Ti3AlC2 MAX Phase Modified Screen-Printed Electrode for the Fabrication of Hydrazine Sensor
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Apparatus
2.3. Fabrication of Sensor
3. Results and Discussion
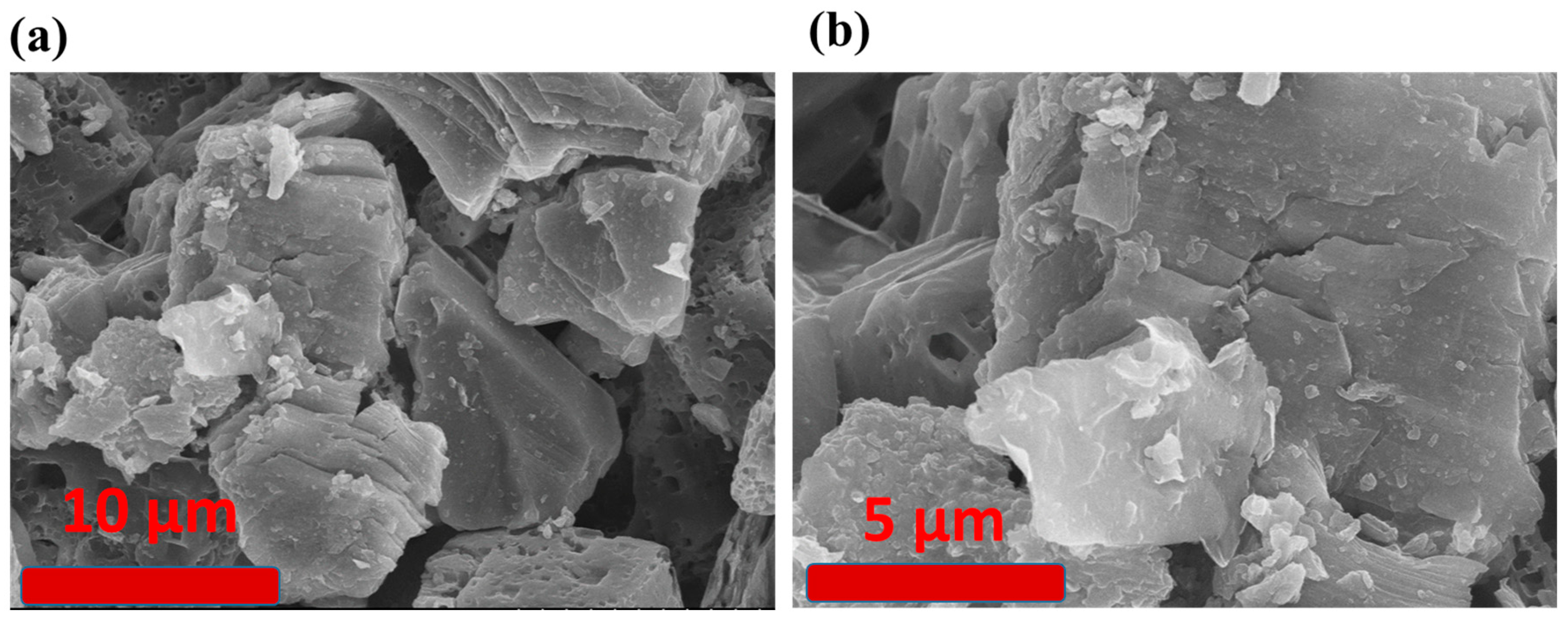
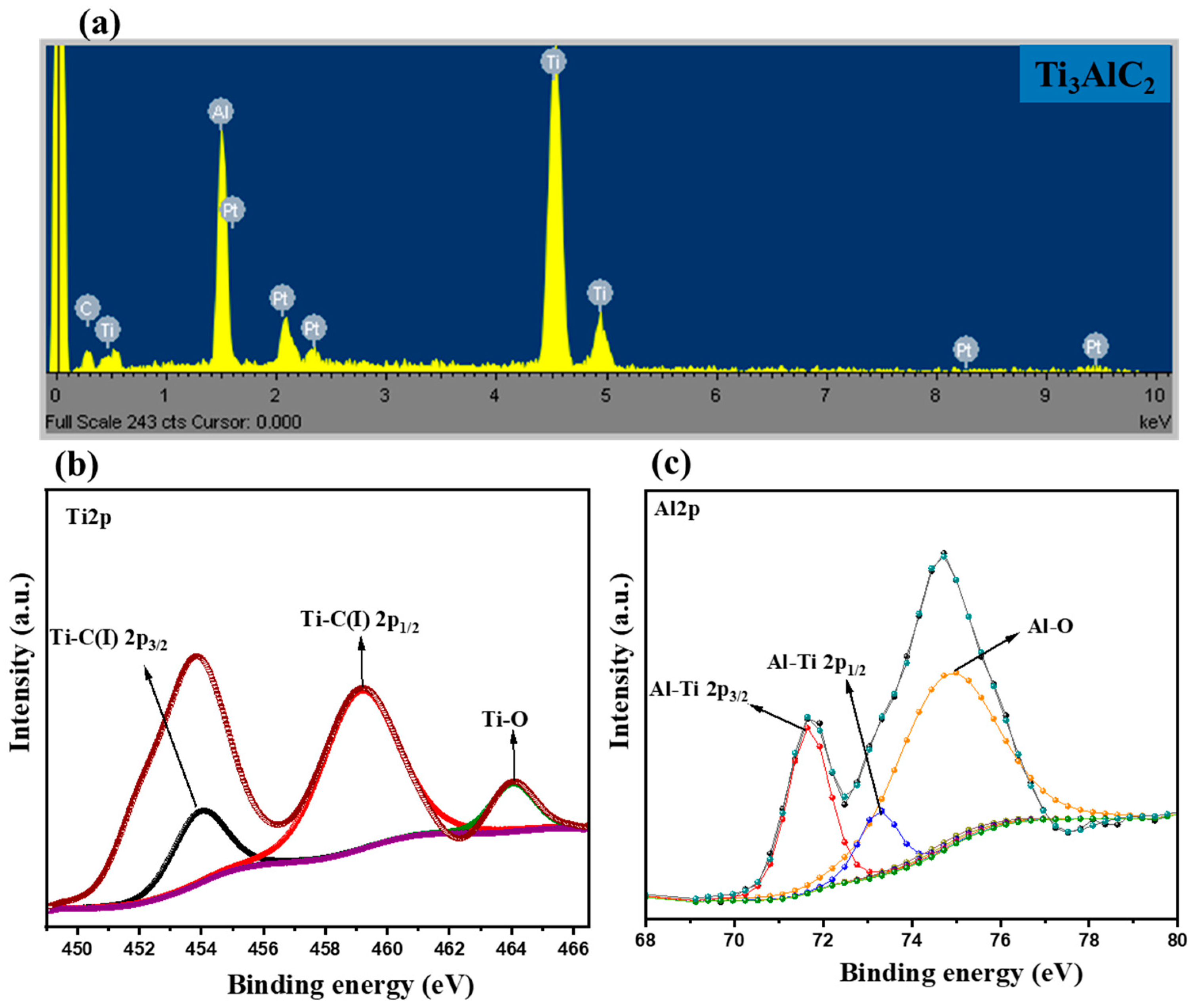
3.1. Materials Characterization
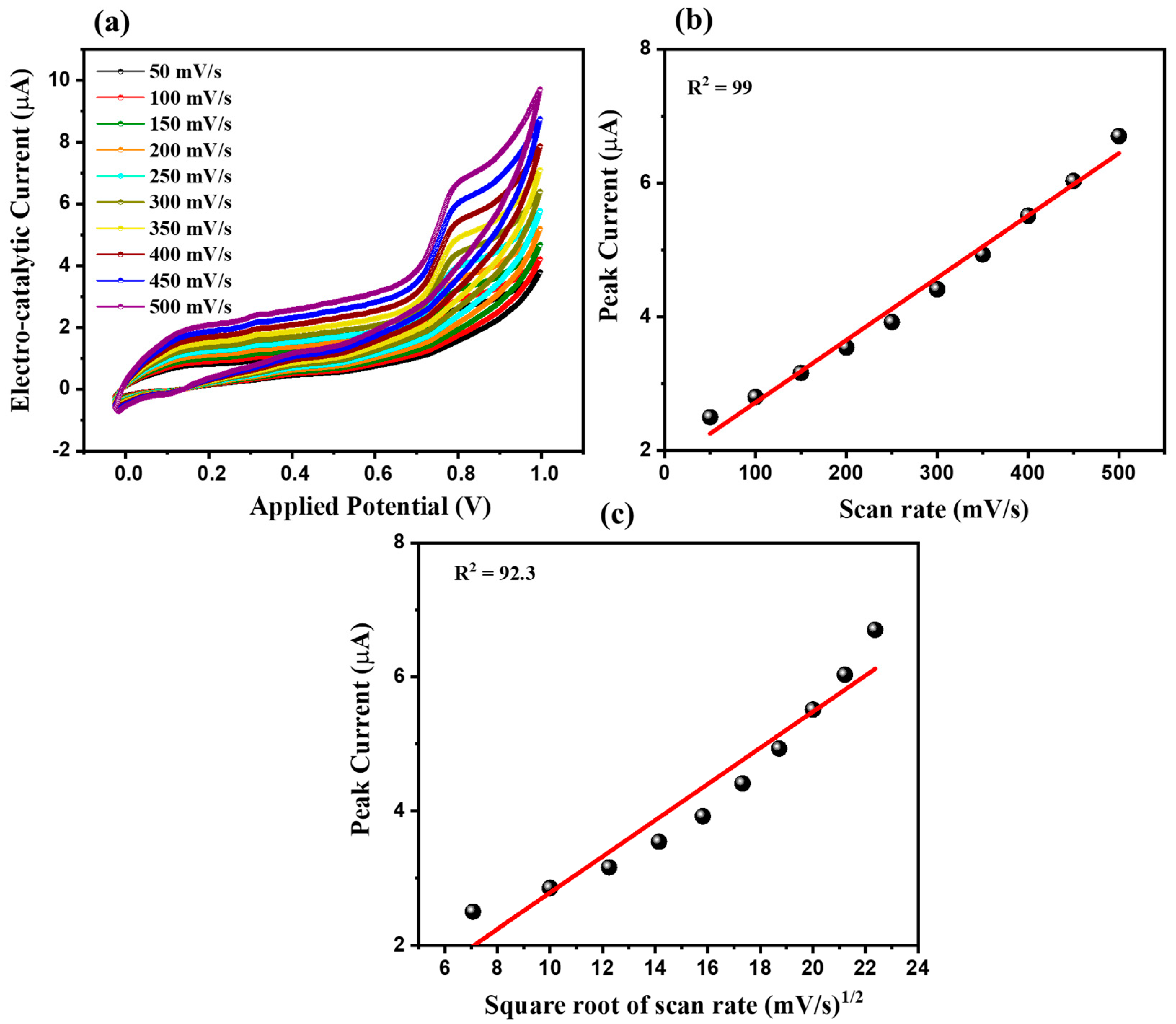
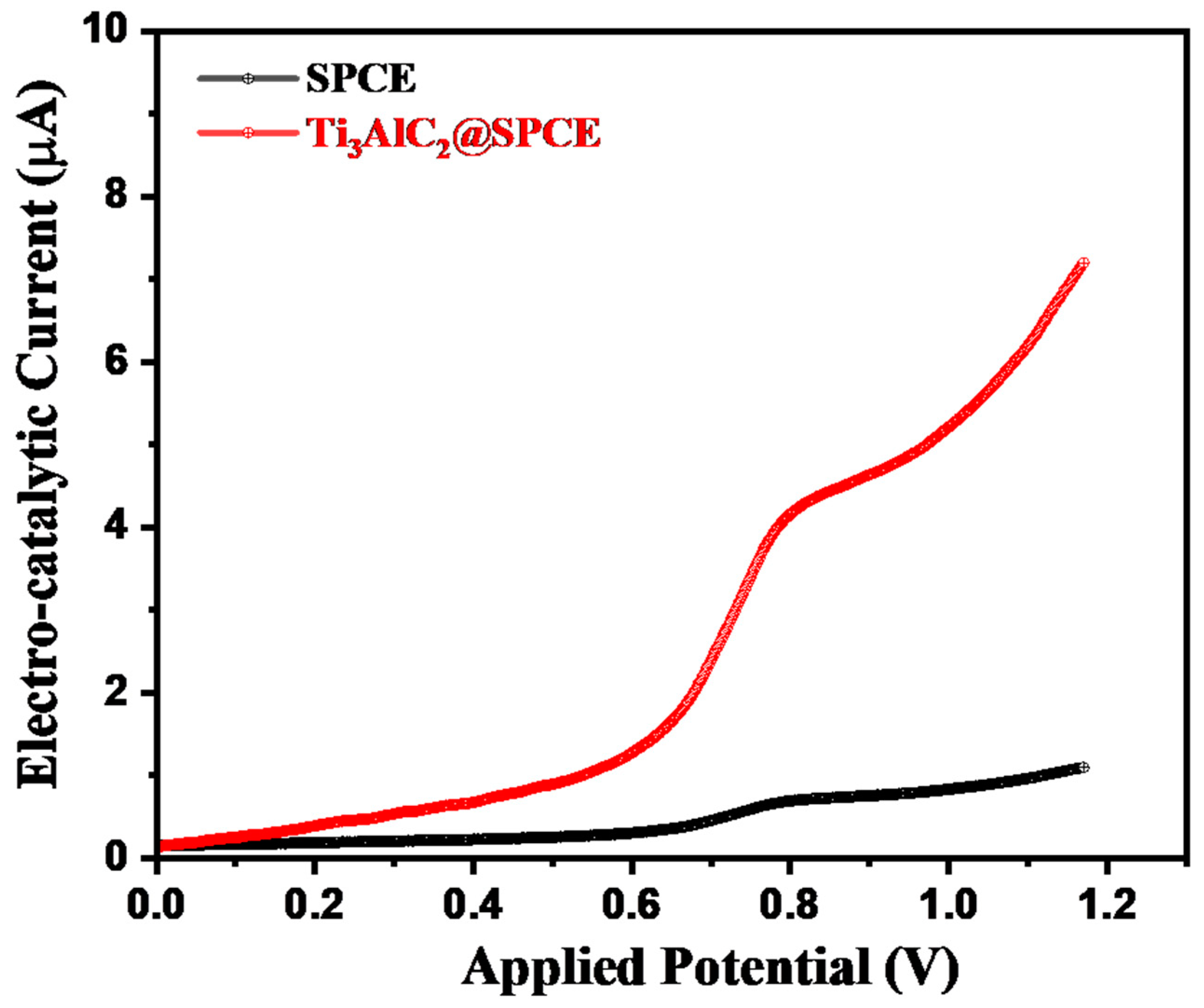
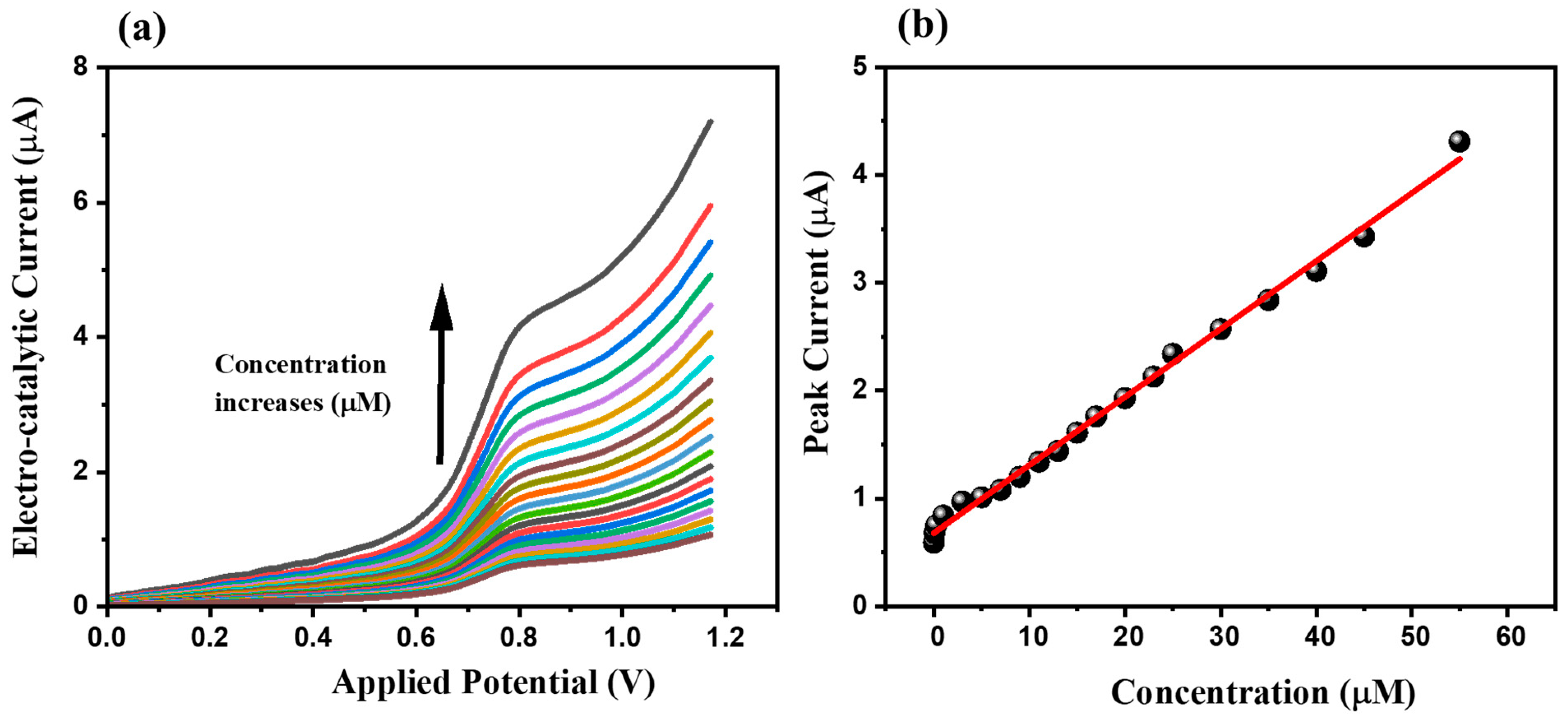
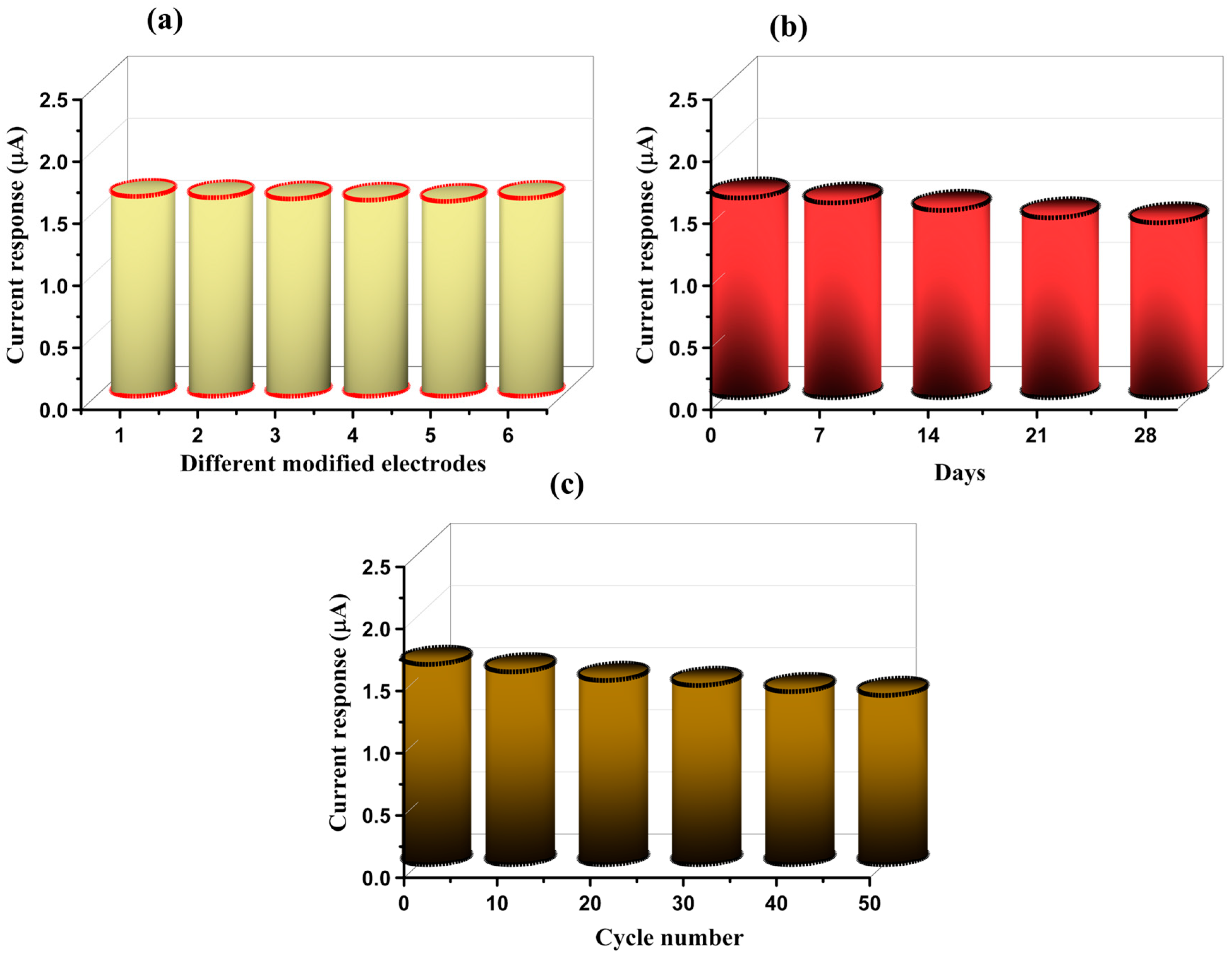
3.2. Electrochemical Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azad, U.P.; Ganesan, V. Determination of Hydrazine by PolyNi(II) Complex Modified Electrodes with a Wide Linear Calibration Range. Electrochim. Acta 2011, 56, 5766–5770. [Google Scholar] [CrossRef]
- Zelnick, S.D.; Mattie, D.R.; Stepaniak, P.C. Occupational Exposure to Hydrazines: Treatment of Acute Central Nervous System Toxicity. Aviat. Space Environ. Med. 2003, 74, 1285–1291. [Google Scholar]
- Casella, I.G.; Guascito, M.R.; Salvi, A.M.; Desimoni, E. Catalytic Oxidation and Flow Detection of Hydrazine Compounds at a Nafion/Ruthenium(III) Chemically Modified Electrode. Anal. Chim. Acta 1997, 354, 333–341. [Google Scholar] [CrossRef]
- Yamazaki, S.I.; Ioroi, T.; Tanimoto, K.; Yasuda, K.; Asazawa, K.; Yamaguchi, S.; Tanaka, H. Electrochemical Oxidation of Hydrazine Derivatives by Carbon-Supported Metalloporphyrins. J. Power Sources 2012, 204, 79–84. [Google Scholar] [CrossRef]
- Li, Z.; Cao, Z.; Grande, C.; Zhang, W.; Dou, Y.; Li, X.; Fu, J.; Shezad, N.; Akhtar, F.; Kaiser, A. A Phase Conversion Method to Anchor ZIF-8 onto a PAN Nanofiber Surface for CO2 capture. RSC Adv. 2021, 12, 664–670. [Google Scholar] [CrossRef]
- Serov, A.; Kwak, C. Direct Hydrazine Fuel Cells: A Review. Appl. Catal. B Environ. 2010, 98, 1–9. [Google Scholar] [CrossRef]
- Ismail, A.A.; Harraz, F.A.; Faisal, M.; El-Toni, A.M.; Al-Hajry, A.; Al-Assiri, M.S. A Sensitive and Selective Amperometric Hydrazine Sensor Based on Mesoporous Au/ZnO Nanocomposites. Mater. Des. 2016, 109, 530–538. [Google Scholar] [CrossRef]
- Kimball, R.F. The Mutagenicity of Hydrazine and Some of Its Derivatives. Mutat. Res. Genet. Toxicol. 1977, 39, 111–126. [Google Scholar] [CrossRef]
- Rahman, M.M.; Balkhoyor, H.B.; Asiri, A.M. Ultrasensitive and Selective Hydrazine Sensor Development Based on Sn/ZnO Nanoparticles. RSC Adv. 2016, 6, 29342–29352. [Google Scholar] [CrossRef]
- Ahmad, R.; Tripathy, N.; Ahn, M.S.; Hahn, Y.B. Highly Stable Hydrazine Chemical Sensor Based on Vertically-Aligned ZnO Nanorods Grown on Electrode. J. Colloid Interface Sci. 2017, 494, 153–158. [Google Scholar] [CrossRef]
- VERNOT, E. Long-Term Inhalation Toxicity of Hydrazine*1. Fundam. Appl. Toxicol. 1985, 5, 1050–1064. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Ahmed, J.; Asiri, A.M.; Siddiquey, I.A.; Hasnat, M.A. Development of Highly-Sensitive Hydrazine Sensor Based on Facile CoS2-CNT Nanocomposites. RSC Adv. 2016, 6, 90470–90479. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, W.; Dou, M.; Wang, F.; Liu, J.; Li, Z.; Ji, J. Nanorod-Constructed Porous Co3O4 Nanowires: Highly Sensitive Sensors for the Detection of Hydrazine. Analyst 2015, 140, 1686–1692. [Google Scholar] [CrossRef] [PubMed]
- Gholivand, M.B.; Azadbakht, A. A Novel Hydrazine Electrochemical Sensor Based on a Zirconium Hexacyanoferrate Film-Bimetallic Au-Pt Inorganic-Organic Hybrid Nanocomposite onto Glassy Carbon-Modified Electrode. Electrochim. Acta 2011, 56, 10044–10054. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, L.; Wang, T.; Han, Q.; Xu, S. A High-Performance Hydrazine Electrochemical Sensor Based on Gold Nanoparticles/Single-Walled Carbon Nanohorns Composite Film. Appl. Surf. Sci. 2016, 369, 36–42. [Google Scholar] [CrossRef]
- Harraz, F.A.; Ismail, A.A.; Al-Sayari, S.A.; Al-Hajry, A.; Al-Assiri, M.S. Highly Sensitive Amperometric Hydrazine Sensor Based on Novel α-Fe2O3/Crosslinked Polyaniline Nanocomposite Modified Glassy Carbon Electrode. Sens. Actuators B Chem. 2016, 234, 573–582. [Google Scholar] [CrossRef]
- Ahmad, K.; Khan, M.Q.; Alsalme, A.; Kim, H. Sulfur-Doped Graphitic-Carbon Nitride (S@g-C3N4) as Bi-Functional Catalysts for Hydrazine Sensing and Hydrogen Production Applications. Synth. Met. 2022, 288, 117100. [Google Scholar] [CrossRef]
- Sajid, M. MXenes: Are They Emerging Materials for Analytical Chemistry Applications?—A Review. Anal. Chim. Acta 2021, 1143, 267–280. [Google Scholar] [CrossRef]
- Raza, W.; Ahmad, K. Room Temperature Gas Sensor Based on Reduce Graphene Oxide for Environmental Monitoring. In Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 1–19. [Google Scholar]
- Mohammad, A.; Khan, M.E.; Cho, M.H. Sulfur-Doped-Graphitic-Carbon Nitride (S-g-C3N4) for Low Cost Electrochemical Sensing of Hydrazine. J. Alloys Compd. 2020, 816, 152522. [Google Scholar] [CrossRef]
- Mohammad, A.; Ahmad, K.; Qureshi, A.; Tauqeer, M.; Mobin, S.M. Zinc Oxide-Graphitic Carbon Nitride Nanohybrid as an Efficient Electrochemical Sensor and Photocatalyst. Sens. Actuators B Chem. 2018, 277, 467–476. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Bai, J.; Jiang, X.; Fan, G. High sensitivity hydrogen peroxideand hydrazine sensor based on silver nanocubes with rich {1 0 0} facets as anenhanced electrochemical sensing platform. Biosens. Bioelectron. 2013, 43, 180–185. [Google Scholar] [CrossRef]
- Rahman, M.M.; Alam, M.M.; Asiri, A.M. Selective Hydrazine Sensor Fabrication with Facile Low-Dimensional Fe2O3/CeO2 Nanocubes. New J. Chem. 2018, 42, 10263–10270. [Google Scholar] [CrossRef]
- Mohammad, A.; Khan, M.E.; Yoon, T.; Hwan Cho, M. Na,O-Co-Doped-Graphitic-Carbon Nitride (Na,O-g-C3N4) for Nonenzymatic Electrochemical Sensing of Hydrogen Peroxide. Appl. Surf. Sci. 2020, 525, 146353. [Google Scholar] [CrossRef]
- Mohammad, A.; Khan, M.E.; Karim, M.R.; Cho, M.H.; Yoon, T. Ag-Modified SnO2-Graphitic-Carbon Nitride Nanostructures for Electrochemical Sensor Applications. Ceram. Int. 2021, 47, 23578–23589. [Google Scholar] [CrossRef]
- Gowthaman, N.S.K.; Ngee Lim, H.; Balakumar, V.; Shankar, S. Ultrasonic Synthesis of CeO2@organic Dye Nanohybrid: Environmentally Benign Rabid Electrochemical Sensing Platform for Carcinogenic Pollutant in Water Samples. Ultrason. Sonochem. 2020, 61, 104828. [Google Scholar] [CrossRef]
- Shukla, S.; Chaudhary, S.; Umar, A.; Chaudhary, G.R.; Mehta, S.K. Tungsten Oxide (WO3) Nanoparticles as Scaffold for the Fabrication of Hydrazine Chemical Sensor. Sens. Actuators B Chem. 2014, 196, 231–237. [Google Scholar] [CrossRef]
- Kim, S.P.; Choi, H.C. Reusable Hydrazine Amperometric Sensor Based on Nafion®-Coated TiO2-Carbon Nanotube Modified Electrode. Sens. Actuators B Chem. 2015, 207, 424–429. [Google Scholar] [CrossRef]
- Ahmad, R.; Tripathy, N.; Jung, D.U.J.; Hahn, Y.B. Highly Sensitive Hydrazine Chemical Sensor Based on ZnO Nanorods Field-Effect Transistor. Chem. Commun. 2014, 50, 1890–1893. [Google Scholar] [CrossRef]
- Alahmadi, N.; Alhasan, H.S.; Gomaa, H.; Abdelwahab, A.A.; Emran, M.Y. Electrochemical sensor design based on CuO nanosheets/ Cellulose derivative nanocomposite for hydrazine monitoring in environmental samples. Microchem. J. 2022, 183, 107909. [Google Scholar] [CrossRef]
- Ali, A.M.; Qreshah, O.; Ismail, A.A.; Harraz, F.A.; Algarni, H.; Assiri, M.A.; Faisal, M.; Chiu, W.S. Morphological and Optical Properties of SnO2 Doped ZnO Nanocomposites for Electrochemical Sensing of Hydrazine. Int. J. Electrochem. Sci. 2019, 14, 1461–1478. [Google Scholar] [CrossRef]
- Raza, W.; Ahmad, K. A Highly Selective Fe@ZnO Modified Disposable Screen Printed Electrode Based Non-Enzymatic Glucose Sensor (SPE/Fe@ZnO). Mater. Lett. 2018, 212, 231–234. [Google Scholar] [CrossRef]
- Ahmad, K.; Raza, W.; Alsulmi, A.; Kim, H. Fabrication of Electrochemical Sensor for Metronidazole Using MoS2/Graphite-like Carbon Nitride Composite Modified Glassy Carbon Electrode. Diam. Relat. Mater. 2023, 138, 110178. [Google Scholar] [CrossRef]
- Kalambate, P.K.; Gadhari, N.S.; Li, X.; Rao, Z.; Navale, S.T.; Shen, Y.; Patil, V.R.; Huang, Y. Recent Advances in MXene–Based Electrochemical Sensors and Biosensors. TrAC Trends Anal. Chem. 2019, 120, 115643. [Google Scholar] [CrossRef]
- Huang, H.; Jiang, R.; Feng, Y.; Ouyang, H.; Zhou, N.; Zhang, X.; Wei, Y. Recent Development and Prospects of Surface Modification and Biomedical Applications of MXenes. Nanoscale 2020, 12, 1325–1338. [Google Scholar] [CrossRef]
- Wang, M.-F.; Li, W.; Hu, P.-J.; He, S.-S.; Yang, H.-M.; Li, X.-Z. A Facile Hydrazine Amperometric Sensor Based on a Glassy Carbon Electrode Modified with Au Nanoparticles-MnO2 Composites. Int. J. Electrochem. Sci. 2016, 11, 1928–1937. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Wu, X.; Ma, P.; Sun, Y.; Du, F.; Song, D.; Xu, G. Application of MXene in Electrochemical Sensors: A Review. Electroanalysis 2021, 33, 1827–1851. [Google Scholar] [CrossRef]
- Yang, M.; Lu, H.; Liu, S. Recent Advances of MXene-Based Electrochemical Immunosensors. Appl. Sci. 2022, 12, 5630. [Google Scholar] [CrossRef]
- Tran, V.A.; Tran, N.T.; Doan, V.D.; Nguyen, T.Q.; Thi, H.H.P.; Vo, G.N.L. Application Prospects of MXenes Materials Modifications for Sensors. Micromachines 2023, 14, 247. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Li, L.; Zhang, Y.; Zhang, X.; Geng, D.; Hu, W. Recent Advances in Growth of Transition Metal Carbides and Nitrides (MXenes) Crystals. Adv. Funct. Mater. 2022, 32, 2111357. [Google Scholar] [CrossRef]
- Bansal, P.; Bhanjana, G.; Prabhakar, N.; Dhau, J.S.; Chaudhary, G.R. Electrochemical sensor based on ZrO2 NPs/Au electrode sensing layer for monitoring hydrazine and catechol in real water samples. J. Mol. Liq. 2017, 248, 651–657. [Google Scholar] [CrossRef]
- Kumar, J.A.; Prakash, P.; Krithiga, T.; Amarnath, D.J.; Premkumar, J.; Rajamohan, N.; Vasseghian, Y.; Saravanan, P.; Rajasimman, M. Methods of Synthesis, Characteristics, and Environmental Applications of MXene: A Comprehensive Review. Chemosphere 2022, 286, 131607. [Google Scholar] [CrossRef] [PubMed]
- Soundiraraju, B.; Anthony, A.M.; Pandurangan, P.; Kattikkanal George, B. Electrochemical behavior of chromium carbide MXene modified electrodes: Hydrazine sensing. Mater. Today Commun. 2022, 32, 103982. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for Synthesis and Processing of Two-Dimensional Titanium Carbide (Ti3C2Tx MXene. Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Ares, P.; Novoselov, K.S. Recent Advances in Graphene and Other 2D Materials. Nano Mater. Sci. 2022, 4, 3–9. [Google Scholar] [CrossRef]
- Eom, W.; Shin, H.; Ambade, R.B.; Lee, S.H.; Lee, K.H.; Kang, D.J.; Han, T.H. Large-Scale Wet-Spinning of Highly Electroconductive MXene Fibers. Nat. Commun. 2020, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lukatskaya, M.R.; Mashtalir, O.; Ren, C.E.; Dall’Agnese, Y.; Rozier, P.; Taberna, P.L.; Naguib, M.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. Cation Intercalation and High Volumetric Capacitance of Two-Dimensional Titanium Carbide. Science 2013, 341, 1502–1505. [Google Scholar] [CrossRef] [PubMed]
- Rhouati, A.; Berkani, M.; Vasseghian, Y.; Golzadeh, N. MXene-Based Electrochemical Sensors for Detection of Environmental Pollutants: A Comprehensive Review. Chemosphere 2022, 291, 132921. [Google Scholar] [CrossRef] [PubMed]
- Osuagwu, B.; Raza, W.; Tesler, A.B.; Schmuki, P. Facile Approach of Direct Sulfidation of FTO to Form Vertically Aligned SnS2Nanoflake Photoanodes for Efficient Photoelectrochemical Water Splitting. ACS Appl. Energy Mater. 2021, 4, 8395–8400. [Google Scholar] [CrossRef]
- Raza, W.; Ahmad, K.; Khan, R.A.; Kim, H. Ag Decorated ZnO for Enhanced Photocatalytic H2 Generation and Pollutant Degradation. Int. J. Hydrogen Energy 2023, 48, 29071–29081. [Google Scholar] [CrossRef]
- Ni, Y.; Zhu, J.; Zhang, L.; Hong, J. Hierarchical ZnO micro/nanoarchitectures: Hydrothermal preparation, characterization and application in the detection of hydrazine. CrystEngComm 2010, 12, 2213–2218. [Google Scholar] [CrossRef]
- Tian, Z.; Tian, H.; Cao, K.; Bai, S.; Peng, Q.; Wang, Y.; Zhu, Q. Facile preparation of Ti3C2Tx sheets by selectively etching in a H2SO4/H2O2 mixture. Front. Chem. 2022, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Gowthaman, N.S.K.; Mohapatra, D.; Arul, P.; Chang, W.S. Ultrasonic-assisted decoration of AuNPs on carbon nano-onions as robust electrochemical scaffold for sensing of carcinogenic hydrazine in industrial effluents. J. Ind. Eng. Chem. 2023, 117, 227–237. [Google Scholar] [CrossRef]
- Gharani, M.; Bahari, A.; Ghasemi, S. Preparation of MoS2-reduced graphene oxide/Au nanohybrid for electrochemical sensing of hydrazine. J. Mater. Sci. Mater. Electron. 2021, 32, 7765–7777. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H.; Hosseinzadeh, R.; Afshar, A.A.; Varma, R.S.; Jang, H.W.; Shokouhimehr, M. Electrochemical Detection of Hydrazine by Carbon Paste Electrode Modified with Ferrocene Derivatives, Ionic Liquid, and CoS2-Carbon Nanotube Nanocomposite. ACS Omega 2021, 6, 4641–4648. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, N.; Majidi, M.R.; Asadpour-Zeynali, K. Synthesis of MoS2-QDs@Fe3O4 nanocomposites decorated on reduced-graphene-oxide: Application in sensitive electrocatalytic determination of hydrazine. Synth. Met. 2023, 296, 117361. [Google Scholar] [CrossRef]
- Rana, D.S.; Kalia, S.; Thakur, N.; Singh, R.K.; Kumar, R.; Singh, D. Synthesis of reduced graphene oxide-molybdenum disulfide nanocomposite as potential scaffold for fabrication of efficient hydrazine sensor. Mater. Chem. Phys. 2023, 294, 127048. [Google Scholar] [CrossRef]
- Ganesha, H.; Veeresh, S.; Nagaraju, Y.S.; Devendrappa, H. A CTAB-assisted PANI-MoS2 nanosheet flower morphology for the highly sensitive electrochemical detection of hydrazine. RSC Adv. 2023, 13, 34891–34903. [Google Scholar]
- Saeb, E.; Asadpour-Zeynali, K. Facile synthesis of TiO2@PANI@Au nanocomposite as an electrochemical sensor for determination of hydrazine. Microchem. J. 2021, 160, 105603. [Google Scholar] [CrossRef]




| Electrode | DL (µM) | Linear Range (µM) | Sensitivity (µA/µM.cm2) | References |
|---|---|---|---|---|
| Ti3AlC2@SPCE | 0.01 | 1–50 | 6.76 | This study |
| ZrO2 NPs/Au electrode | 1.05 | - | 8.99 | [42] |
| WO3 | 144 | - | - | [27] |
| Cr2CTx MXene | 0.66 | 1.99–200 | 1.81 | [44] |
| ZnO | 2.1 | - | - | [52] |
| Erbium-doped Nb2CTx | 67 | - | - | [44] |
| PVP-AgNCs/GCE | 1.1 | - | - | [22] |
| CuO nanosheets (CuO NSs)/Cellulose acetate butyrate (CAB) | 0.15 | - | - | [30] |
| SnO2/ZnO | 0.36 | 2.5–25 | 1.16 | [31] |
| Au NPs/MnO2 Composites | 1.7 | - | - | [36] |
| Au@ carbon nano-onions/GCE | 0.012 | 0.05–1000 | - | [54] |
| MoS2/rGO/Au | 0.5 | 2–30 | - | [55] |
| Ionic liquid/CoS2-CNT/CP | 0.015 | 0.03–500 | 0.073 | [56] |
| MoS2-QDs@Fe3O4/rGO | 0.12 | 0.8–2190 | 0.035 | [57] |
| rGO-MoS2 | 0.132 | - | 89.89 | [58] |
| PANI-MoS2 | 0.40 | 10–100 | 7.23 | [59] |
| TiO2@PANI/GCE | 0.15 | - | - | [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, K.; Raza, W.; Khan, R.A. Ti3AlC2 MAX Phase Modified Screen-Printed Electrode for the Fabrication of Hydrazine Sensor. Micromachines 2024, 15, 633. https://doi.org/10.3390/mi15050633
Ahmad K, Raza W, Khan RA. Ti3AlC2 MAX Phase Modified Screen-Printed Electrode for the Fabrication of Hydrazine Sensor. Micromachines. 2024; 15(5):633. https://doi.org/10.3390/mi15050633
Chicago/Turabian StyleAhmad, Khursheed, Waseem Raza, and Rais Ahmad Khan. 2024. "Ti3AlC2 MAX Phase Modified Screen-Printed Electrode for the Fabrication of Hydrazine Sensor" Micromachines 15, no. 5: 633. https://doi.org/10.3390/mi15050633
APA StyleAhmad, K., Raza, W., & Khan, R. A. (2024). Ti3AlC2 MAX Phase Modified Screen-Printed Electrode for the Fabrication of Hydrazine Sensor. Micromachines, 15(5), 633. https://doi.org/10.3390/mi15050633






