Designing a Simple Electrochemical Genosensor for the Detection of Urinary PCA3, a Prostate Cancer Biomarker
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instrumentation
2.3. Urine Sample Collection and Processing
2.4. Total RNA Extraction from Urine Sediments
2.5. Reverse Transcription of RNA into Complementary DNA
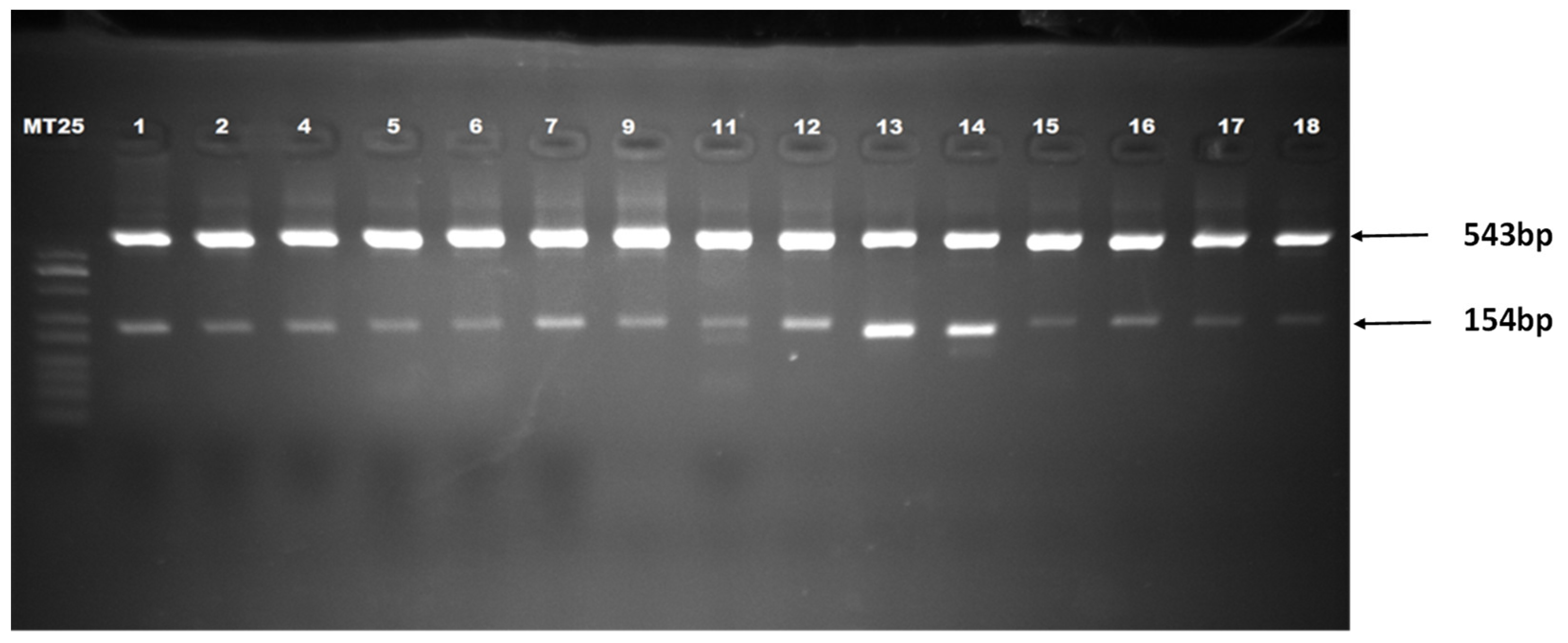
2.6. PCA3 Amplification
2.7. Genosensor Design
3. Results
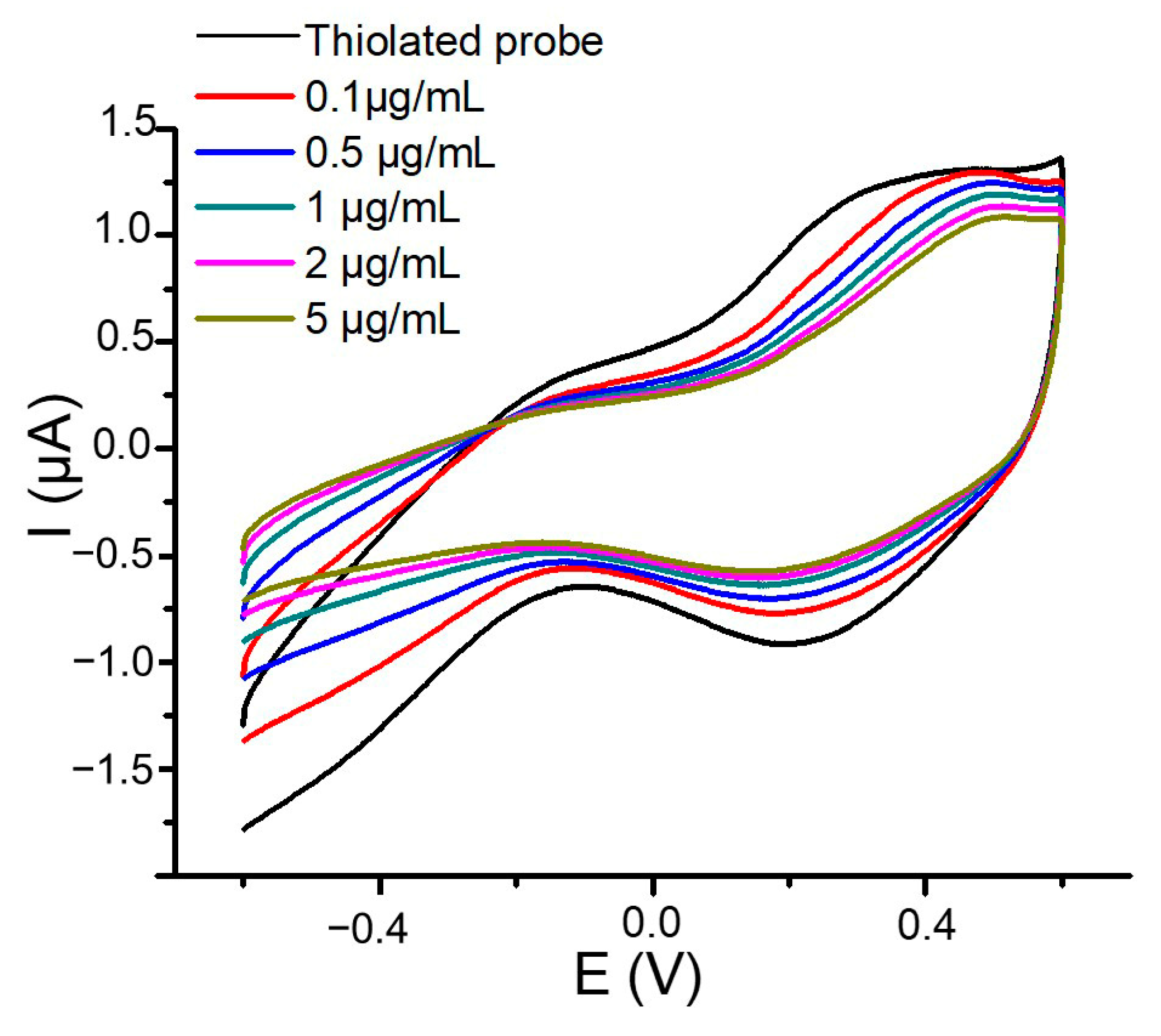
3.1. Optimization of the Electrochemical Parameters for the Genosensor’s Design
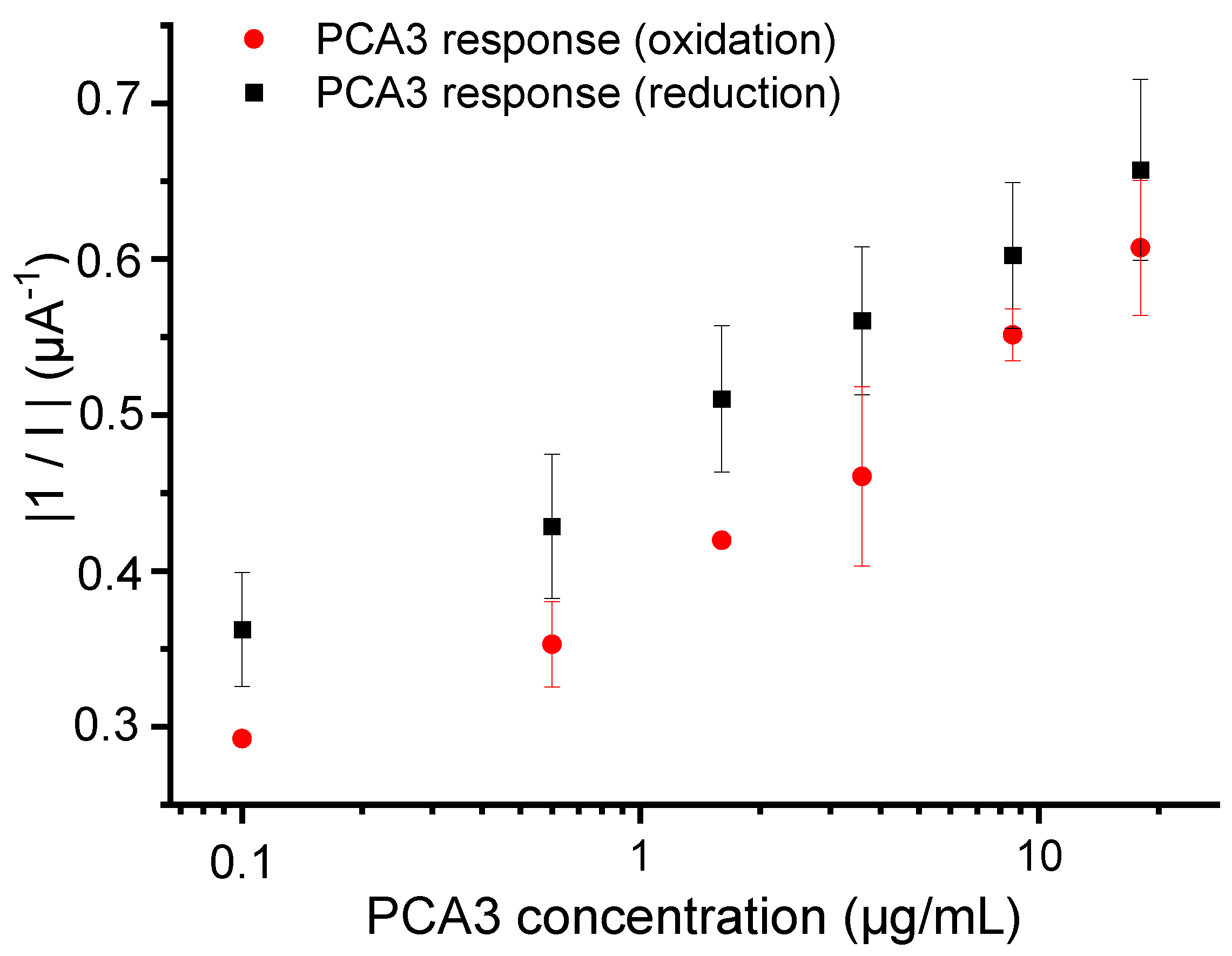
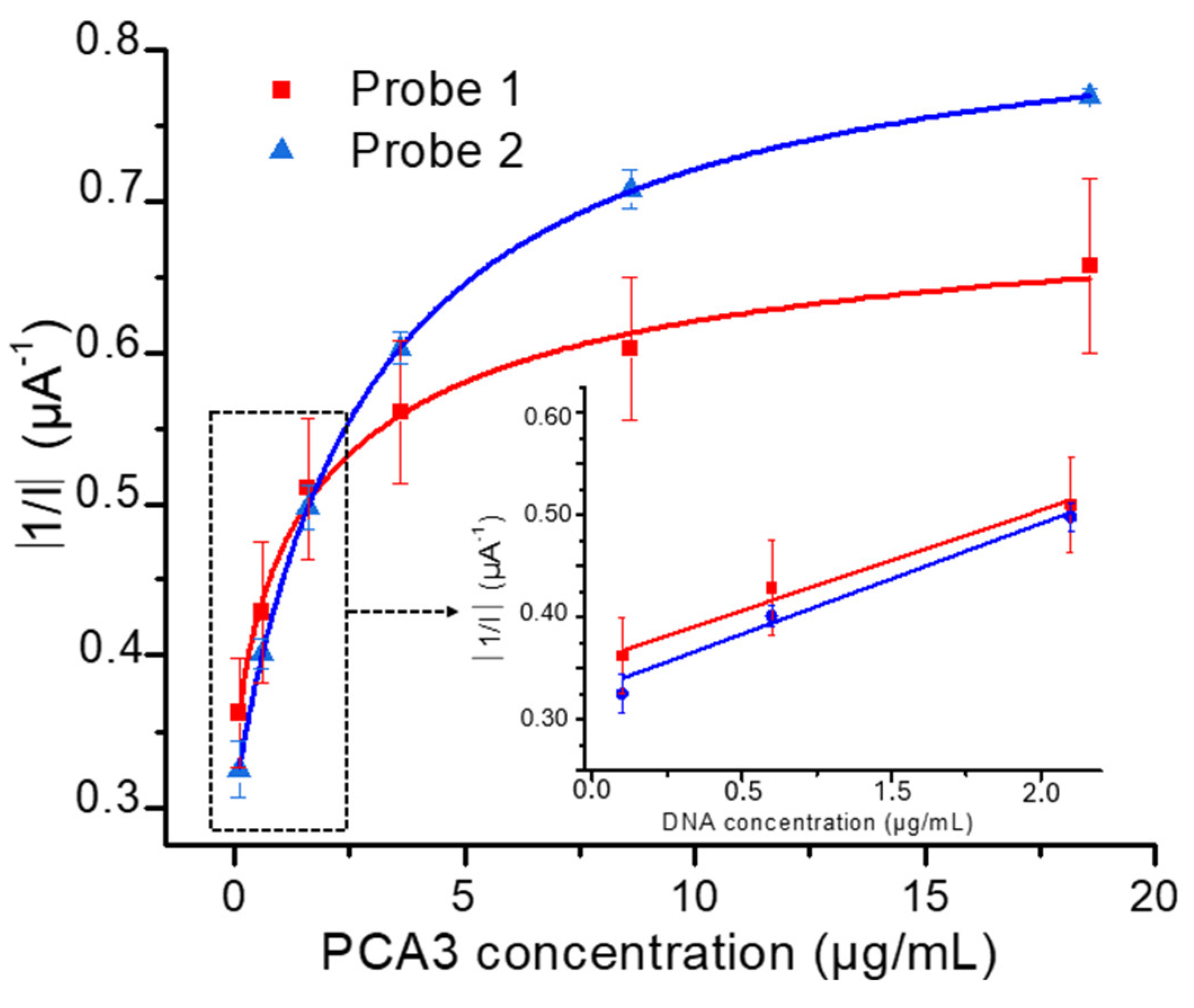
3.2. Influence of Probe’s Type on the Hybridization Process
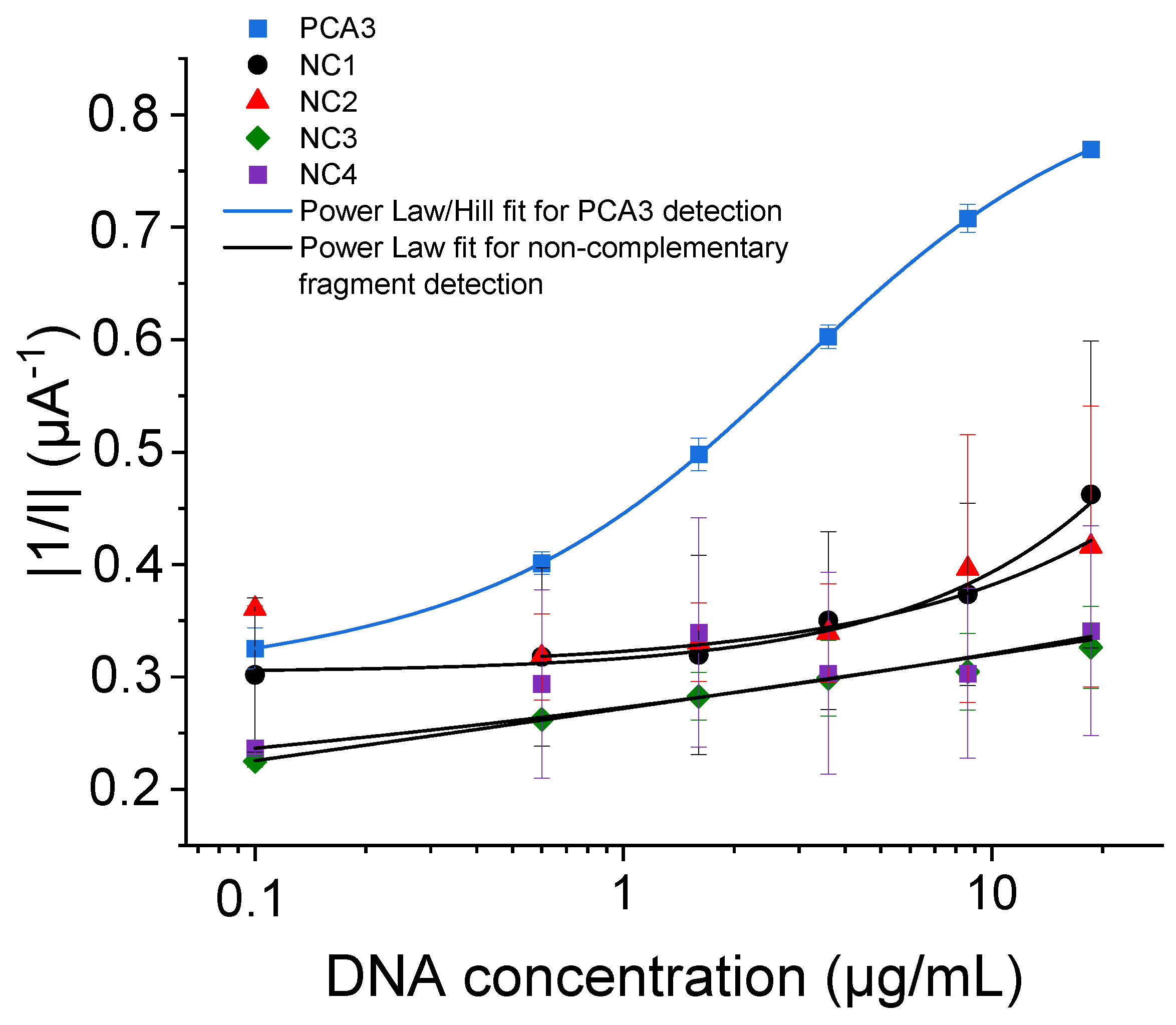
3.3. Specificity Tests
4. Outlook
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Globocan Cancer Today. Available online: https://gco.iarc.who.int/today/ (accessed on 14 March 2024).
- Jackson, S.D.; de la Rue, M.R.; Greenslade, T.P.; John, A.M.; Wahid, S.; Martin, R.M.; Williams, N.J.; Turner, E.L. Screening asymptomatic men for prostate cancer: A comparison of international guidelines on prostate-specific antigen testing. J. Med. Screen. 2022, 29, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Liang, Z.; Feng, T.; Mai, Z.; Jin, S.; Wu, L.; Zhou, H.; Chen, Y.; Yan, W. Up-to-Date Imaging and Diagnostic Techniques for Prostate Cancer: A Literature Review. Diagnostics 2023, 13, 2283. [Google Scholar] [CrossRef]
- Pulumati, A.; Pulumati, A.; Dwarakanath, B.S.; Verma, A.; Papineni, R.V. Technological advancements in cancer diagnostics: Improvements and limitations. Cancer Rep. 2023, 6, e1764. [Google Scholar] [CrossRef]
- Duffy, M.J. Biomarkers for prostate cancer: Prostate-specific antigen and beyond. Clin. Chem. Lab. Med. 2020, 58, 326–339. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Soriano, M.C.; Parker, L.A.; López-Garrigos, M.; Hernández-Aguado, I.; Caballero-Romeu, J.P.; Gómez-Pérez, L.; Alfayate-Guerra, R.; Pastor-Valero, M.; Garcia, N.; Lumbreras, B. Factors associated with false negative and false positive results of prostate-specific antigen (PSA) and the impact on patient health: Cohort study protocol. Medicine 2019, 98, e17451. [Google Scholar] [CrossRef] [PubMed]
- Mokni, M.; Fourati, N.; Zerrouki, C.; Othmane, A.; Omezzine, A.; Bouslama, A. Review on recent advances in urinary biomarkers based electrochemical sensors for prostate cancer detection. In Advanced Sensors for Biomedical Applications; Kanoun, O., Derbel, N., Eds.; Springer: Cham, Switzerland, 2021; pp. 123–136. [Google Scholar]
- Liao, C.; Wu, Z.; Lin, C.; Chen, X.; Zou, Y.; Zhao, W.; Li, X.; Huang, G.; Xu, B.; Briganti, G.E.; et al. Nurturing the marriages of urinary liquid biopsies and nano-diagnostics for precision urinalysis of prostate cancer. Smart Med. 2023, 2, e20220020. [Google Scholar] [CrossRef]
- Jordaens, S.; Zwaenepoel, K.; Tjalma, W.; Deben, C.; Beyers, K.; Vankerckhoven, V.; Pauwels, P.; Vorsters, A. Urine biomarkers in cancer detection: A systematic review of preanalytical parameters and applied methods. Int. J. Cancer 2023, 152, 2186–2205. [Google Scholar] [CrossRef] [PubMed]
- Vlaeminck-Guillem, V.; Devonec, M.; Champetier, D.; Decaussi-Petrucci, M.; Paparel, P.; Perrin, P.; Ruffion, A. Test urinaire PCA3 et diagnostic du cancer prostatique: Étude à partir de 1015 patients. Prog. Urol. 2015, 25, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- Hessels, D.; Gunnewiek, J.M.K.; van Oort, I.; Karthaus, H.F.; van Leenders, G.J.; van Balken, B.; Kiemeney, L.A.; Witjes, J.A.; Schalken, J.A. DD3PCA3-based molecular urine analysis for the diagnosis of prostate cancer. Eur. Urol. 2003, 44, 8–16. [Google Scholar] [CrossRef]
- Nakanishi, H.; Groskopf, J.; Fritsche, H.A.; Bhadkamkar, V.; Blase, A.; Kumar, S.V.; Davis, J.W.; Troncoso, P.; Rittenhouse, H.; Babaian, R.J. PCA3 molecular urine assay correlates with prostate cancer tumor volume: Implication in selecting candidates for active surveillance. J. Urol. Balt. 2008, 179, 1804–1810. [Google Scholar] [CrossRef]
- Whitman, E.J.; Groskopf, J.; Ali, A.; Chen, Y.; Blase, A.; Furusato, B.; Petrovics, G.; Ibrahim, M.; Elsamanoudi, S.; Cullen, J. PCA3 score before radical prostatectomy predicts extracapsular extension and tumor volume. J. Urol. Balt. 2008, 180, 1975–1979. [Google Scholar] [CrossRef] [PubMed]
- Lemos, A.E.G.; da Rocha Matos, A.; Ferreira, L.B.; Gimba, E.R.P. The long non-coding RNA PCA3: An update of its functions and clinical applications as a biomarker in prostate cancer. Oncotarget 2019, 10, 6589. [Google Scholar] [CrossRef]
- Tosoian, J.J.; Patel, H.D.; Mamawala, M.; Landis, P.; Wolf, S.; Elliott, D.J.; Epstein, J.I.; Carter, H.B.; Ross, A.E.; Sokoll, L.J.; et al. Longitudinal assessment of urinary PCA3 for predicting prostate cancer grade reclassification in favorable-risk men during active surveillance. Prostate Cancer Prostatic Dis. 2017, 20, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Qu, X.; Jiang, J.; Gao, P.; Zhao, D.; Lian, X.; Li, X. Diagnostic significance of urinary long non-coding PCA3 RNA in prostate cancer. Oncotarget 2017, 8, 58577. [Google Scholar] [CrossRef] [PubMed]
- Truong, M.; Yang, B.; Jarrard, D.F. Toward the Detection of Prostate Cancer in Urine: A Critical Analysis. J. Urol. Balt. 2013, 189, 422–429. [Google Scholar] [CrossRef]
- Lee, D.; Shim, S.R.; Ahn, S.T.; Oh, M.M.; Moon, D.G.; Park, H.S.; Cheon, J.; Kim, J.W. Diagnostic performance of the prostate cancer antigen 3 test in Prostate Cancer: A systematic review and meta-analysis. Clin. Genitourin. Cancer 2020, 18, 402–408.e5. [Google Scholar] [CrossRef] [PubMed]
- Khalilzadeh, B.; Rashidi, M.; Soleimanian, A.; Tajalli, H.; Kanberoglu, G.S.; Baradaran, B.; Rashidi, M.R. Development of a reliable microRNA based electrochemical genosensor for monitoring of miR-146a, as key regulatory agent of neurodegenerative disease. Int. J. Biol. Macromol. 2019, 134, 695–703. [Google Scholar] [CrossRef]
- Azimi Sanavi, M.; Mahdavian, F.; Dorosti, N.; Karami, N.; Karami, S.; Khatami, S.H.; Vakili, O.; Taheri-Anganeh, M.; Karima, S.; Movahedpour, A. A review of highly sensitive electrochemical genosensors for microRNA detection: A novel diagnostic platform for neurodegenerative diseases diagnostics. Biotechnol. Appl. Biochem. 2023, 70, 1044–1056. [Google Scholar] [CrossRef] [PubMed]
- Pallares-Rusiñol, A.; Moura, S.L.; Martí, M.; Pividori, M.I. Electrochemical genosensing of overexpressed GAPDH transcripts in breast cancer exosomes. Anal. Chem. 2023, 95, 2487–2495. [Google Scholar] [CrossRef]
- Khosravi, F.; Rahaie, M.; Ghaani, M.R.; Azimzadeh, M.; Mostafavi, E. Ultrasensitive electrochemical miR-155 nanocomposite biosensor based on functionalized/conjugated graphene materials and gold nanostars. Sens. Actuators B 2023, 375, 132877. [Google Scholar] [CrossRef]
- Fortunati, S.; Giliberti, C.; Giannetto, M.; Bertucci, A.; Capodaglio, S.; Ricciardi, E.; Giacomini, P.; Bianchi, V.; Boni, A.; De Munari, I. A highly sensitive electrochemical magneto-genosensing assay for the specific detection of a single nucleotide variation in the KRAS oncogene in human plasma. Biosens. Bioelectron. X 2023, 15, 100404. [Google Scholar] [CrossRef]
- Jaapar, F.N.; Parmin, N.; Halim, N.H.A.; Hashim, U.; Gopinath, S.C.; Ruslinda, A.R.; Nadzirah, S.; Voon, C.; Uda, M.; Ang, W.C. Facile Electrical DNA Genosensor for Human Papillomavirus (HPV 58) for Early Detection of Cervical Cancer. Int. J. Nanoelectron. Mater. 2023, 16, 101760. [Google Scholar]
- Rawat, R.; Singh, S.; Roy, S.; Kumar, A.; Goswami, T.; Mathur, A. Design and development of an electroanalytical genosensor based on Cu-PTCA/rGO nanocomposites for the detection of cervical cancer. Mater. Chem. Phys. 2023, 295, 127050. [Google Scholar] [CrossRef]
- Shahbazi-Derakhshi, P.; Mahmoudi, E.; Majidi, M.M.; Sohrabi, H.; Amini, M.; Majidi, M.R.; Niaei, A.; Shaykh-Baygloo, N.; Mokhtarzadeh, A. An ultrasensitive miRNA-based genosensor for detection of microRNA 21 in gastric cancer cells based on functional signal amplifier and synthesized perovskite-graphene oxide and AuNPs. Biosensors 2023, 13, 172. [Google Scholar] [CrossRef]
- Kuche-Meshki, M.; Zare, H.R.; Akbarnia, A.; Moshtaghioun, S.M. A sensitive electrochemical genosensor for measuring gastric cancer biomarker, microRNA-106b, based on asparagine polymerization on carbon quantum dots modified electrode. Microchem. J. 2023, 191, 108846. [Google Scholar] [CrossRef]
- Sánchez-Salcedo, R.; Miranda-Castro, R.; de-los-Santos-Álvarez, N.; Fernández-Martínez, D.; García-Flórez, L.J.; Lobo-Castañón, M.J. An electrochemical genosensing platform for the relative quantification of the circulating long noncoding RNA CCAT1 to aid in the diagnosis of colorectal cancer. Sens. Actuators B 2023, 376, 132940. [Google Scholar] [CrossRef]
- Mazouz, Z.; Fourati, N.; Zerrouki, C.; Ommezine, A.; Rebhi, L.; Yaakoubi, N.; Kalfat, R.; Othmane, A. Discriminating DNA mismatches by electrochemical and gravimetric techniques. Biosens. Bioelectron. 2013, 48, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Morais, S.L.; Magalhães, J.M.; Domingues, V.F.; Delerue-Matos, C.; Ramos-Jesus, J.; Ferreira-Fernandes, H.; Pinto, G.R.; Santos, M.; Barroso, M.F. Development of an electrochemical DNA-based biosensor for the detection of the cardiovascular pharmacogenetic-altering SNP CYP2C9* 3. Talanta 2023, 264, 124692. [Google Scholar] [CrossRef] [PubMed]
- Arishi, W.A.; Eissa, S.; Al-Kattan, K.; Zourob, M. Aptamer-based label-free electrochemical biosensors for the diagnosis of sickle cell anemia. Biosens. Bioelectron. X 2023, 14, 100389. [Google Scholar] [CrossRef]
- Thamwarokun, A.; Kaset, C.; Karuwan, C.; Kamsong, W.; Attapong, J.; Chomean, S. A label-free electrochemical biosensor for the detection of alpha-thalassemia 1 (SEA deletion) carriers using screen-printed carbon electrodes. Biosens. Bioelectron. X 2023, 14, 100385. [Google Scholar] [CrossRef]
- Liu, S.; Shi, J.; Lin, Y.; Luo, H.; Wu, Y.; Yan, J.; Tan, X.; Huang, K.-J. A sandwich-type dual-mode biosensor based on graphdiyne and DNA nanoframework for ultra-sensitive detection of CD142 gene. Biosens. Bioelectron. 2024, 248, 115962. [Google Scholar] [CrossRef] [PubMed]
- da Silva Catunda, L.G.; do Prado, T.M.; de Oliveira, T.R.; Dos Santos, D.J.A.; Gomes, N.O.; Correa, D.S.; Faria, R.C.; Machado, S.A.S. SARS-CoV-2 detection enabled by a portable and label-free photoelectrochemical genosensor using graphitic carbon nitride and gold nanoparticles. Electrochim. Acta 2023, 451, 142271. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.; Gan, K.B.; Yusof, N.Y.M.; Goh, C.T.; Tan, L.L. Electrochemical genosensor based on RNA-responsive human telomeric G-quadruplex DNA: A proof-of-concept with SARS-CoV-2 RNA. Talanta 2024, 247, 125916. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Dhanda, M.; Arora, R.; Singh, G.; Mohan, H.; Lata, S. A TiO2-adenine nanocomposite as modification material for screen-printed gold electrode to detect H1N1 (swine flu) virus: A disposable genosensor. Microchem. J. 2024, 197, 109831. [Google Scholar] [CrossRef]
- Yan, J.; Cheng, Q.; Liu, H.; Wang, L.; Yu, K. Sensitive and rapid detection of influenza A virus for disease surveillance using dual-probe electrochemical biosensor. Bioelectrochemistry 2023, 153, 108497. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, H.; Majidi, M.R.; Asadpour-Zeynali, K.; Khataee, A.; Mokhtarzadeh, A. Self-assembled monolayer-assisted label-free electrochemical genosensor for specific point-of-care determination of Haemophilus influenzae. Microchim. Acta 2023, 190, 112. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, M.; Patel, M.; Bisht, N.; Shruti; Das Mukherjee, M.; Tiwari, A.; Mondal, D.; Srivastava, A.K.; Dwivedi, N.; Dhand, C. Reduced graphene oxide-polydopamine-gold nanoparticles: A ternary nanocomposite-based electrochemical genosensor for rapid and early Mycobacterium tuberculosis detection. Biosensors 2023, 13, 342. [Google Scholar] [CrossRef] [PubMed]
- Taufiq, S.; Waqar, M.; Sharif, M.N.; Abbas, S.R. Towards portable rapid TB biosensor: Detecting Mycobacterium tuberculosis in raw sputum samples using functionalized screen printed electrodes. Bioelectrochemistry 2023, 150, 108353. [Google Scholar] [CrossRef]
- Sánchez-Salcedo, R.; Miranda-Castro, R.; de-Los-Santos-Álvarez, N.; Lobo-Castañón, M.J. Dual electrochemical genosensor for early diagnosis of prostate cancer through lncRNAs detection. Biosens. Bioelectron. 2021, 192, 113520. [Google Scholar] [CrossRef]
- Takita, S.; Nabok, A.; Lishchuk, A.; Mussa, M.H.; Smith, D. Enhanced performance electrochemical biosensor for detection of prostate cancer biomarker PCA3 using specific aptamer. Eng 2023, 4, 367–379. [Google Scholar] [CrossRef]
- Nabok, A.; Abu-Ali, H.; Takita, S.; Smith, D.P. Electrochemical Detection of Prostate Cancer Biomarker PCA3 Using Specific RNA-Based Aptamer Labelled with Ferrocene. Chemosensors 2021, 9, 59. [Google Scholar] [CrossRef]
- Rodrigues, V.C.; Soares, J.C.; Soares, A.C.; Braz, D.C.; Melendez, M.E.; Ribas, L.C.; Scabini, L.F.S.; Bruno, O.M.; Carvalho, A.L.; Reis, R.M.; et al. Electrochemical and optical detection and machine learning applied to images of genosensors for diagnosis of prostate cancer with the biomarker PCA3. Talanta 2021, 222, 121444. [Google Scholar] [CrossRef]
- Soares, J.C.; Soares, A.C.; Rodrigues, V.C.; Melendez, M.E.; Santos, A.C.; Faria, E.F.; Reis, R.M.; Carvalho, A.L.; Oliveira, O.N. Detection of the Prostate Cancer Biomarker PCA3 with Electrochemical and Impedance-Based Biosensors. ACS Appl. Mater. Interfaces 2019, 11, 46645–46650. [Google Scholar] [CrossRef]
- Bogomolova, A.; Komarova, E.; Reber, K.; Gerasimov, T.; Yavuz, O.; Bhatt, S.; Aldissi, M. Challenges of electrochemical impedance spectroscopy in protein biosensing. Anal. Chem. 2009, 81, 3944–3949. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Horiuchi, T.; Morita, M.; Niwa, O. Electrochemical reaction of Fe(CN)3−/4−6 on gold electrodes analyzed by surface plasmon resonance. Surf Sci. 1999, 427, 195–198. [Google Scholar] [CrossRef]
- Lazar, J.; Schnelting, C.; Slavcheva, E.; Schnakenberg, U. Hampering of the stability of gold electrodes by ferri-/ferrocyanide redox couple electrolytes during electrochemical impedance spectroscopy. Anal. Chem. 2016, 88, 682–687. [Google Scholar] [CrossRef]
- Hai, X.; Li, Y.; Zhu, C.; Song, W.; Cao, J.; Bi, S. DNA-based label-free electrochemical biosensors: From principles to applications. Trends Analyt. Chem. 2020, 133, 116098. [Google Scholar] [CrossRef]
- Labuda, J.; Brett, A.M.O.; Evtugyn, G.; Fojta, M.; Mascini, M.; Ozsoz, M.; Palchetti, I.; Paleček, E.; Wang, J. Electrochemical nucleic acid-based biosensors: Concepts, terms, and methodology (IUPAC Technical Report). Pure Appl. Chem. 2010, 82, 1161–1187. [Google Scholar] [CrossRef]
- Tinzl, M.; Marberger, M.; Horvath, S.; Chypre, C. DD3PCA3 RNA analysis in urine–a new perspective for detecting prostate cancer. Eur. Urol. 2004, 46, 182–187. [Google Scholar] [CrossRef]
- Mearini, E.; Antognelli, C.; Del Buono, C.; Cochetti, G.; Giannantoni, A.; Nardelli, E.; Talesa, V.N. The combination of urine DD3PCA3 mRNA and PSA mRNA as molecular markers of prostate cancer. Biomarkers 2009, 14, 235–243. [Google Scholar] [CrossRef]
- Bussemakers, M.J.; Van Bokhoven, A.; Verhaegh, G.W.; Smit, F.P.; Karthaus, H.F.; Schalken, J.A.; Debruyne, F.M.; Ru, N.; Isaacs, W.B. DD3:: A new prostate-specific gene, highly overexpressed in prostate cancer. Cancer Res. 1999, 59, 5975–5979. [Google Scholar] [PubMed]
- Mokni, M.; Tlili, A.; Attia, G.; Khaoulani, S.; Zerrouki, C.; Omezzine, A.; Othmane, A.; Bouslama, A.; Fourati, N. Novel sensitive immunosensor for the selective detection of Engrailed 2 urinary prostate cancer biomarker. Biosens. Bioelectron. 2022, 217, 114678. [Google Scholar] [CrossRef] [PubMed]
- Prediger, E. How to Design Primers and Probes for PCR and qPCR. Available online: https://www.idtdna.com/pages/education/decoded/article/designing-pcr-primers-and-probes (accessed on 18 March 2024).
- Jaworska, A.; Jablonska, A.; Wilanowski, T.; Palys, B.; Sek, S.; Kudelski, A. Influence of amine and thiol modifications at the 3′ ends of single stranded DNA molecules on their adsorption on gold surface and the efficiency of their hybridization. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 203, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Rashid, J.I.A.; Yusof, N.A. The strategies of DNA immobilization and hybridization detection mechanism in the construction of electrochemical DNA sensor: A review. Sens. Bio-Sens. Res. 2017, 16, 19–31. [Google Scholar] [CrossRef]
- Nimse, S.B.; Song, K.; Sonawane, M.D.; Sayyed, D.R.; Kim, T. Immobilization techniques for microarray: Challenges and applications. Sensors 2014, 14, 22208–22229. [Google Scholar] [CrossRef] [PubMed]
- Revenga-Parra, M.; García, T.; Pariente, F.; Lorenzo, E.; Alonso, C. Effects of ionic strength and probe DNA length on the electrochemical impedance spectroscopic response of biosensors. Electroanalysis 2011, 23, 100–107. [Google Scholar] [CrossRef]
- Paleček, E.; Fojta, M.; Tomschik, M.; Wang, J. Electrochemical biosensors for DNA hybridization and DNA damage. Biosens. Bioelectron. 1998, 13, 621–628. [Google Scholar] [CrossRef]
- Paleček, E. Fifty years of nucleic acid electrochemistry. Electroanalysis 2009, 21, 239–251. [Google Scholar] [CrossRef]
- Miranda-Castro, R.; de-los-Santos-Álvarez, N.; Lobo-Castañón, M.J.; Miranda-Ordieres, A.J.; Tuñón-Blanco, P. Structured nucleic acid probes for electrochemical devices. Electroanalysis 2009, 21, 2077–2090. [Google Scholar] [CrossRef]
- Jin, Y.; Yao, X.; Liu, Q.; Li, J. Hairpin DNA probe based electrochemical biosensor using methylene blue as hybridization indicator. Biosens. Bioelectron. 2007, 22, 1126–1130. [Google Scholar] [CrossRef]
- Singhal, P.; Kuhr, W.G. Ultrasensitive voltammetric detection of underivatized oligonucleotides and DNA. Anal. Chem. 1997, 69, 4828–4832. [Google Scholar] [CrossRef] [PubMed]
- Takita, S.; Nabok, A.; Lishchuk, A.; Smith, D. Optimization of apta-sensing platform for detection of prostate cancer marker PCA3. Int. J. Mol. Sci. 2021, 22, 12701. [Google Scholar] [CrossRef]
- Crulhas, B.P.; Basso, C.R.; Castro, G.R.; Pedrosa, V.A. Detection of Prostate Cancer Biomarker PCA3 by Using Aptasensors. Curr. Med. Chem. 2022, 29, 5895–5902. [Google Scholar] [CrossRef] [PubMed]
- Takita, S.; Nabok, A.; Lishchuk, A.; Mussa, M.H.; Smith, D. Detection of Prostate Cancer Biomarker PCA3 with Electrochemical Apta-Sensor. Eng. Proc. 2022, 16, 8. [Google Scholar] [CrossRef]
- Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef] [PubMed]
- Ermini, M.; Scarano, S.; Bini, R.; Banchelli, M.; Berti, D.; Mascini, M.; Minunni, M. A rational approach in probe design for nucleic acid-based biosensing. Biosens. Bioelectron. 2011, 26, 4785–4790. [Google Scholar] [CrossRef]
- Mazouz, Z.; Mokni, M.; Fourati, N.; Zerrouki, C.; Barbault, F.; Seydou, M.; Kalfat, R.; Yaakoubi, N.; Omezzine, A.; Bouslema, A.; et al. Computational approach and electrochemical measurements for protein detection with MIP-based sensor. Biosens. Bioelectron. 2020, 151, 111978. [Google Scholar] [CrossRef]



| Sequence | |
|---|---|
| Probe 1 | H-S-(CH2)6-5′-AGATCTTCCTGGTCTCCCTCG GCTGCAGCCACACAAATCT-3′ |
| Probe 2 | H-S-(CH2)6-5′-GGGAAG GAC CTG ATG ATA CAG AGG AAT TAC AACACATATA-3′ |
| Target 1 | 5′-AGATTTGTGTGGCTGCAGCCGAGGGAGACCAGGAAGATCT-3′ |
| Target 2 | 5′-TATATGTGTTGTAATTCCTCTGTATCATCAGGTCCTTCCC-3′ |
| Surface Modification | Redox Probe Use | Concentration Range | LOD pM | Ref. |
|---|---|---|---|---|
| RNA aptamer | Ferrocene | 0.1 × 10−3 to 1 µg/mL | 400 | [43] |
| Thiolated DNA aptamer | Methylene blue | 0 to 150 ng/mL | 9200 | [66] |
| CHT/MWCNT/SsDNA | K3/4[Fe(CN)6] | 10−16 to 10−6 M | 1280 | [45] |
| RNA aptamer | Ferrocene | 0 to 10 nM | 10 | [67] |
| AuNPs/chondroitin sulfate/SsDNA | K3/4[Fe(CN)6] | 10−6 to 10 μM | 83 | [44] |
| Thiolated DNA (probe 1) | Without redox probe | 0.1 to 10 µg/mL | 13 | The present work |
| Thiolated DNA (probe 2) | Without redox probe | 0.1 to 10 µg/mL | 9 |
| Sequence | Description | |
|---|---|---|
| NC1 | 5′-AGATTTGTGTGGCTGCAGCCGAGG GAGACCAGGAAGATCT-3′ | The first 40 nucleotides of the amplified PCA3 fragment |
| NC2 | 5′-GATGACCCAAGATGGCGGC-3′ | A PCA3 portion from bases 486 to 507 |
| NC3 | 5′-CCTCCTGAAGAATCGATTCCT-3′ | A portion from PSA gene |
| NC4 | 5′-ATGGATGAAACCCAGACACA -3′ | A portion from beta-2 microglobulin gene |
| Sensitivity (µA−1·µg−1·mL) | |
|---|---|
| PCA3 | 0.10 ± 0.01 |
| NC1 | 0.011 ± 0.007 |
| NC2 | 0.006 ± 0.002 |
| NC3 | 0.014± 0.002 |
| NC4 | 0.07 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokni, M.; Tlili, A.; Khalij, Y.; Attia, G.; Zerrouki, C.; Hmida, W.; Othmane, A.; Bouslama, A.; Omezzine, A.; Fourati, N. Designing a Simple Electrochemical Genosensor for the Detection of Urinary PCA3, a Prostate Cancer Biomarker. Micromachines 2024, 15, 602. https://doi.org/10.3390/mi15050602
Mokni M, Tlili A, Khalij Y, Attia G, Zerrouki C, Hmida W, Othmane A, Bouslama A, Omezzine A, Fourati N. Designing a Simple Electrochemical Genosensor for the Detection of Urinary PCA3, a Prostate Cancer Biomarker. Micromachines. 2024; 15(5):602. https://doi.org/10.3390/mi15050602
Chicago/Turabian StyleMokni, Meriem, Amal Tlili, Yassine Khalij, Ghada Attia, Chouki Zerrouki, Wissem Hmida, Ali Othmane, Ali Bouslama, Asma Omezzine, and Najla Fourati. 2024. "Designing a Simple Electrochemical Genosensor for the Detection of Urinary PCA3, a Prostate Cancer Biomarker" Micromachines 15, no. 5: 602. https://doi.org/10.3390/mi15050602
APA StyleMokni, M., Tlili, A., Khalij, Y., Attia, G., Zerrouki, C., Hmida, W., Othmane, A., Bouslama, A., Omezzine, A., & Fourati, N. (2024). Designing a Simple Electrochemical Genosensor for the Detection of Urinary PCA3, a Prostate Cancer Biomarker. Micromachines, 15(5), 602. https://doi.org/10.3390/mi15050602







