Development and Calibration of a Microfluidic, Chip-Based Sensor System for Monitoring the Physical Properties of Water Samples in Aquacultures
Abstract
1. Introduction
2. Materials and Methods
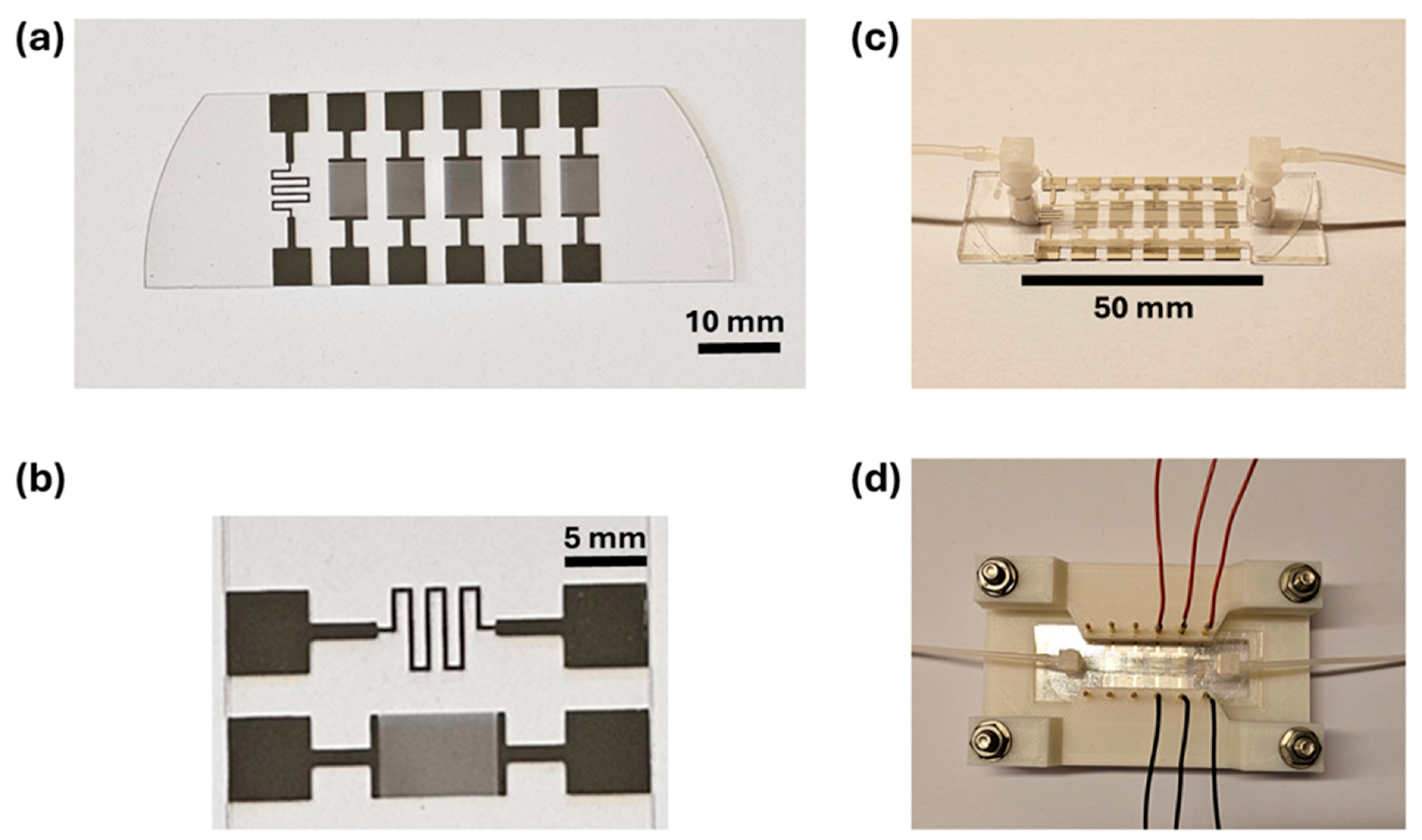
2.1. Sensing Elements and Microfluidic Channel Device
2.2. Impedance Measurements and Reference Electrolytes
2.3. Temperature Calibration Measurements
2.4. Water Samples under Study
3. Results and Discussion
3.1. Electrical Conductivity of the Reference Electrolytes
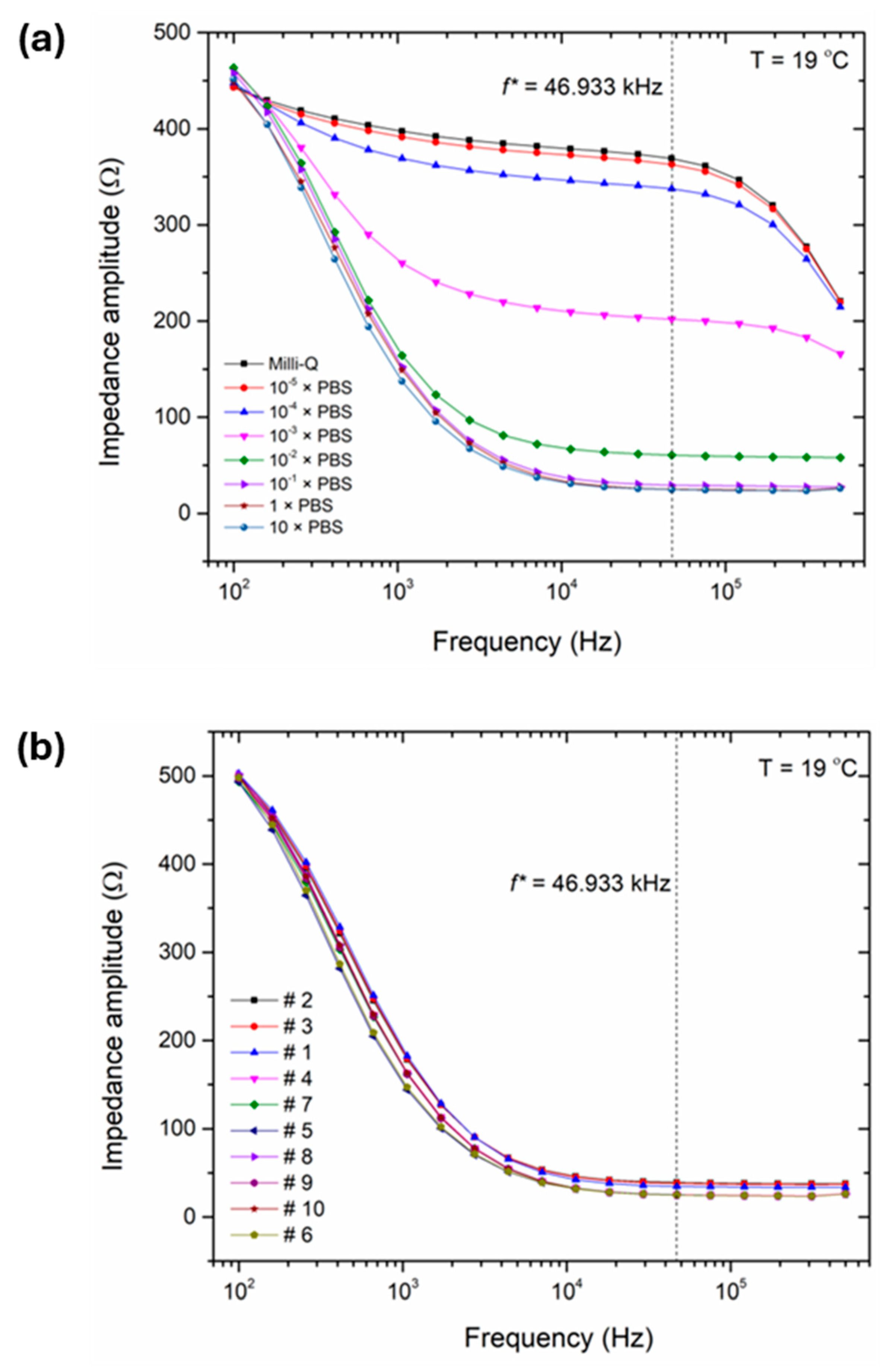
3.2. Impedance Spectra of Water Samples and Reference Electrolytes with Chip-Based Sensor Setup
3.3. Single-Frequency Algorithm for Conductivity Measurements with the IDES Sensor

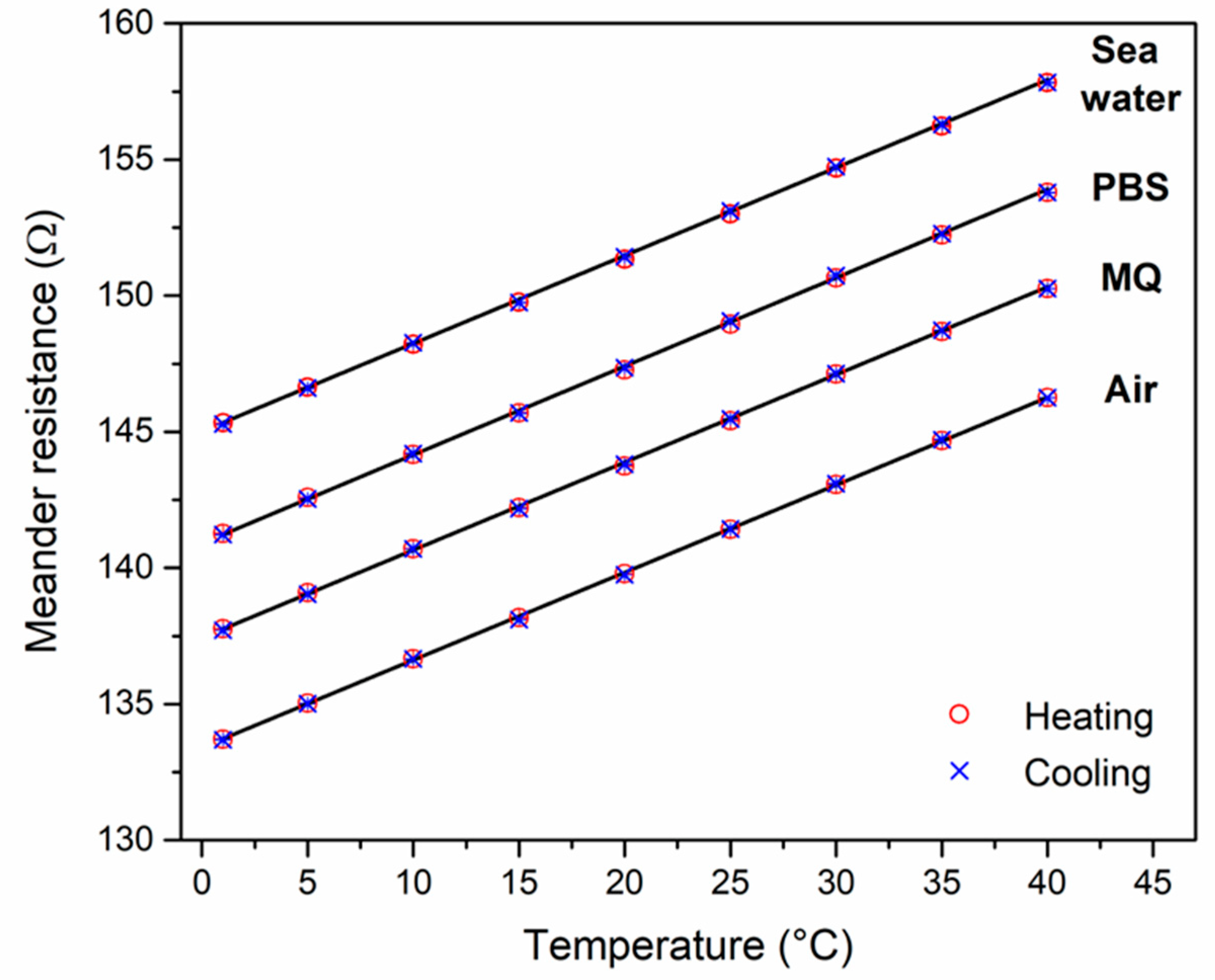
3.4. Calibration of the Chip-Based Platinum RTD
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Online Document 52021DC0236, Released by the European Commission on 12 May 2021. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM:2021:236:FIN (accessed on 8 April 2024).
- Ahmed, N.; Turchini, G.M. Recirculating Aquaculture Systems (RAS): Environmental Solution and Climate Change Adaptation. J. Clean. Prod. 2021, 297, 126604. [Google Scholar] [CrossRef]
- Suantika, G.; Situmorang, M.L.; Kurniawan, J.B.; Pratiwi, S.A.; Aditiawati, P.; Astutia, D.I.; Azizah, F.F.N.; Djohan, Y.A.; Zuhri, U.; Simatupang, T.M. Development of a Zero Water Discharge (ZWD)—Recirculating Aquaculture System (RAS) Hybrid System for Super Intensive White Shrimp (Litopenaeus vannamei) Culture Under Low Salinity Conditions and its Industrial Trial in Commercial Shrimp Urban Farming in Gresik, East Java, Indonesia. Aquacult. Eng. 2018, 82, 12–24. [Google Scholar] [CrossRef]
- Thornber, K.; Bashar, A.; Ahmed, M.S.; Bell, A.; Trew, J.; Hasan, M.; Hasan, N.A.; Alam, M.M.; Chaput, D.L.; Haque, M.M.; et al. Antimicrobial Resistance in Aquaculture Environments: Unravelling the Complexity and Connectivityof the Underlying Societal Drivers. Environ. Sci. Technol. 2022, 56, 14891–14903. [Google Scholar] [CrossRef]
- Schar, D.; Zhao, C.; Wang, Y.; Larsson, D.G.J.; Gilbert, M.; Van Boeckel, T.P. Twenty-Year Trends in Antimicrobial Resistance from Aquaculture and Fisheries in Asia. Nat. Commun. 2021, 12, 5384. [Google Scholar] [CrossRef]
- Pepi, M.; Focardi, S. Antibiotic-Resistant Bacteria in Aquaculture and Climate Change: A Challenge for Health in the Mediterranean Area. Int. J. Environ. Res. Public Health 2021, 18, 5723. [Google Scholar] [CrossRef]
- Webpage of the Eranet Project ARENA. Available online: https://www.jpi-arena.eu/en/ (accessed on 16 October 2023).
- Huck, C.; Poghossian, A.; Bäcker, M.; Chaudhuri, S.; Zander, W.; Schubert, J.; Begoyan, V.K.; Buniatyan, V.V.; Wagner, P.; Schöning, M.J. Capacitively Coupled Electrolyte-Conductivity Sensor Based on High-k Material of Barium Strontium Titanate. Sens. Actuators B Chem. 2014, 198, 102–109. [Google Scholar] [CrossRef]
- Beutner, A.; Scherer, B.; Matysik, F.-M. Dual Detection for Non-Aqueous Capillary Electrophoresis Combining Contactless Conductivity Detection and Mass Spectrometry. Talanta 2018, 183, 33–38. [Google Scholar] [CrossRef]
- Katz, E.; Willner, I. Probing Biomolecular Interactions at Conductive and Semiconductive Surfaces by Impedance Spectroscopy: Routes to Impedimetric Immunosensors, DNA-Sensors, and Enzyme Biosensors. Electroanalysis 2003, 15, 913–947. [Google Scholar] [CrossRef]
- Lisdat, F.; D Schäfer, D. The Use of Electrochemical Impedance Spectroscopy for Biosensing. Anal. Bioanal. Chem. 2008, 391, 1555–1567. [Google Scholar] [CrossRef]
- Lazanas, A.C.; Prodromidis, M.I. Electrochemical Impedance Spectroscopy—A Tutorial. ACS Meas. Sci. Au 2023, 3, 162–193. [Google Scholar] [CrossRef]
- Poghossian, A.; Schöning, M.J. Capacitive Field-Effect EIS Chemical Sensors and Biosensors: A Status Report. Sensors 2020, 20, 5639. [Google Scholar] [CrossRef]
- Stilman, W.; Campolim Lenzi, M.; Wackers, G.; Deschaume, O.; Yongabi, D.; Mathijssen, G.; Bartic, C.; Gruber, J.; Wübbenhorst, M.; Heyndrickx, M.; et al. Low Cost, Sensitive Impedance Detection of E. coli Bacteria in Food-Matrix Samples Using Surface-Imprinted Polymers as Whole-Cell Receptors. Phys. Stat. Sol. A 2022, 219, 2100405. [Google Scholar] [CrossRef]
- Cornelis, P.; Givanoudi, S.; Yongabi, D.; Iken, H.; Duwé, S.; Deschaume, O.; Robbens, J.; Dedecker, P.; Bartic, C.; Wübbenhorst, M.; et al. Sensitive and Specific Detection of E. coli Using Biomimetic Receptors in Combination with a Modified Heat-Transfer Method. Biosens. Bioelectron. 2019, 136, 97–105. [Google Scholar] [CrossRef]
- Cardoso, A.R.; Tavares, A.P.M.; Goreti, F.; Sales, M. In-situ Generated Molecularly Imprinted Material for Chloramphenicol Electrochemical Sensing in Waters Down to the Nanomolar Level. Sens. Actuators B Chem. 2018, 256, 420–428. [Google Scholar] [CrossRef]
- Vu, Q.T.; Nguyen, Q.H.; Nguy Phan, T.; Luong, T.T.; Eersels, K.; Wagner, P.; Truong, L.T.N. Highly Sensitive Molecularly Imprinted Polymer-Based Electrochemical Sensors Enhanced by Gold Nanoparticles for Norfloxacin Detection in Aquaculture Water. ACS Omega 2023, 8, 2887–2896. [Google Scholar] [CrossRef] [PubMed]
- Web Page The Engineering ToolBox. Available online: https://www.engineeringtoolbox.com/resistivity-conductivity-d_418.html (accessed on 10 October 2023).
- Cahill, D.G. Thermal Conductivity Measurement from 30 to 750 K: The 3ω Method. Rev. Sci. Instrum. 1990, 61, 802–808. [Google Scholar] [CrossRef]
- Khorshid, M.; Bakhshi Sichani, S.; Cornelis, P.; Wackers, G.; Wagner, P. The Hot-Wire Concept: Towards a One-Element Thermal Biosensor Platform. Biosens. Bioelectron. 2021, 179, 113043. [Google Scholar] [CrossRef]
- Clausen, C.; Pedersen, T.; Bentien, A. The 3-Omega Method for the Measurement of Fouling Thickness, the Liquid Flow Rate, and Surface Contact. Sensors 2017, 17, 552. [Google Scholar] [CrossRef]
- Sadiq, F.A.; De Reu, K.; Burmølle, M.; Maes, S.; Heyndrickx, M. Synergistic Interactions in Multispecies Biofilm Combinations of Bacterial Isolates Recovered from Diverse Food Processing Industries. Front. Microbiol. 2023, 14, 1159434. [Google Scholar] [CrossRef]
- Bates, N.R.; Best, M.H.P.; Neely, K.; Garley, R.; Dickson, A.G.; Johnson, R.J. Detecting Anthropogenic Carbon Dioxide Uptake and Ocean Acidification in the North Atlantic Ocean. Biogeosciences 2012, 9, 2509–2522. [Google Scholar] [CrossRef]
- Eslek, Z.; Tulpar, A. Solution Preparation and Conductivity Measurements: An Experiment for Introductory Chemistry. J. Chem. Educ. 2013, 90, 1665–1667. [Google Scholar] [CrossRef]
- Srinivasa, M.K.; Lee, J.; Hyun, K.; Yoo, H.D. Modifying Kohlrausch’s Law to Describe Nonaqueous Electrolytes for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2023, 15, 59296–59308. [Google Scholar] [CrossRef] [PubMed]
- Webpage The Engineering ToolBox. Available online: https://www.engineeringtoolbox.com/conductors-d_1381.html (accessed on 10 October 2023).
- Golnabi, H.; Matloob, M.R.; Bahar, M.; Sharifian, M. Investigation of Electrical Conductivity of Different Water Liquids and Electrolyte Solutions. Iran. Phys. J. 2009, 3, 24–28. [Google Scholar]
- Swertz, O.; Colijn, F.; Hofstraat, H.W.; Althuis, B.A. Temperature, Salinity, and Fluorescence in Southern North Sea: High-Resolution Data Sampled from a Ferry. Environ. Manag. 1999, 23, 527–538. [Google Scholar] [CrossRef]
- McAdams, E.T.; Jossinet, J.; Subramanian, R.; McCauley, R.G.E. Characterization of Gold Electrodes in Phosphate Buffered Saline Solution by Impedance and Noise Measurements for Biological Applications. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2006, 1–15, 4594–4597. [Google Scholar] [CrossRef] [PubMed]
- van Grinsven, B.; Vandenryt, T.; Duchateau, S.; Gaulke, A.; Grieten, L.; Thoelen, R.; Ingebrandt, S.; De Ceuninck, W.; Wagner, P. Customized Impedance Spectroscopy Device as Possible Sensor Platform for Biosensor Applications. Phys. Stat. Sol. A 2010, 207, 919–923. [Google Scholar] [CrossRef]
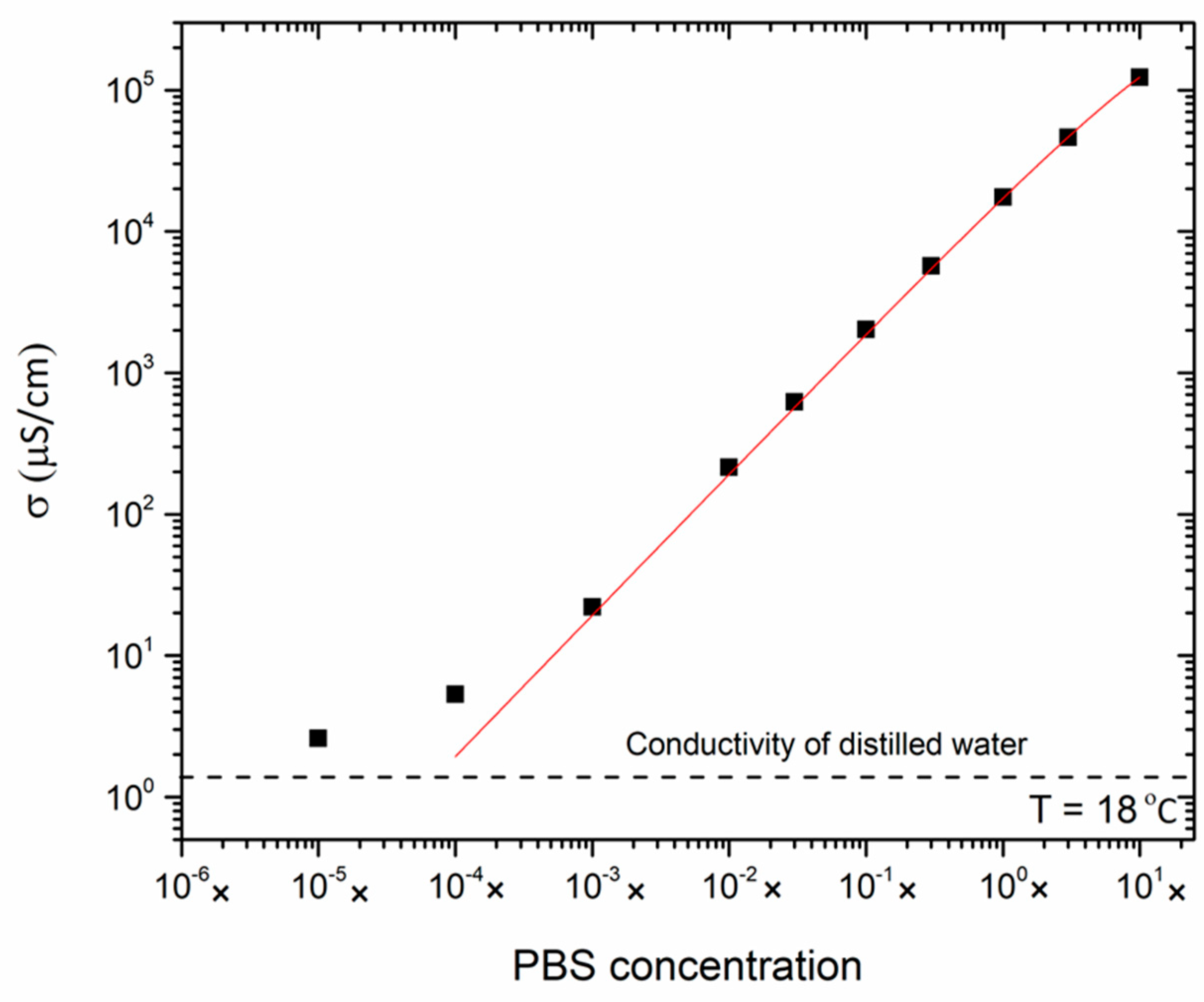
| PBS Concentration | pH Value | Conductivity σ (μS cm−1) |
|---|---|---|
| 10.0× | 6.78 ± 0.01 | (1.233 ± 0.002) × 105 |
| 3.0× | 7.19 ± 0.01 | (4.624 ± 0.009) × 104 |
| 1.0× | 7.43 ± 0.01 | (1.749 ± 0.004) × 104 |
| 0.3× | 7.56 ± 0.01 | (5.679 ± 0.009) × 103 |
| 0.1× | 7.55 ± 0.01 | (2.032 ± 0.008) × 103 |
| 3.0 × 10−2× | 7.42 ± 0.01 | 621.5 ± 1.1 |
| 1.0 × 10−2× | 6.93 ± 0.01 | 215.1 ± 0.2 |
| 1.0 × 10−3× | 6.01 ± 0.00 | 22.17 ± 0.12 |
| 1.0 × 10−4× | 5.84 ± 0.02 | 5.340 ± 0.013 |
| 1.0 × 10−5× | 5.73 ± 0.00 | 2.614 ± 0.009 |
| Milli-Q water | 6.08 ± 0.07 | 2.211 ± 0.034 |
| Sample Type | Sample Number | Place, Description | pH Value |
|---|---|---|---|
| Surface waters | # 1 | Dyle river (Leuven, Arenberg castle) | 8.08 ± 0.01 |
| # 2 | Leuven-Dyle channel (Mechelen, Stuyvenbergvaart) | 7.89 ± 0.01 | |
| # 3 | Brussels-Scheldt sea channel (Grimbergen, Verbrande Brug) | 7.84 ± 0.01 | |
| Recirculating aquaculture systems RAS | # 4 | Mullet (Mugil cephalus), cultivated in brackish water | 7.35 ± 0.01 |
| # 5 | Sea bream (Abramis brama) | 5.76 ± 0.01 | |
| # 6 | Sea bream (Abramis brama), newly placed filters | 5.82 ± 0.01 | |
| # 7 | Sole (Solea solea), lightly brackish water | 8.11 ± 0.01 | |
| # 8 | Gray shrimp (Crangon crangon) | 7.54 ± 0.01 | |
| Ocean waters | # 9 | Sea water from Eastern Scheldt (The Netherlands) | 7.97 ± 0.01 |
| # 10 | Atlantic ocean water from Ostend Harbor | 7.86 ± 0.01 |
| Sample Number | Conductivity σ (μS cm−1) Reference Instrument | Conductivity σ (μS cm−1) Impedimetric Sensor | Relative Deviation |
|---|---|---|---|
| # 1 | 855.8 ± 0.5 | 854 ± 77 | −0.2% |
| # 2 | 574.6 ± 0.8 | 589 ± 48 | +2.5% |
| # 3 | 616.2 ± 0.9 | 626 ± 44 | +1.6% |
| # 4 | (21.40 ± 0.03) × 103 | (21.38 ± 0.64) × 103 | −0.1% |
| # 5 | (48.80 ± 0.05) × 103 | (46.89 ± 1.39) × 103 | −3.9% |
| # 6 | (50.86 ± 0.08) × 103 | (50.68 ± 2.70) × 103 | −0.4% |
| # 7 | (41.18 ± 0.07) × 103 | (41.20 ± 1.08) × 103 | <+0.1% |
| # 8 | (49.21 ± 0.06) × 103 | (49.38 ± 1.23) × 103 | +0.3% |
| # 9 | (49.62 ± 0.07) × 103 | (49.81 ± 1.56) × 103 | +0.4% |
| # 10 | (50.20 ± 0.03) × 103 | (50.24 ± 2.86) × 103 | <+0.1% |
| Medium | Resistance at 20 °C (Ω) | TCR (10−3 K−1) | R2 Linear Fit |
|---|---|---|---|
| Air | 139.779 ± 0.002 | 2.303 | 0.9999 |
| Milli-Q | 139.741 ± 0.001 | 2.273 | 0.9996 |
| 1 × PBS | 139.274 ± 0.004 | 2.301 | 0.9998 |
| Sea water (# 9) | 139.329 ± 0.005 | 2.303 | 0.9999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aliazizi, F.; Özsoylu, D.; Bakhshi Sichani, S.; Khorshid, M.; Glorieux, C.; Robbens, J.; Schöning, M.J.; Wagner, P. Development and Calibration of a Microfluidic, Chip-Based Sensor System for Monitoring the Physical Properties of Water Samples in Aquacultures. Micromachines 2024, 15, 755. https://doi.org/10.3390/mi15060755
Aliazizi F, Özsoylu D, Bakhshi Sichani S, Khorshid M, Glorieux C, Robbens J, Schöning MJ, Wagner P. Development and Calibration of a Microfluidic, Chip-Based Sensor System for Monitoring the Physical Properties of Water Samples in Aquacultures. Micromachines. 2024; 15(6):755. https://doi.org/10.3390/mi15060755
Chicago/Turabian StyleAliazizi, Fereshteh, Dua Özsoylu, Soroush Bakhshi Sichani, Mehran Khorshid, Christ Glorieux, Johan Robbens, Michael J. Schöning, and Patrick Wagner. 2024. "Development and Calibration of a Microfluidic, Chip-Based Sensor System for Monitoring the Physical Properties of Water Samples in Aquacultures" Micromachines 15, no. 6: 755. https://doi.org/10.3390/mi15060755
APA StyleAliazizi, F., Özsoylu, D., Bakhshi Sichani, S., Khorshid, M., Glorieux, C., Robbens, J., Schöning, M. J., & Wagner, P. (2024). Development and Calibration of a Microfluidic, Chip-Based Sensor System for Monitoring the Physical Properties of Water Samples in Aquacultures. Micromachines, 15(6), 755. https://doi.org/10.3390/mi15060755








