The Impact of Targeted Therapies on Red Blood Cell Aggregation in Patients with Chronic Lymphocytic Leukemia Evaluated Using Software Image Flow Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Groups and Ethics Statement
2.2. Blood Collection and Sample Preparations
2.3. Stimulation of RBC Aggregation
2.4. Viscosity Measurements
2.5. Microfluidic System Description and Experiments
2.6. Design of the Experiments
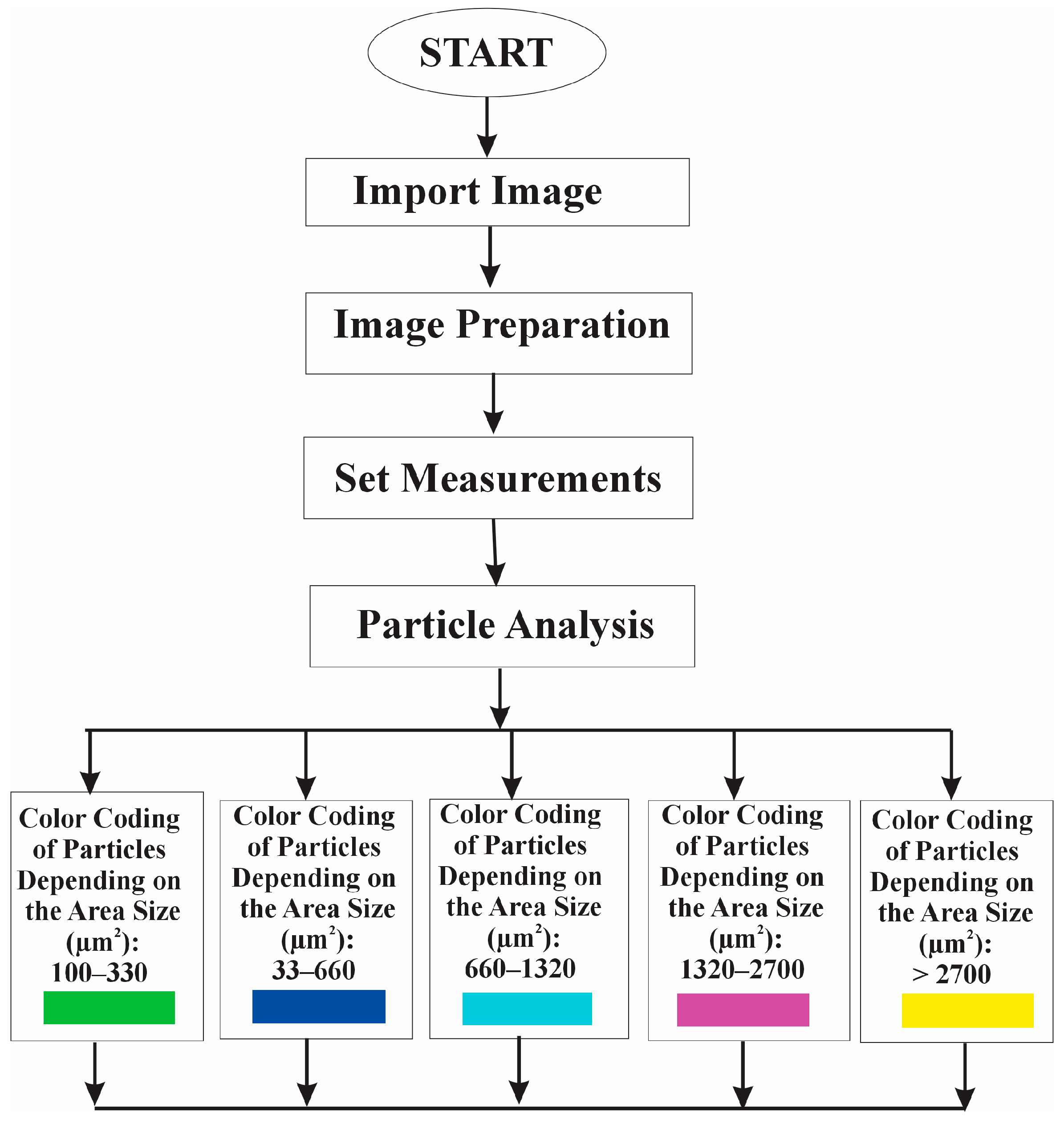
2.7. Algorithm for Software Image Flow Analysis of RBC Aggregates
- Detecting particles based on their area interval (50 µm2 and infinity).
- Adding the detected particles to the Region of Interest (ROI) Manager tool for subsequent analysis and processing.
2.8. RBC Aggregation Parameters
2.9. Optical Microscopy
2.10. Statistics
3. Results
3.1. Clinical Characteristics of the CLL Patients and Healthy Controls
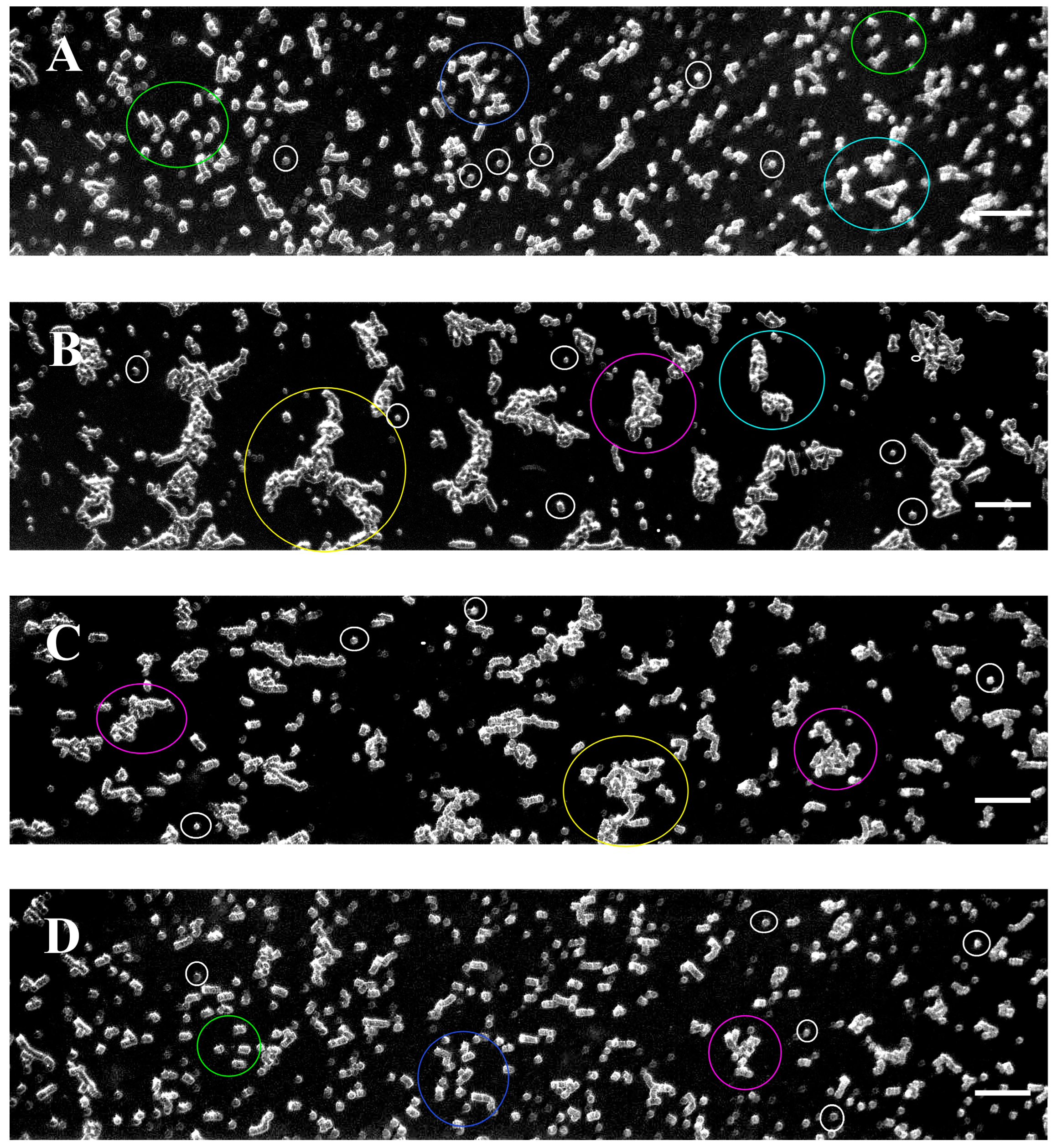
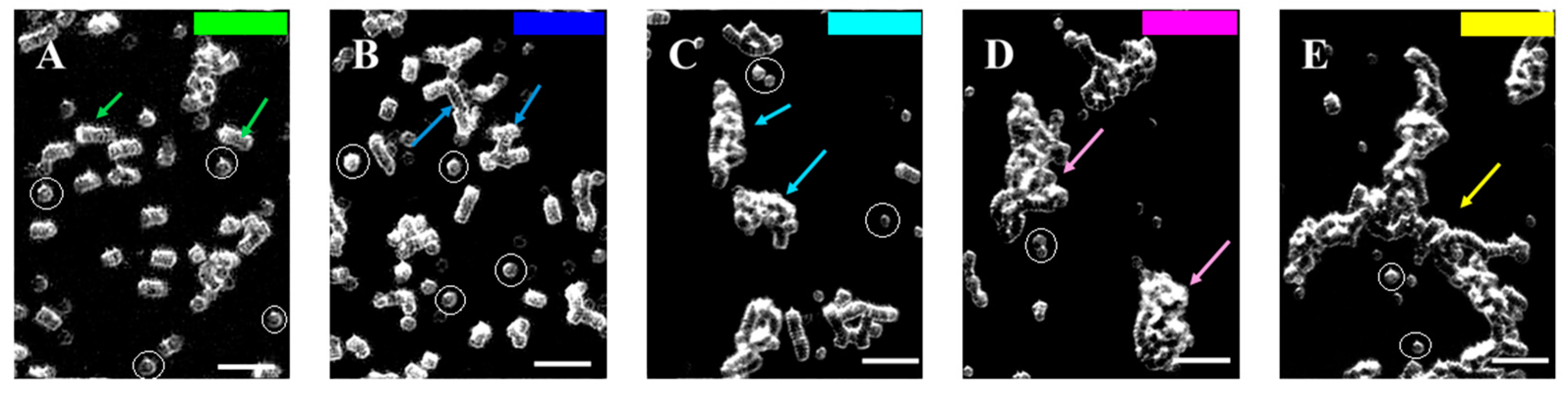
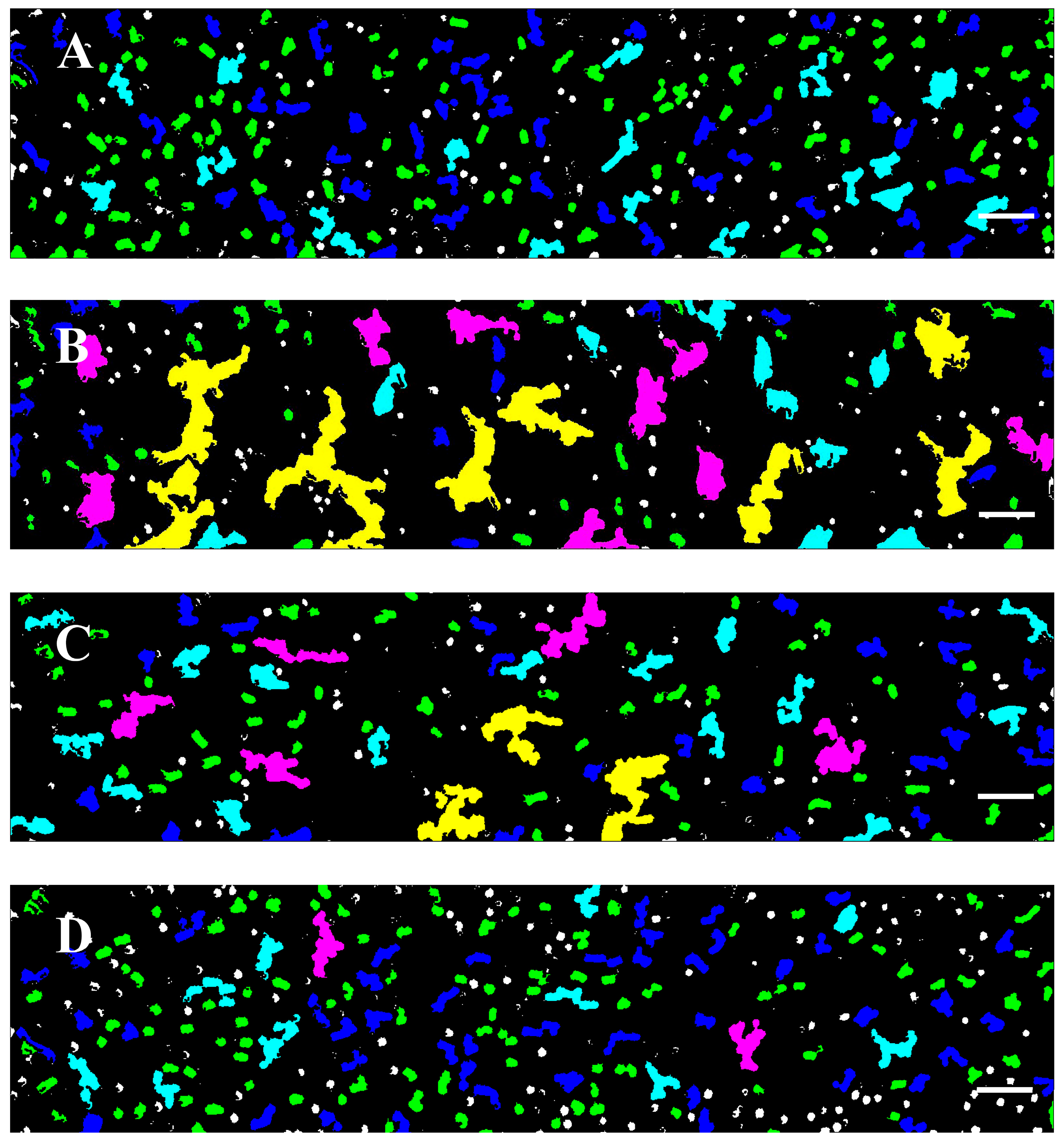
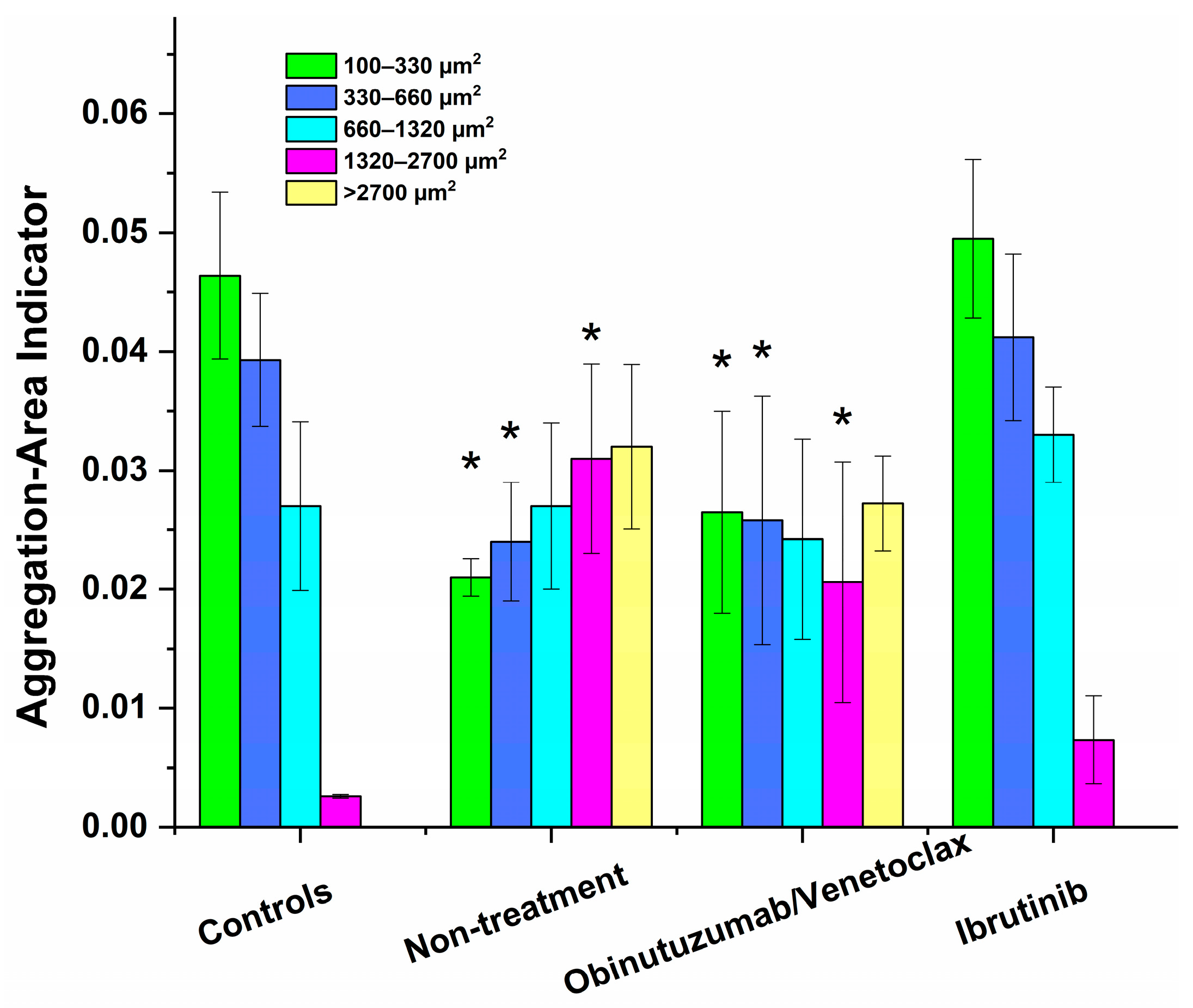
3.2. Software Image Flow Analysis of RBC Aggregation of Healthy Control and CLL Patient Groups
3.3. RBC Aggregation of Healthy Subjects and CLL Patients, Assessed Using a Microfluidic System
3.4. Effect of In Vivo Target Therapies on RBC Aggregation
3.5. Aggregation Indices Calculated for the Aggregation State of RBCs from Healthy Subjects and CLL Patients
3.6. Morphology of RBCs Derived from Healthy Individuals and CLL Patients
4. Discussion
4.1. Alterations in RBCs’ Rheological Properties in Untreated CLL Patients
4.2. Effect of Targeted Therapies on RBC Aggregation in CLL
5. Conclusions
- (1)
- The ability to classify RBC aggregates based on their size and to capture subtle differences in their dimensions, thus providing information on their contribution to various diseases;
- (2)
- Differentiation of aggregate types: the ability to distinguish between different aggregate types (such as rouleaux formations vs. three-dimensional cluster networks) offers deeper insight into how aggregation mechanisms might change under pathological conditions.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics. CA Cancer J. Clin. 2021, 71, 7. [Google Scholar] [CrossRef] [PubMed]
- John, C.B.; Kittai, A.S.; Farrukh, T.A. Chronic Lymphocytic Leukemia. In Williams Hematology, 10th ed.; Kaushansky, K., Lichtman, M.A., Prchal, J.T., Levi, M.M., Press, O.W., Burns, L.J., Caligiuri, M., Eds.; McGraw-Hill Education: New York, NY, USA, 2021; Available online: https://accessmedicine.mhmedical.com/content.aspx?bookid=2962§ionid=252533788 (accessed on 29 November 2024).
- Fecteau, J.F.; Kipps, T.J. Structure and function of the hematopoietic cancer niche: Focus on chronic lymphocytic leukemia. Front. Biosci. (Schol Ed). 2012, 4, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Glennie, M.J.; French, R.R.; Cragg, M.S.; Taylor, R.P. Mechanisms of killing by anti-CD20 monoclonal antibodies. Mol. Immunol. 2007, 44, 3823–3837. [Google Scholar] [CrossRef]
- Reed, J.C.; Pellecchia, M. Apoptosis-based therapies for hematologic malignancies. Blood 2005, 106, 408–418. [Google Scholar] [CrossRef]
- Parmar, S.; Patel, K.; Pinilla-Ibarz, J. Ibrutinib (imbruvica): A novel targeted therapy for chronic lymphocytic leukemia. Pharm. Ther. 2014, 39, 483–519. [Google Scholar]
- Shatzel, S.R.; Olson, D.L.; Tao, O.J.T.; McCarty, A.V.; Danilov, T.G. DeLoughery, Ibrutinib-associated bleeding: Pathogenesis, management and risk reduction strategies. J. Thromb. Haemost. 2017, 15, 835–847. [Google Scholar] [CrossRef]
- Gramley, F.; Lorenzen, J.; Jedamzik, B.; Gatter, K.; Koellensperger, E.; Munzel, T.; Pezzella, F. Atrial fibrillation is associated with cardiac hypoxia. Cardiovasc. Pathol. 2010, 19, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Davidkin, I.L.; Kusmina, T.P.; Naumova, K.V.; Khayretdinov, R.K.; Danilova, O.E.; Stepanova, T.Y.; Osadchuk, A.M.; Mordvinova, E.V. Endothelial dysfunction in patients with lymphoproliferative disorders, and its changes in the course of polychemotherapy. Russ. Open Med. J. 2020, 9, e0309. [Google Scholar] [CrossRef]
- Terwoord, J.D.; Beyer, A.M.; Gutterman, D.D. Endothelial dysfunction as a complication of anti-cancer therapy. Pharm. Ther. 2022, 237, 108116. [Google Scholar] [CrossRef]
- Baskurt, O.K.; Meiselman, H.J. Comparative hemorheology. Clin. Hemorheol. Microcirc. 2013, 53, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Baskurt, O.; Meiselman, H. Blood rheology and hemodynamics. Semin. Thromb. Hemost. 2003, 29, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Nader, E.; Skinner, S.; Romana, M.; Fort, R.; Lemonne, N.; Guillot, N.; Gauthier, A.; Antoine-Jonville, S.; Renoux, C.; Hardy-Dessources, M.D.; et al. Blood Rheology: Key Parameters, Impact on Blood Flow, Role in Sickle Cell Disease and Effects of Exercise. Front. Physiol. 2019, 10, 1329. [Google Scholar] [CrossRef]
- Wiewiora, M.; Jopek, J.; Świętochowska, E.; Sławomir, G.; Piecuch, J.; Gąska, M.; Piecuch, J. Blood-based protein biomarkers and red blood cell aggregation in pancreatic cancer. Clin. Hemorheol. Microcirc. 2023, 85, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Alexandrova-Watanabe, A.; Abadjieva, E.; Giosheva, I.; Langari, A.; Tiankov, T.; Gartchev, E.; Komsa-Penkova, R.; Todinova, S. Assessment of Red Blood Cell Aggregation in Preeclampsia by Microfluidic Image Flow Analysis—Impact of Oxidative Stress on Disease Severity. Int. J. Mol. Sci. 2024, 25, 3732. [Google Scholar] [CrossRef] [PubMed]
- Alexandrova-Watanabe, A. Study of Rheological, Mechanical and Morphological Properties of Blood, Its Formal Elements and Parameters of Hemocoagulation in Type 2 Diabetes Mellitus. Ph.D. Thesis, Institute of Mechanics, Bulgarian Academy of Sciences, Sofia, Bulgaria, 2021. [Google Scholar]
- Majumder, D.; Banerjee, D.; Chandra, S.; Banerjee, S.; Chakrabarti, A. Red cell morphology in leukemia, hypoplastic anemia and myelodysplastic syndrome. Pathophysiology 2006, 13, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, F. A Review of Microfluidic Devices for Rheological Characterization. Micromachines 2022, 13, 167. [Google Scholar] [CrossRef]
- Bezelya, A.; Küçüktürkmen, B.; Bozkır, A. Microfluidic Devices for Precision Nanoparticle Production. Micro 2023, 3, 822–866. [Google Scholar] [CrossRef]
- Preetam, S.; Nahak, B.K.; Patra, S.; Toncu, D.C.; Park, S.; Syväjärvi, M.; Orive, G.; Tiwari, A. Emergence of microfluidics for next generation biomedical devices. Biosens. Bioelectron. X 2022, 10, 100106. [Google Scholar] [CrossRef]
- Pinho, D.; Carvalho, V.; Gonçalves, I.M.; Teixeira, S.; Lima, R. Visualization and Measurements of Blood Cells Flowing in Microfluidic Systems and Blood Rheology: A Personalized Medicine Perspective. J. Pers. Med. 2020, 10, 249. [Google Scholar] [CrossRef]
- Mehri, R.; Mavriplis, C.; Fenech, M. Red blood cell aggregates and their effect on non-Newtonian blood viscosity at low hematocrit in a two-fluid low shear rate microfluidic system. PLoS ONE 2018, 13, e0199911. [Google Scholar] [CrossRef] [PubMed]
- Foresto, P.; D’Arrigo, M.; Carreras, L.; Cuezzo, R.E.; Valverde, J.; Rasia, R. Evaluation of red blood cell aggregation in diabetes by computerized image analysis. Medicina (B Aires) 2000, 60 Pt 1, 570–572. [Google Scholar]
- Kaliviotis, E.; Dusting, J.; Balabani, S. Spatial variation of blood viscosity: Modelling using shear fields measured by a μPIV based technique. Med. Eng. Phys. 2011, 33, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Dusting, J.; Kaliviotis, E.; Balabani, S.; Yianneskis, M. Coupled human erythrocyte velocity field and aggregation measurements at physiological hematocrit levels. J. Biomech. 2009, 42, 1438–1443. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Gavish, B.; Zhang, S.; Mahler, Y.; Yedgar, S. Monitoring of erythrocyte aggregate morphology under flow by computerized image analysis. Biorheology 1995, 32, 487–496. [Google Scholar] [PubMed]
- Muravyov, A.V.; Tikhomirova, I.A.; Zamyshlyaev, A.V.; Mikhailov, P.V.; Petrochenko, E.P.; Petrochenko, A.S. Characteristics of the aggregoscopy method for recording the microrheology of erythrocytes. Thromb. Hemost. Rheol. 2017, 4, 39–44. [Google Scholar]
- Mehri, R.; Niazi, E.; Mavriplis, C.; Fenech, M. An automated method for dynamic red blood cell aggregate detection in microfluidic flow. Physiol. Meas. 2018, 39, 01NT02. [Google Scholar] [CrossRef] [PubMed]
- Hallek, M.; Cheson, B.D.; Catovsky, D.; Caligaris-Cappio, F.; Dighiero, G.; Döhner, H.; Hillmen, P.; Keating, M.; Montserrat, E.; Chiorazzi, N.; et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018, 131, 2745–2760. [Google Scholar] [CrossRef]
- Hallek, M.; Cheson, B.D.; Catovsky, D.; Caligaris-Cappio, F.; Dighiero, G.; Döhner, H.; Hillmen, P.; Keating, M.J.; Montserrat, E.; Rai, K.R.; et al. International Workshop on Chronic Lymphocytic Leukemia. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: A report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008, 111, 5446–5456. [Google Scholar] [PubMed]
- Šimkovič, M.; Vodárek, P.; Motyčková, M.; Belada, D.; Vrbacký, F.; Žák, P.; Smolej, L. Venous thromboembolism in patients with chronic lymphocytic leukemia. Thromb Res. 2015, 136, 1082–1086. [Google Scholar] [CrossRef]
- Whittle, A.M.; Allsup, D.J.; Bailey, J.R. Chronic lymphocytic leukaemia is a risk factor for venous thromboembolism. Leuk Res. 2011, 35, 419–421. [Google Scholar] [CrossRef]
- Alexy, T.; Detterich, J.; Connes, P.; Toth, K.; Nader, E.; Kenyeres, P.; Arriola-Montenegro, J.; Ulker, P.; Simmonds, M.J. Physical Properties of Blood and their Relationship to Clinical Conditions. Front. Physiol. 2022, 13, 906768. [Google Scholar] [CrossRef]
- Gade, I.L.; Riddersholm, S.J.; Christiansen, I.; Rewes, A.; Frederiksen, M.; Enggaard, L.; Poulsen, C.B.; Bergmann, O.J.; Gillström, D.B.; Pedersen, R.S.; et al. Venous thromboembolism in chronic lymphocytic leukemia: A Danish nationwide cohort study. Blood Adv. 2018, 2, 3025–3034. [Google Scholar] [CrossRef]
- Assiri, A.H.; Lamba, M.; Veinot, J.P. Chronic lymphocytic leukemia involving the coronary arteries with accompanying acute myocardial infarction. Cardiovasc. Pathol. 2005, 14, 324–326. [Google Scholar] [CrossRef]
- Bhuyan, A.; Hazarika, P.; Deka, R. Clinicopathological Features of Pancytopenia in Adults and the Role of Bone Marrow Study in Etiological Categorization: A One-Year Cross-Sectional Study. Med. J. Babylon 2022, 19, 415–421. [Google Scholar] [CrossRef]
- Bortolato, S.A.; Canales, M.A.M.; Riquelme, B.D.; Raviola, M.; Leguto, A.J.; Rebechi, J.P.; Ponce de León, P.; Korol, A.M. New insights into the analysis of red blood cells from leukemia and anemia patients: Nonlinear quantifiers, fractal mathematics, and Wavelet Transform. Phys. A Stat. Mech. Its Appl. 2021, 567, 125645. [Google Scholar] [CrossRef]
- Kundu, M.; Basu, J.; Rakshit, M.M.; Chakrabarti, P. Abnormalities in erythrocyte membrane band 3 in chronic myelogenous leukemia. Biochim. Biophys. Acta (BBA) Biomembr. 1989, 985, 97–100. [Google Scholar] [CrossRef]
- Barshtein, G.; Arbell, D.; Gural, A.; Livshits, L. Distribution of Red Blood Cells Deformability: Study on Density-Separated Cell Subpopulations. Colloids Interfaces 2024, 8, 47. [Google Scholar] [CrossRef]
- McMahon, T.J. Red Blood Cell Deformability, Vasoactive Mediators, and Adhesion. Front. Physiol. 2019, 10, 1417. [Google Scholar] [CrossRef] [PubMed]
- Barshtein, G.; Gural, A.; Arbell, D.; Barkan, R.; Livshits, L.; Pajic-Lijakovic, I.; Yedgar, S. Red Blood Cell Deformability Is Expressed by a Set of Interrelated Membrane Proteins. Int. J. Mol. Sci. 2023, 24, 12755. [Google Scholar] [CrossRef]
- Hon, G.M.; Hassan, M.S.; van Rensburg, S.J.; Abel, S.; van Jaarsveld, P.; Erasmus, R.T.; Matsha, T. Red blood cell membrane fluidity in the etiology of multiple sclerosis. J. Membr. Biol. 2009, 232, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Tse, W.T.; Lux, S.E. Red blood cell membrane disorders. Br. J. Haematol. 1999, 104, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Puthumana, M.M.; Maîtrejean, G.; Wagner, C.; Podgorski, T. Influence of cell density and in-vivo aging on erythrocyte aggregability: Dissociation dynamics in extensional flow. arXiv 2024, arXiv:2409.08877v2. [Google Scholar] [CrossRef]
- Lee, J.M.; Suh, J.S.; Kim, Y.K. Red Blood Cell Deformability and Distribution Width in Patients with Hematologic Neoplasms. Clin. Lab. 2022, 68, 2118. [Google Scholar] [CrossRef]
- Rozovski, U.; Keating, M.J.; Estrov, Z. Targeting inflammatory pathways in chronic lymphocytic leukemia. Crit. Rev. Oncol. Hematol. 2013, 88, 655–666. [Google Scholar] [CrossRef]
- Yan, X.J.; Dozmorov, I.; Li, W.; Yancopoulos, S.; Sison, C.; Centola, M.; Jain, P.; Allen, S.L.; Kolitz, J.E.; Rai, K.R.; et al. Identification of outcome-correlated cytokine clusters in chronic lymphocytic leukemia. Blood 2011, 118, 5201–5210. [Google Scholar] [CrossRef]
- Fayad, L.; Keating, M.J.; Reuben, J.M.; O′Brien, S.; Lee, B.N.; Lerner, S.; Kurzrock, R. Interleukin-6 and interleukin-10 levels in chronic lymphocytic leukemia: Correlation with phenotypic characteristics and outcome. Blood 2001, 97, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Thisdelle, J.; Hou, S.; Fajardo-Despaigne, E.J.; Gibson, S.B.; Johnston, J.B.; Dawe, D.E.; Banerji, V.; Marshall, A.J. Elevated expression of interleukin 16 in chronic lymphocytic leukemia is associated with disease burden and abnormal immune microenvironment. Leuk. Res. 2023, 131, 107315. [Google Scholar] [CrossRef]
- O′Donnell, A.; Pepper, C.; Mitchell, S.; Pepper, A. NF-kB and the CLL microenvironment. Front. Oncol. 2023, 13, 1169397. [Google Scholar] [CrossRef]
- Nagarsheth, N.; Wicha, M.S.; Zou, W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat. Rev. Immunol. 2017, 17, 559–572. [Google Scholar] [CrossRef]
- van Atteku, M.H.; Eldering, E.; Kater, A.P. Chronic lymphocytic leukemia cells are active participants in microenvironmental cross-talk. Haematologica 2017, 102, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Nader, E.; Romana, M.; Connes, P. The Red Blood Cell—Inflammation Vicious Circle in Sickle Cell Disease. Front. Immunol. 2020, 11, 454. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Z.; Song, G.; Wang, H.; Long, M.; Cai, S. Effects of oxidative damage of membrane protein thiol groups on erythrocyte membrane viscoelasticities. Clin. Hemorheol. Microcirc. 1999, 21, 137–146. [Google Scholar] [PubMed]
- Nagababu, E.; Gulyani, S.; Earley, C.J.; Cutler, R.G.; Mattson, M.P.; Rifkind, J.M. Iron-deficiency anaemia enhances red blood cell oxidative stress. Free Radic Res. 2008, 42, 824–829. [Google Scholar] [CrossRef]
- Liu, Y.; Song, Y.; Yin, Q. Effects of ibrutinib on T-cell immunity in patients with chronic lymphocytic leukemia. Front. Immunol. 2022, 13, 962552. [Google Scholar] [CrossRef]
- Popov, V.M.; Matei, C.O.; Omer, M.; Onisai, M.; Matei, M.B.; Savopol, T.; Bumbea, H.; Moisescu, M.G. Effects of Ibrutinib on biophysical parameters of platelet in patients with chronic lymphocytic leukaemia. Am. J. Blood Res. 2020, 10, 311–319. [Google Scholar]
- Svanberg, R.; Janum, S.; Patten, P.E.M.; Ramsay, A.G.; Niemann, C.U. Targeting the tumor microenvironment in chronic lymphocytic leukemia. Haematologica 2021, 106, 2312–2324. [Google Scholar] [CrossRef] [PubMed]
- Niemann, C.U.; Herman, S.E.; Maric, I.; Gomez-Rodriguez, J.; Biancotto, A.; Chang, B.Y.; Martyr, S.; Stetler-Stevenson, M.; Yuan, C.M.; Calvo, K.R.; et al. Disruption of in vivo Chronic Lymphocytic Leukemia Tumor-Microenvironment Interactions by Ibrutinib--Findings from an Investigator-Initiated Phase II Study. Clin. Cancer Res. 2016, 22, 1572–1582. [Google Scholar] [CrossRef]
- Karel, M.F.A.; Tullemans, B.M.E.; D′Italia, G.; Lemmens, T.P.; Claushuis, T.A.M.; Kuijpers, M.J.E.; Cosemans, J.M.E.M. The effect of Bruton’s tyrosine kinase inhibitor Ibrutinib on atherothrombus formation under stenotic flow conditions. Thromb. Res. 2022, 212, 72–80. [Google Scholar] [CrossRef]
- Tullemans, B.M.E.; Karel, M.F.A.; Léopold, V.; Ten Brink, M.S.; Baaten, C.C.F.M.J.; Maas, S.L.; de Vos, A.F.; Eble, J.A.; Nijziel, M.R.; van der Vorst, E.P.C.; et al. Comparison of inhibitory effects of irreversible and reversible Btk inhibitors on platelet function. EJHaem 2021, 2, 685–699. [Google Scholar] [CrossRef]
- Goel, M.S.; Diamond, S.L. Adhesion of normal erythrocytes at depressed venous shear rates to activated neutrophils, activated platelets, and fibrin polymerized from plasma. Blood 2002, 100, 3797–3803. [Google Scholar] [CrossRef] [PubMed]
- Swanepoelp, A.C.; Pretorius, E. Erythrocyte-platelet interaction in uncomplicated pregnancy. Microsc. Microanal. 2014, 20, 1848–1860. [Google Scholar] [CrossRef]
- Trejo-Soto, C.; Hernández-Machado, A. Normalization of Blood Viscosity According to the Hematocrit and the Shear Rate. Micromachines 2022, 13, 357. [Google Scholar] [CrossRef]
- Deng, Y.; Papageorgiou, D.P.; Li, X.; Perakakis, N.; Mantzoros, C.S.; Dao, M.; Karniadakis, G.E. Quantifying Fibrinogen-Dependent Aggregation of Red Blood Cells in Type 2 Diabetes Mellitus. Biophys. J. 2020, 119, 900–912. [Google Scholar] [CrossRef]
- Li, Q.; Li, L.; Li, Y. Enhanced RBC Aggregation in Type 2 Diabetes Patients. J. Clin. Lab. Anal. 2015, 29, 387–389. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zennadi, R. The Role of RBC Oxidative Stress in Sickle Cell Disease: From the Molecular Basis to Pathologic Implications. Antioxidants 2021, 10, 1608. [Google Scholar] [CrossRef] [PubMed]
- Bosek, M.; Ziomkowska, B.; Pyskir, J.; Wybranowski, T.; Pyskir, M.; Cyrankiewicz, M.; Napiórkowska, M.; Durmowicz, M.; Kruszewski, S. Relationship between red blood cell aggregation and dextran molecular mass. Sci. Rep. 2022, 12, 19751. [Google Scholar] [CrossRef]
| Characteristics | Groups | ||||
|---|---|---|---|---|---|
| Reference Values | Healthy Controls (n = 13) | CLL Patients Without Treatment (n = 6) | CLL Patients Receiving Obinutuzumab/ Venetoclax (n = 10) | CLL Patients Receiving Ibrutinib Treatment (n = 5) | |
| age (years) | - | 58.00 ± 7.4 | 57.75 ± 14.0 | 69.11 ± 7.8 | 63.4 ± 10.8 |
| gender (F/M) | 8/5 | 2/4 | 4/6 | 2/3 | |
| RBC count (T/L) | 4.60–6.20 | 4.97 ± 0.23 | 4.97 ± 0.41 | 4.62 ± 0.45 | 4.80 ± 0.20 |
| Hb (g/L) | 140.00–180.00 | 161.40 ± 8.09 | 150.50 ± 12.82 | 136.70 ± 12.94 * | 141.80 ± 5.84 |
| Ht (L/L) | 0.40–0.54 | 0.48 ± 0.01 | 0.45 ± 0.02 | 0.41 ± 0.03 | 0.43 ± 0.02 |
| MCV (fL) | 80.00–95.00 | 89.10 ± 3.74 | 90.70 ± 4.69 | 88.99 ± 5.96 | 89.74 ± 1.81 |
| MCH (Pg/L) | 27.00–32.00 | 30.55 ± 1.33 | 30.30 ± 1.46 | 29.69 ± 2.39 | 29.54 ± 1.18 |
| MCHC (g/L) | 320.00–360.00 | 344.75 ± 4.15 | 334.50 ± 11.67 | 333.40 ± 13.93 | 329.60 ± 17.51 |
| RDW % | 11.60–14.80 | 13.88 ± 0.80 | 14.60 ± 1.54 | 14.26 ± 1.06 | 13.90 ± 0.57 |
| WBC | 3.50–10.50 | 6.3 ± 1.1 | 11.7 ÷ 157.5 * | 3.88 ± 1.00 | 6.43 ± 1.34 |
| total bilirubin (umol/L) | 3.40–20.50 | 15.73 ± 11.46 | 15.73 ± 11.46 | 17.98 ± 14.04 | 19.33 ± 11.06 |
| lymphocytes (ABS) | 1.10–3.80 | 1.91 ± 0.17 | 6.70 ÷ 144.28 * | 1.31 ± 0.49 | 2.08 ± 1.19 |
| platelet count ×109/L | 142.00–440.00 | 301.67 ± 92.63 | 208.25 ± 31.27 | 170.70 ± 43.19 | 166.80 ± 20.74 |
| Parameters | Groups | |||
|---|---|---|---|---|
| Healthy Controls | CLL Patients Without Treatment | CLL Patients Receiving Obinutuzumab/ Venetoclax | CLL Patients Receiving Ibrutinib Treatment | |
| AAI1 | 0.046 ± 0.007 | 0.022 ± 0.009 * | 0.026 ± 0.01 * | 0.054 ± 0.011 ♦ |
| NA1 | 106 ± 10.6 | 45.5 ± 23 * | 58.3 ± 24.3 * | 119 ± 38.5 ♦ |
| AAI2 | 0.039 ± 0.008 | 0.024 ± 0.006 * | 0.026 ± 0.009 * | 0.040 ± 0.007 ♦ |
| NA2 | 34.7 ± 7.4 | 20.5 ± 6.2 * | 22.2 ± 9.8 * | 35 ± 7.8 ♦ |
| AAI3 | 0.027 ± 0.01 | 0.027 ± 0.008 | 0.024 ± 0.008 | 0.030 ± 0.006 |
| NA3 | 10.3 ± 3.2 | 12.5 ± 3.6 | 10.6 ± 4.1 | 13.8 ± 3.0 |
| AAI4 | 0.0026 ± 0.0002 | 0.031 ± 0.008 * | 0.021 ± 0.009 * | 0.007 ± 0.003 |
| NA4 | 1.0 ± 0.7 | 8.3 ± 1.2 * | 4.6 ± 2.7 * | 1.8 ± 0.8 ♦ |
| AAI5 | - | 0.032 ± 0.009 | 0.021 ± 0.004 | - |
| NA5 | - | 3.5 ± 0.9 | 2.1 ± 1.6 | - |
| Healthy Controls | CLL Patients Without Treatment | CLL Patients Receiving Obinutuzumab/ Venetoclax | CLL Patients Receiving Ibrutinib Treatment |
|---|---|---|---|
| 0.015 ± 0.002 | 0.0063 ± 0.001 | 0.0078 ± 0.001 | 0.014 ± 0.004 |
| Groups | RBC Morphological Types (%) | ||
|---|---|---|---|
| Biconcave | Echinocytes | Spherocytes | |
| Healthy controls | 83.3 ± 4.3 | 14.9 ± 3.7 | 1.8 ± 0.9 |
| Untreated CLL patients | 73.2 ± 6.6 * | 21.2 ± 3.9 * | 5.6 ± 2.0 * |
| CLL patients receiving Obinutuzumab/Venetoclax | 71.7 ± 9.7 * | 21.5 ± 8.9 * | 6.8 ± 2.3 * |
| CLL patients receiving Ibrutinib treatment | 81.4 ± 2.6 | 15.1 ± 4.5 | 3.5 ± 2.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexandrova-Watanabe, A.; Abadjieva, E.; Gartcheva, L.; Langari, A.; Ivanova, M.; Guenova, M.; Tiankov, T.; Strijkova, V.; Krumova, S.; Todinova, S. The Impact of Targeted Therapies on Red Blood Cell Aggregation in Patients with Chronic Lymphocytic Leukemia Evaluated Using Software Image Flow Analysis. Micromachines 2025, 16, 95. https://doi.org/10.3390/mi16010095
Alexandrova-Watanabe A, Abadjieva E, Gartcheva L, Langari A, Ivanova M, Guenova M, Tiankov T, Strijkova V, Krumova S, Todinova S. The Impact of Targeted Therapies on Red Blood Cell Aggregation in Patients with Chronic Lymphocytic Leukemia Evaluated Using Software Image Flow Analysis. Micromachines. 2025; 16(1):95. https://doi.org/10.3390/mi16010095
Chicago/Turabian StyleAlexandrova-Watanabe, Anika, Emilia Abadjieva, Lidia Gartcheva, Ariana Langari, Miroslava Ivanova, Margarita Guenova, Tihomir Tiankov, Velichka Strijkova, Sashka Krumova, and Svetla Todinova. 2025. "The Impact of Targeted Therapies on Red Blood Cell Aggregation in Patients with Chronic Lymphocytic Leukemia Evaluated Using Software Image Flow Analysis" Micromachines 16, no. 1: 95. https://doi.org/10.3390/mi16010095
APA StyleAlexandrova-Watanabe, A., Abadjieva, E., Gartcheva, L., Langari, A., Ivanova, M., Guenova, M., Tiankov, T., Strijkova, V., Krumova, S., & Todinova, S. (2025). The Impact of Targeted Therapies on Red Blood Cell Aggregation in Patients with Chronic Lymphocytic Leukemia Evaluated Using Software Image Flow Analysis. Micromachines, 16(1), 95. https://doi.org/10.3390/mi16010095









