Clarifying the Dominant Role of Crystallinity and Molecular Orientation in Differently Processed Thin Films of Regioregular Poly(3-hexylthiophene)
Abstract
1. Introduction
2. Materials and Methods
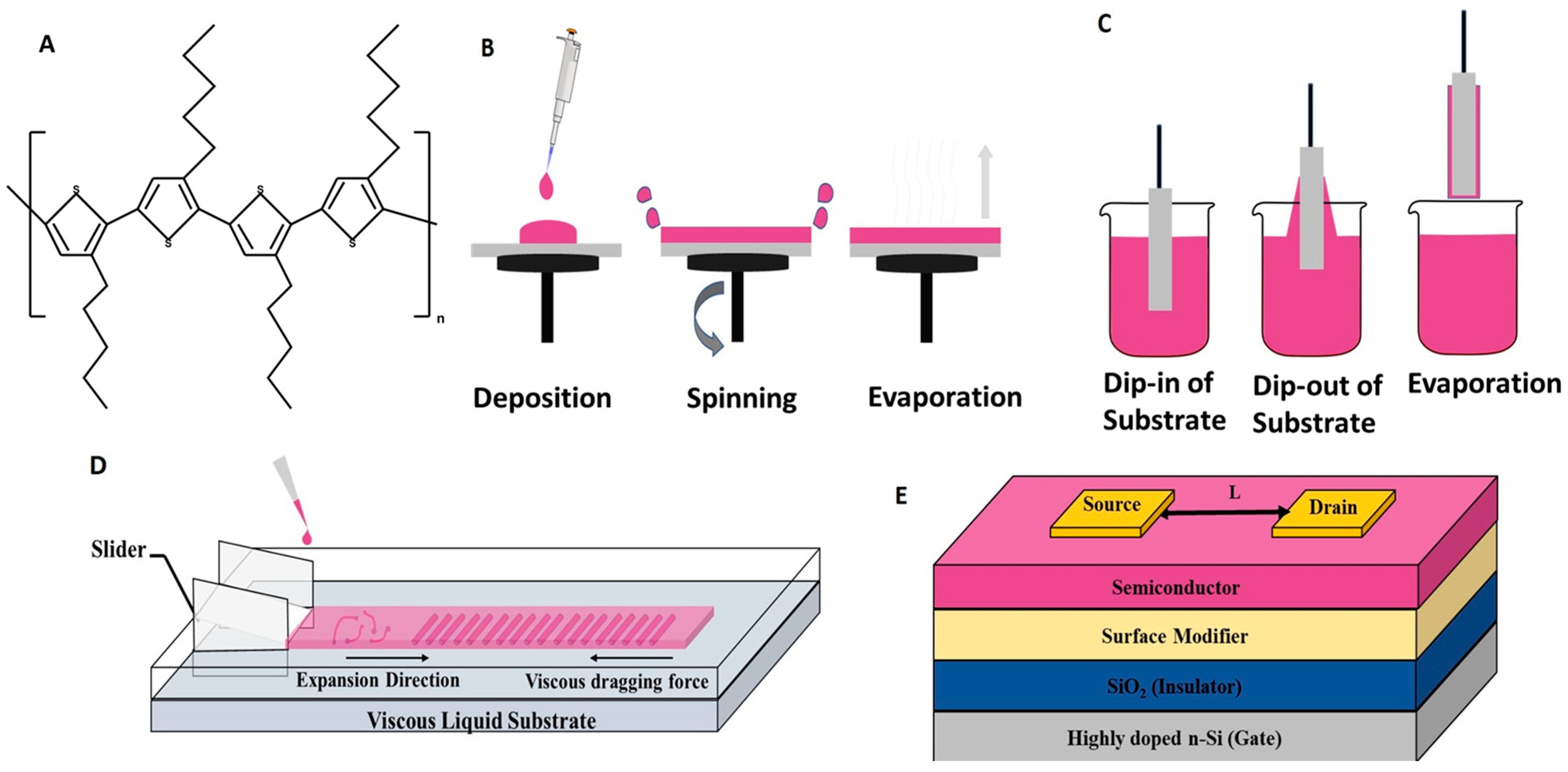
2.1. Materials
2.2. Thin Film and Device Fabrication
2.2.1. Spin Coating
2.2.2. Dip Coating
2.2.3. Unidirectional Floating Film Transfer Method
2.3. Thin Film and Device Characterizations
2.3.1. Optical and Microstructural Characterizations
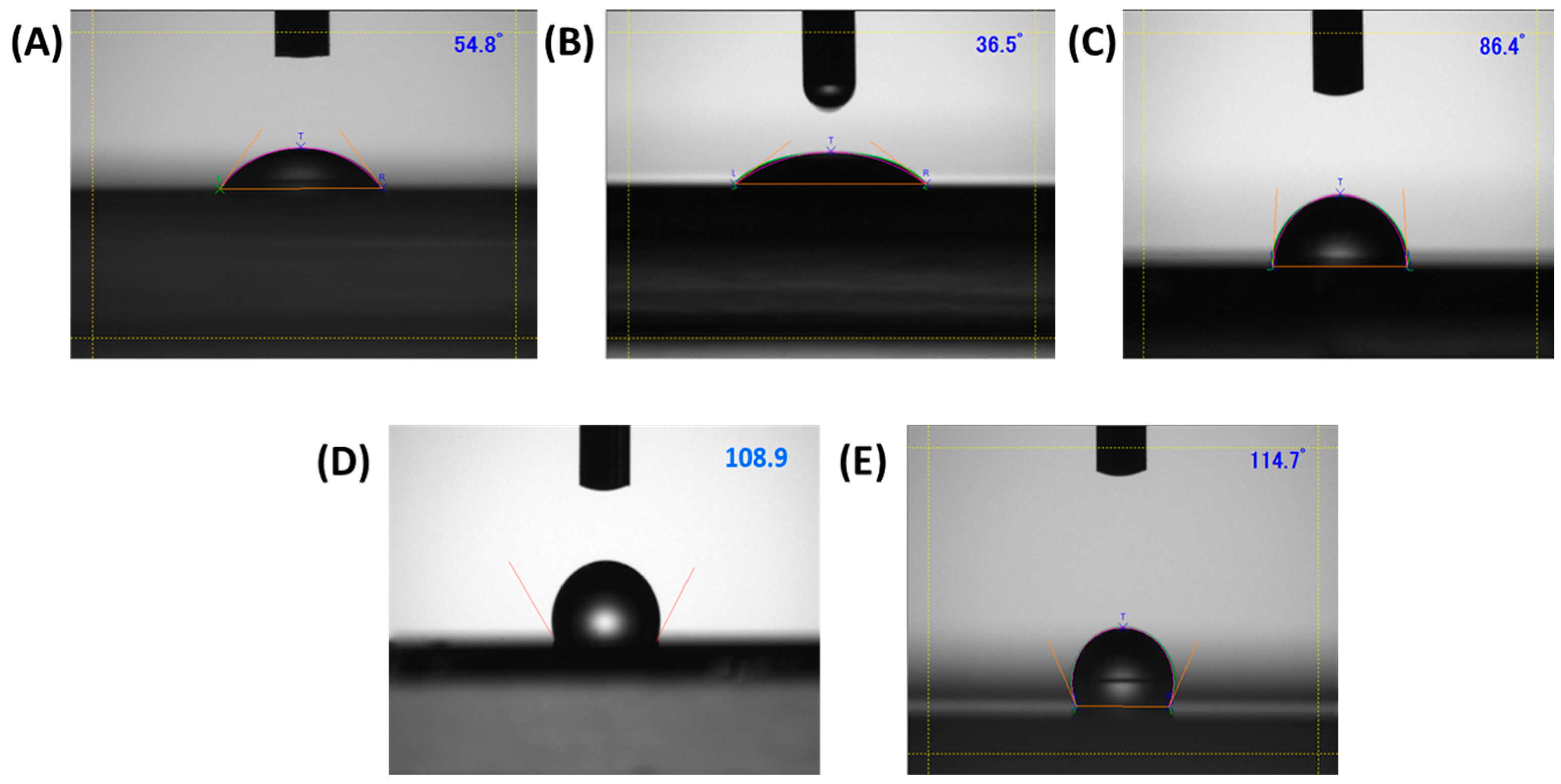
2.3.2. Contact Angle Measurement
2.3.3. Electrical Characterizations
3. Results
3.1. Contact Angle Goniometry
3.2. Optimization of Thin Film Fabrication
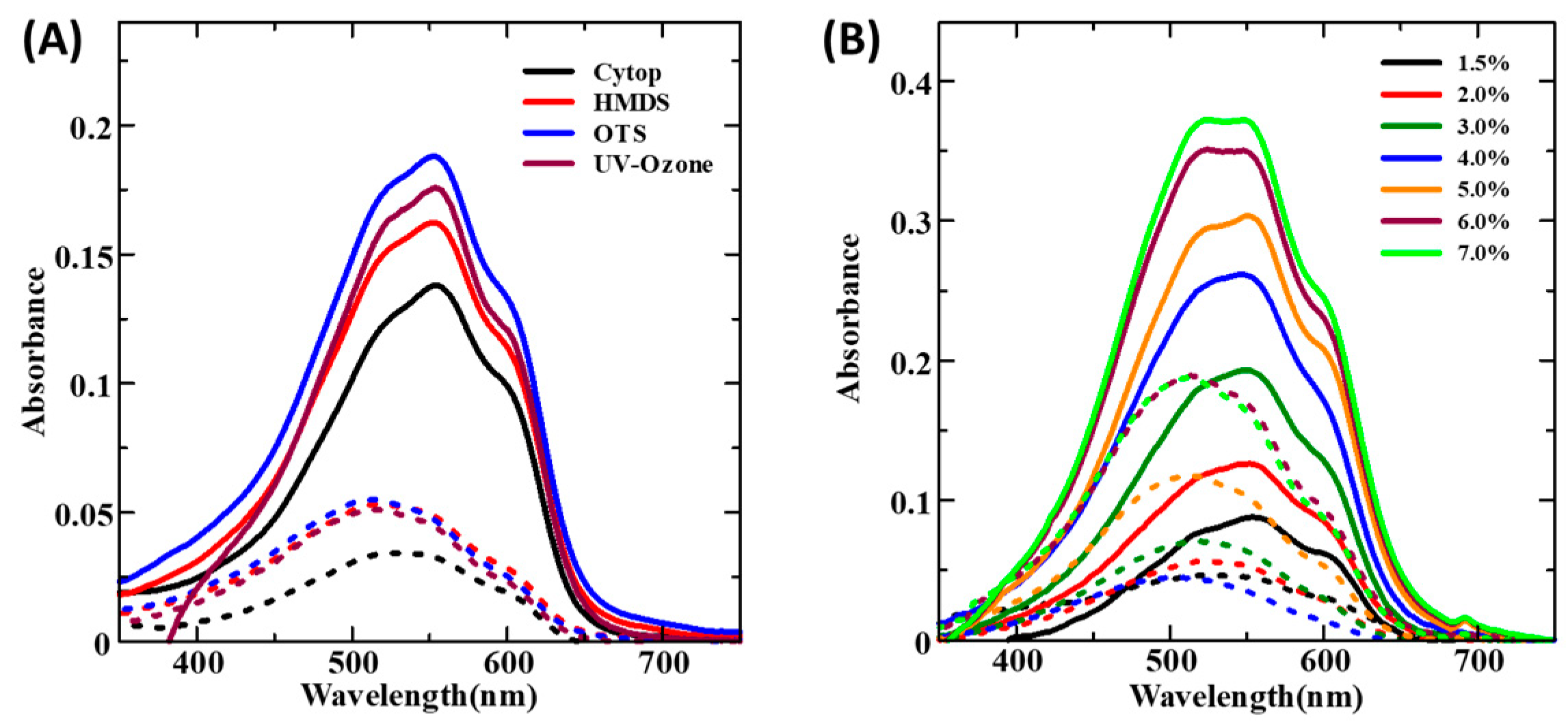
3.2.1. Spin-Coated Thin Films
3.2.2. Dip-Coated Thin Films
3.2.3. UFTM Fabricated Thin Films
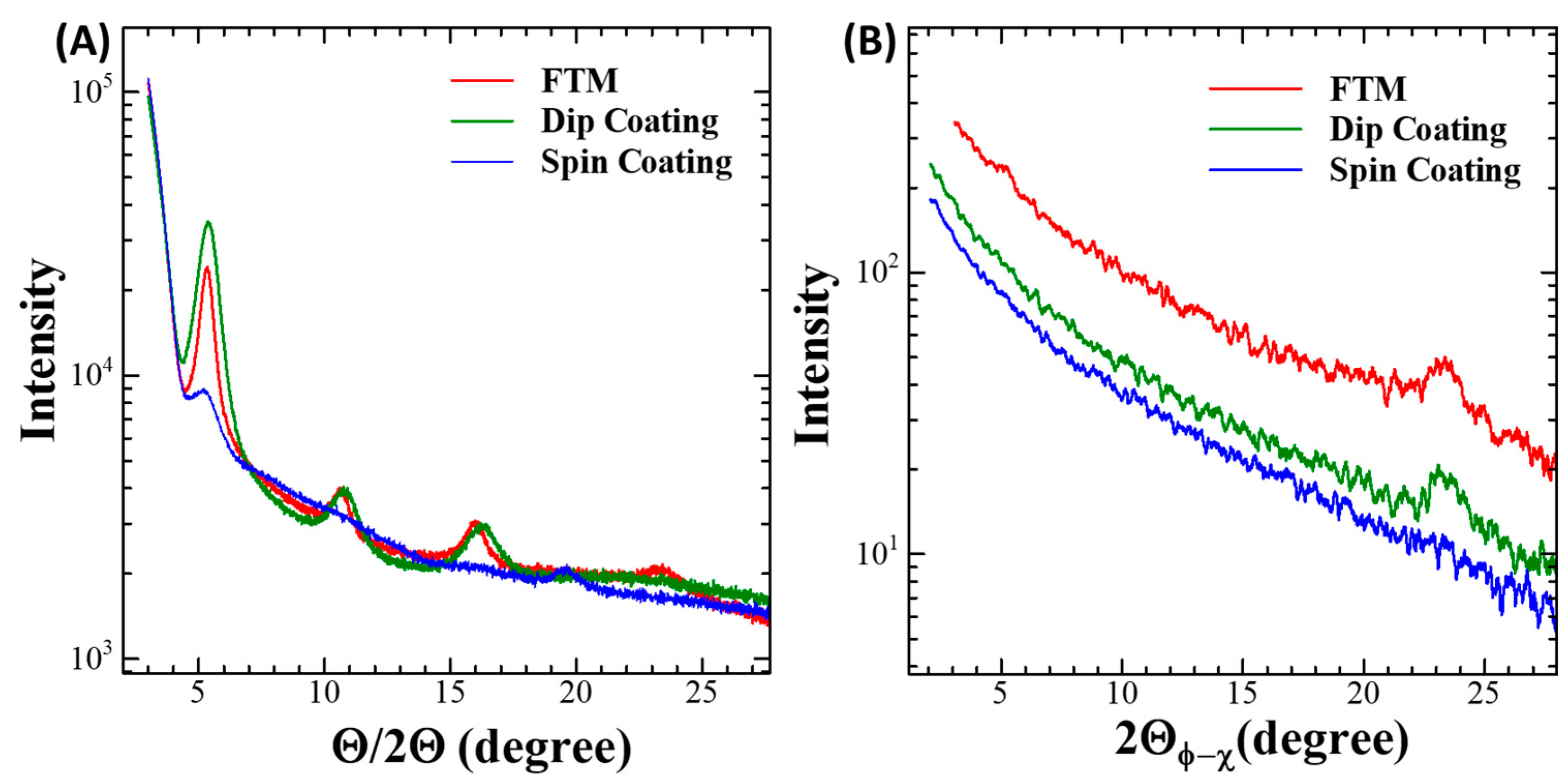
3.3. Microstructural Characterization
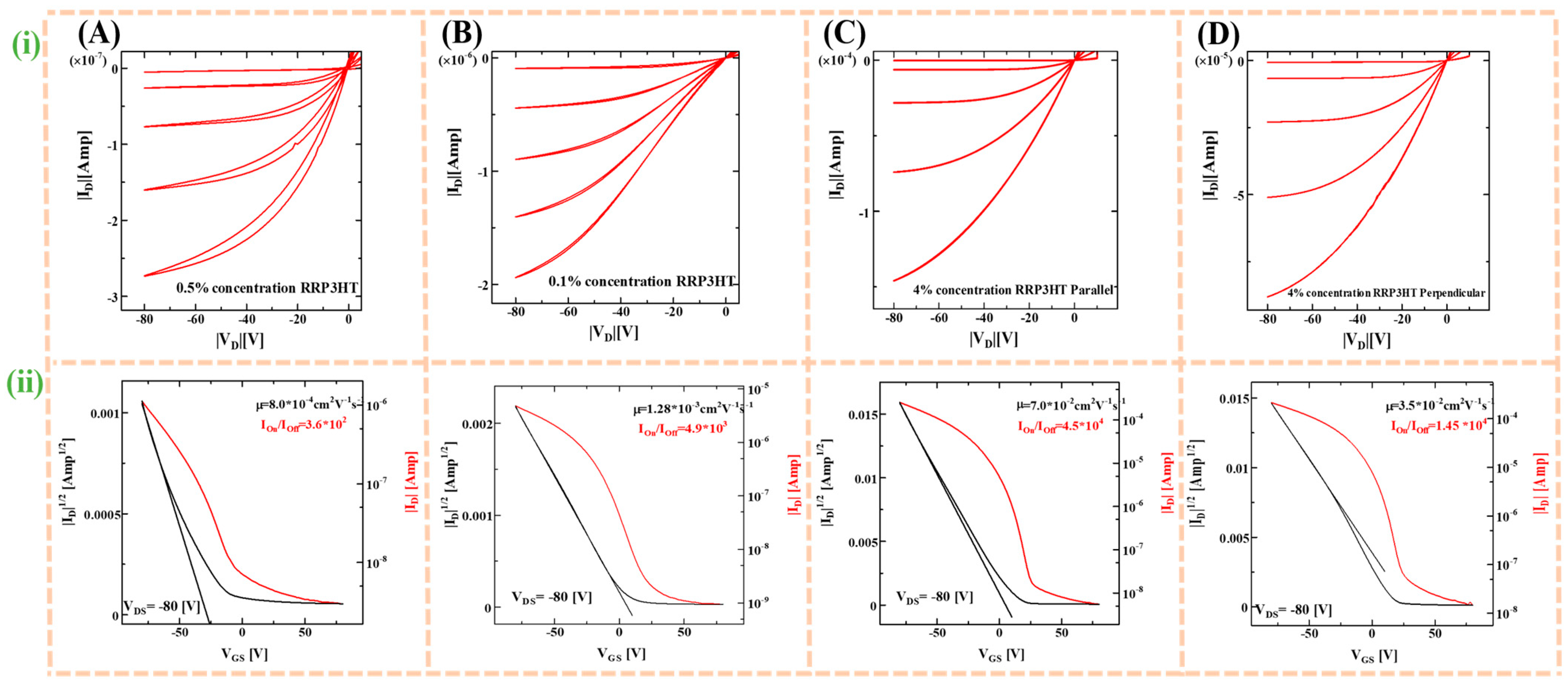
3.4. Electrical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arden, W.M. The International Technology Roadmap for Semiconductors—Perspectives and challenges for the next 15 years. Curr. Opin. Solid. State Mater. Sci. 2002, 6, 371–377. [Google Scholar] [CrossRef]
- Petty, M.C. Molecular Electronics; John Wiley & Sons, Ltd.: Chichester, UK, 2007. [Google Scholar] [CrossRef]
- Ikawa, M.; Yamada, T.; Matsui, H.; Minemawari, H.; Tsutsumi, J.; Horii, Y.; Chikamatsu, M.; Azumi, R.; Kumai, R.; Hasegawa, T. Simple push coating of polymer thin-film transistors. Nat. Commun. 2012, 3, 1176. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Kurosawa, Y.; Ito, H.; Makishima, Y.; Kita, H.; Yoshida, T.; Suzuri, Y. Solution processing of alternating PDMS/SiOx multilayer for encapsulation of organic light emitting diodes. Org. Electron. 2019, 64, 176–180. [Google Scholar] [CrossRef]
- Sandström, A.; Dam, H.F.; Krebs, F.C.; Edman, L. Ambient fabrication of flexible and large-area organic light-emitting devices using slot-die coating. Nat. Commun. 2012, 3, 1002. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Liu, S.; Chen, W.; Fan, F.; Liu, G. Organic Memory and Memristors: From Mechanisms, Materials to Devices. Adv. Electron. Mater. 2021, 7, 2100432. [Google Scholar] [CrossRef]
- Marrocchi, A.; Lanari, D.; Facchetti, A.; Vaccaro, L. Poly(3-hexylthiophene): Synthetic methodologies and properties in bulk heterojunction solar cells. Energy Environ. Sci. 2012, 5, 8457. [Google Scholar] [CrossRef]
- Xu, J.; Wu, H.-C.; Zhu, C.; Ehrlich, A.; Shaw, L.; Nikolka, M.; Wang, S.; Molina-Lopez, F.; Gu, X.; Luo, S.; et al. Multi-scale ordering in highly stretchable polymer semiconducting films. Nat. Mater. 2019, 18, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Y.; Rondeau-Gagné, S.; Chiu, Y.-C.; Chortos, A.; Lissel, F.; Wang, G.-J.N.; Schroeder, B.C.; Kurosawa, T.; Lopez, J.; Katsumata, T.; et al. Intrinsically stretchable and healable semiconducting polymer for organic transistors. Nature 2016, 539, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Sirringhaus, H. 25th Anniversary Article: Organic Field-Effect Transistors: The Path Beyond Amorphous Silicon. Adv. Mater. 2014, 26, 1319–1335. [Google Scholar] [CrossRef]
- Sirringhaus, H. Device Physics of Solution-Processed Organic Field-Effect Transistors. Adv. Mater. 2005, 17, 2411–2425. [Google Scholar] [CrossRef]
- Mi, J.L.; Zhao, X.B.; Zhu, T.J.; Tu, J.P. Improved thermoelectric figure of merit in n-type CoSb3 based nanocomposites. Appl. Phys. Lett. 2007, 91, 172116. [Google Scholar] [CrossRef]
- Chang, J.-F.; Sun, B.; Breiby, D.W.; Nielsen, M.M.; Sölling, T.I.; Giles, M.; McCulloch, I.; Sirringhaus, H. Enhanced Mobility of Poly(3-hexylthiophene) Transistors by Spin-Coating from High-Boiling-Point Solvents. Chem. Mater. 2004, 16, 4772–4776. [Google Scholar] [CrossRef]
- Noh, J.; Jeong, S.; Lee, J.-Y. Ultrafast formation of air-processable and high-quality polymer films on an aqueous substrate. Nat. Commun. 2016, 7, 12374. [Google Scholar] [CrossRef] [PubMed]
- Yabuuchi, Y.; Uzurano, G.; Nakatani, M.; Fujii, A.; Ozaki, M. Uniaxial orientation of poly(3-hexylthiophene) thin films fabricated by the bar-coating method. Jpn. J. Appl. Phys. 2019, 58, SBBG04. [Google Scholar] [CrossRef]
- Brinkmann, M.; Hartmann, L.; Biniek, L.; Tremel, K.; Kayunkid, N. Orienting Semi-Conducting π-Conjugated Polymers. Macromol. Rapid Commun. 2014, 35, 9–26. [Google Scholar] [CrossRef]
- Nagamatsu, S.; Takashima, W.; Kaneto, K.; Yoshida, Y.; Tanigaki, N.; Yase, K.; Omote, K. Backbone Arrangement in “Friction-Transferred” Regioregular Poly(3-alkylthiophene)s. Macromolecules 2003, 36, 5252–5257. [Google Scholar] [CrossRef]
- Richard, M.; Ajaji, A.A.; Ren, S.; Foti, A.; Tran, J.; Frigoli, M.; Gusarv, B.; Bonnassieux, Y.; Caurel, E.G.; Bulkin, P.; et al. Yassar: Large-scale patterning of π-conjugated materials by meniscus guided coating methods. Adv. Colloid. Interface 2020, 275, 102080. [Google Scholar] [CrossRef]
- Salleo, A.; Kline, R.J.; DeLongchamp, D.M.; Chabinyc, M.L. Microstructural Characterization and Charge Transport in Thin Films of Conjugated Polymers. Adv. Mater. 2010, 22, 3812–3838. [Google Scholar] [CrossRef]
- Noriega, R.; Rivnay, J.; Vandewal, K.; Koch, F.P.V.; Stingelin, N.; Smith, P.; Toney, M.F.; Salleo, A. A general relationship between disorder, aggregation and charge transport in conjugated polymers. Nat. Mater. 2013, 12, 1038–1044. [Google Scholar] [CrossRef]
- Puetz, J.; Aegerter, M.A.; Technique, D.C. Sol-Gel Technologies for Glass Producers and Users; Springer: Boston, MA, USA, 2004; pp. 37–48. [Google Scholar] [CrossRef]
- Rai, H.; Gaurav, K.V.; Pradhan, S.; Desu, M.; Sharma, S.; Nagamatsu, S.; Pandey, S.S. Vertical Distribution of Molecular Orientation and Its Implication on Charge Transport in Floating Films of Conjugated Polymers. Phys. Status Solidi 2023, 220, 2300236. [Google Scholar] [CrossRef]
- Pandey, M.; Kumari, N.; Nagamatsu, S.; Pandey, S.S. Recent advances in the orientation of conjugated polymers for organic field-effect transistors. J. Mater. Chem. C Mater. 2019, 7, 13323–13351. [Google Scholar] [CrossRef]
- Pandey, M.; Nagamatsu, S.; Pandey, S.S.; Hayase, S.; Takashima, W. Orientation Characteristics of Non-regiocontrolled Poly (3-hexyl-thiophene) Film by FTM on Various Liquid Substrates. J. Phys. Conf. Ser. 2016, 704, 012005. [Google Scholar] [CrossRef]
- Förch, R.; Schönherr, H.; Jenkins, A.T.A. (Eds.) Appendix C: Contact Angle Goniometry. In Surface Design: Applications in Bioscience and Nanotechnology; Wiley: Hoboken, NJ, USA, 2009; pp. 471–473. [Google Scholar] [CrossRef]
- Zan, H.-W.; Chou, C.-W. Effect of Surface Energy on Pentacene Thin-Film Growth and Organic Thin Film Transistor Characteristics. Jpn. J. Appl. Phys. 2009, 48, 031501. [Google Scholar] [CrossRef]
- Lim, S.C.; Kim, S.H.; Lee, J.H.; Kim, M.K.; Kim, D.J.; Zyung, T. Surface-treatment effects on organic thin-film transistors. Synth. Met. 2005, 148, 75–79. [Google Scholar] [CrossRef]
- Griffin, J.; Spooner, E.; Hassan, H. Spin Coating: Complete Guide to Theory and Techniques, Ossila (n.d.). Available online: https://www.ossila.com/pages/spin-coating#:~:text=As%20mentioned%20above%2C%20spin%20coating,at%20the%20expense%20of%20consistency (accessed on 20 February 2024).
- Förch, R.; Schönherr, H.; Jenkins, A.T.A. (Eds.) Surface Design: Applications in Bioscience and Nanotechnology; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Spano, F.C. Modeling disorder in polymer aggregates: The optical spectroscopy of regioregular poly(3-hexylthiophene) thin films. J. Chem. Phys. 2005, 122, 234701, Erratum in J. Chem. Phys. 2007, 126, 159901. [Google Scholar] [CrossRef]
- Spano, F.C.; Silva, C. H- and J-Aggregate Behavior in Polymeric Semiconductors. Annu. Rev. Phys. Chem. 2014, 65, 477–500. [Google Scholar] [CrossRef] [PubMed]
- Baghgar, M.; Labastide, J.A.; Bokel, F.; Hayward, R.C.; Barnes, M.D. Effect of Polymer Chain Folding on the Transition from H- to J-Aggregate Behavior in P3HT Nanofibers. J. Phys. Chem. C 2014, 118, 2229–2235. [Google Scholar] [CrossRef]
- Clark, J.; Chang, J.-F.; Spano, F.C.; Friend, R.H.; Silva, C. Determining exciton bandwidth and film microstructure in polythiophene films using linear absorption spectroscopy. Appl. Phys. Lett. 2009, 94, 163306. [Google Scholar] [CrossRef]
- Wang, G.; Hirasa, T.; Moses, D.; Heeger, A.J. Fabrication of regioregular poly(3-hexylthiophene) field-effect transistors by dip-coating. Synth. Met. 2004, 146, 127–132. [Google Scholar] [CrossRef]
- Sharma, S.; Vats, A.K.; Tang, L.; Kaishan, F.; Toyoda, J.; Nagamatsu, S.; Ando, Y.; Tamagawa, M.; Tanaka, H.; Pandey, M.; et al. High field-effect mobility in oriented thin films of D-A type semiconducting polymers by engineering stable interfacial system. Chem. Eng. J. 2023, 469, 143932. [Google Scholar] [CrossRef]
- Pandey, M.; Sugita, Y.; Toyoda, J.; Katao, S.; Abe, R.; Cho, Y.; Benten, H.; Nakamura, M. Unidirectionally Aligned Donor–Acceptor Semiconducting Polymers in Floating Films for High-Performance Unipolar n -Channel Organic Transistors. Adv. Electron. Mater. 2023, 9, 2201043. [Google Scholar] [CrossRef]
- Goh, R.G.S.; Waclawik, E.R.; Motta, N.; Bell, J.M. Influence of Dispersed Carbon Nanotubes on the Optical and Structural Properties of a Conjugated Polymer; Chiao, J.-C., Dzurak, A.S., Jagadish, C., Thiel, D.V., Eds.; SPIE: Bellingham, WA, USA, 2005; p. 60370Z. [Google Scholar] [CrossRef]
- Sugiyama, K.; Kojima, T.; Fukuda, H.; Yashiro, H.; Matsuura, T.; Shimoyama, Y. ESR and X-ray diffraction studies on thin films of poly-3-hexylthiophene: Molecular orientation and magnetic interactions. Thin Solid. Films 2008, 516, 2691–2694. [Google Scholar] [CrossRef]
- Yang, H.; LeFevre, S.W.; Ryu, C.Y.; Bao, Z. Solubility-driven thin film structures of regioregular poly(3-hexyl thiophene) using volatile solvents. Appl. Phys. Lett. 2007, 90, 172116. [Google Scholar] [CrossRef]
- DeLongchamp, D.M.; Vogel, B.M.; Jung, Y.; Gurau, M.C.; Richter, C.A.; Kirillov, O.A.; Obrzut, J.; Fischer, D.A.; Sambasivan, S.; Richter, L.J.; et al. Variations in Semiconducting Polymer Microstructure and Hole Mobility with Spin-Coating Speed. Chem. Mater. 2005, 17, 5610–5612. [Google Scholar] [CrossRef]
- Sharma, S.; Vats, A.K.; Pandey, M.; Nagamatsu, S.; Chen, J.-C.; Pandey, S.S. Unraveling the Implications of Macromolecular Orientation on the Planar and Vertical Charge Transport in Organic Electronic Devices. ACS Appl. Polym. Mater. 2024, 4, 83158323. [Google Scholar] [CrossRef]
- Xue, L.; Gao, X.; Zhao, K.; Liu, J.; Yu, X.; Han, Y. The formation of different structures of poly(3-hexylthiophene) film on a patterned substrate by dip coating from aged solution. Nanotechnology 2010, 21, 145303. [Google Scholar] [CrossRef]

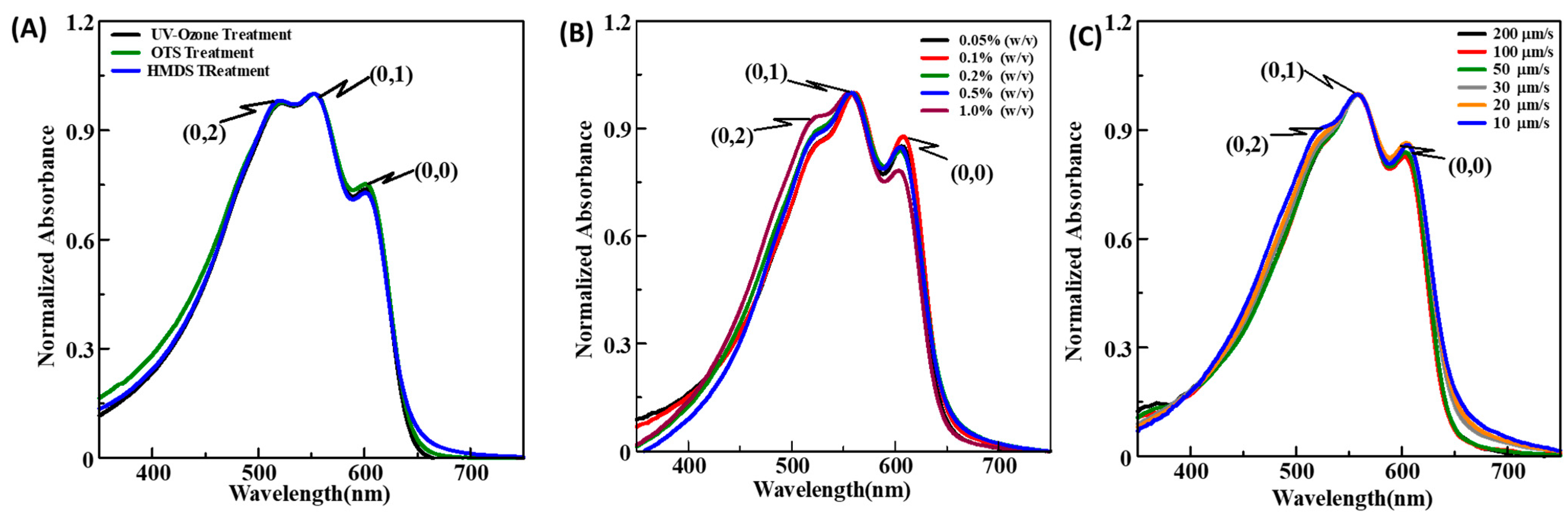
| CP Conc. (w/v) | A0-0 | A0-1 | A0-2 | A0-0/A0-1 | A0-1/A0-2 | Exciton Bandwidth (meV) |
|---|---|---|---|---|---|---|
| 0.1% | 0.033 | 0.053 | 0.050 | 0.624 | 0.655 | 127 |
| 0.2% | 0.060 | 0.097 | 0.094 | 0.620 | 0.636 | 128 |
| 0.5% | 0.129 | 0.200 | 0.195 | 0.647 | 0.665 | 118 |
| 0.8% | 0.192 | 0.288 | 0.284 | 0.667 | 0.676 | 110 |
| 1.0% | 0.217 | 0.322 | 0.318 | 0.674 | 0.682 | 108 |
| CP Conc. (w/v) | A0-0 | A0-1 | A0-2 | A0-0/A0-1 | A0-1/A0-2 | Exciton Bandwidth (meV) |
|---|---|---|---|---|---|---|
| 0.05% | 0.089 | 0.105 | 0.089 | 0.850 | 0.999 | 45 |
| 0.1% | 0.166 | 0.189 | 0.161 | 0.878 | 1.035 | 36 |
| 0.2% | 0.231 | 0.276 | 0.245 | 0.837 | 0.943 | 49 |
| 0.5% | 0.356 | 0.420 | 0.369 | 0.846 | 0.964 | 46 |
| 1.0% | 0.453 | 0.579 | 0.538 | 0.782 | 0.841 | 68 |
| Polymer Conc. (w/v) | UV-Ozone | HMDS | OTS | CYTOP | Exciton Bandwidth (meV) |
|---|---|---|---|---|---|
| 1.5% | 1.8 | ||||
| 2.0% | 2.5 | ||||
| 3.0% | 3.1 | ||||
| 4.0% | 4.2 | 4.1 | 4.0 | 4.3 | 102 |
| 5.0% | 3.5 | ||||
| 6.0% | 2.3 | ||||
| 7.0% | 2.1 |
| Fabrication Techniques | Concentration (w/v) | Thickness (nm) | Carrier Mobility (cm2V−1s−1) | On-Off Ratio |
|---|---|---|---|---|
| Spin coating | 0.5% | 34 | 8.0 × 10−4 | 3.6 × 102 |
| Dip coating | 0.1% | 29 | 1.3 × 10−3 | 4.9 × 103 |
| UFTM | 4.0% | 23 | 7.0 × 10−2 | 4.5 × 104 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaurav, K.V.; Rai, H.; Singh, K.R.; Sharma, S.; Ando, Y.; Pandey, S.S. Clarifying the Dominant Role of Crystallinity and Molecular Orientation in Differently Processed Thin Films of Regioregular Poly(3-hexylthiophene). Micromachines 2024, 15, 677. https://doi.org/10.3390/mi15060677
Gaurav KV, Rai H, Singh KR, Sharma S, Ando Y, Pandey SS. Clarifying the Dominant Role of Crystallinity and Molecular Orientation in Differently Processed Thin Films of Regioregular Poly(3-hexylthiophene). Micromachines. 2024; 15(6):677. https://doi.org/10.3390/mi15060677
Chicago/Turabian StyleGaurav, Kumar Vivek, Harshita Rai, Kshitij RB Singh, Shubham Sharma, Yoshito Ando, and Shyam S. Pandey. 2024. "Clarifying the Dominant Role of Crystallinity and Molecular Orientation in Differently Processed Thin Films of Regioregular Poly(3-hexylthiophene)" Micromachines 15, no. 6: 677. https://doi.org/10.3390/mi15060677
APA StyleGaurav, K. V., Rai, H., Singh, K. R., Sharma, S., Ando, Y., & Pandey, S. S. (2024). Clarifying the Dominant Role of Crystallinity and Molecular Orientation in Differently Processed Thin Films of Regioregular Poly(3-hexylthiophene). Micromachines, 15(6), 677. https://doi.org/10.3390/mi15060677








