Electric-Field-Assisted Synthesis of Cu/MoS2 Nanostructures for Efficient Hydrogen Evolution Reaction
Abstract
1. Introduction
2. Material and Method
2.1. Chemicals and Reagents
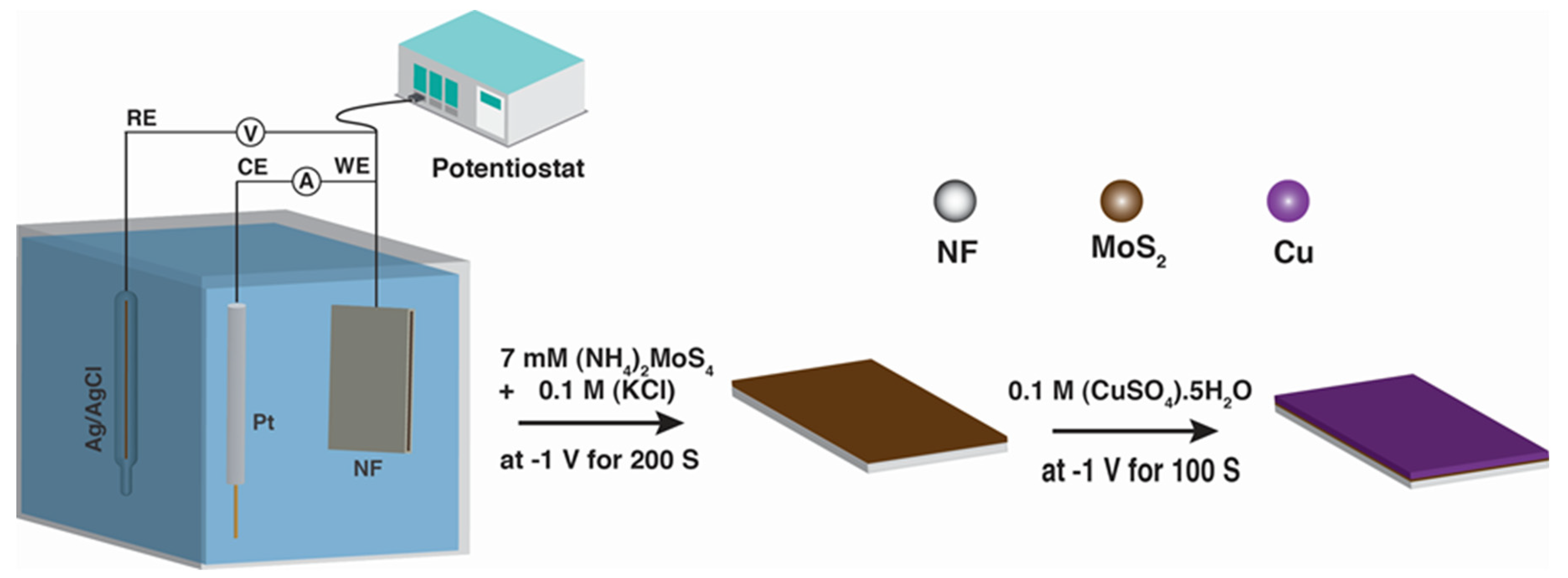
2.2. Synthesis of MoS2 and Cu/MoS2 Electrocatalysts
2.3. Characterization of CMS Nanostructure
2.4. Electrochemical Measurements
3. Result and Discussion
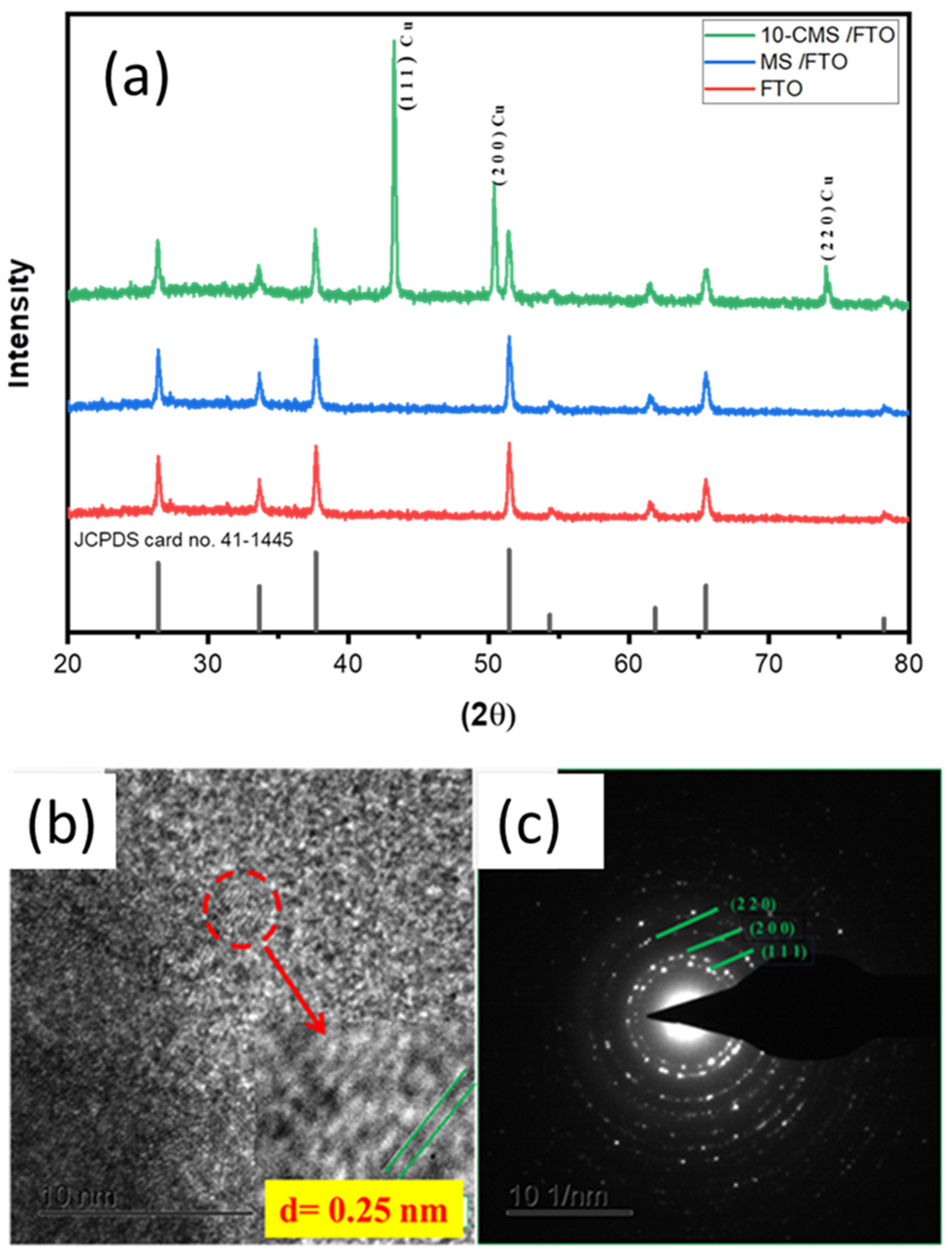
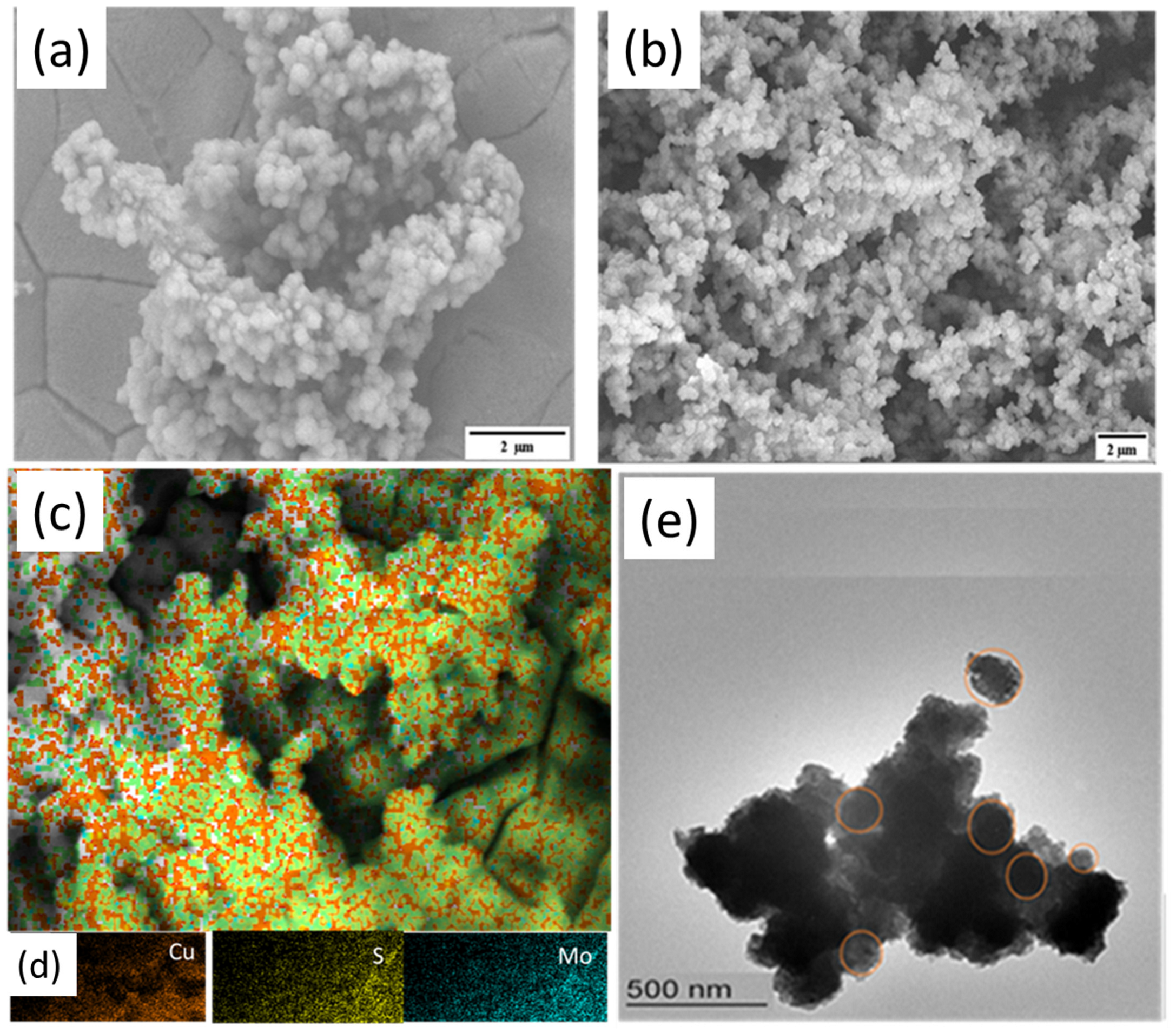
3.1. Fabrication and Characterization of CMS Nanostructures
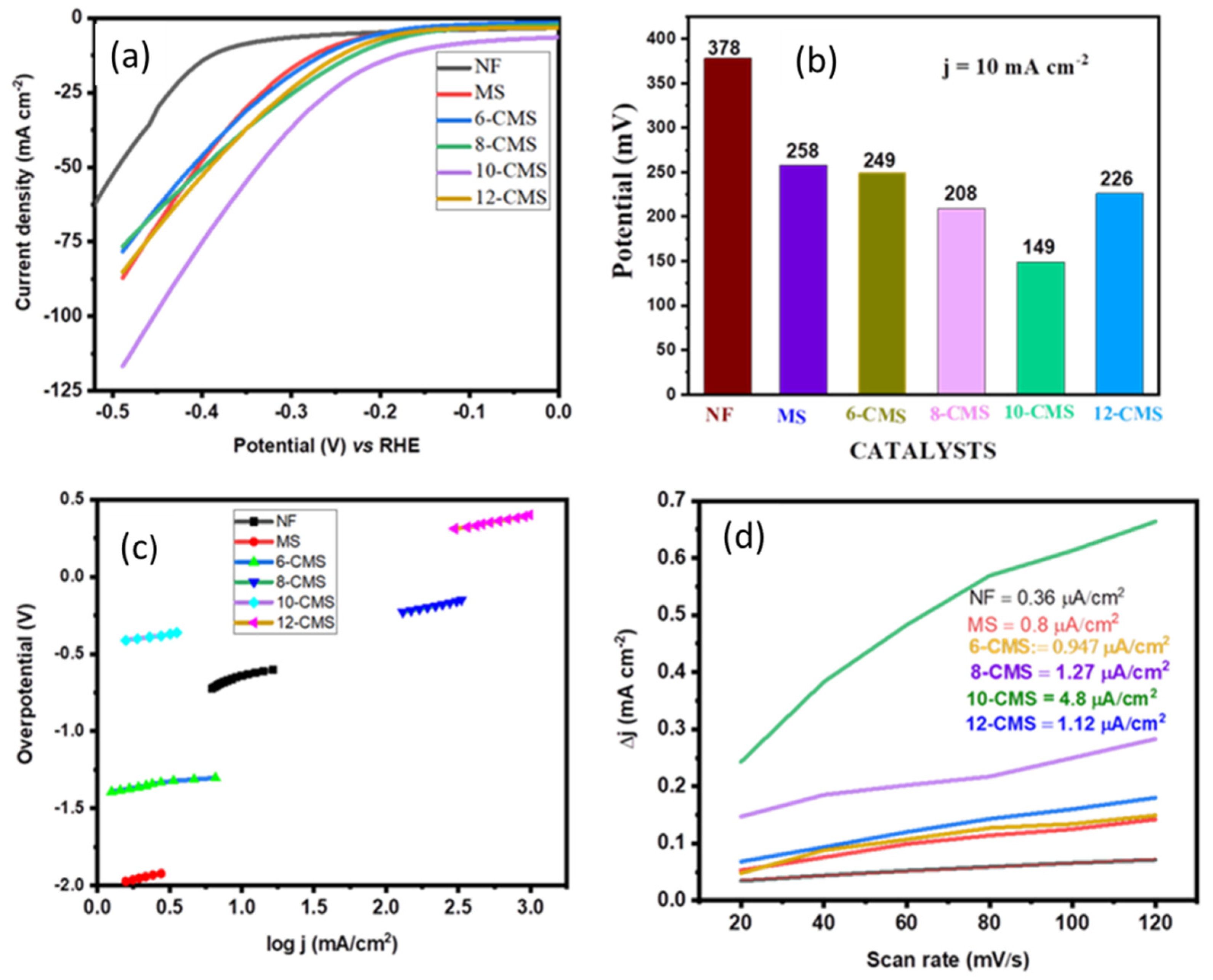
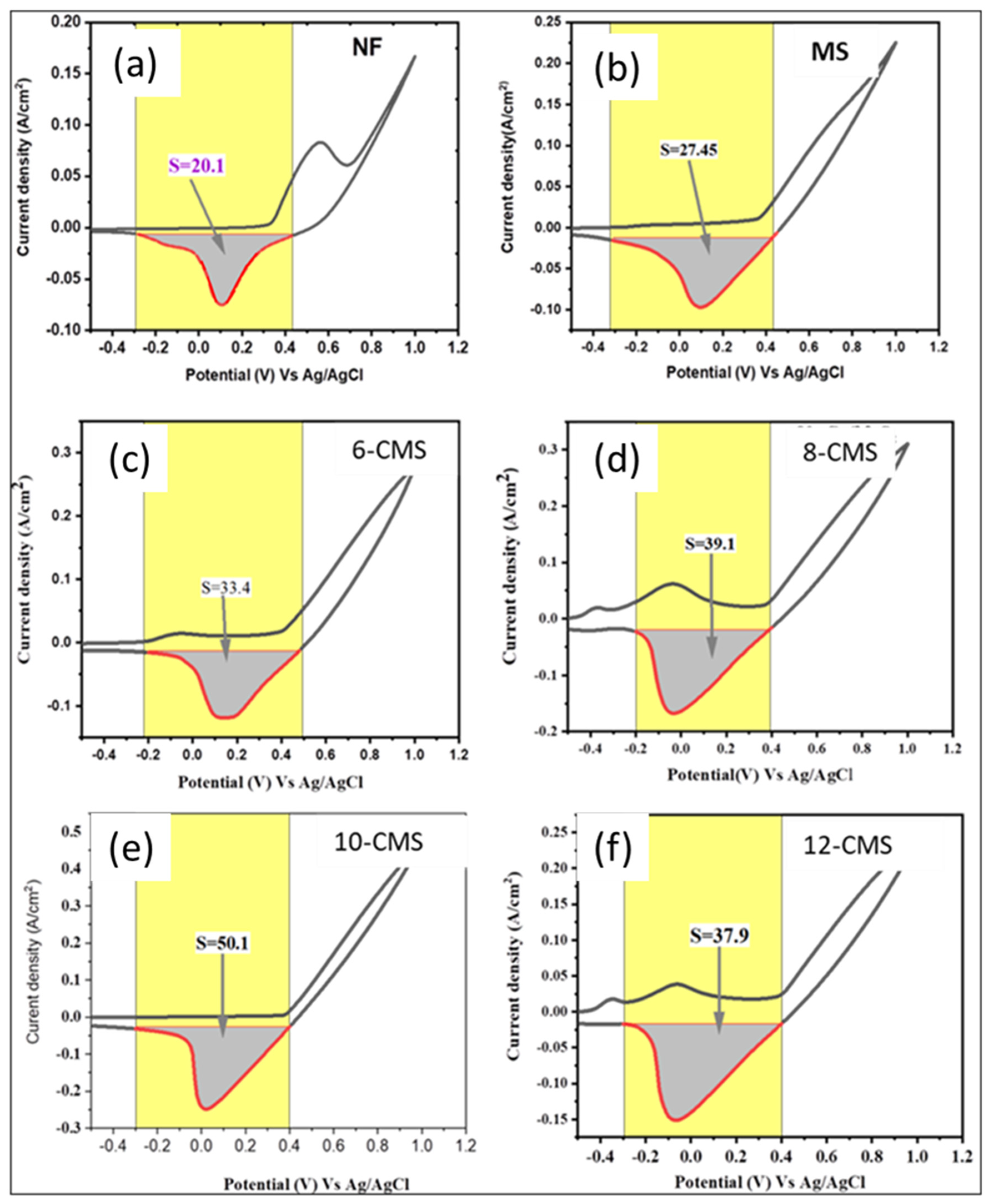
3.2. HER Catalytic Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Samantara, A.K.; Ratha, S. Mechanism and Key Parameters for Catalyst Evaluation. In Metal Oxides/Chalcogenides and Composites. Springer Briefs in Materials; Springer: Cham, Switzerland, 2019; pp. 11–29. [Google Scholar]
- Alonso-Vante, N.; Roldán, C.A.C.; Huerta, R.d.G.G.; Sánchez, G.R.; Robledo, A.M. Fundamentals of Electrocatalyst Materials and Interfacial Characterization; Wiley: Hoboken, NJ, USA, 2019. [Google Scholar] [CrossRef]
- Tiwari, A.P.; Novak, T.G.; Bu, X.; Ho, J.C.; Jeon, S. Layered Ternary and Quaternary Transition Metal Chalcogenide Based Catalysts for Water Splitting. Catalysts 2018, 8, 551. [Google Scholar] [CrossRef]
- Herbaut, M.; Siaj, M.; Claverie, J.P. Nanomaterials-Based Water Splitting: How Far Are We from a Sustainable Solution? ACS Appl. Nano Mater. 2021, 4, 907–910. [Google Scholar] [CrossRef]
- Samantara, A.K.; Ratha, S. Materials Development for Active/Passive Components of a Supercapacitor: Background, Present Status and Future Perspective; Springer: Singapore, 2018; Available online: https://books.google.co.uk/books?hl=en&lr=&id=dfVADwAAQBAJ&oi=fnd&pg=PR5&dq=Aneeya+K.+Samantara+Satyajit+Ratha+materials+development&ots=v5UOewLdnC&sig=n8TXALS8quiE6cLC7v7g-xO5WpQ#v=onepage&q=AneeyaK.SamantaraSatyajitRathamaterialsdevelopment&f=fals (accessed on 23 March 2024). [CrossRef]
- Bhat, K.S.; Nagaraja, H.S. Recent trends and insights in nickel chalcogenide nanostructures for water-splitting reactions. Mater. Res. Innov. 2021, 25, 29–52. [Google Scholar] [CrossRef]
- Cao, J.; Zhou, J.; Zhang, Y.; Liu, X. A facile one-step fabrication of a novel Cu/MoS2 nano-assembled structure for enhanced hydrogen evolution reaction performance. RSC Adv. 2017, 7, 25867–25871. [Google Scholar] [CrossRef]
- Pataniya, P.M.; Sumesh, C.K. MoS2 nanosheets on Cu-foil for rapid electrocatalytic hydrogen evolution reaction. J. Electroanal. Chem. 2022, 912, 116270. [Google Scholar] [CrossRef]
- Ilyas, T.; Raziq, F.; Ali, S.; Zada, A.; Ilyas, N.; Shaha, R.; Wang, Y.; Qiao, L. Facile synthesis of MoS2/Cu as trifunctional catalyst for electrochemical overall water splitting and photocatalytic CO2 conversion. Mater. Des. 2021, 204, 109674. [Google Scholar] [CrossRef]
- Wang, F.; Shifa, T.A.; Zhan, X.; Huang, Y.; Liu, K.; Cheng, Z.; Jiang, C.; He, J. Recent advances in transition-metal dichalcogenide based nanomaterials for water splitting. Nanoscale 2015, 7, 19764–19788. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Meng, X. Modulating the Electronic Properties of MoS2Nanosheets for Electrochemical Hydrogen Production: A Review. ACS Appl. Nano Mater. 2021, 4, 11413–11427. [Google Scholar] [CrossRef]
- Sharma, M.D.; Mahala, C.; Modak, B.; Pande, S.; Basu, M. Doping of MoS2by ‘cu’ and ‘v’: An Efficient Strategy for the Enhancement of Hydrogen Evolution Activity. Langmuir 2021, 37, 4847–4858. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, L.; Li, J.; Lin, X.; Li, X.; Fang, Y.; Huang, J.; Li, W.; Tian, M.; Jin, J.; et al. Synthesis of Cu e MoS2/rGO hybrid as non-noble metal electrocatalysts for the hydrogen evolution reaction. J. Power Sources 2015, 292, 15–22. [Google Scholar] [CrossRef]
- Gudal, C.C.; Pan, U.N.; Paudel, D.R.; Kandel, M.R.; Kim, N.H.; Lee, J.H. Bifunctional P-Intercalated and Doped Metallic (1T)-Copper Molybdenum Sul fi de Ultrathin 2D-Nanosheets with Enlarged Interlayers for Efficient Overall Water Splitting. ACS Appl. Mater. Interfaces 2022, 14, 14492–14503. [Google Scholar] [CrossRef] [PubMed]
- Quy, V.H.V.; Vijayakumar, E.; Ho, P.; Park, J.-H.; Rajesh, J.A.; Kwon, J.; Chae, J.; Kim, J.-H.; Kang, S.-H.; Ahn, K.-S. Electrodeposited MoS2 as electrocatalytic counter electrode for quantum dot- and dye-sensitized solar cells. Electrochim. Acta 2018, 260, 716–725. [Google Scholar] [CrossRef]
- Gicha, B.B.; Tufa, L.T.; Kang, S.; Goddati, M.; Bekele, E.T.; Lee, J. Transition metal-based 2D layered double hydroxide nanosheets: Design strategies and applications in oxygen evolution reaction. Nanomaterials 2021, 11, 1388. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhou, H.; Sun, J.; Qin, F.; Luo, D.; Xie, L.; Yu, F.; Bao, J.; Li, Y.; Yu, Y.; et al. Hierarchical Cu@CoFe layered double hydroxide core-shell nanoarchitectures as bifunctional electrocatalysts for efficient overall water splitting. Nano Energy 2017, 41, 327–336. [Google Scholar] [CrossRef]
- Choudhury, B.J.; Roy, K.; Moholkar, V.S. Improvement of Supercapacitor Performance through Enhanced Interfacial Interactions Induced by Sonication. Ind. Eng. Chem. Res. 2021, 60, 7611–7623. [Google Scholar] [CrossRef]
- Yu, L.; Sun, S.; Li, H.; Xu, Z.J. Effects of catalyst mass loading on electrocatalytic activity: An example of oxygen evolution reaction. Fundam. Res. 2021, 1, 448–452. [Google Scholar] [CrossRef]
- Lee, J.H.; Jang, W.S.; Han, S.W.; Baik, H.K. Efficient hydrogen evolution by mechanically strained MoS2 nanosheets. Langmuir 2014, 30, 9866–9873. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, J.; Lin, X.; Li, X.; Fang, Y.; Jiao, L.; An, X.; Fu, Y.; Jin, J.; Li, R. Designed synthesis of multi-walled carbon nanotubes@Cu@MoS2 hybrid as advanced electrocatalyst for highly efficient hydrogen evolution reaction. J. Power Sources 2015, 300, 301–308. [Google Scholar] [CrossRef]
- Han, D.; Luo, Z.; Li, Y.; Gao, N.; Ge, J.; Liu, C.; Xing, W. Synergistic engineering of MoS2 via dual-metal doping strategy towards hydrogen evolution reaction. Appl. Surf. Sci. 2020, 529, 147117. [Google Scholar] [CrossRef]
- Liu, Y.; Guan, S.; Du, X.; Chen, Y.; Yang, Y.; Chen, K.; Zheng, Z.; Wang, X.; Shen, X.; Hu, C.; et al. S-Vacancy Defect and Transition-Metal Atom Doping to Trigger Hydrogen Evolution of Two-Dimensional MoS 2. Energy Fuels 2023, 37, 5370–5377. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, H.; Hao, J.; Lu, S.; Duan, F.; Xu, F.; Du, M. One-dimensional, space-confined, solid-phase growth of the Cu9S5@MoS2 core–shell heterostructure for electrocatalytic hydrogen evolution. J. Colloid. Interface Sci. 2021, 595, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Connor, P.; Schuch, J.; Kaiser, B.; Jaegermann, W. The Determination of Electrochemical Active Surface Area and Specific Capacity Revisited for the System MnOx as an Oxygen Evolution Catalyst. Z. Fur Phys. Chem. 2020, 234, 979–994. [Google Scholar] [CrossRef]
- Duan, J.; Chen, S.; Vasileff, A.; Qiao, S.Z. Anion and Cation Modulation in Metal Compounds for Bifunctional Overall Water Splitting. ACS Nano 2016, 10, 8738–8745. [Google Scholar] [CrossRef] [PubMed]
- Anantharaj, S.; Ede, S.R.; Sakthikumar, K.; Karthick, K.; Mishra, S.; Kundu, S. Recent Trends and Perspectives in Electrochemical Water Splitting with an Emphasis on Sulfide, Selenide, and Phosphide Catalysts of Fe, Co, and Ni: A Review. ACS Catal. 2016, 6, 8069–8097. [Google Scholar] [CrossRef]
- Tong, J.; Li, Y.; Bo, L.; Li, W.; Li, T.; Zhang, Q.; Kong, D.; Wang, H.; Li, C. CoP/N-Doped Carbon Hollow Spheres Anchored on Electrospinning Core-Shell N-Doped Carbon Nanofibers as Efficient Electrocatalysts for Water Splitting. ACS Sustain. Chem. Eng. 2019, 7, 17432–17442. [Google Scholar] [CrossRef]
- Aguedo, J.; Lorencova, L.; Barath, M.; Farkas, P.; Tkac, J. Electrochemical impedance spectroscopy on 2D nanomaterial mxene modified interfaces: Application as a characterization and transducing tool. Chemosensors 2020, 8, 127. [Google Scholar] [CrossRef]

| Prepared Sample | Electrolyte | Over-Potential η (mV) | Synthesis Method | Ref |
|---|---|---|---|---|
| 10-CMS | KOH | 149 | Electrodeposition Method | This work |
| MWCNTs@Cu@MoS2 | H2SO4 | 146 | Solvothermal Method | [21] |
| MoS2/Cu | KOH | 160 | Etching Method | [9] |
| Ag-Ag2S/MoS2 | H2SO4 | 150 | Microwave-assisted technique | [12] |
| Etched MoS2 | H2SO4 | 316 | One-step microwave-assisted method | [7] |
| MoS2 Quantum Dot | H2SO4 | 190 | Hydrothermal Method | [22] |
| MoS2NTs array | H2SO4 | 296 | Electrophoretic Deposition | [8] |
| Cu-MoS2-SV | H2SO4 | 197 | Chemical Etching Method | [23] |
| Cu9S5@MoS2 | KOH | 146 | Chemical Vapor Deposition (CVD) | [24] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yonas, S.; Gicha, B.B.; Adhikari, S.; Sabir, F.K.; Tran, V.T.; Nwaji, N.; Gonfa, B.A.; Tufa, L.T. Electric-Field-Assisted Synthesis of Cu/MoS2 Nanostructures for Efficient Hydrogen Evolution Reaction. Micromachines 2024, 15, 495. https://doi.org/10.3390/mi15040495
Yonas S, Gicha BB, Adhikari S, Sabir FK, Tran VT, Nwaji N, Gonfa BA, Tufa LT. Electric-Field-Assisted Synthesis of Cu/MoS2 Nanostructures for Efficient Hydrogen Evolution Reaction. Micromachines. 2024; 15(4):495. https://doi.org/10.3390/mi15040495
Chicago/Turabian StyleYonas, Surra, Birhanu Bayissa Gicha, Samir Adhikari, Fedlu Kedir Sabir, Van Tan Tran, Njemuwa Nwaji, Bedasa Abdisa Gonfa, and Lemma Teshome Tufa. 2024. "Electric-Field-Assisted Synthesis of Cu/MoS2 Nanostructures for Efficient Hydrogen Evolution Reaction" Micromachines 15, no. 4: 495. https://doi.org/10.3390/mi15040495
APA StyleYonas, S., Gicha, B. B., Adhikari, S., Sabir, F. K., Tran, V. T., Nwaji, N., Gonfa, B. A., & Tufa, L. T. (2024). Electric-Field-Assisted Synthesis of Cu/MoS2 Nanostructures for Efficient Hydrogen Evolution Reaction. Micromachines, 15(4), 495. https://doi.org/10.3390/mi15040495









