Research Progress of Electrochemical Machining Technology in Surface Processing: A Review
Abstract
1. Introduction
1.1. Technical Background and Challenges of Surface Treatment
1.2. Classification System of ECM Technologies for Surface Treatment
1.2.1. Technological Pathways for Surface Quality Enhancement
1.2.2. Multi-Scale Technological Pathways for Material Shaping
- (1)
- Macro-Scale Material Shaping
- (2)
- Micro-Scale Material Shaping
1.2.3. Conclusions
2. Research Progress in Electrochemical Technology for Surface Processing
2.1. Surface Quality Improvement ECM Technology
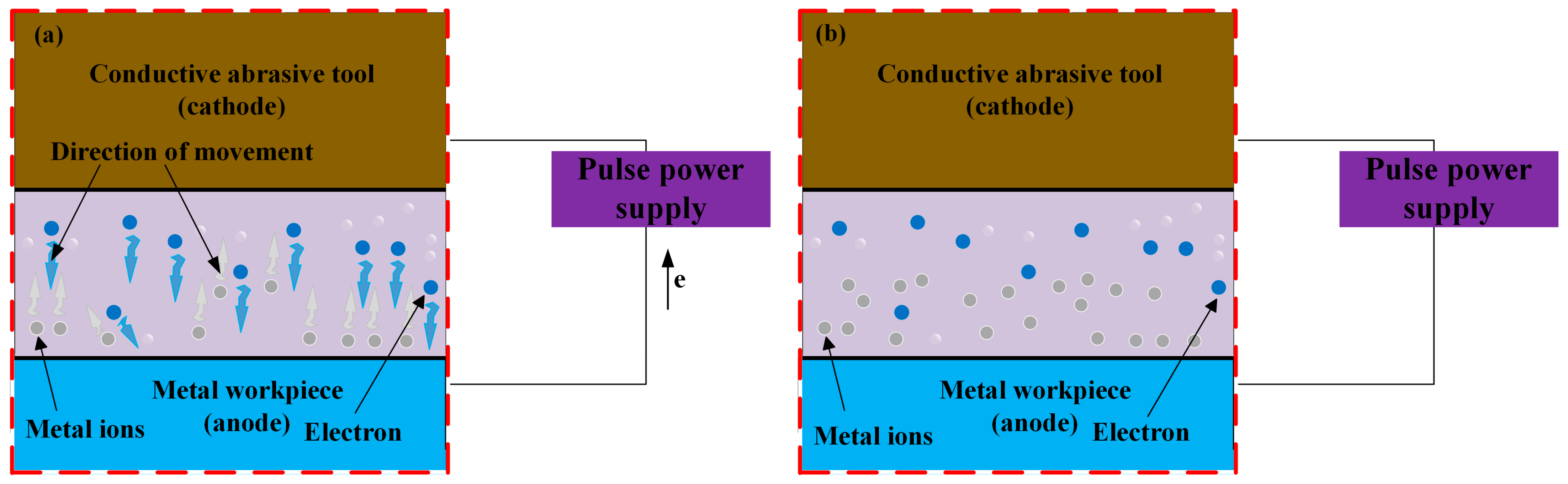
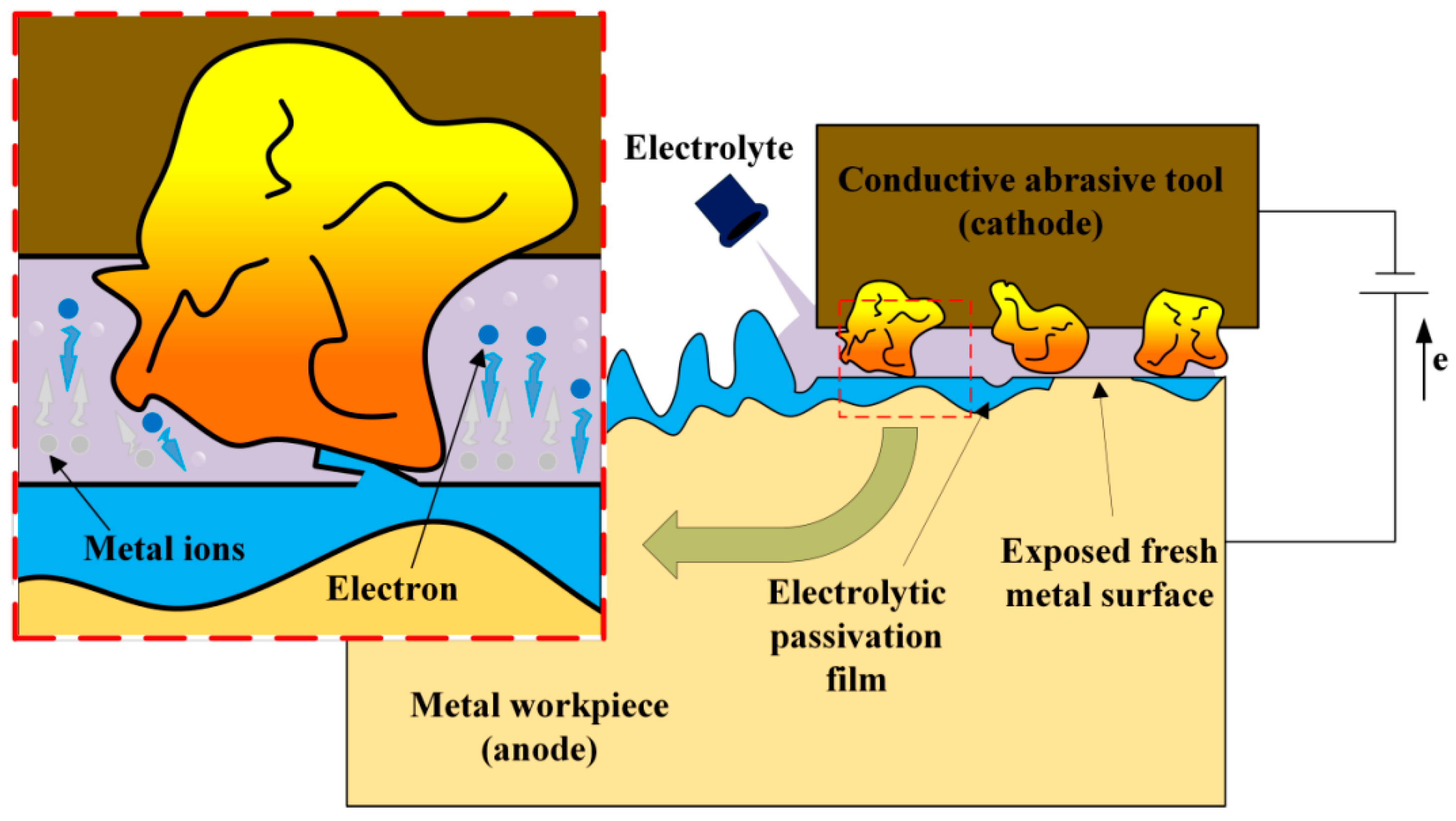
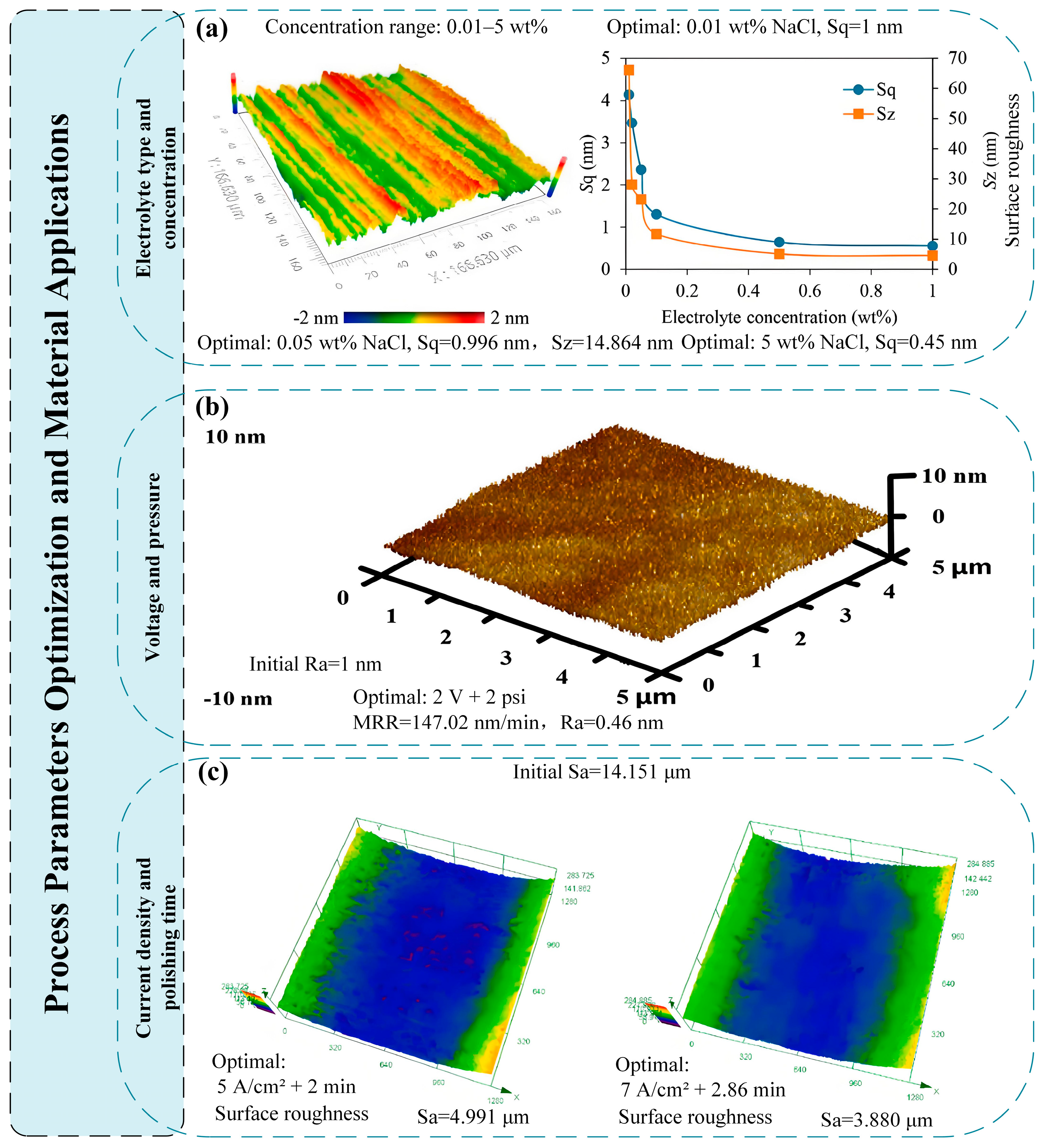
2.1.1. Pulsed Electrochemical Polishing
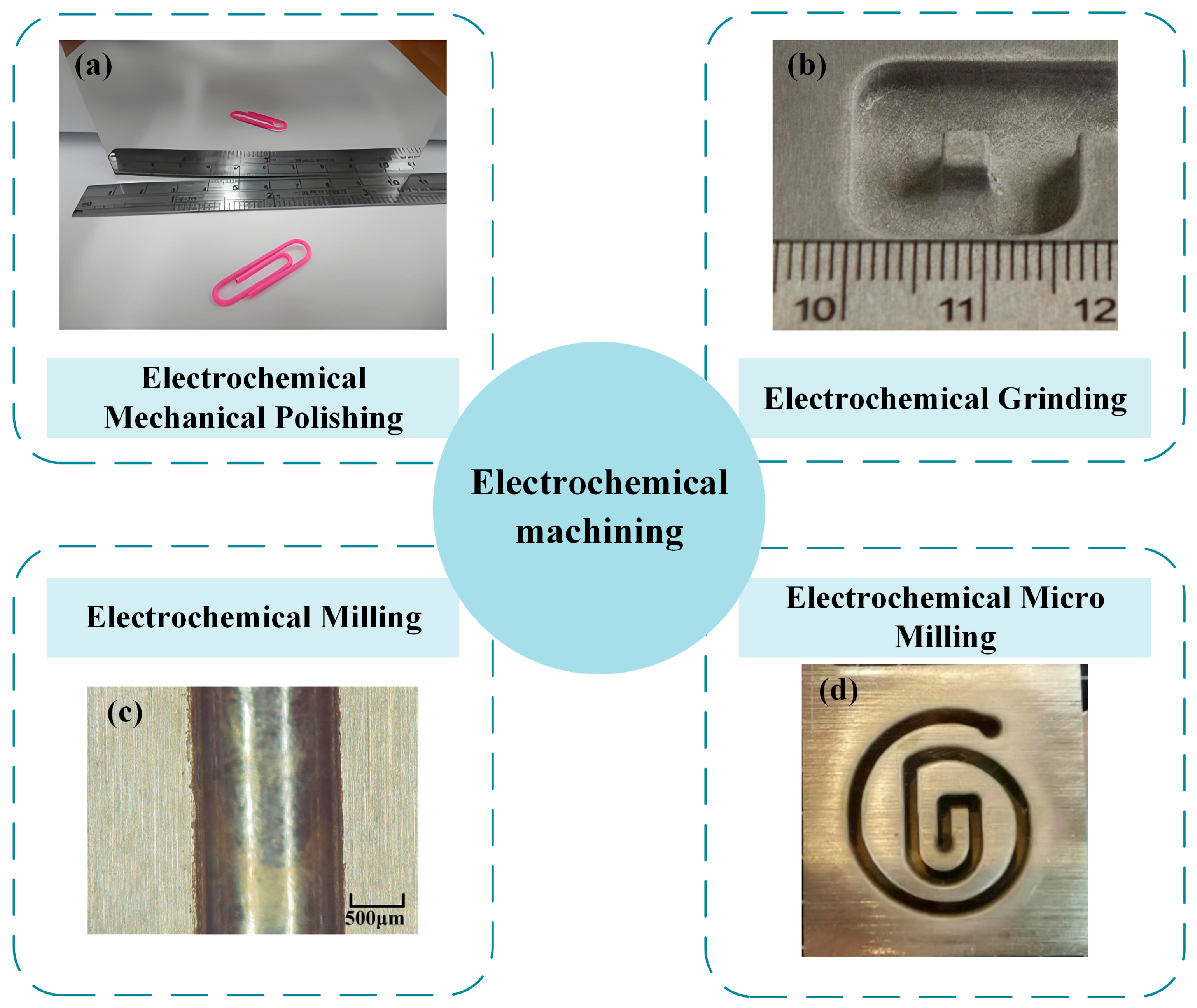
2.1.2. Electrochemical Mechanical Polishing
2.2. Macroscopic Material Forming ECM Technology
2.2.1. Electrochemical Grinding

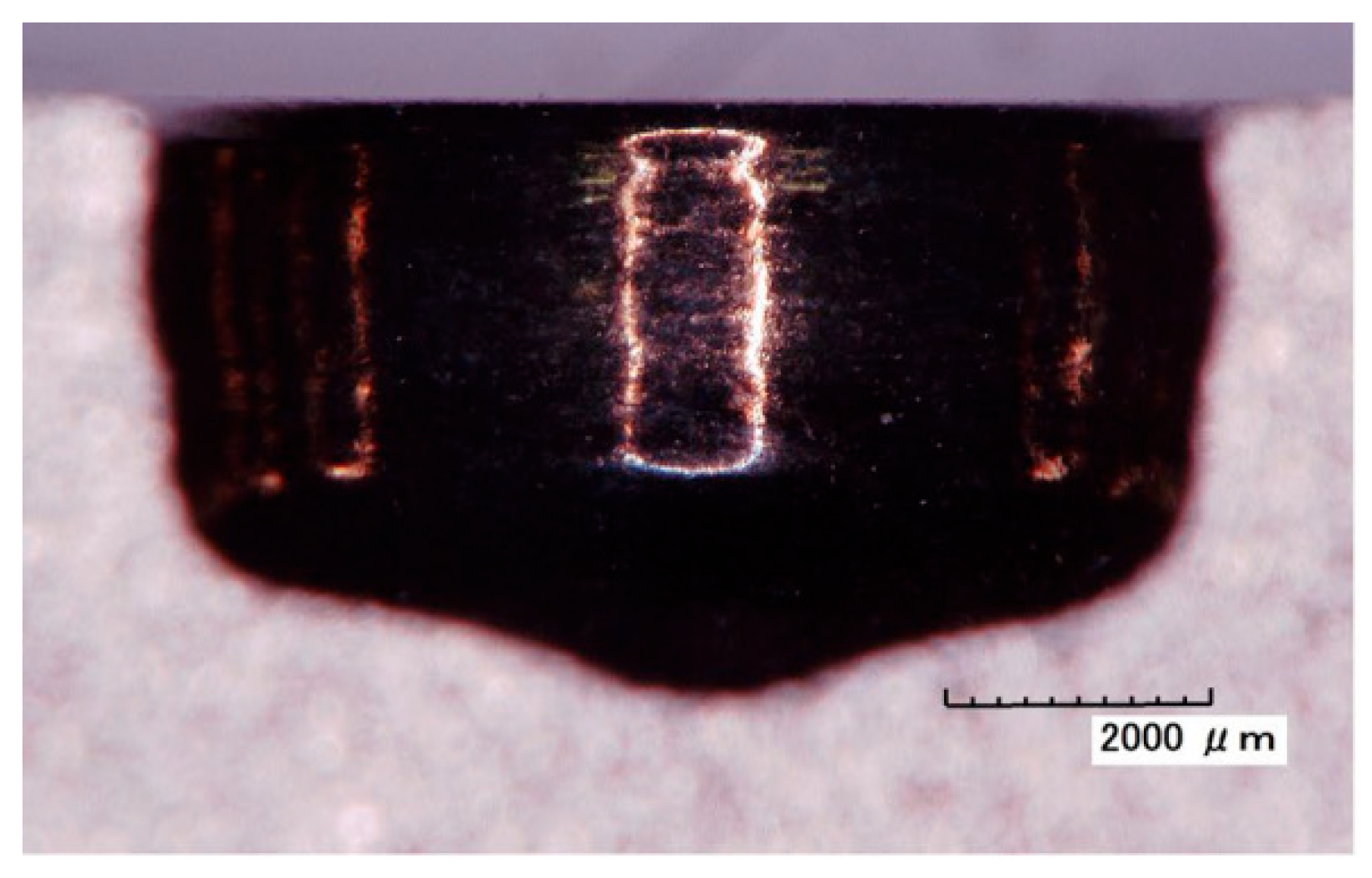
2.2.2. Electrochemical Milling-Grinding
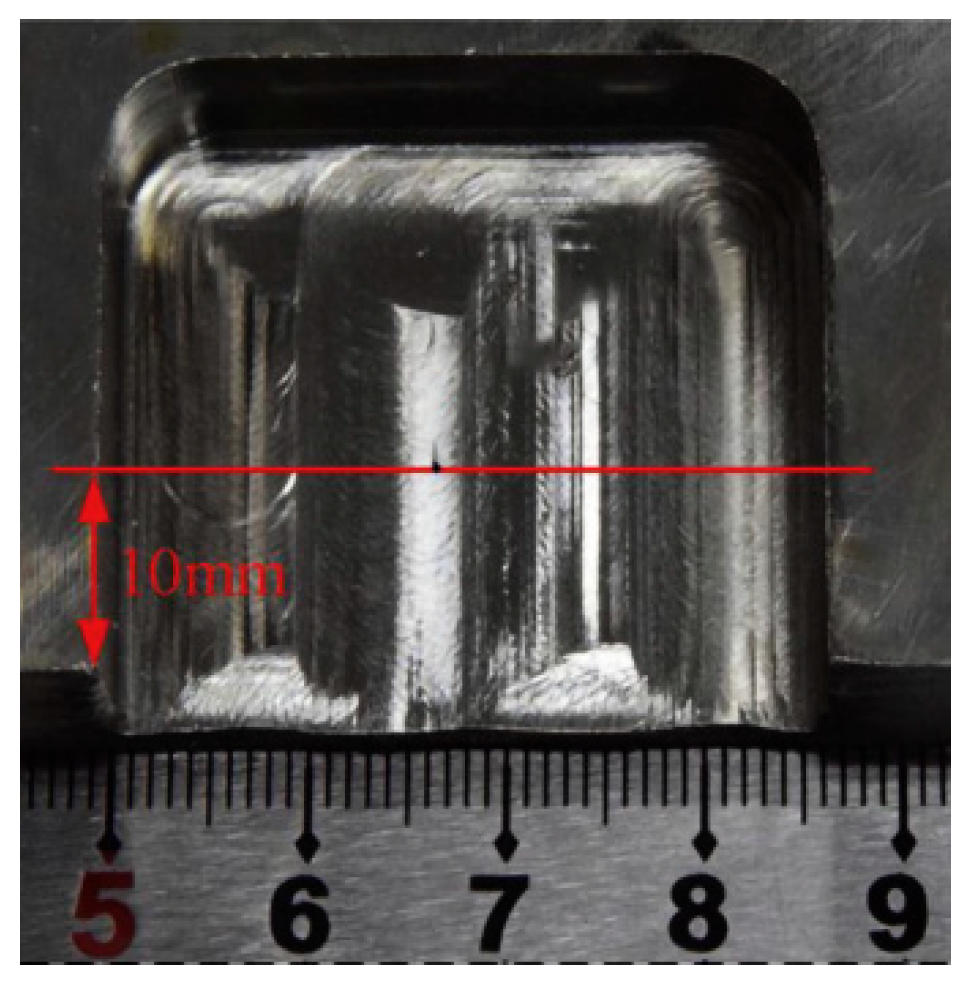
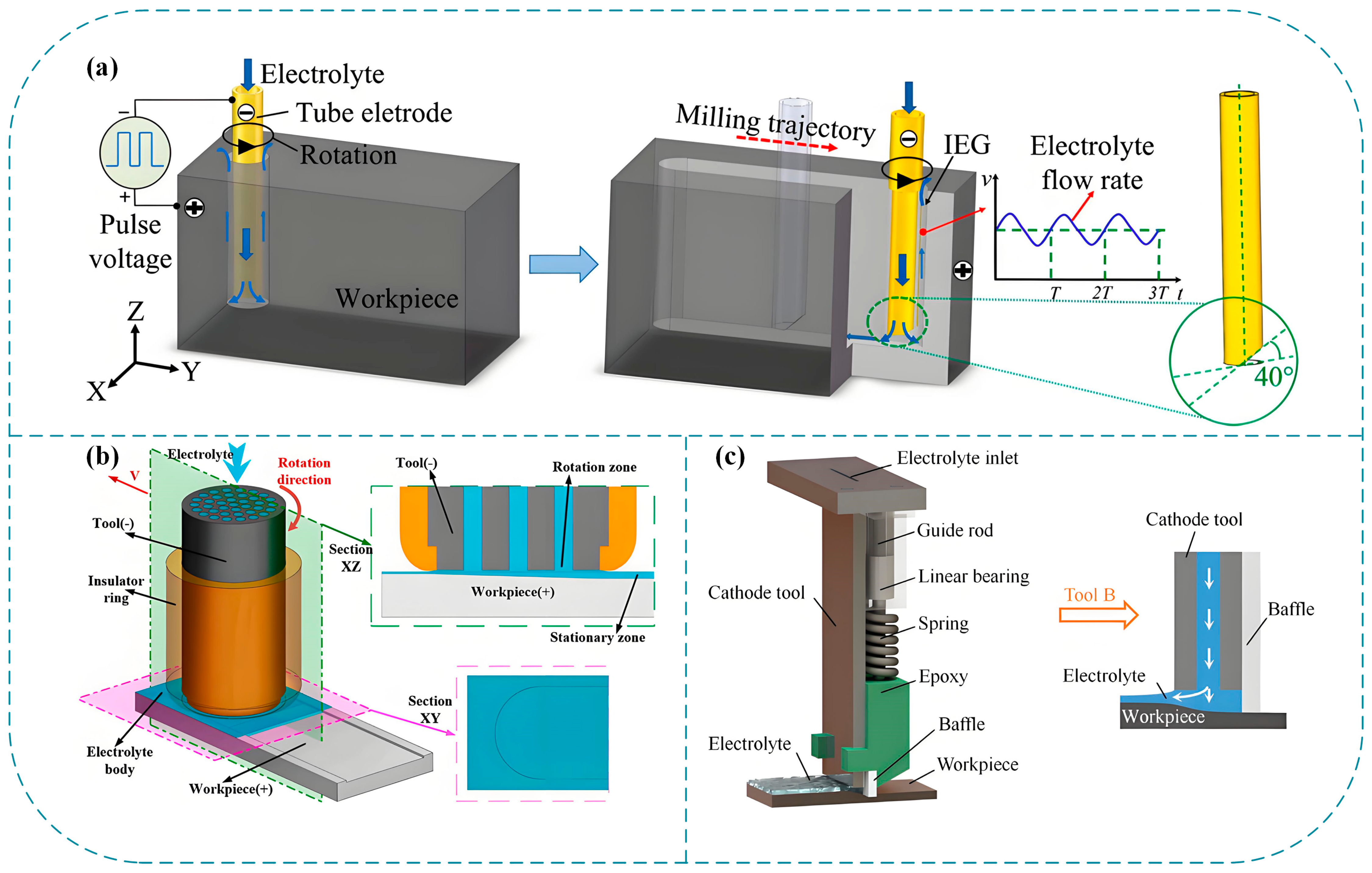
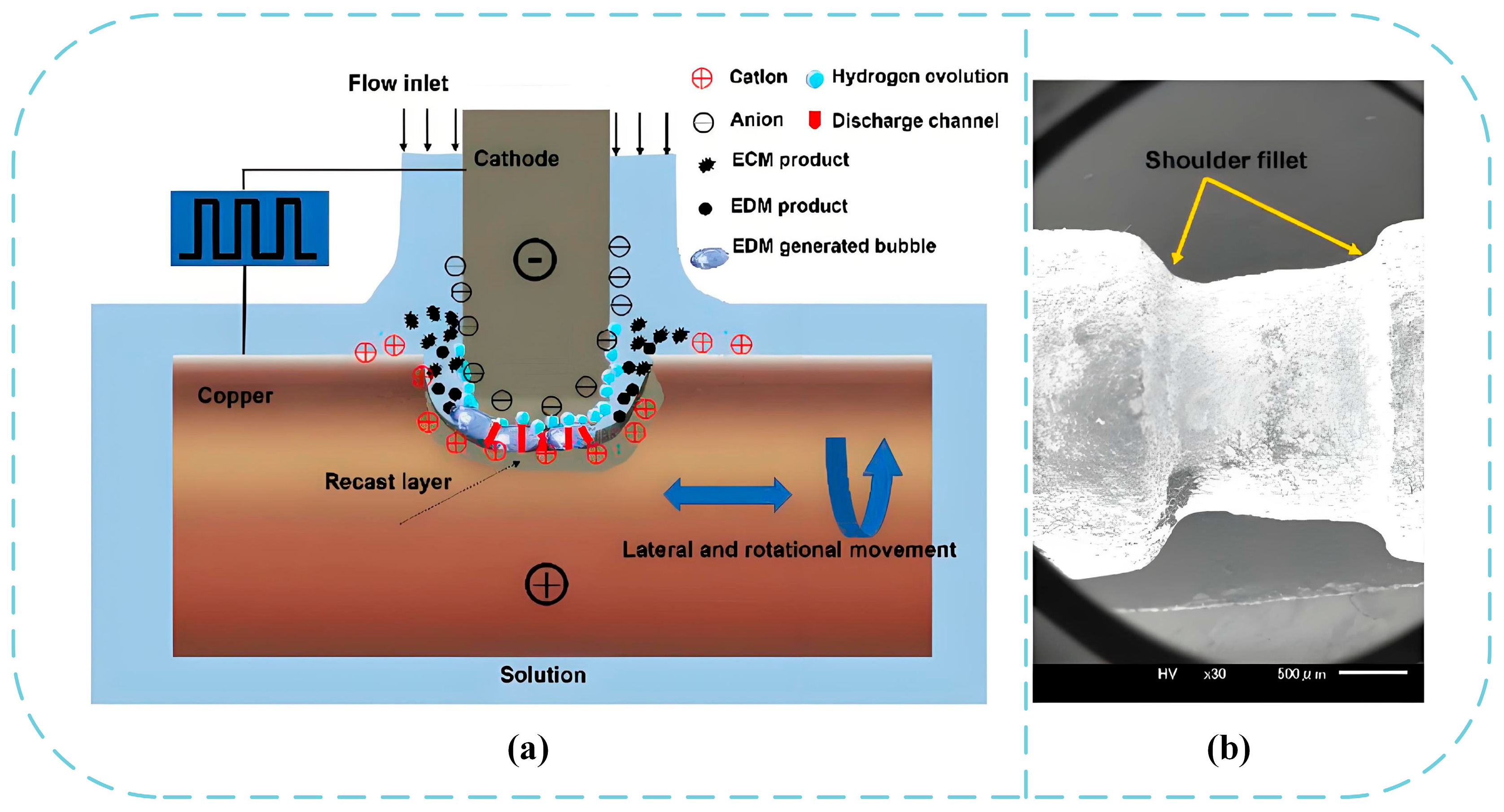
2.2.3. Electrochemical Milling
2.2.4. Electrochemical Turning
2.3. Microscopic Material Forming ECM Technology
2.3.1. Wire Electrochemical Turning
2.3.2. Electrochemical Micro-Milling
2.3.3. Electrochemical Micro-Turning
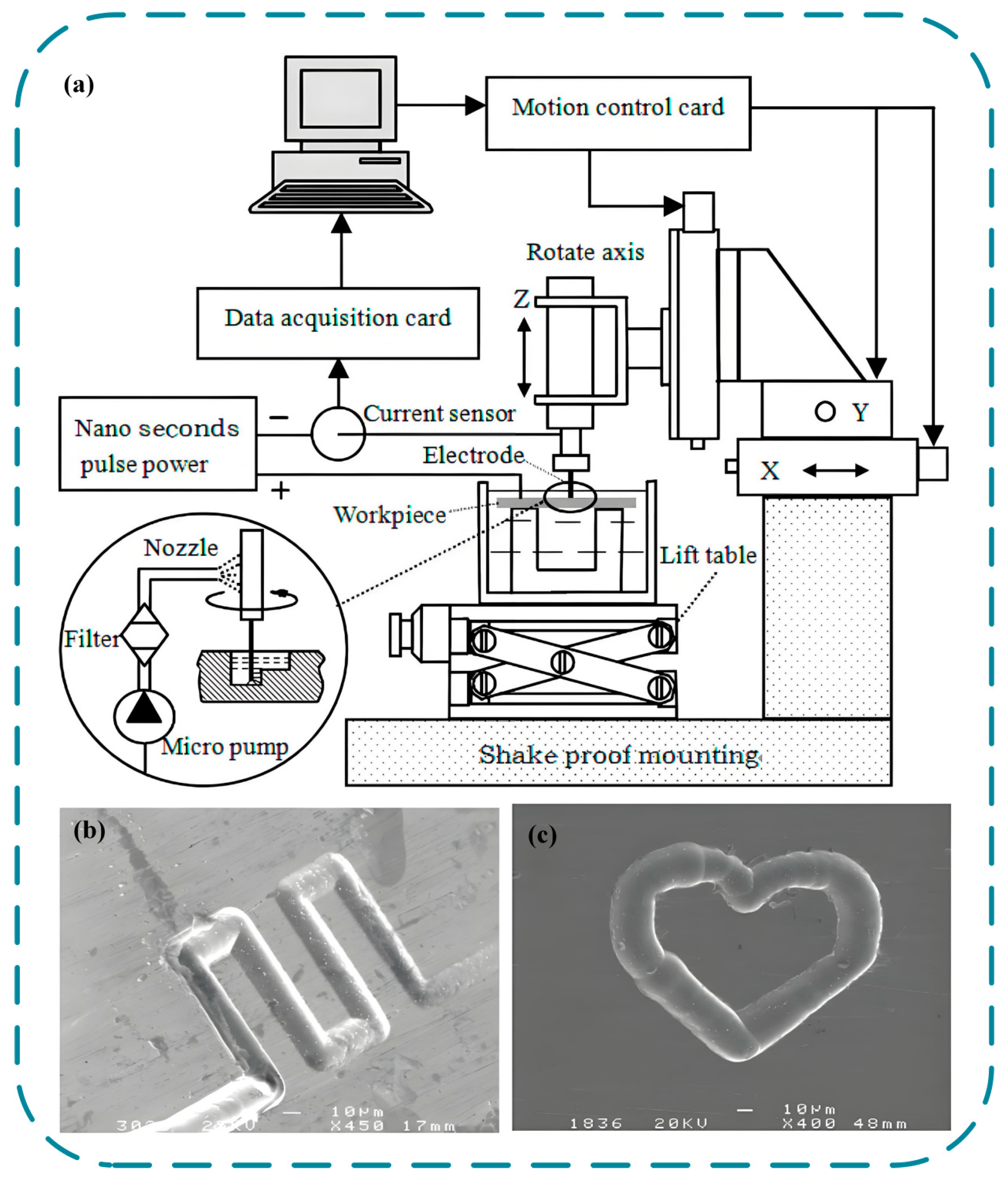
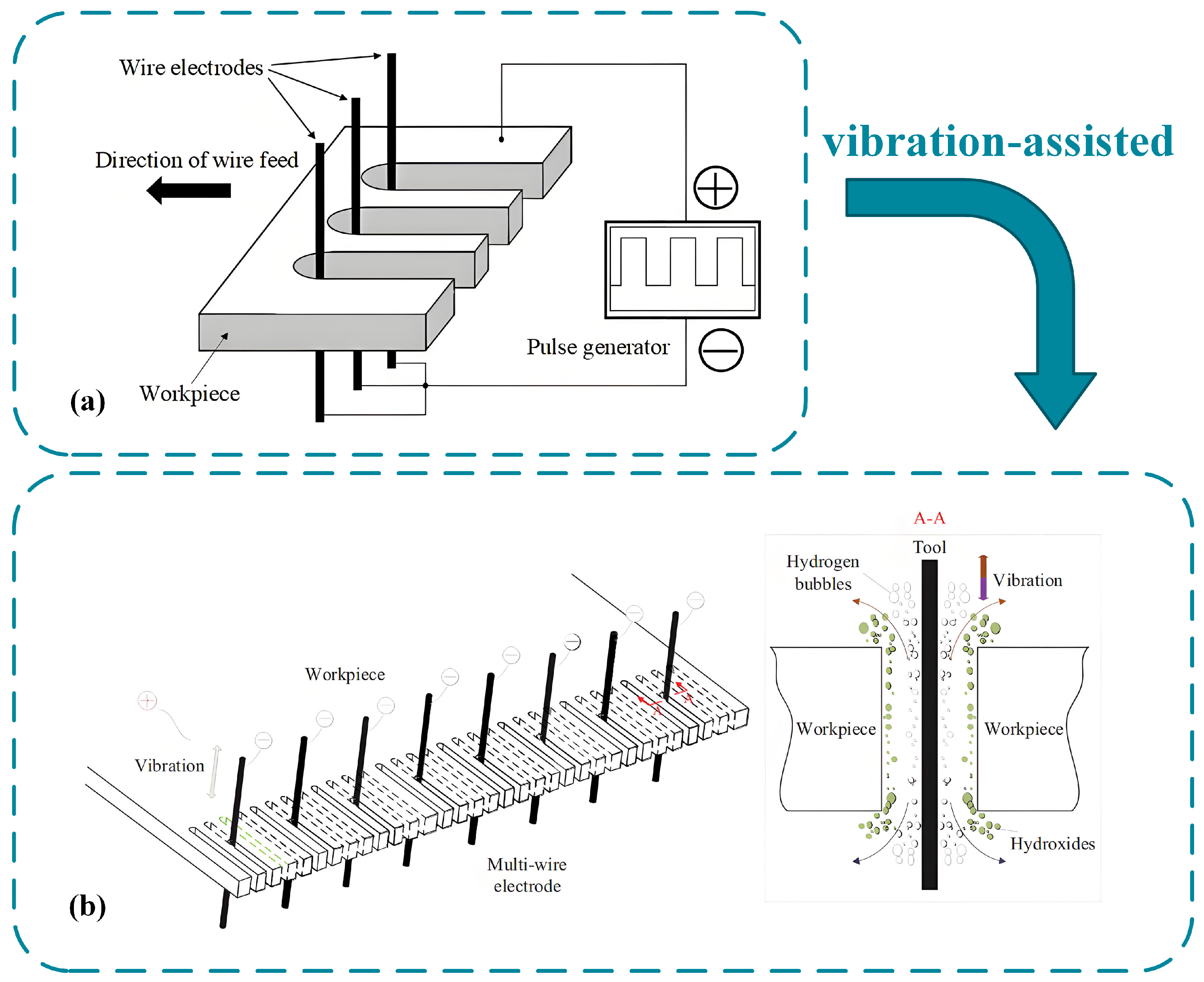
2.3.4. Wires Electrochemical Cutting
2.3.5. Mask Electrochemical Machining
2.3.6. Jet Electrochemical Machining
2.4. Summary of Typical ECM Technologies
3. Typical Applications and Case Analysis
3.1. Typical Applications of Surface Quality Improvement ECM
3.1.1. Medical Devices
3.1.2. Energy Industry
3.1.3. Technological Significance of Surface Quality Improvement ECM
3.2. Typical Applications of ECM in Macroscopic Material Forming
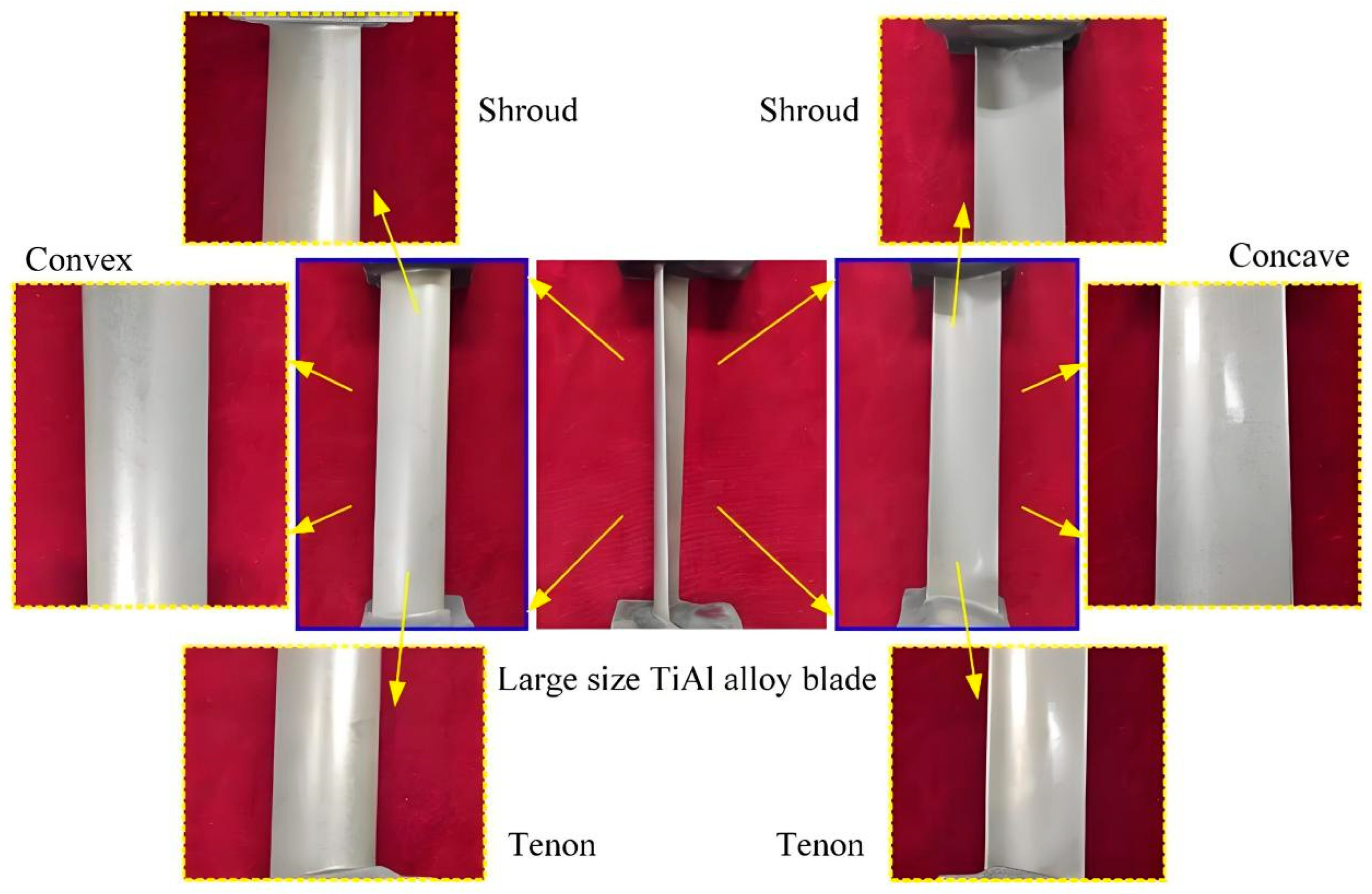
3.2.1. Aerospace Field: Turbine Blades
3.2.2. Aerospace Field: Thin Walled Receiver
3.2.3. Technological Significance of ECM in Macroscopic Material Forming
3.3. Typical Applications of ECM in Microscopic Material Forming
3.3.1. Micro Electrode Fabrication for Precision Machining
3.3.2. Microfluidic Device Fabrication on Printed Circuit Boards
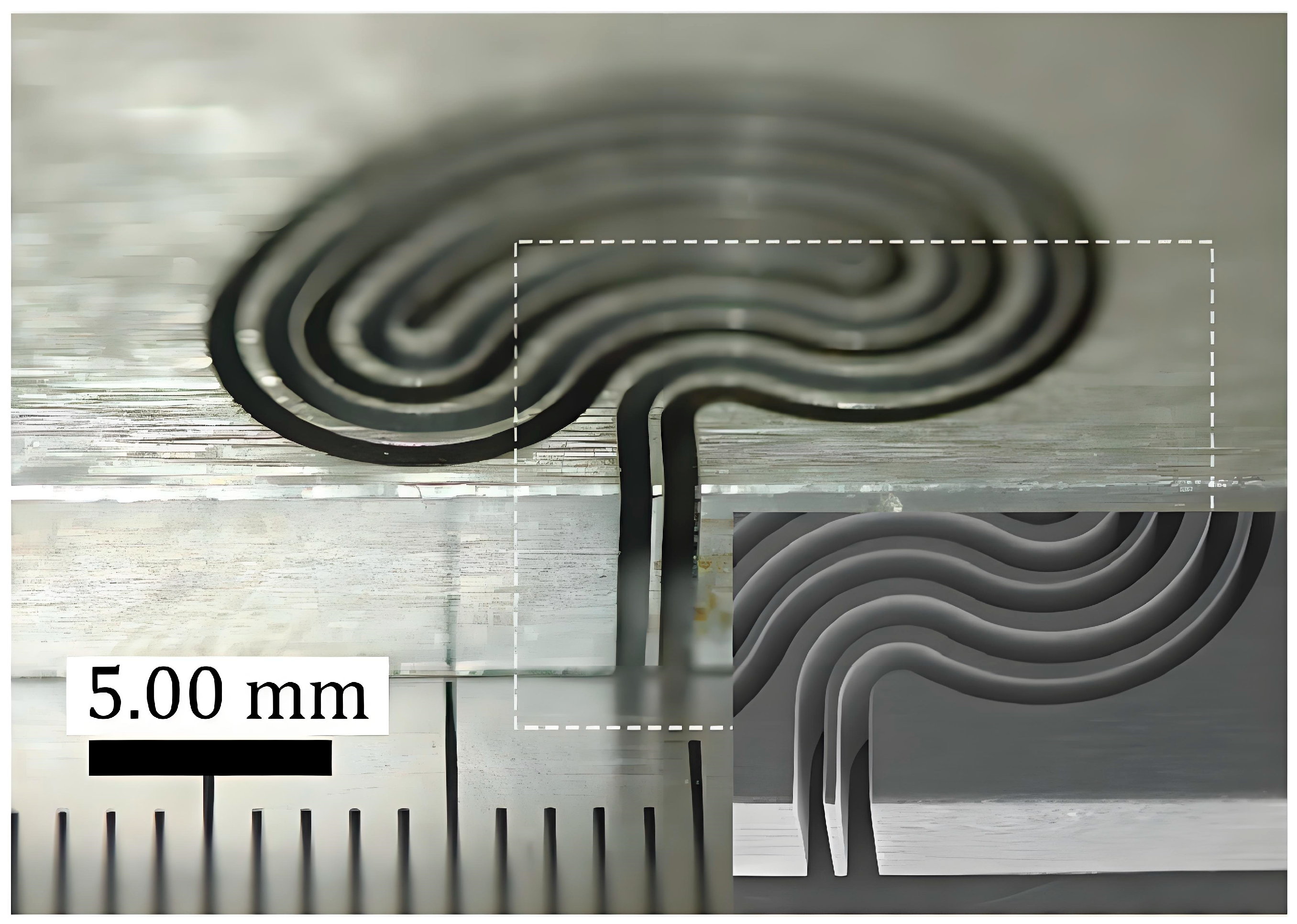
3.3.3. Micro-Channel Array Fabrication for Fuel Cell Bipolar Plates
3.3.4. Technological Significance of ECM in Microscopic Material Forming
4. Challenges and Development Directions
4.1. Existing Technological Bottlenecks
4.1.1. Dynamic Gap Control Challenges
4.1.2. Environmental Limitations
4.1.3. Abrasive Tool Degradation
4.2. Frontier Research Directions
4.2.1. Green Processing Technology
4.2.2. Intelligent Control Strategy
4.2.3. Cross Scale Processing
4.2.4. Multi Energy Field Combination
5. Conclusions and Prospect
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ezugwu, E.O. Key improvements in the machining of difficult-to-cut aerospace superalloys. Int. J. Mach. Tools Manuf. 2005, 45, 1353–1367. [Google Scholar] [CrossRef]
- Zinkle, S.J.; Was, G.S. Materials challenges in nuclear energy. Acta Mater. 2013, 61, 735–758. [Google Scholar] [CrossRef]
- El-Genk, M.S.; Tournier, J.M. A review of refractory metal alloys and mechanically alloyed-oxide dispersion strengthened steels for space nuclear power systems. J. Nucl. Mater. 2005, 340, 93–112. [Google Scholar] [CrossRef]
- Ezugwu, E.O.; Wang, Z.M. Titanium alloys and their machinability—A review. J. Mater. Process. Technol. 1997, 68, 262–274. [Google Scholar] [CrossRef]
- Liao, Z.; Xu, D.D.; García Luna, G.; Axinte, D.; Augustinavicius, G.; Sarasua, J.A.; Wretland, A. Influence of surface integrity induced by multiple machining processes upon the fatigue performance of a nickel based superalloy. J. Mater. Process. Technol. 2021, 298, 117313. [Google Scholar] [CrossRef]
- Saxena, K.K.; Qian, J.; Reynaerts, D. A review on process capabilities of electrochemical micromachining and its hybrid variants. Int. J. Mach. Tools Manuf. 2018, 127, 28–56. [Google Scholar] [CrossRef]
- Wang, Y.D.; Xu, Z.Y.; Hu, J.C.; Zhang, A. Surface integrity analysis of electrochemical machining of γ-TiAl alloys. Mater. Today Commun. 2020, 25, 101686. [Google Scholar] [CrossRef]
- Lee, S.-J.; Chen, Y.-H.; Liu, C.P.; Fan, T.J. Electrochemical mechanical polishing of flexible stainless steel substrate for thin-film solar cells. Int. J. Electrochem. Sci. 2013, 8, 6878–6888. [Google Scholar] [CrossRef]
- Li, H.S.; Niu, S.; Zhang, Q.L.; Fu, S.X.; Qu, N.S. Investigation of material removal in inner-jet electrochemical grinding of GH4169 alloy. Sci. Rep. 2017, 7, 3482. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qu, N.; Fang, X. Reducing stray corrosion in jet electrochemical milling by adjusting the jet shape. J. Mater. Process. Technol. 2019, 264, 240–248. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Yao, J.L.; Zhang, C.Y.; Chen, X.L.; Liu, J.W.; Zhang, Y.J. Electrochemical milling of narrow grooves with high aspect ratio using a tube electrode. J. Mater. Process. Technol. 2020, 282, 116695. [Google Scholar] [CrossRef]
- Gao, B.; Zhai, W.J.; Zhai, Q.; Xia, Y.L.; Wang, C.; Peng, K.X. Electro-chemical mechanical polishing of 4H-SiC for scratch-free surfaces with less oxide layer at high efficiency. ECS J. Solid State Sci. Technol. 2019, 8, P677. [Google Scholar] [CrossRef]
- Ma, G.L.; Li, S.J.; Liu, X.; Yin, X.C.; Jia, Z.; Liu, F.L. Combination of plasma electrolytic processing and mechanical polishing for single-crystal 4H-SiC. Micromachines 2021, 12, 606. [Google Scholar] [CrossRef]
- Yang, X.; Yang, X.Z.; Kawai, K.; Arima, K.; Yamamura, K. Novel SiC wafer manufacturing process employing three-step slurryless electrochemical mechanical polishing. J. Manuf. Process. 2021, 70, 350–360. [Google Scholar] [CrossRef]
- Tsuji, A.; Jia, P.F.; Takizawa, M.; Murata, J. Improvement in the polishing characteristics of titanium-based materials using electrochemical mechanical polishing. Surf. Interfaces 2022, 35, 102490. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, Y.M.; Du, M.F. The polishing mechanism of electrochemical mechanical polishing technology. J. Mater. Process. Technol. 2003, 140, 280–286. [Google Scholar] [CrossRef]
- Klocke, F.; Harst, S.; Ehle, L.; Zeis, M.; Klink, A. Surface integrity in electrochemical machining processes: An analysis on material modifications occurring during electrochemical machining. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2018, 232, 578–585. [Google Scholar] [CrossRef]
- Sagathiya, N.; Sharma, V.; Ramkumar, J. Electrochemical turning operation: A state of the art review. J. Manuf. Process. 2024, 113, 131–151. [Google Scholar] [CrossRef]
- Vanderauwera, W.; Vanloffelt, M.; Perez, R.; Lauwers, B. Investigation on the performance of macro electrochemical milling. Procedia Cirp 2013, 6, 356–361. [Google Scholar] [CrossRef]
- Liu, G.D.; Karim, M.R.; Arshad, M.H.; Saxena, K.K.; Liang, W.; Tong, H.; Li, Y.; Yang, Y.X.; Li, C.J.; Reynaerts, D. Tooling aspects of micro electrochemical machining (ECM) technology: Design, functionality, and fabrication routes. J. Mater. Process. Technol. 2023, 320, 118098. [Google Scholar] [CrossRef]
- Wang, Y.D.; Xu, Z.Y.; Meng, D.M.; Liu, L.; Fang, Z.D. Multi-physical field coupling simulation and experiments with pulse electrochemical machining of large size TiAl intermetallic blade. Metals 2023, 13, 985. [Google Scholar] [CrossRef]
- Chen, Y.; Fang, M.; Jiang, L. Multiphysics simulation of the material removal process in pulse electrochemical machining (PECM). Int. J. Adv. Manuf. Technol. 2017, 91, 2455–2464. [Google Scholar] [CrossRef]
- Chun, K.H.; Kim, S.H.; Lee, E.S. Analysis of the relationship between electrolyte characteristics and electrochemical machinability in PECM on invar (Fe-Ni) fine sheet. Int. J. Adv. Manuf. Technol. 2016, 87, 3009–3017. [Google Scholar] [CrossRef]
- Wang, J.T.; Xu, Z.Y.; Geng, T.Y.; Zhu, D. Dependency of the pulsed electrochemical machining characteristics of Inconel 718 in NaNO3 solution on the pulse current. Sci. China Technol. Sci. 2022, 65, 2485–2502. [Google Scholar] [CrossRef]
- Fang, M.; Chen, Y.L.; Jiang, L.J.; Su, Y.S.; Liang, Y. Optimal design of cathode based on iterative solution of multi-physical model in pulse electrochemical machining (PECM). Int. J. Adv. Manuf. Technol. 2019, 105, 3261–3270. [Google Scholar] [CrossRef]
- Zhang, Z.; De Meter, E.; Basu, S. Use of bidirectional electrolyte flow to improve PECM uniformity. Int. J. Adv. Manuf. Technol. 2023, 127, 2843–2859. [Google Scholar] [CrossRef]
- Boxhammer, M.; Altmannshofer, S. Model predictive control in pulsed electrochemical machining. J. Process Control 2014, 24, 296–303. [Google Scholar] [CrossRef]
- Zhu, R.; Zhang, T.Y.; Yao, Q.Y.; Peng, Y.; Cheng, F.; Wang, Z.R.; Wang, Y.G.; Lu, X.L.; Wang, C.Y.; Zhao, Y.W. Atomistic mechanisms of SiC electrochemical mechanical polishing in aqueous H2O2: A ReaxFF molecular dynamics study. J. Manuf. Process. 2025, 136, 56–67. [Google Scholar] [CrossRef]
- Gao, F.; Liang, H. Material removal mechanisms in electrochemical–mechanical polishing of tantalum. Electrochim. Acta 2009, 54, 6808–6815. [Google Scholar] [CrossRef]
- Yang, X.; Yang, X.; Yamamura, K. Effects of electrolyte type and concentration on the anodic oxidation of 4H-SiC (0001) in slurryless electrochemical mechanical polishing. Electrochim. Acta 2024, 474, 143531. [Google Scholar] [CrossRef]
- Xie, F.; Zhong, M.; Xu, W. Investigation of electrochemical behavior and polishing mechanism in electrochemical mechanical polishing of cobalt. Mater. Sci. Semicond. Process. 2024, 169, 107899. [Google Scholar] [CrossRef]
- An, L.; Wang, D.; Zhu, D. Combined electrochemical and mechanical polishing of interior channels in parts made by additive manufacturing. Addit. Manuf. 2022, 51, 102638. [Google Scholar] [CrossRef]
- Zhao, C.; Qu, N.; Tang, X. Electrochemical mechanical polishing of internal holes created by selective laser melting. J. Manuf. Process. 2021, 64, 1544–1562. [Google Scholar] [CrossRef]
- Hung, J.C.; Yang, P.J.; Lin, X.H.; Jian, S.Y.; Kao, C.H.; Ferng, Y.C.; Huang, Y.S.; Jen, K.K. Surface passivation and brightening of titanium-based AM materials using a robotic electrochemical mechanical polishing system. Int. J. Adv. Manuf. Technol. 2024, 134, 4339–4352. [Google Scholar] [CrossRef]
- Murata, J.; Hayama, K.; Takizawa, M. Environment-friendly electrochemical mechanical polishing using solid polymer electrolyte/CeO2 composite pad for highly efficient finishing of 4H-SiC (0001) surface. Appl. Surf. Sci. 2023, 625, 157190. [Google Scholar] [CrossRef]
- Inada, N.; Takizawa, M.; Adachi, M.; Murata, J. Sustainable electrochemical mechanical polishing (ECMP) for 4H-SiC wafer using chemical-free polishing slurry with hydrocarbon-based solid polymer electrolyte. Appl. Surf. Sci. 2024, 664, 160241. [Google Scholar] [CrossRef]
- Xue, L.; Qu, N.; Ding, W. Impact of electrochemical machining parameters on the electrochemical grinding process. Mater. Manuf. Process. 2025, 40, 594–602. [Google Scholar] [CrossRef]
- Yehia, H.M.; Hakim, M.; El-Assal, A. Effect of the Al2O3 powder addition on the metal removal rate and the surface roughness of the electrochemical grinding machining. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2020, 234, 1538–1548. [Google Scholar] [CrossRef]
- Niu, S.; Qu, N.; Li, H. Investigation of electrochemical mill-grinding using abrasive tools with bottom insulation. Int. J. Adv. Manuf. Technol. 2018, 97, 1371–1382. [Google Scholar] [CrossRef]
- Li, H.S.; Fu, S.X.; Zhang, Q.L.; Niu, S.; Qu, N.S. Simulation and experimental investigation of inner-jet electrochemical grinding of GH4169 alloy. Chin. J. Aeronaut. 2018, 31, 608–616. [Google Scholar] [CrossRef]
- Wang, H.Z.; Man, H.; Yang, J.H.; Zang, J.H.; Che, R.C.; Wang, F.; Sun, D.L.; Fang, F. Self-adapting electrochemical grinding strategy for stable silicon anode. Adv. Funct. Mater. 2022, 32, 2109887. [Google Scholar] [CrossRef]
- Ma, X.S.; Jiao, F.; Niu, Y.; Wang, X.; Hu, Z.Z.; Bie, W.B.; Yang, G.B. Modeling of the material removal rate in internal cylindrical plunge electrochemical grinding. J. Manuf. Process. 2023, 92, 89–106. [Google Scholar] [CrossRef]
- Wang, J.C.; Zhang, L.W.; Cheng, J.H.; Liu, J.; Zou, Y.C.; Wei, D.Q.; Cheng, S.; Wang, Y.M. A precise machining surface obtained electrolyte plasma polishing on Ti–6Al–4V alloy: Microstructure and in-situ mechanical behaviors. J. Manuf. Process. 2025, 133, 979–991. [Google Scholar] [CrossRef]
- Niu, S.; Qu, N.S.; Yue, X.K.; Li, H.S. Effect of tool-sidewall outlet hole design on machining performance in electrochemical mill-grinding of Inconel 718. J. Manuf. Process. 2019, 41, 10–22. [Google Scholar] [CrossRef]
- Niu, S.; Qu, N.S.; Yue, X.K.; Liu, G.Q.; Li, H.S. Combined rough and finish machining of Ti–6Al–4V alloy by electrochemical mill-grinding. Mach. Sci. Technol. 2020, 24, 621–637. [Google Scholar] [CrossRef]
- Yue, X.; Qu, N.; Niu, S.; Li, H. Improving the machined bottom surface in electrochemical mill-grinding by adjusting the electrolyte flow field. J. Mater. Process. Technol. 2019, 276, 116413. [Google Scholar] [CrossRef]
- Li, J.; Li, H.S.; Hu, X.Y.; Niu, S.; Xu, G.L. Simulation analysis and experimental validation of cathode tool in electrochemical mill-grinding of Ti6Al4V. Appl. Sci. 2020, 10, 1941. [Google Scholar] [CrossRef]
- Wang, F.; Zhou, J.; Wu, S.Y.; Kang, X.M.; Zhao, W.S. Study on material removal mechanism of photocatalytic-assisted electrochemical milling-grinding SiCp/Al. Int. J. Adv. Manuf. Technol. 2023, 124, 817–832. [Google Scholar] [CrossRef]
- Niu, S.; Huang, K.Q.; Ming, P.M.; Qin, G.; Peng, Y.S. Electrochemical Mill Grinding of (TiB+ TiC)/Ti6Al4V Composites Using Abrasive Tool with Bottom Outlet Holes. Micromachines 2024, 15, 1410. [Google Scholar] [CrossRef]
- Qu, N.S.; Fang, X.L.; Zhang, J.Z.; Kong, H.H.; Liu, Y.; Wang, M.L.; Yue, X.K.; Ma, Y.H.; Shen, Z.H.; Chen, J.J. Macro electrochemical milling and its hybrid variants. Chin. J. Aeronaut. 2024, 37, 1–35. [Google Scholar] [CrossRef]
- Mishra, K.; Dey, D.; Sarkar, B.R.; Bhattacharyya, B. Experimental investigation into electrochemical milling of Ti6Al4V. J. Manuf. Process. 2017, 29, 113–123. [Google Scholar] [CrossRef]
- Wang, B.; Yang, H.; Feng, Y.; Zeng, S.; Tan, L.; Xu, R.; Wang, L.; Hu, R.; Yu, Y. Boosting low-temperature sodium/potassium storage performance of Bi via novel electrochemical milling process. Mater. Today Energy 2021, 20, 100627. [Google Scholar] [CrossRef]
- Niu, S.; Qu, N.S.; Fu, S.X.; Fang, X.L.; Li, H.S. Investigation of inner-jet electrochemical milling of nickel-based alloy GH4169/Inconel 718. Int. J. Adv. Manuf. Technol. 2017, 93, 2123–2132. [Google Scholar] [CrossRef]
- Mishra, K.; Sarkar, B.R.; Bhattacharyya, B. Influence of inner-spraying rotating tool during electrochemical milling of Nimonic-263 alloy. Mater. Manuf. Process. 2019, 34, 807–813. [Google Scholar] [CrossRef]
- Kong, H.; Qu, N. Jet electrochemical milling of Ti-6Al-4V alloy with ultra-high current density. Int. J. Adv. Manuf. Technol. 2023, 129, 4091–4100. [Google Scholar] [CrossRef]
- Liu, Y.; Qu, N. Electrochemical milling of TB6 titanium alloy in NaNO3 solution. J. Electrochem. Soc. 2019, 166, E35. [Google Scholar] [CrossRef]
- Chen, X.L.; Ye, Z.S.; Li, G.J.; Saxena, K.K.; Zhang, C.Y.; Zhang, Y.J. Electrochemical milling of deep-narrow slots with a pulsating electrolyte flow field. CIRP J. Manuf. Sci. Technol. 2022, 39, 244–260. [Google Scholar] [CrossRef]
- Shen, Z.H.; Li, H.S.; Zhang, J.Z.; Qu, N.S. Improving flatness of machined surface in rotating cathode electrochemical milling. J. Manuf. Process. 2024, 116, 60–76. [Google Scholar] [CrossRef]
- Kong, H.; Qu, N. Flat jet electrochemical milling of TC4 alloy with tailoring backward parallel flow. Chin. J. Aeronaut. 2024, 37, 574–592. [Google Scholar] [CrossRef]
- Van Camp, D.; Qian, J.; Vleugels, J.; Lauwers, B. Experimental investigation of the process behaviour in Mechano-Electrochemical Milling. Cirp Ann. 2018, 67, 217–220. [Google Scholar] [CrossRef]
- Kong, H.; Qu, N.; Chen, J. Machining behaviour modulation of electrochemical milling via manipulation of inter-electrode gap: From electrochemical machining to electrochemical discharge machining. J. Mater. Process. Technol. 2024, 333, 118584. [Google Scholar] [CrossRef]
- Wang, M.; Qu, N. Investigation on material removal mechanism in mechano-electrochemical milling of TC4 titanium alloy. J. Mater. Process. Technol. 2021, 295, 117206. [Google Scholar] [CrossRef]
- Yu, L.; Fang, M.; Jiang, L.J.; Chu, X.F.; Hou, L.L.; Cheng, X.; Wang, J.L. Gap compensation control method for robotic electrochemical milling machining based on multi-physics field coupling. Int. J. Adv. Manuf. Technol. 2024, 135, 395–407. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Zhao, C.H.; Qu, N.S.; Shen, Z.H. Enhancement of surface quality in the electrochemical milling of 316L using a novel cathode structure. Precis. Eng. 2022, 78, 134–145. [Google Scholar] [CrossRef]
- Yahyavi Zanjani, M.; Ghattan Kashani, H.; Mirahmadi, A. Improvement of electrochemical turning for machining complex shapes using a simple gap size sensor and a tubular shape tool. Int. J. Adv. Manuf. Technol. 2013, 69, 375–381. [Google Scholar] [CrossRef]
- Ma, X.; Hu, X.Y.; Cao, X.B.; Lü, Z.H.; Shen, J.Z.; Li, H.S. Optimizing electrochemical turning of titanium matrix composites: Enhancing efficiency with inclined cathode tools. CIRP J. Manuf. Sci. Technol. 2025, 59, 34–45. [Google Scholar] [CrossRef]
- Ebeid, S.J.; Ei-Taweel, T.A. Surface improvement through hybridization of electrochemical turning and roller burnishing based on the Taguchi technique. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2005, 219, 423–430. [Google Scholar] [CrossRef]
- Liu, Y.; Qu, N.; Qiu, Z. Flow field simulation and experimental investigation on macro electrolyte jet electrochemical turning of TB6 titanium alloy. Int. J. Adv. Manuf. Technol. 2022, 120, 2617–2632. [Google Scholar] [CrossRef]
- Ge, Y.C.; Zhu, Z.W.; Ma, Z.; Wang, D.Y. Tool design and experimental study on electrochemical turning of nickel-based cast superalloy. J. Electrochem. Soc. 2018, 165, E162. [Google Scholar] [CrossRef]
- Ge, Y.C.; Zhu, Z.W.; Ma, Z.; Wang, D.Y. Large allowance electrochemical turning of revolving parts using a universal cylindrical electrode. J. Mater. Process. Technol. 2018, 258, 89–96. [Google Scholar] [CrossRef]
- Han, W.; Kunieda, M. Precision electrochemical machining of tungsten micro-rods using wire electrochemical turning method. Int. J. Adv. Manuf. Technol. 2020, 111, 295–307. [Google Scholar] [CrossRef]
- El-Taweel, T.A.; Gouda, S.A. Performance analysis of wire electrochemical turning process—RSM approach. Int. J. Adv. Manuf. Technol. 2011, 53, 181–190. [Google Scholar] [CrossRef]
- El-Taweel, T.A.; Haridy, S. An application of fractional factorial design in wire electrochemical turning process. Int. J. Adv. Manuf. Technol. 2014, 75, 1207–1218. [Google Scholar] [CrossRef]
- Mishra, K.; Gupta, S.; Bhattacharyya, B. Problematic areas in micro-electrochemical milling of HSTR alloys. Int. J. Adv. Manuf. Technol. 2020, 111, 1015–1036. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, D.; Zhu, L. Micro electrochemical milling of complex structures by using in situ fabricated cylindrical electrode. Int. J. Adv. Manuf. Technol. 2012, 60, 977–984. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, Y.; Guo, C.S.; Deng, S.H.; Kong, H.H. Experimental research on machining localization and surface quality in micro electrochemical milling of nickel-based superalloy. Micromachines 2018, 9, 402. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.D.; Guo, C.S.; Kong, H.H. Analysis on machining performance of nickel-base superalloy by electrochemical micro-milling with high-speed spiral electrode. Micromachines 2019, 10, 476. [Google Scholar] [CrossRef]
- Skoczypiec, S.; Grabowski, M.; Ruszaj, A. The impact of electrochemical assistance on the microturning process. Int. J. Adv. Manuf. Technol. 2016, 86, 1873–1880. [Google Scholar] [CrossRef]
- Grabowski, M.; Skoczypiec, S.; Wyszynski, D. A Study on microturning with electrochemical assistance of the cutting process. Micromachines 2018, 9, 357. [Google Scholar] [CrossRef]
- Wang, C.; Sasaki, T.; Hirao, A. Micro shaft turning by electrochemical discharge machining using a sandwich cathode. Precis. Eng. 2025, 94, 344–357. [Google Scholar] [CrossRef]
- Zeng, Y.B.; Yu, Q.; Fang, X.L.; Xu, K.; Li, H.S.; Qu, N.S. Wire electrochemical machining with monodirectional traveling wire. Int. J. Adv. Manuf. Technol. 2015, 78, 1251–1257. [Google Scholar] [CrossRef]
- Debnath, S.; Masanta, M.; Bhattacharyya, B. Wire Electrochemical Machining employing newly developed tungsten micro wire with repeatedly similar cross sectional variations. J. Manuf. Process. 2022, 74, 535–543. [Google Scholar] [CrossRef]
- He, H.D.; Qu, N.S.; Zeng, Y.B.; Fang, X.L.; Yao, Y.Y. Machining accuracy in pulsed wire electrochemical machining of γ-TiAl alloy. Int. J. Adv. Manuf. Technol. 2016, 86, 2353–2359. [Google Scholar] [CrossRef]
- Wu, X.Y.; Li, S.J.; Zhao, W.; Tang, L.; Li, Z.P. Experiment investigation of using wire electrochemical machining in deionized water to reduce the wire electrical discharge machining surface roughness. Int. J. Adv. Manuf. Technol. 2019, 102, 343–353. [Google Scholar] [CrossRef]
- Singh, T.; Arab, J.; Chen, S.C. Improvement on surface quality of Inconel-718 slits via laser cutting and wire electrochemical machining processes. Opt. Laser Technol. 2023, 167, 109637. [Google Scholar] [CrossRef]
- Zou, X.H.; Fang, X.L.; Chen, M.; Zhu, D. Investigation on mass transfer and dissolution localization of wire electrochemical machining using vibratory ribbed wire tools. Precis. Eng. 2018, 51, 597–603. [Google Scholar] [CrossRef]
- Fang, X.L.; Zou, X.H.; Chen, M.; Zhu, D. Study on wire electrochemical machining assisted with large-amplitude vibrations of ribbed wire electrodes. CIRP Ann. 2017, 66, 205–208. [Google Scholar] [CrossRef]
- Klocke, F.; Herrig, T.; Zeis, M.; Klink, A. Experimental investigations of cutting rates and surface integrity in wire electrochemical machining with rotating electrode. Procedia CIRP 2018, 68, 725–730. [Google Scholar] [CrossRef]
- Maity, S.; Debnath, S.; Bhattacharyya, B. Modeling and investigation on multi-wire electrochemical machining (MWEC) assisted with different flushing strategies. J. Manuf. Process. 2020, 57, 857–870. [Google Scholar] [CrossRef]
- He, H.D.; Zeng, Y.B.; Yao, Y.Y.; Qu, N.S. Improving machining efficiency in wire electrochemical micromachining of array microstructures using axial vibration-assisted multi-wire electrodes. J. Manuf. Process. 2017, 25, 452–460. [Google Scholar] [CrossRef]
- Besekar, N.; Bhattacharyya, B. Experimental investigation and characterization of NiTinol shape memory alloy during wire electrochemical machining. J. Manuf. Process. 2022, 81, 346–361. [Google Scholar] [CrossRef]
- He, H.; Zeng, Y.; Qu, N. An investigation into wire electrochemical micro machining of pure tungsten. Precis. Eng. 2016, 45, 285–291. [Google Scholar] [CrossRef]
- Qu, N.S.; Ji, H.J.; Zeng, Y.B. Wire electrochemical machining using reciprocated traveling wire. Int. J. Adv. Manuf. Technol. 2014, 72, 677–683. [Google Scholar] [CrossRef]
- Wang, G.Q.; Li, H.S.; Qu, N.S.; Zhu, D. Improvement of electrolyte flow field during through-mask electrochemical machining by changing mask wall angle. J. Manuf. Process. 2017, 25, 246–252. [Google Scholar] [CrossRef]
- Li, H.S.; Wang, G.Q.; Qu, N.S.; Zhu, D. Through-mask electrochemical machining of a large-area hole array in a serpentine flow channel. Int. J. Adv. Manuf. Technol. 2017, 89, 933–940. [Google Scholar] [CrossRef]
- Wang, G.; Li, H.; Zhu, D. Improvement of machining consistency during through-mask electrochemical large-area machining. Chin. J. Aeronaut. 2019, 32, 1051–1058. [Google Scholar] [CrossRef]
- Yang, X.C.; Du, L.Q.; Li, A.Q.; Wu, M.X.; Wu, C.H.; Li, J.M. A novel method of induction electrode through-mask electrochemical micromachining. Int. J. Mach. Tools Manuf. 2024, 203, 104221. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, X.H.; Wu, C.D.; Han, G.F.; Zhang, J. A novel mask electrochemical additive and subtractive combined manufacturing technique for microstructures with high machining performance. Int. J. Adv. Manuf. Technol. 2023, 124, 2863–2875. [Google Scholar] [CrossRef]
- Chen, X.L.; Fan, G.C.; Lin, C.H.; Dong, B.Y.; Guo, Z.N.; Fang, X.L.; Qu, N.S. Investigation on the electrochemical machining of micro groove using masked porous cathode. J. Mater. Process. Technol. 2020, 276, 116406. [Google Scholar] [CrossRef]
- Miao, G.D.; Ao, S.; Chen, X.L.; Fang, X.L.; Zhu, D. Fabrication of semicircular micro-grooves on Ti6Al4V by through-mask scanning electrochemical machining. Int. J. Adv. Manuf. Technol. 2023, 126, 3175–3192. [Google Scholar] [CrossRef]
- Chen, X.L.; Zhu, J.J.; Xu, Z.Z.; Su, G.K. Modeling and experimental research on the evolution process of micro through-slit array generated with masked jet electrochemical machining. J. Mater. Process. Technol. 2021, 298, 117304. [Google Scholar] [CrossRef]
- Kendall, T.; Bartolo, P.; Gillen, D.; Diver, C. A review of physical experimental research in jet electrochemical machining. Int. J. Adv. Manuf. Technol. 2019, 105, 651–667. [Google Scholar] [CrossRef]
- Chen, X.L.; Dong, B.Y.; Fan, G.C.; Zhang, C.Y.; Liu, J.W.; Zhang, Y.J.; Guo, Z.N. Investigation on modified jet electrochemical machining of micro-channel. Int. J. Adv. Manuf. Technol. 2019, 104, 4433–4443. [Google Scholar] [CrossRef]
- Liu, W.D.; Ao, S.S.; Li, Y.; Liu, Z.M.; Wang, Z.M.; Luo, Z.; Wang, Z.P.; Song, R.F. Jet electrochemical machining of TB6 titanium alloy. Int. J. Adv. Manuf. Technol. 2017, 90, 2397–2409. [Google Scholar] [CrossRef]
- Malik, A.; Manna, A. Investigation on the laser-assisted jet electrochemical machining process for improvement in machining performance. Int. J. Adv. Manuf. Technol. 2018, 96, 3917–3932. [Google Scholar] [CrossRef]
- Guo, C.; Qian, J.; Reynaerts, D. A three-dimensional FEM model of channel machining by scanning micro electrochemical flow cell and jet electrochemical machining. Precis. Eng. 2018, 52, 507–519. [Google Scholar] [CrossRef]
- Luo, J.; Fang, X.; Zhu, D. Jet electrochemical machining of multi-grooves by using tube electrodes in a row. J. Mater. Process. Technol. 2020, 283, 116705. [Google Scholar] [CrossRef]
- Wang, L.N.; Jin, M.; Zheng, Y.D.; Guan, Y.P.; Lu, X.; Luo, J.L. Nanotubular surface modification of metallic implants via electrochemical anodization technique. Int. J. Nanomed. 2014, 9, 4421–4435. [Google Scholar] [CrossRef]
- Gao, A.; Hang, R.Q.; Bai, L.; Tang, B.; Chu, P.K. Electrochemical surface engineering of titanium-based alloys for biomedical application. Electrochim. Acta 2018, 271, 699–718. [Google Scholar] [CrossRef]
- Al-Hashedi, A.A.; Laurenti, M.; Abdallah, M.-N.; Albuquerque, R.F., Jr.; Tamimi, F. Electrochemical treatment of contaminated titanium surfaces in vitro: An approach for implant surface decontamination. ACS Biomater. Sci. Eng. 2016, 2, 1504–1518. [Google Scholar] [CrossRef]
- da Silva Oliveira, A.; Pamplona de Sousa, G.C.; Araújo Valença, A.K.; da Silva Neto, J.F.; Gomes, K.C. Effect of surface treatments on absorptance and morphology of molybdenum and silica absorbing thin films. Sol. Energy 2023, 254, 158–167. [Google Scholar] [CrossRef]
- Lee, S.J.; Chen, Y.H.; Hu, S.C.; Lin, Y.C.; Chang, J.W.; Poon, T.L.; Ke, W.C. Improved performance of amorphous Si thin-film solar cells on 430 stainless steel substrate by an electrochemical mechanical polishing process. J. Alloys Compd. 2013, 558, 95–98. [Google Scholar] [CrossRef]
- Klocke, F.; Klink, A.; Veselovac, D.; Aspinwall, D.K.; Soo, S.L.; Schmidt, M.; Schilp, J.; Levy, G.; Kruth, J.P. Turbomachinery component manufacture by application of electrochemical, electro-physical and photonic processes. CIRP Ann. 2014, 63, 703–726. [Google Scholar] [CrossRef]
- Ren, M.Z.; Zhu, D.; Hou, Z.H.; Lei, G.P. Investigation on multi-physical field simulations of blade ECM using vertical flow. Int. J. Adv. Manuf. Technol. 2022, 123, 4251–4263. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Y. Electrochemical machining of complex components of aero-engines: Developments, trends, and technological advances. Chin. J. Aeronaut. 2021, 34, 28–53. [Google Scholar] [CrossRef]
- Cao, W.J.; Wang, D.Y.; Cui, G.W.; Zhang, J.; Zhu, D. Improvement on the machining accuracy of titanium alloy casing during counter-rotating electrochemical machining by using an insulation coating. Surf. Coat. Technol. 2022, 443, 128585. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, D.; Zhu, D. Electrochemical machining of complex structures on thin-walled plates using pure rolling cathode tool. Int. J. Adv. Manuf. Technol. 2024, 135, 1685–1696. [Google Scholar] [CrossRef]
- Zhou, S.F.; Wang, D.Y.; Cui, G.W.; Zhang, J.; Zhu, D. Co-rotating electrochemical machining of convex structures on the inner surface of annular parts using a flexible cathode tool. Precis. Eng. 2024, 89, 55–69. [Google Scholar] [CrossRef]
- Saha, S.; Ball, A.K.; Mukherjee, A.; Das, A.; Halder, S.; Hanumaiah, N. Optimization of electrochemical etching process for manufacturing of micro electrodes for micro-EDM application. Proc. Inst. Mech. Eng. 2021, 235, 925–940. [Google Scholar] [CrossRef]
- Singh, J.; Bhattacharya, S. Fabrication of micro-mixer on printed circuit board using electrochemical micromachining. J. Micromanuf. 2019, 2, 85–94. [Google Scholar] [CrossRef]
- Chen, X.L.; Dong, B.Y.; Zhang, C.Y.; Luo, H.P.; Liu, J.W.; Zhang, Y.J.; Guo, Z.N. Electrochemical direct-writing machining of micro-channel array. J. Mater. Process. Technol. 2018, 265, 138–149. [Google Scholar] [CrossRef]
- Dadi, S.S.O.; Patel, D.S.; Patil, G.; Garg, G.K. Eco-friendly tool-based electrochemical polishing of additively manufactured metallic components. J. Manuf. Process. 2023, 108, 252–267. [Google Scholar] [CrossRef]
- Singh, M.; Singh, S.; Kumar, S. Environmental aspects of various electrolytes used in electrochemical discharge machining process. J. Braz. Soc. Mech. Sci. Eng. 2020, 42, 395. [Google Scholar] [CrossRef]
- Chuang, M.C.; Jan, C.M.; Wang, Y.J.; Hsu, Y.L. Intelligent Monitoring and Compensation between EDM and ECM. Appl. Sci. 2023, 13, 927. [Google Scholar] [CrossRef]
- Tao, J.; Ren, W.F.; Deng, H.R.; Sun, L.H.; Xu, J.K. Cross-scale electrochemical machining of superhydrophobic end face cavities via microstructured surface cathodes. Appl. Surf. Sci. 2025, 682, 161659. [Google Scholar] [CrossRef]
- Zhu, X.M.; Liu, Y.; Zhang, J.H.; Wang, K.; Kong, H.H. Ultrasonic-assisted electrochemical drill-grinding of small holes with high-quality. J. Adv. Res. 2020, 23, 151–161. [Google Scholar] [CrossRef]
- Zhang, C.X.; Xu, Z.Y.; Zhang, X.Y.; Zhang, J.Y. Surface integrity of holes machined by electrochemical discharge drilling method. CIRP J. Manuf. Sci. Technol. 2020, 31, 643–651. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, H.R.; Wang, S.H.; Wang, K.; Li, M.H.; Peng, T.F. Micro electrochemical milling of micro metal parts with rotating ultrasonic electrode. Sensors 2020, 20, 6617. [Google Scholar] [CrossRef]




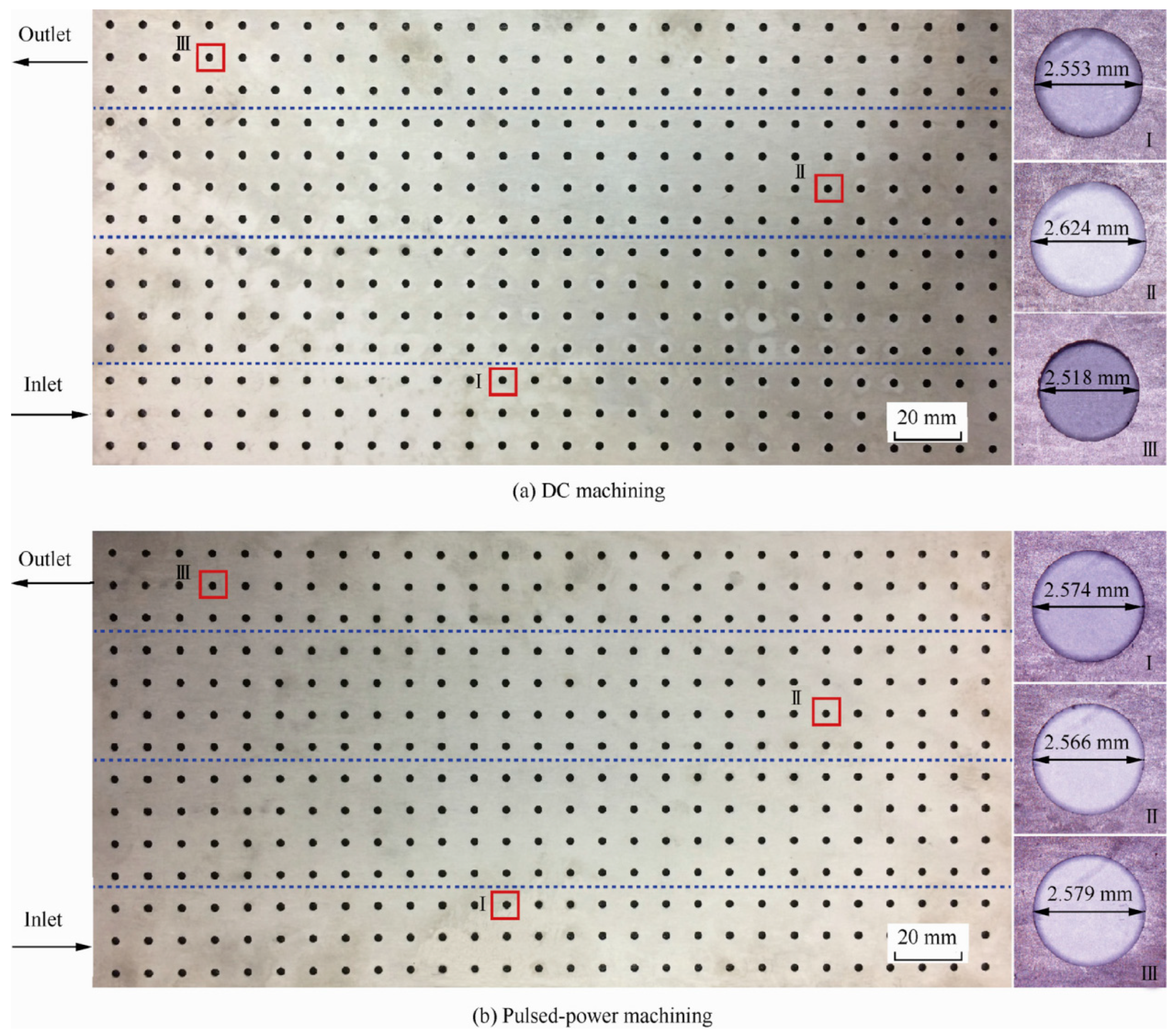
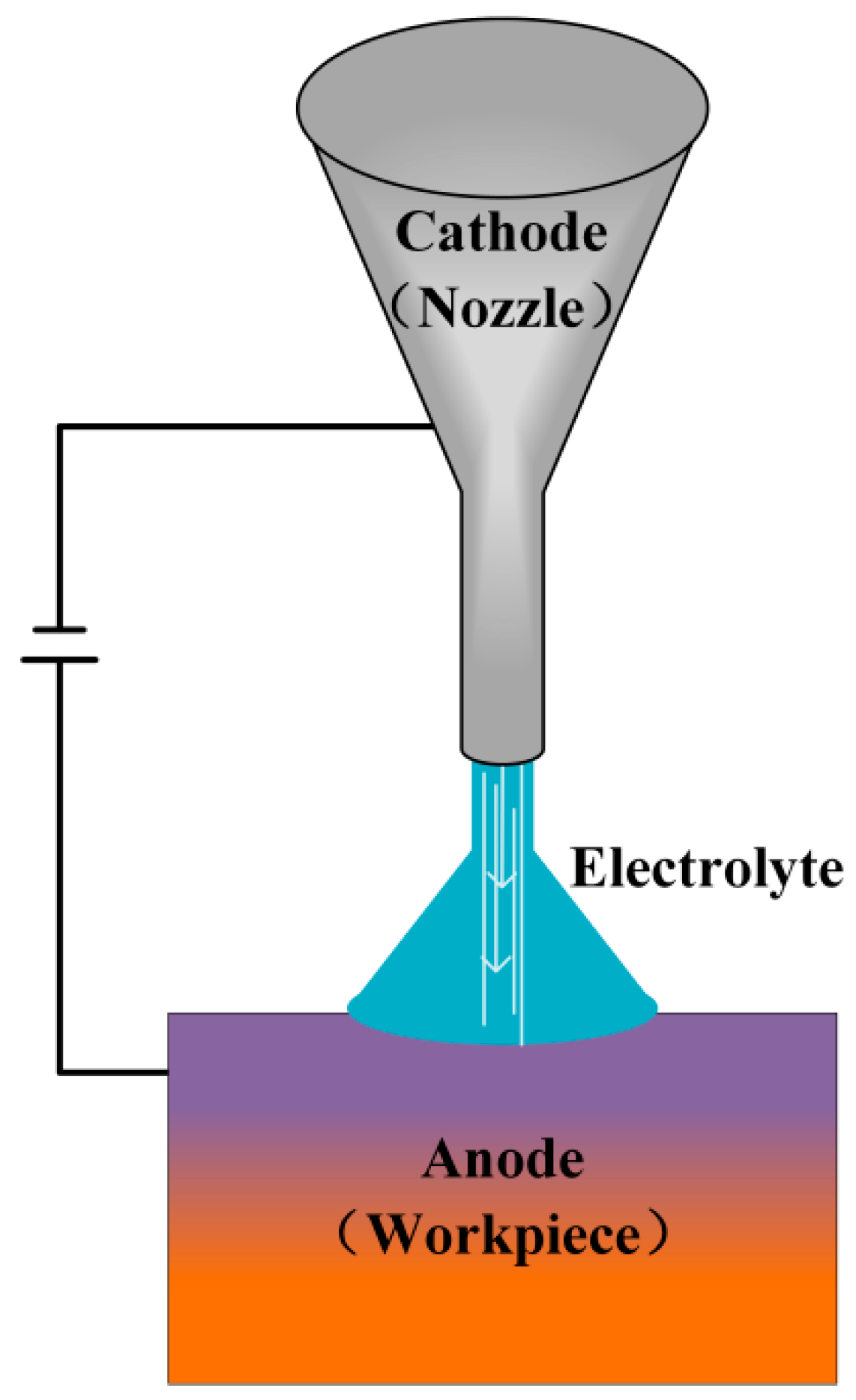
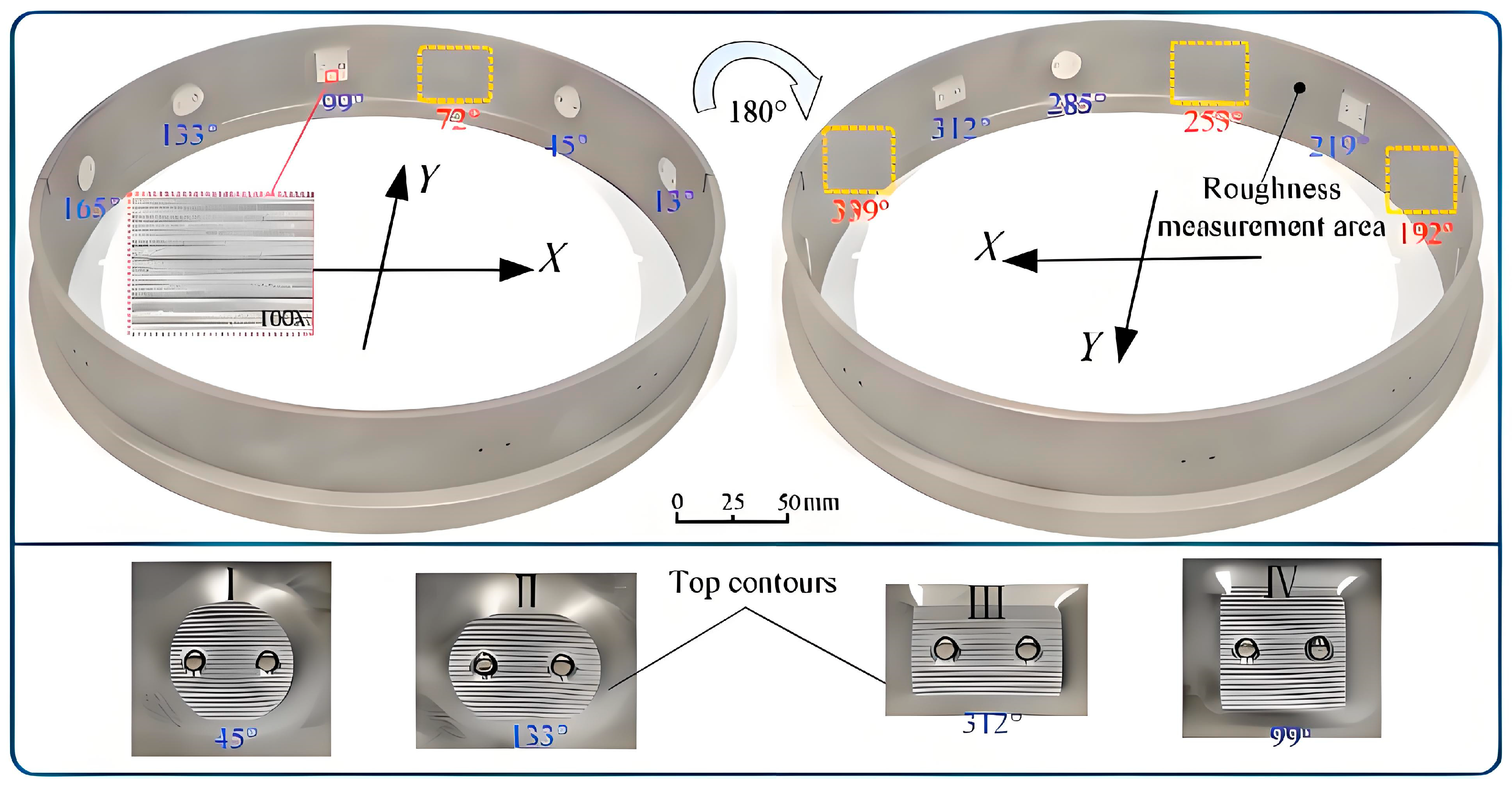
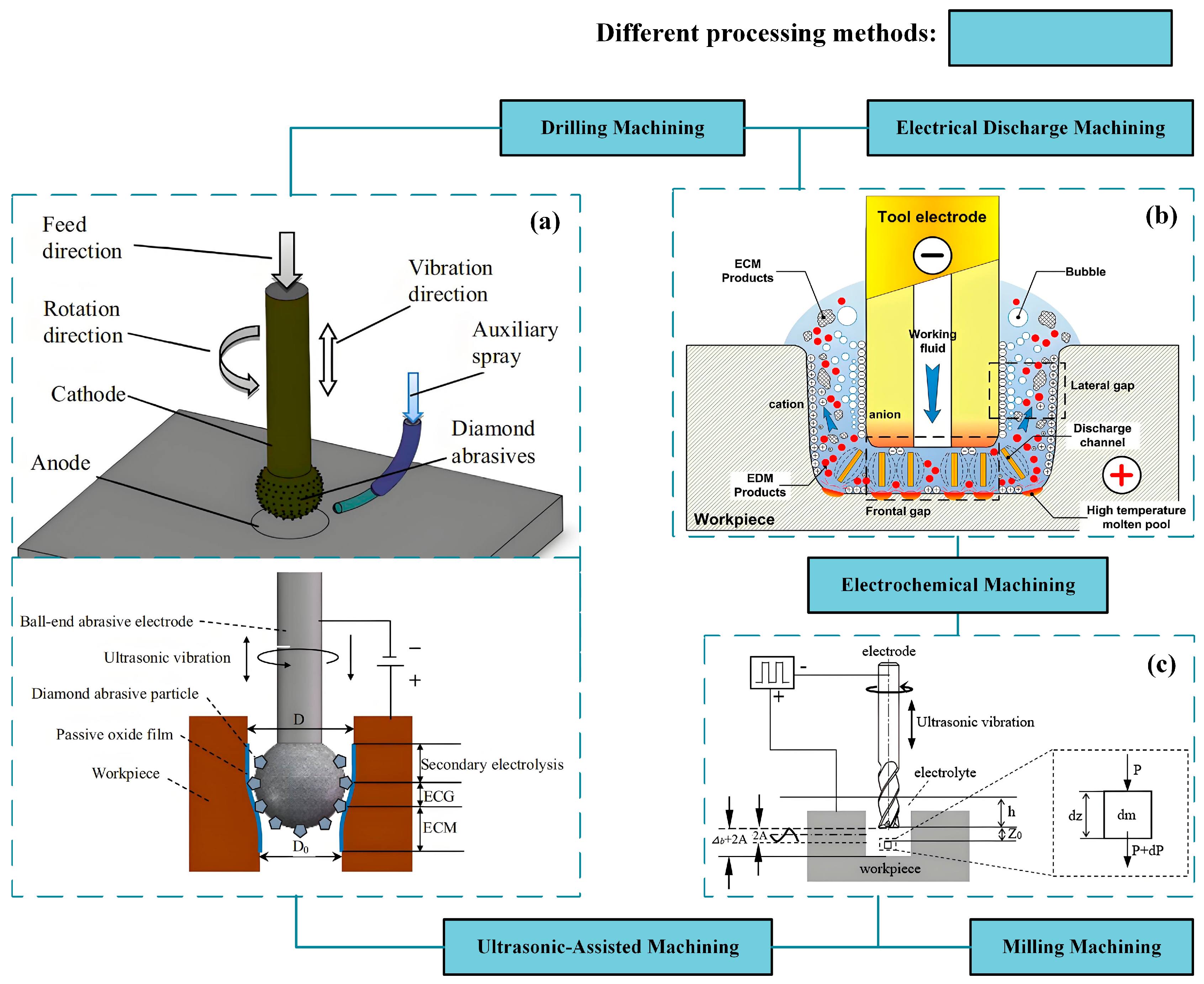
| No. | Technology Name | Machining Performance | Applicable Materials | Key Technical Limitations | Typical Applications |
|---|---|---|---|---|---|
| 1 | Pulsed Electrochemical Polishing | Surface Roughness: Low (Ra 0.06–0.3 μm for Inconel 718/Nimonic-263) MRR: Medium (10–20% higher than DC electrochemical polishing) | Nickel-based superalloys (Inconel 718, Nimonic-263), Invar sheets, stainless steels (304) | Dynamic IEG control lags 15–20 ms for sudden disturbances Electrolyte machinability depends on passivation capacity | Precision molds, aerospace turbine blade sealing surfaces, high-gloss metal parts |
| 2 | Electrochemical Mechanical Polishing | Surface Roughness: Low (Ra 0.449 nm for 4H-SiC, Sa 0.5–0.66 nm for Ti-6Al-4V) MRR: Slow (2.3 μm/h for 4H-SiC, 147.02 nm/min for cobalt) | Third-generation semiconductors (4H-SiC, GaN), titanium alloys (pure Ti, Ti-6Al-4V), stainless steels (304, 316L), tantalum, AM components (17-4PH, Ti-6Al-4V AM parts) | Difficult to control electric field distribution uniformity High cost of solid electrolytes (e.g., Nafion/CeO2 pad) | Semiconductor substrates (4H-SiC wafers), titanium medical implants, precision optical components |
| 3 | Electrochemical Grinding | Surface Roughness: Medium (Ra 0.35–1.8 μm for GH4169/Ti-6Al-4V) MRR: Fast (10–30 mm3/min for GH4169/Inconel 718) | Difficult-to-machine alloys (GH4169, Ti-6Al-4V, Inconel 718), cemented carbides, ceramics, quenched steels (42CrMo4) | Abrasive tool degradation (Cu-based binder corrosion, 8–12% wear rate) Non-uniform electrolyte flow causes local overcutting | Aerospace turbine blades, precision gears, silicon anodes for batteries |
| 4 | Electrochemical Milling-Grinding | Surface Roughness: Medium (Ra 1.06 μm for Ti-6Al-4V, Ra 0.37 μm for titanium matrix composites) MRR: Fast (216.6 mm3/min for Inconel 718, 248.3 mm3/min for Ti-6Al-4V) | Nickel-based alloys (Inconel 718), titanium alloys (Ti-6Al-4V), SiCP/Al composites, (TiB+TiC)/Ti6Al4V composites | Uneven electrolyte distribution in deep grooves Reinforced phases (SiC/TiB) cause local tool wear | Aerospace complex 3D grooves, thin-walled components, titanium matrix composite parts |
| 5 | Electrochemical Milling | Surface Roughness: Medium–Low (Ra 0.24–0.75 μm for Ti-6Al-4V/316L, Ra 0.06–0.08 μm for Nimonic-263) MRR: Fast (20–282.9 mg/min for Ti-6Al-4V with ultra-high current density) | Titanium alloys (Ti-6Al-4V, TB6), nickel-based superalloys (GH4169, Inconel 718, Nimonic-263), stainless steels (316L, 304) | Unstable dynamic IEG (80–150 μm fluctuation) Edge stray corrosion (needs insulated cathode) | Aerospace thin-walled casings, deep-narrow grooves, TB6 titanium alloy flat surfaces |
| 6 | Electrochemical Turning | Surface Roughness: Medium–Low (Ra 0.222–2.414 μm for Ti matrix composites/TB6, Ra 0.315 μm for Ni-based alloys) MRR: Medium–Fast (167.1% higher single-cycle removal for Ti composites) | Titanium alloys (Ti-6Al-4V, TB6), nickel-based cast superalloys, titanium matrix composites | Shaft shoulder overcutting (needs sandwich cathode) Cannot machine non-rotationally symmetric structures | Aerospace engine shafts, cylindrical TB6 components, large-allowance revolving parts |
| 7 | Wire Electrochemical Turning | Surface Roughness: Medium–Low (micron-scale precision for tungsten micro-rods) MRR: Slow (micro-scale parts, no high MRR data) | Tungsten, difficult-to-machine micro-scale metals | Difficult to control wire tension (affects precision) High requirement for bipolar pulse matching | Tungsten micro-rods (φ11 μm, aspect ratio 36), micro-scale revolving parts |
| 8 | Electrochemical Micro-Milling | Surface Roughness: Low (Ra 0.1 μm for Haynes-188, Ra 0.125 μm for nickel-based alloys) MRR: Slow (aspect ratio 6.1 blind grooves) | Nickel-based alloys (GH3030, Haynes-188), titanium alloys, cemented carbides | Hard to prepare micro-electrodes (needs in-situ 10 μm cylindrical electrodes) Slow electrolyte renewal in microcavities | MEMS 2D shapes (25 μm-wide), 3D stepped structures (45 μm-deep), high-aspect-ratio microgrooves |
| 9 | Electrochemical Micro-Turning | Surface Roughness: Low (Ra 0.08–0.15 μm for 1.4301 stainless steel, Ra 0.15–0.5 μm for micro-shafts) MRR: Slow (5–65% cutting force reduction) | Stainless steels (1.4301), titanium alloys (Ti-6Al-4V), micro-scale difficult-to-machine metals | Poor synergy between workpiece rotation and pulse parameters Micro-shaft shoulder overcutting | Micro-shafts (20% less shoulder overcut), medical micro-devices |
| 10 | Wire Electrochemical Cutting | Surface Roughness: Low (Ra 1.01 μm for 304 stainless steel, Ra 0.86 μm for Inconel-718, Ra 0.108 μm for NiTi) MRR: Medium–Slow (1.7–5.0 μm/s multi-wire feed) | Titanium alloys (Ti-6Al-4V, γ-TiAl), NiTi shape memory alloys, pure tungsten, stainless steels (304, 1.4301) | Poor multi-wire processing consistency (±1.5 μm slit width deviation) Needs precise wire vibration control (2.5–15 mm amplitude) | Fuel cell micro-slit arrays, pure tungsten X-ray gratings, NiTi medical stents |
| 11 | Mask Electrochemical Machining | Surface Roughness: Medium–Low (Ra 0.108 μm for NiTi, burr-free stainless steel micro-slits) MRR: Slow (large-area array single-step forming) | Stainless steels (304, 1.4301), titanium alloys (Ti-6Al-4V), monocrystalline silicon, MEMS materials | Uneven large-area flow field (needs serpentine channels) Limited mask service life for batch processing | MEMS arrays, aero-engine cooling hole arrays, fuel cell flow channels |
| 12 | Jet Electrochemical Machining | Surface Roughness: Medium (Ra 2.414 μm for TB6 titanium alloy, Ra 0.31 μm for 304 stainless steel) MRR: Medium (14.3 μm/h for 4H-SiC) | Titanium alloys (TB6, Ti-6Al-4V), nickel-based alloys (Inconel-718), stainless steels (304), SiC | Micro-scale forming limitation (nozzle ≥ 130 μm) Stray corrosion in non-jet areas | Microfluidic microchannels, heat sink multi-groove arrays, TB6 curved components |
| Application | ECM Technology | Key Quantitative Metrics |
|---|---|---|
| Ti-6Al-4V Implants | ECMP | Surface roughness (Sa): 0.5–0.66 nm; No subsurface damage |
| SS304 Solar Substrates | ECMP | Ra reduced from 35 nm to 10 nm; Solar cell efficiency: 5.1–5.4% |
| Aerospace Component | ECM Technology | Key Quantitative Metrics |
|---|---|---|
| Inconel 718 Turbine Blades | Pulsed ECM (Vertical Flow) | Profile accuracy: ±0.1 mm; Ra: ≤0.35 μm; MRR: 25 mm3/min |
| Ti-6Al-4V Thin-Walled Casing | Counter-Rotating ECM | Sidewall taper: 1.11° (vs. 25.5° by traditional ECM); Thickness error: ±0.05 mm |
| Micro-Manufacturing Goal | ECM Technology | Key Quantitative Metrics |
|---|---|---|
| Tungsten Micro-Electrodes | Electrochemical Etching | Minimum diameter: 3.33 μm; Diameter deviation: ±0.2 μm |
| SS304 Fuel Cell Micro-Channels | Masked Pulsed ECM | Channel width: 302 ± 3.53 μm; Depth: 95.9 ± 1.34 μm; Sidewall Perpendicularity: 95% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Yang, Y.; Han, C.; Pang, G.; Fan, S.; Xu, Y.; He, Z.; Fang, J. Research Progress of Electrochemical Machining Technology in Surface Processing: A Review. Micromachines 2025, 16, 1174. https://doi.org/10.3390/mi16101174
Wang Y, Yang Y, Han C, Pang G, Fan S, Xu Y, He Z, Fang J. Research Progress of Electrochemical Machining Technology in Surface Processing: A Review. Micromachines. 2025; 16(10):1174. https://doi.org/10.3390/mi16101174
Chicago/Turabian StyleWang, Yiran, Yong Yang, Chaoyang Han, Guibing Pang, Shuangjiao Fan, Yunchao Xu, Zhen He, and Jianru Fang. 2025. "Research Progress of Electrochemical Machining Technology in Surface Processing: A Review" Micromachines 16, no. 10: 1174. https://doi.org/10.3390/mi16101174
APA StyleWang, Y., Yang, Y., Han, C., Pang, G., Fan, S., Xu, Y., He, Z., & Fang, J. (2025). Research Progress of Electrochemical Machining Technology in Surface Processing: A Review. Micromachines, 16(10), 1174. https://doi.org/10.3390/mi16101174






