.-I.; Veca, L.M. 3D Graphene Foam by Chemical Vapor Deposition: Synthesis, Properties, and Energy-Related Applications. Molecules 2022, 27, 3634. [Google Scholar] [CrossRef]
- Kataria, S.; Wagner, S.; Ruhkopf, J.; Gahoi, A.; Pandey, H.; Bornemann, R.; Vaziri, S.; Smith, A.D.; Ostling, M.; Lemme, M.C. Chemical vapor deposited graphene: From synthesis to applications. Phys. Status Solidi (a) 2014, 211, 2439–2449. [Google Scholar] [CrossRef]
- Liu, L.; Wu, J.; Wu, L.; Ye, M.; Liu, X.; Wang, Q.; Hou, S.; Lu, P.; Sun, L.; Zheng, J.; et al. Phase-selective synthesis of 1T′ MoS2 monolayers and heterophase bilayers. Nat. Mater. 2018, 17, 1108–1114. [Google Scholar] [CrossRef]
- Empante, T.A.; Zhou, Y.; Klee, V.; Nguyen, A.E.; Lu, I.H.; Valentin, M.D.; Naghibi Alvillar, S.A.; Preciado, E.; Berges, A.J.; Merida, C.S.; et al. Chemical Vapor Deposition Growth of Few-Layer MoTe2 in the 2H, 1T′, and 1T Phases: Tunable Properties of MoTe2 Films. ACS Nano 2017, 11, 900–905. [Google Scholar] [CrossRef]
- Kang, T.; Tang, T.W.; Pan, B.; Liu, H.; Zhang, K.; Luo, Z. Strategies for Controlled Growth of Transition Metal Dichalcogenides by Chemical Vapor Deposition for Integrated Electronics. ACS Mater. Au 2022, 2, 665–685. [Google Scholar] [CrossRef]
- Napari, M.; Huq, T.N.; Hoye, R.L.Z.; MacManus-Driscoll, J.L. Nickel oxide thin films grown by chemical deposition techniques: Potential and challenges in next-generation rigid and flexible device applications. InfoMat 2021, 3, 536–576. [Google Scholar] [CrossRef]
- Duncan, J.A.; Azim, F.; Dhakal, A.; Pokhrel, H.; Mishra, S.R.; Pollard, S.D. Direct chemical vapor deposition of CoO on Ni-foam for supercapacitor electrode applications. Next Mater. 2025, 8, 100570. [Google Scholar] [CrossRef]
- Bláha, M.; Bouša, M.; Valeš, V.; Frank, O.; Kalbáč, M. Two-Dimensional CVD-Graphene/Polyaniline Supercapacitors: Synthesis Strategy and Electrochemical Operation. ACS Appl. Mater. Interfaces 2021, 13, 34686–34695. [Google Scholar] [CrossRef]
- Tai, H.; Shi, J.; Xu, D.; Liang, K.; Wang, X.; Shi, L.; Liu, Z. Towards enhancing performance of supercapacitors: Nanostructure engineering and optimal controlling crystal phase of NiS/Ni3S4 from metal-organic frameworks precursor. J. Energy Storage 2024, 94, 112397. [Google Scholar] [CrossRef]
- Shinde, P.A.; Seo, Y.; Lee, S.; Kim, H.; Pham, Q.N.; Won, Y.; Chan Jun, S. Layered manganese metal-organic framework with high specific and areal capacitance for hybrid supercapacitors. Chem. Eng. J. 2020, 387, 122982. [Google Scholar] [CrossRef]
- Wang, F.; Li, P.; Li, W.; Wang, D. Electrochemical Synthesis of Multidimensional Nanostructured Silicon as a Negative Electrode Material for Lithium-Ion Battery. ACS Nano 2022, 16, 7689–7700. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Li, X.; Wang, Y.; Zhang, Y.; Jiang, Y.; He, Y.-S.; Niu, C.; Che, H.; Li, L.; Ma, Z.-F. Self-limited and reversible surface hydration of Na2Fe(SO4)2 cathodes for long-cycle-life Na-ion batteries. Energy Storage Mater. 2025, 74, 103882. [Google Scholar] [CrossRef]
- Zhao, T.; Wang, J.; Du, C.; Li, W.; Zhao, M.; Wang, R.; Xin, Y.; Zhou, K.; Zhang, Z. Modifying Separators with a Multistrategy-Constructed (ZnCo)3S4–MoS2 Heterostructure for High-Performance Lithium–Sulfur Batteries. ACS Appl. Nano Mater. 2025, 8, 8220–8230. [Google Scholar] [CrossRef]
- Lahtinen, K.; Rautama, E.-L.; Jiang, H.; Räsänen, S.; Kallio, T. Reuse of LiCoO2 Electrodes Collected from Spent Li-Ion Batteries after Electrochemical Re-Lithiation of the Electrode. ChemSusChem 2021, 14, 2434–2444. [Google Scholar] [CrossRef]
- Lung-Hao Hu, B.; Wu, F.-Y.; Lin, C.-T.; Khlobystov, A.N.; Li, L.-J. Graphene-modified LiFePO4 cathode for lithium ion battery beyond theoretical capacity. Nat. Commun. 2013, 4, 1687. [Google Scholar] [CrossRef]
- Egorov, K.; Zhao, W.; Knemeyer, K.; Filippin, A.N.; Giraldo, A.; Battaglia, C. Mitigating First-Cycle Capacity Losses in NMC811 via Lithicone Layers Grown by Molecular Layer Deposition. ACS Appl. Mater. Interfaces 2023, 15, 20075–20080. [Google Scholar] [CrossRef] [PubMed]
- Jo, E.; Park, J.-H.; Park, J.; Hwang, J.; Chung, K.Y.; Nam, K.-W.; Kim, S.M.; Chang, W. Different thermal degradation mechanisms: Role of aluminum in Ni-rich layered cathode materials. Nano Energy 2020, 78, 105367. [Google Scholar] [CrossRef]
- Solchenbach, S.; Wetjen, M.; Pritzl, D.; Schwenke, K.U.; Gasteiger, H.A. Lithium oxalate as capacity and cycle-life enhancer in LNMO/graphite and LNMO/SiG full cells. J. Electrochem. Soc. 2018, 165, A512–A524. [Google Scholar] [CrossRef]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The success story of graphite as a lithium-ion anode material–Fundamentals, remaining challenges, and recent developments including silicon (oxide) composites. Sustain. Energy Fuels 2020, 4, 5387–5416. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, D.; Qiu, X.; Ma, Y.; Kong, D.; Müllen, K.; Li, X.; Zhi, L. Stable high-capacity and high-rate silicon-based lithium battery anodes upon two-dimensional covalent encapsulation. Nat. Commun. 2020, 11, 3826. [Google Scholar] [CrossRef]
- Qian, J.; Henderson, W.A.; Xu, W.; Bhattacharya, P.; Engelhard, M.; Borodin, O.; Zhang, J.-G. High rate and stable cycling of lithium metal anode. Nat. Commun. 2015, 6, 6362. [Google Scholar] [CrossRef]
- Tang, Q.; Wang, L.; Xue, Z.; Li, C.; Lv, D.; Zhang, N.; Zhu, K. Free-Standing Sulfur/Carbon Nanocomposite Cathodes for Lithium–Sulfur Rechargeable Batteries. ACS Appl. Nano Mater. 2025, 8, 863–870. [Google Scholar] [CrossRef]
- Pandit, B.; Johansen, M.; Susana Martínez-Cisneros, C.; Naranjo-Balseca, J.M.; Levenfeld, B.; Ravnsbæk, D.B.; Varez, A. Na3V2(PO4)3 Cathode for Room-Temperature Solid-State Sodium-Ion Batteries: Advanced In Situ Synchrotron X-ray Studies to Understand Intermediate Phase Evolution. Chem. Mater. 2024, 36, 2314–2324. [Google Scholar] [CrossRef]
- Ding, W.; Ye, Z.; Huang, Z.; Hu, H.; Liu, L. Unlocking the High Rate Performance of Na0.44MnO2 with a Boron Doping Strategy. Batter. Supercaps 2025, 8, e202500029. [Google Scholar] [CrossRef]
- Wang, K.; Jin, Y.; Sun, S.; Huang, Y.; Peng, J.; Luo, J.; Zhang, Q.; Qiu, Y.; Fang, C.; Han, J. Low-Cost and High-Performance Hard Carbon Anode Materials for Sodium-Ion Batteries. ACS Omega 2017, 2, 1687–1695. [Google Scholar] [CrossRef] [PubMed]
- Sanna, S.; Orgiani, P.; Krymskaya, O.; Di Castro, D.; Galdi, A.; Tkalčević, M.; Aruta, C.; Tebano, A. Epitaxial growth mechanism and structural characterization of spinel-type LixMn2O4 electrodes realized via pulsed laser deposition. Materialia 2025, 39, 102382. [Google Scholar] [CrossRef]
- Li, M.; Gai, Y.; Deng, T.; Xing, L.; Zeng, R.; Lu, D. In-situ electrochemical decomposition of tetraethylsilane/triethyl(ethynyl)silane additives for simultaneous H2O/HF removal and electrode/electrolyte interface stabilization in LiNi0.8Co0.1Mn0.1O2/mesocarbon microbeads Li-ion cells. Electrochim. Acta 2025, 539, 147062. [Google Scholar] [CrossRef]
- Palanisamy, R.; Pavadai, N.; Pavadai, R.; Saito, N.; Pattananuwat, P.; Sujaridworakun, P. Enhanced energy storage efficiency of an innovative three-dimensional nickel cobalt metal organic framework nanocubes with molybdenum disulphide electrode material as a battery-like supercapacitor. J. Alloys Compd. 2024, 1009, 176991–177002. [Google Scholar] [CrossRef]


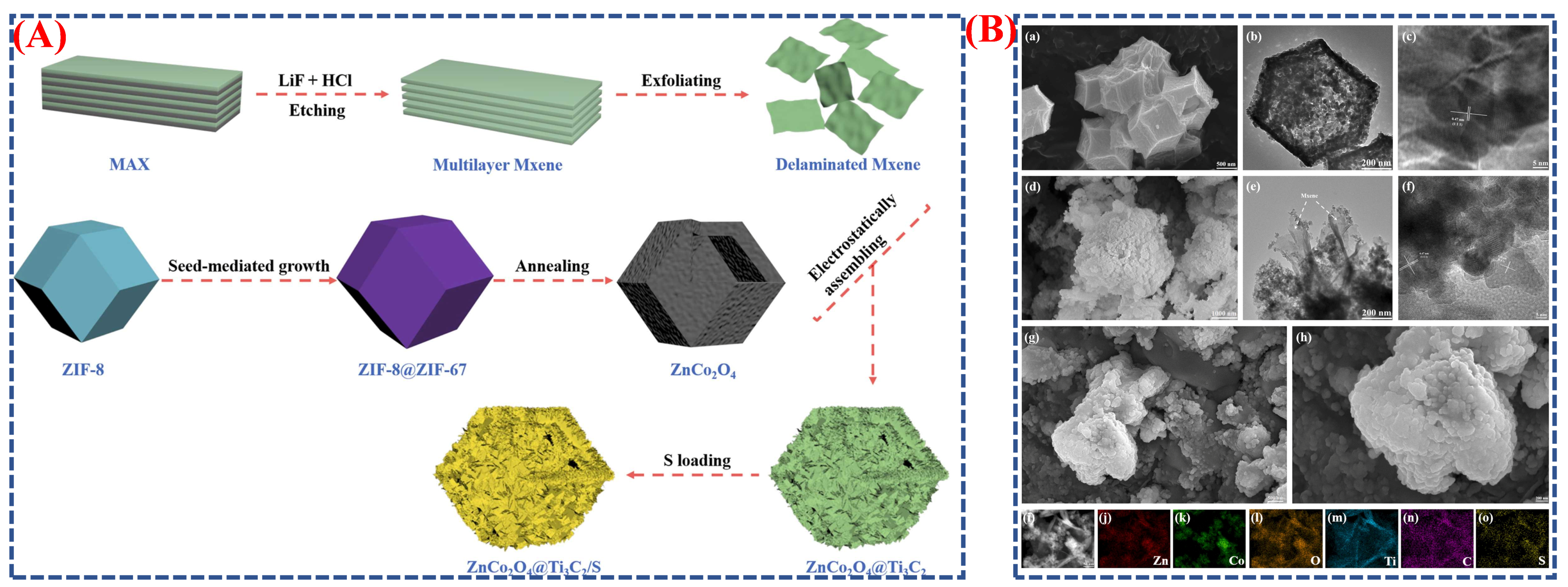
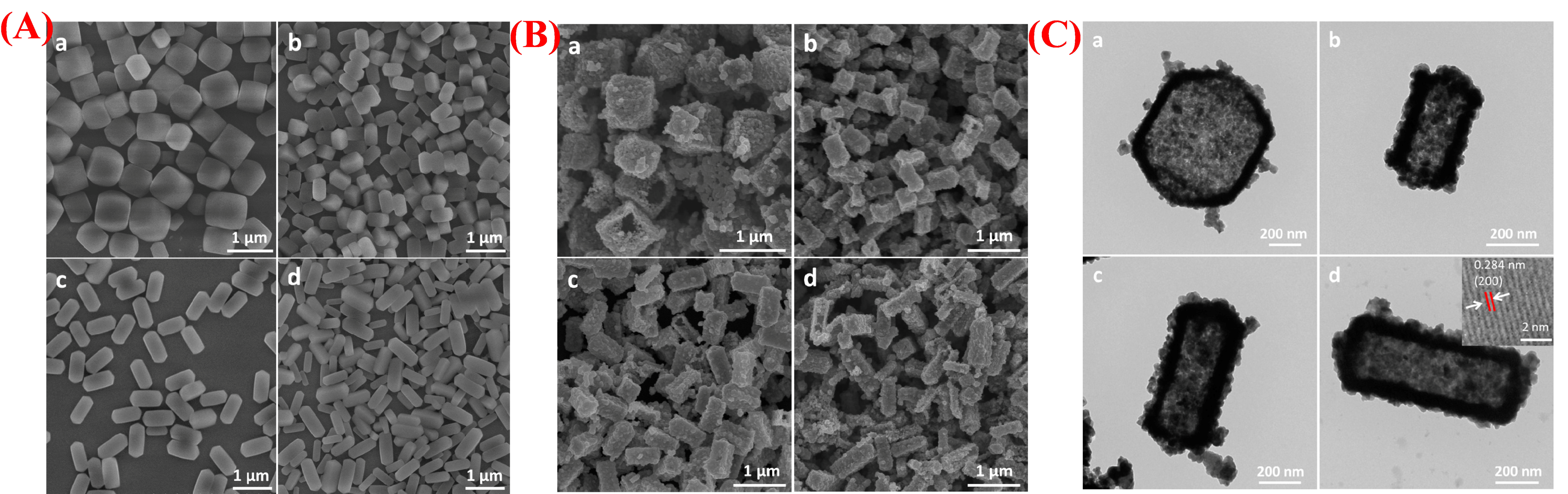
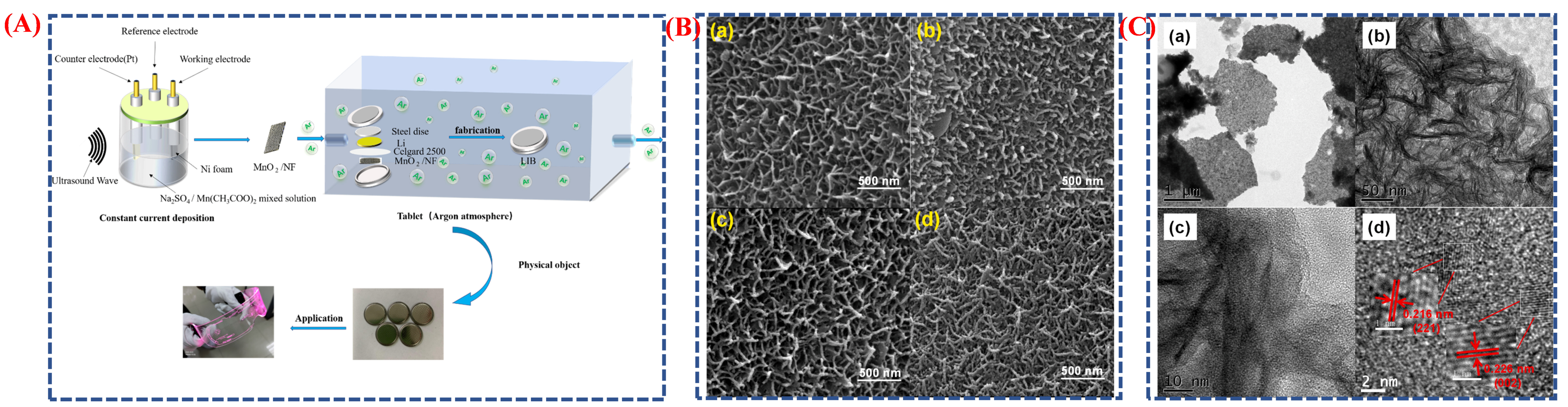

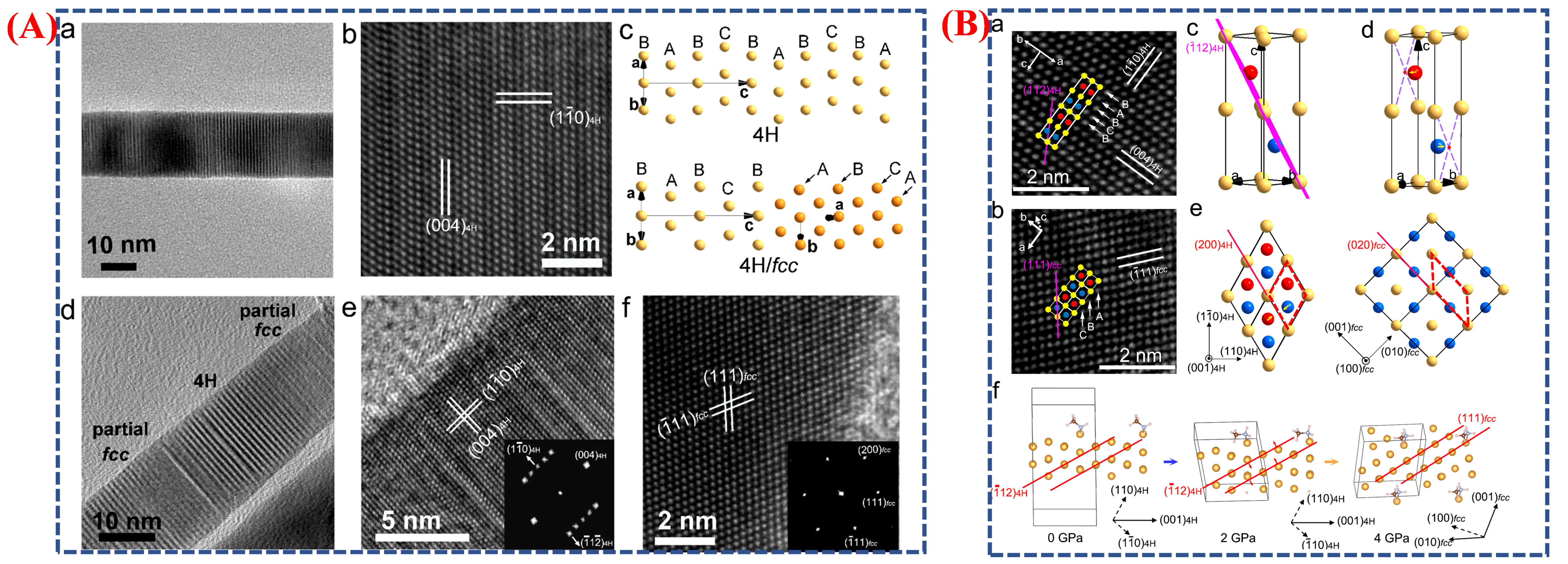



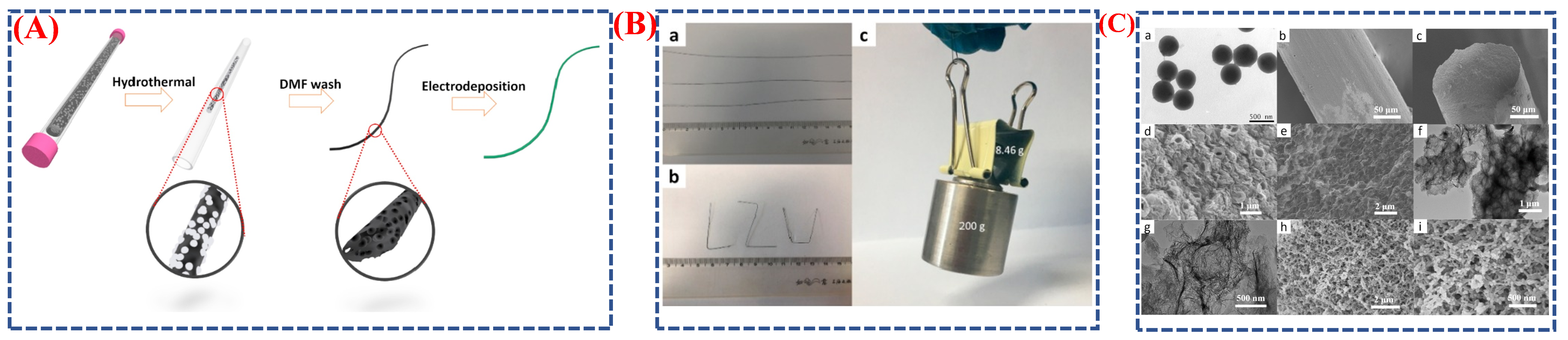
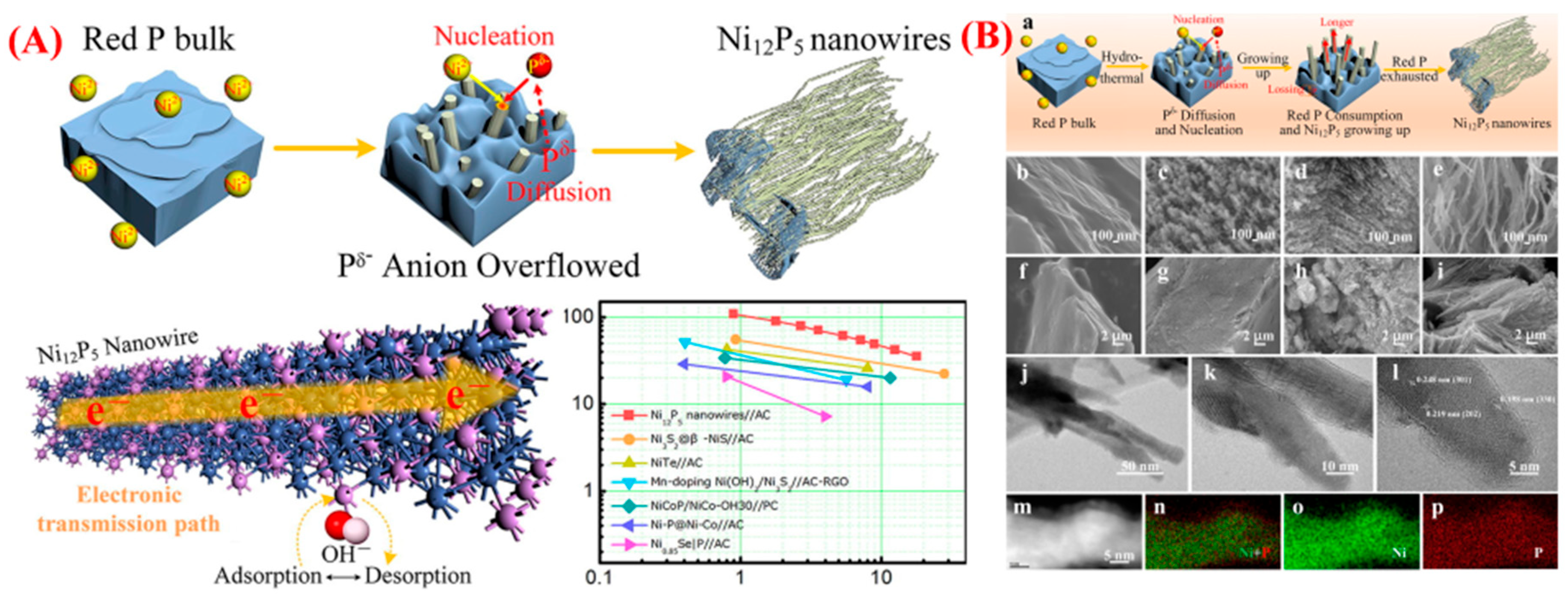
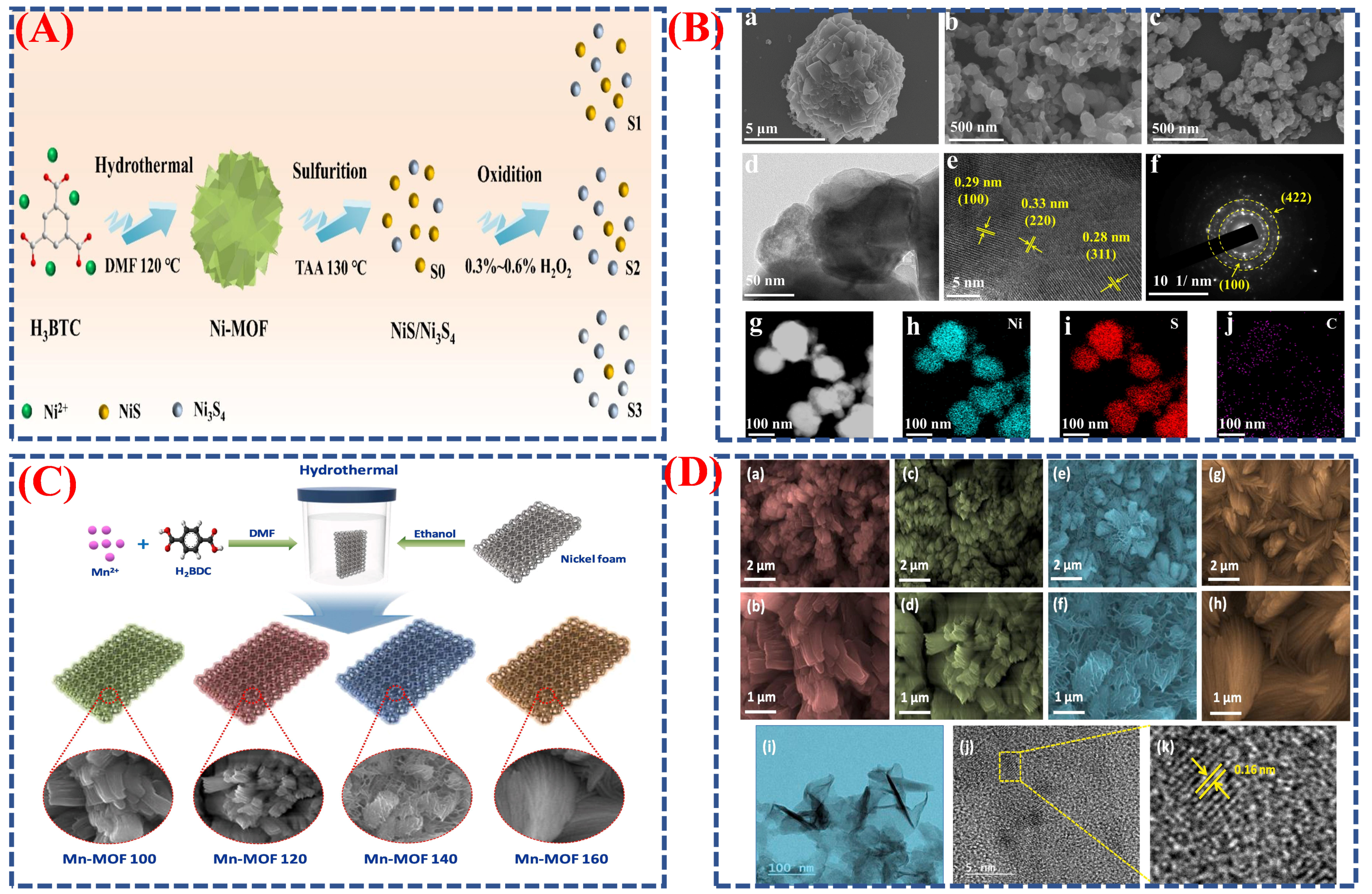
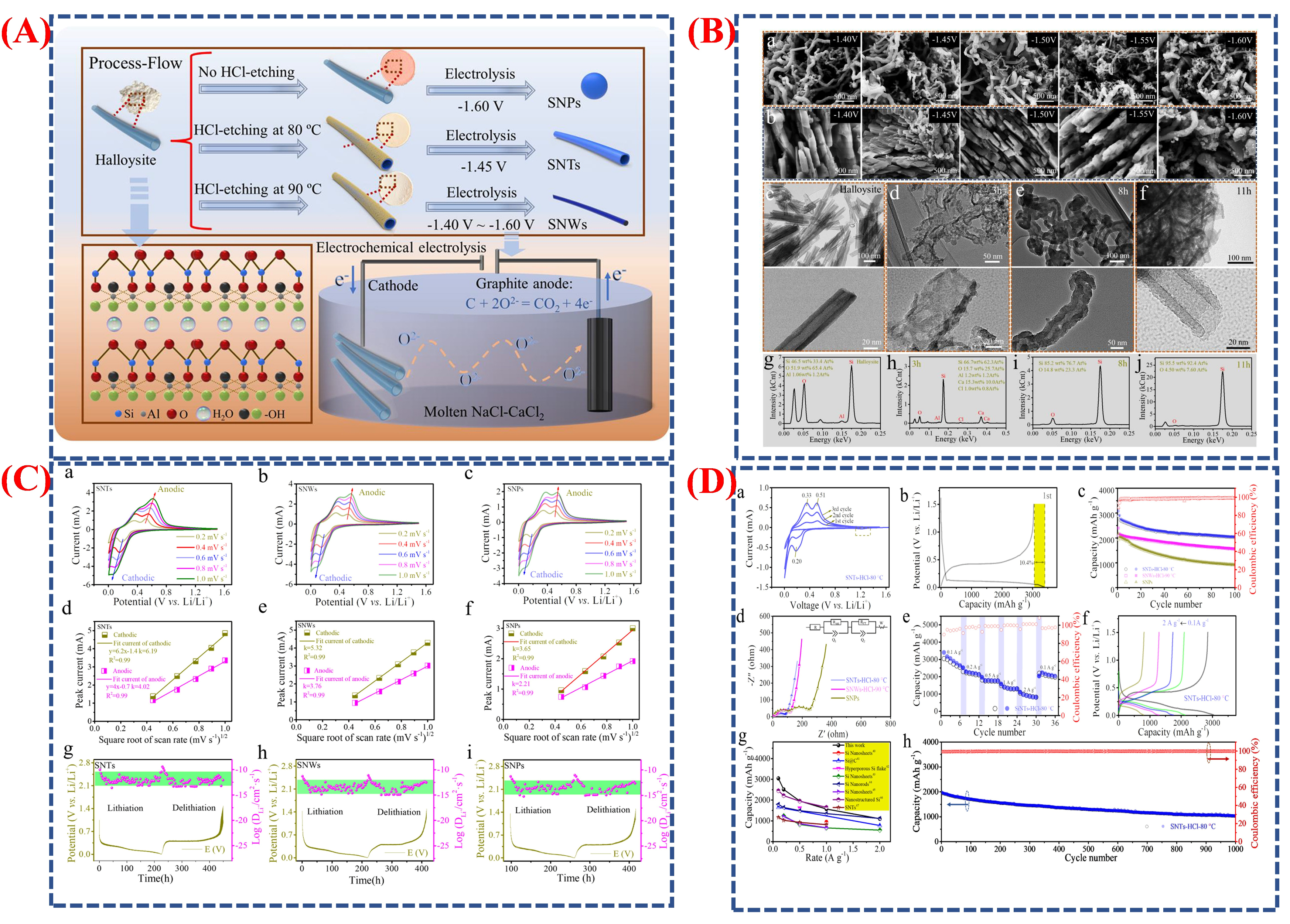


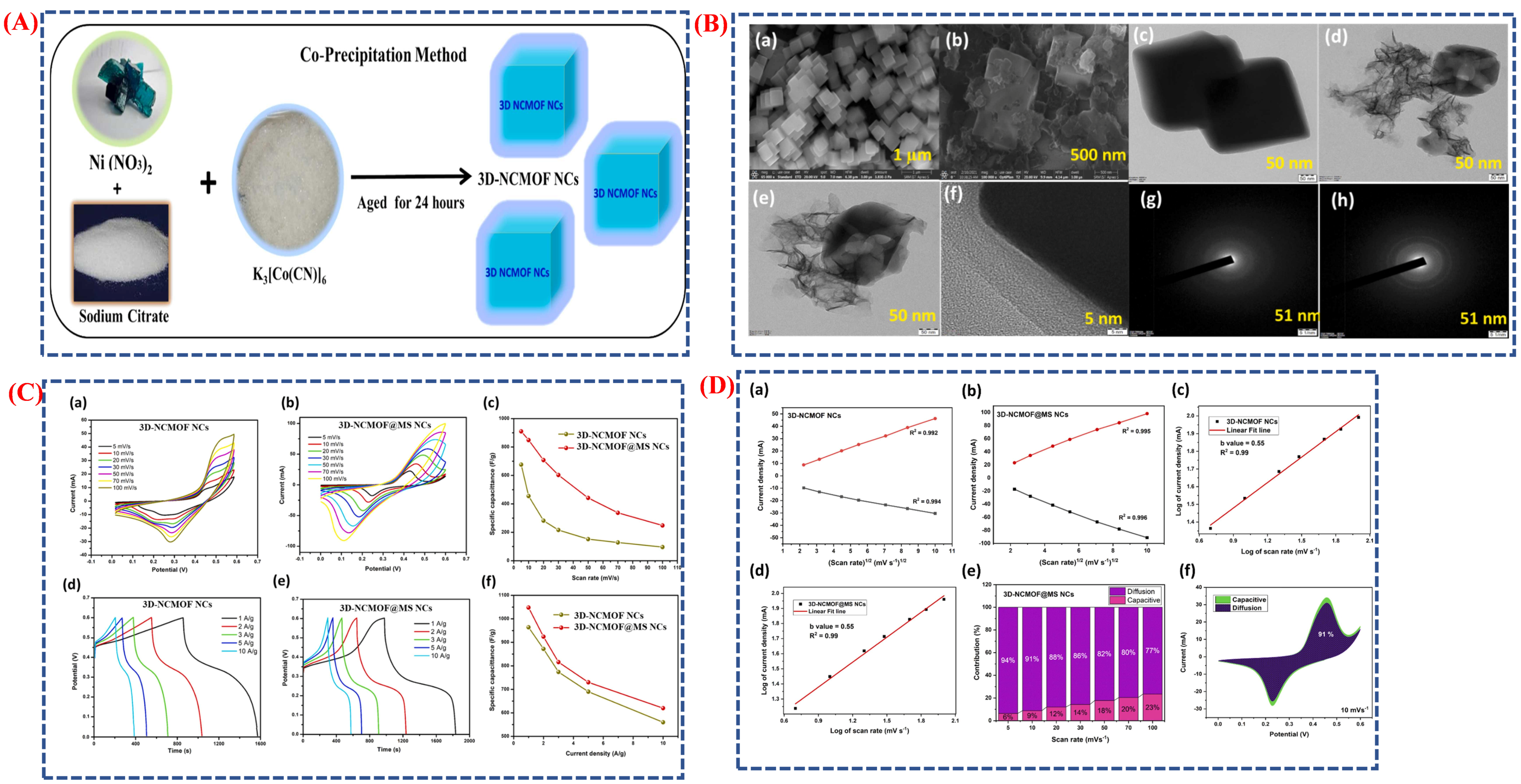
| Synthesis Method | Metal | Unconventional Phase | Reference |
|---|---|---|---|
| In situ growth on graphene-oxide template (solution) | Au | Hexagonal close-packed (hcp, 2H) Au square sheets | [24] |
| High-yield colloidal (solution) synthesis of nanoribbons | Au | 4H hexagonal Au (4H Au nanoribbons) | [25] |
| Solution-phase epitaxial coating on 4H Au template | Ir, Rh, Os, Ru, Cu | 4H hexagonal (epitaxial 4H Ir, Rh, Os, Ru, Cu) | [26] |
| Ligand-protected single-crystal cluster synthesis (X-ray) | Au (nanocluster) | Body-centered cubic (bcc) Au38 nanocluster | [27] |
| Atomically precise clusters/spectroscopy | Au (atom-precise NCs) | hcp Au30 and bcc Au38 nanoclusters | [28] |
| Solvothermal/electron-beam decomposition | Rh | Hexagonal close-packed (hcp) Rh nanoparticles | [29] |
| Liquid-cell in situ TEM (H supply control) | Pd → Pd hydride | Metastable hcp palladium hydride (PdHx) | [30] |
| Chemical reduction/shape control (citric acid, seed-mediated) | Ag | Metastable hexagonal polytypes of Ag (2H/4H) | [31] |
| Template-free colloidal growth (nanoplates) | Ag | 2H and related metastable Ag structures | [32] |
| DC magnetron/high-pressure sputtering | Ag | Unusual hexagonal (4H) Ag observed | [33] |
| Gas + e-beam in situ TEM | Au | fcc → metasTable 4H phase | [34] |
| Pechini/sol–gel heat treatment | Ni | Hexagonal close-packed (hcp) Ni nanoparticles | [35] |
| Colloidal/polyol/PEG reduction | Ni | hcp Ni synthesized in colloidal/PEG systems | [36] |
| No. | Material | Specific Capacity (mAh g−1) | Energy Density (Wh kg−1) | Stability/Notes | Reference |
|---|---|---|---|---|---|
| 1 | LiCoO2 | 150 | 555.0 | Typical practical values: retained capacity ~150 mAh g−1. | [99] |
| 2 | LiFePO4 (carbon/graphene-modified) | 208 | 707.2 | High reversible capacity; graphene-modified electrode. | [100] |
| 3 | NMC811 (LiNi0.8Mn0.1Co0.1O2) | ≈200 | 760.0 | Depends on rate and SOC window; first-cycle losses are common. | [101] |
| 4 | NCA (LiNi-Co-Al) | ≈200 | 740.0 | High capacity; stability improved by Al doping. | [102] |
| 5 | LNMO/LiNi0.5Mn1.5O4 | 147 | 690.9 | High voltage (~4.7 V); electrolyte decomposition limits cycle life. | [103] |
| 6 | Graphite | 372 | 37.2 | Excellent cyclability; baseline anode. | [104] |
| 7 | Si–C encapsulated Si (SF@G) | 2646/2194 | 1058.4/877.6 | Stable 500 cycles; high Coulombic efficiency. | [105] |
| 8 | Li metal (theoretical) | 3860 | — | Very high capacity; dendrite and CE limitations. | [106] |
| 9 | Li–S (sulfur cathode) | 1661 | 3488.1 | High capacity; shuttle effect mitigated by host design. | [107] |
| 10 | Na3V2(PO4)3 (NVP) | 110 | 374.0 | Stable cycling; good ion conductivity. | [108] |
| 11 | Na0.44MnO2 | 108 | 324.0 | Good rate capability; moderate cycle retention. | [109] |
| 12 | Hard carbon (Na anode) | 250–350 | — | Common Na-ion anode; 300+ cycles with 80–90% retention. | [110] |
| 13 | LiMn2O4 (spinel) | 147 | 588.0 | Good power; capacity fades at high T/current. | [111] |
| 14 | NMC811 (operando studies) | 159–200 | 606.4–760.0 | Studied diffusion limits and phase evolution. | [112] |
| 15 | Na0.44MnO2/other Na cathodes | 100–120 | 300–420 | Excellent cycling (e.g., 105 → 102 mAh g−1 after 100 cycles). | [109] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangiri, R.; Reddy, N.P.; Bae, J. Phase Engineering of Nanomaterials: Tailoring Crystal Phases for High-Performance Batteries and Supercapacitors. Micromachines 2025, 16, 1289. https://doi.org/10.3390/mi16111289
Mangiri R, Reddy NP, Bae J. Phase Engineering of Nanomaterials: Tailoring Crystal Phases for High-Performance Batteries and Supercapacitors. Micromachines. 2025; 16(11):1289. https://doi.org/10.3390/mi16111289
Chicago/Turabian StyleMangiri, Ramanadha, Nandarapu Purushotham Reddy, and Joonho Bae. 2025. "Phase Engineering of Nanomaterials: Tailoring Crystal Phases for High-Performance Batteries and Supercapacitors" Micromachines 16, no. 11: 1289. https://doi.org/10.3390/mi16111289
APA StyleMangiri, R., Reddy, N. P., & Bae, J. (2025). Phase Engineering of Nanomaterials: Tailoring Crystal Phases for High-Performance Batteries and Supercapacitors. Micromachines, 16(11), 1289. https://doi.org/10.3390/mi16111289





