One-Step Dry Coating of Hybrid ZnO–WO3 Nanosheet Photoanodes for Photoelectrochemical Water Splitting with Composition-Dependent Performance
Abstract
:1. Introduction
2. Experimental
2.1. Material Details
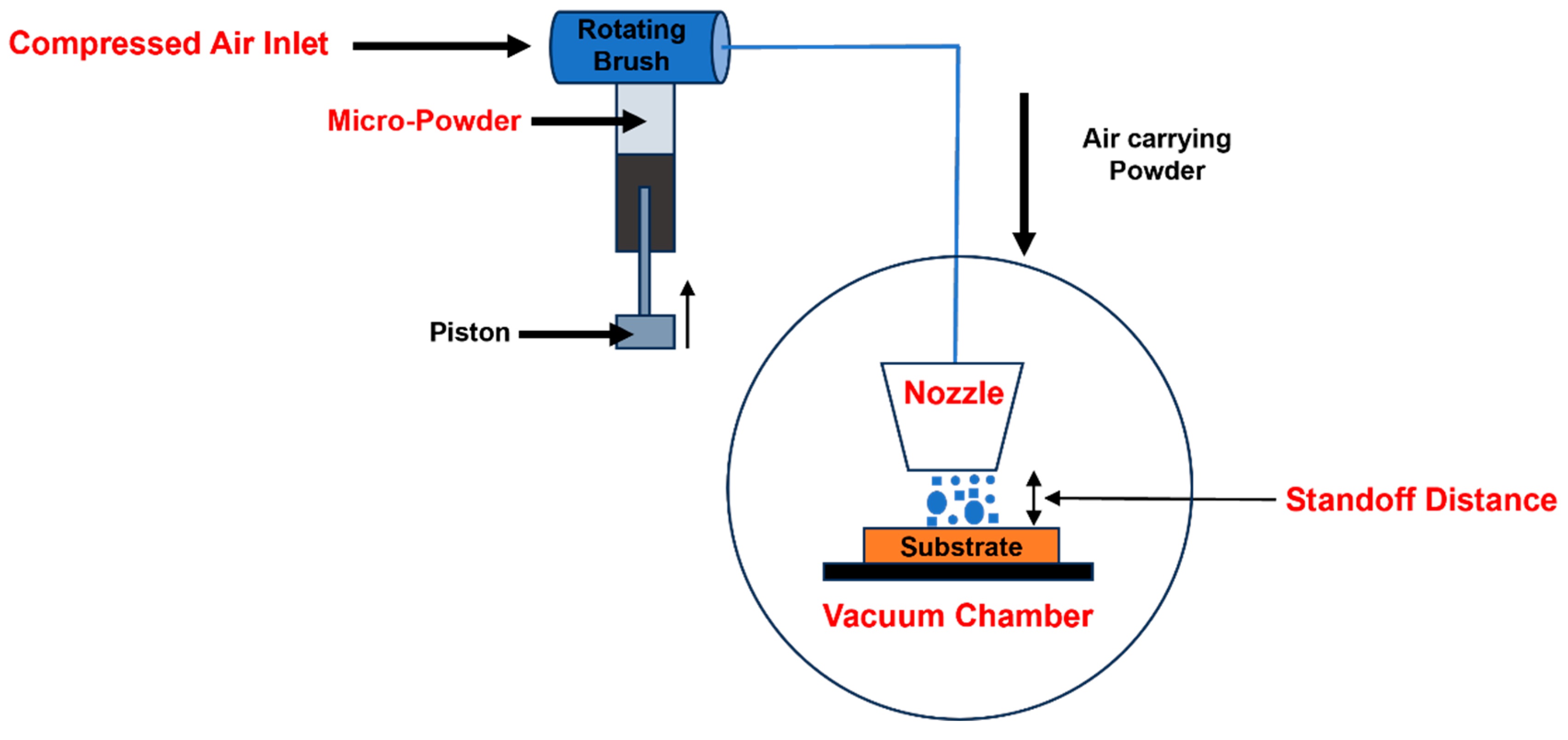
2.2. Dry Coating of ZnO–WO3 Thin Films by the NPDS
2.3. Photoelectrochemical Water Splitting Characteristics
2.4. Material Characterization
3. Results and Discussion
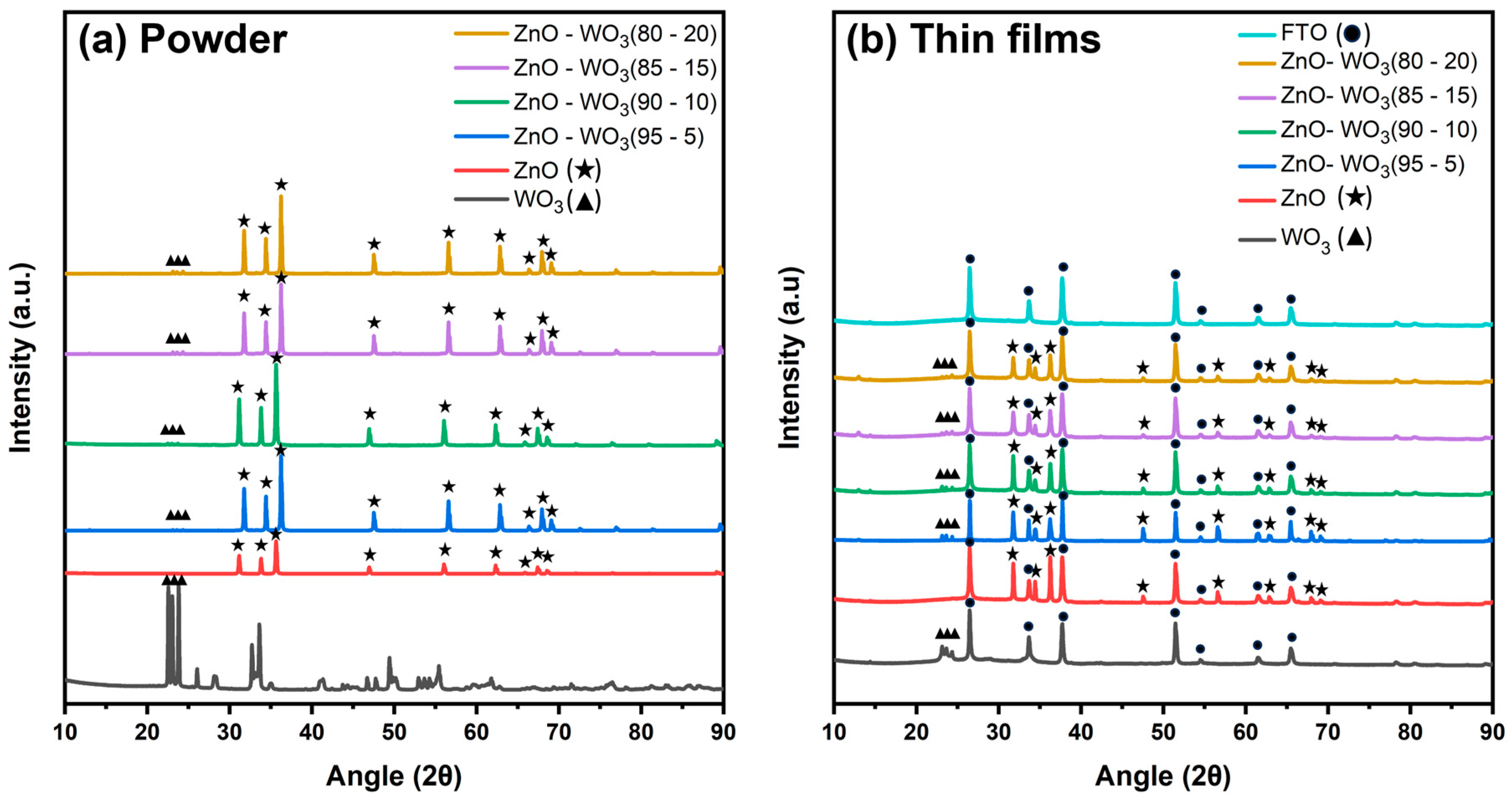
3.1. XRD Analysis
3.2. Raman Spectra Analysis
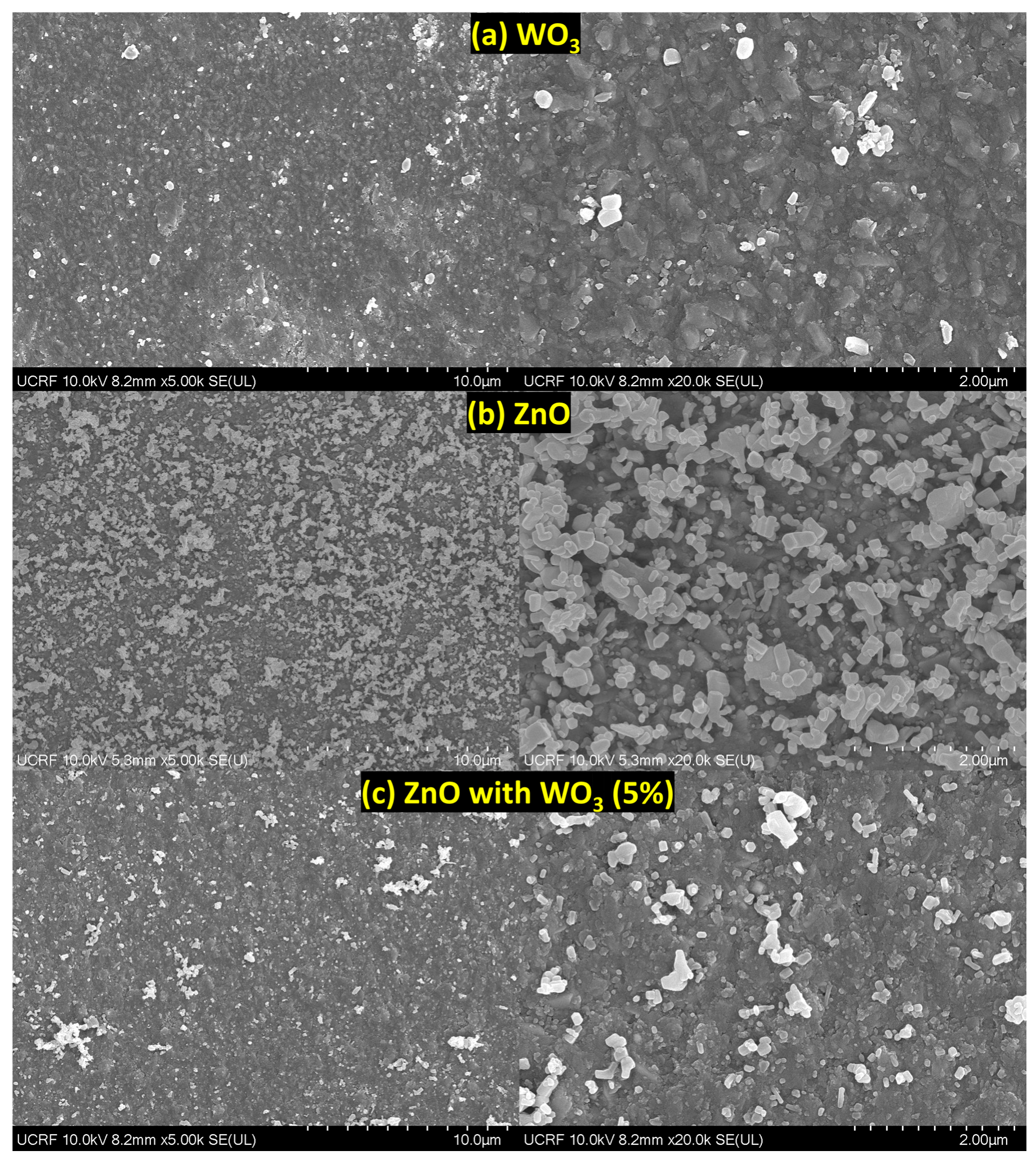
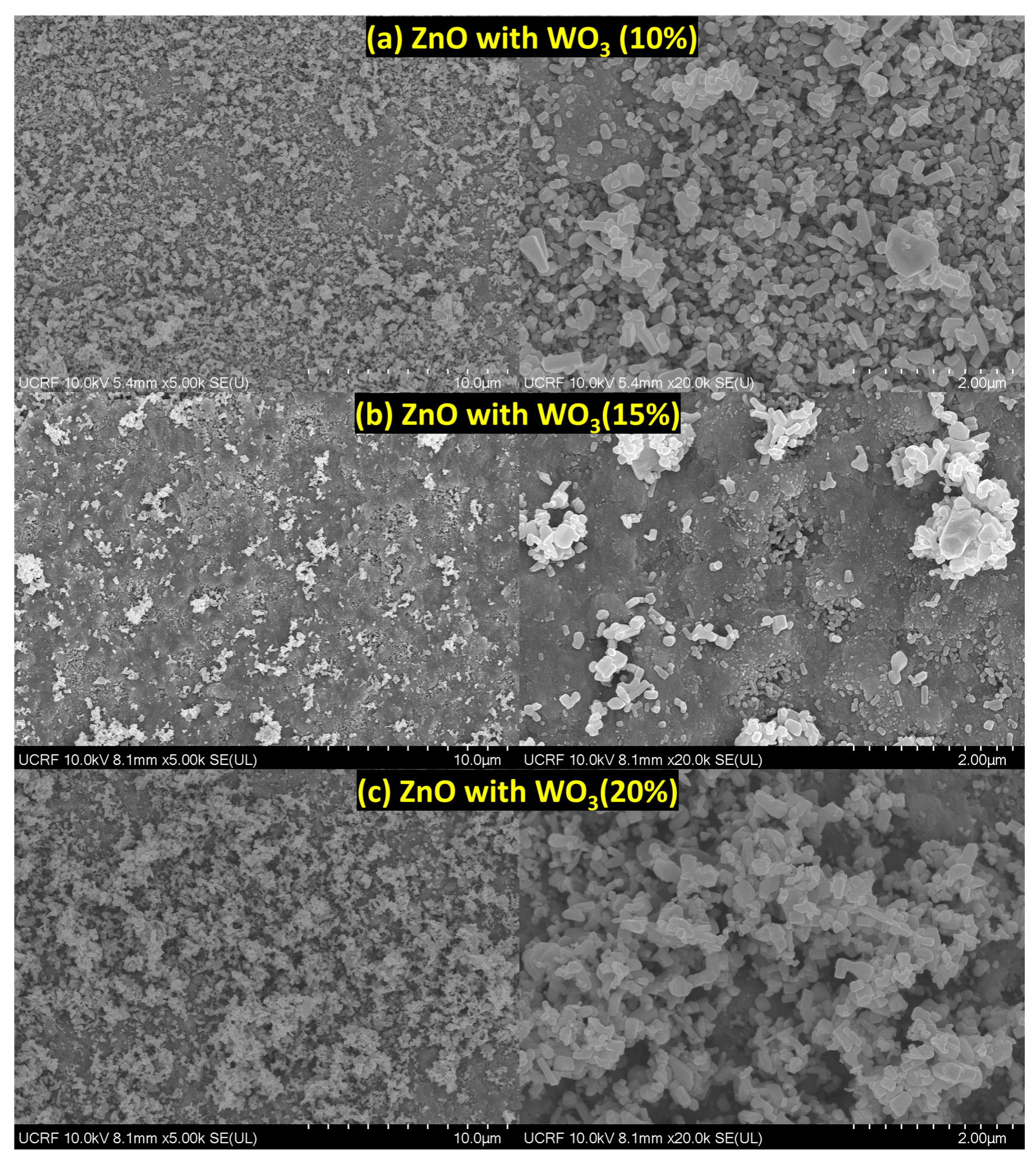
3.3. SEM Analysis
3.4. XPS Analysis of ZnO–WO3 NCs
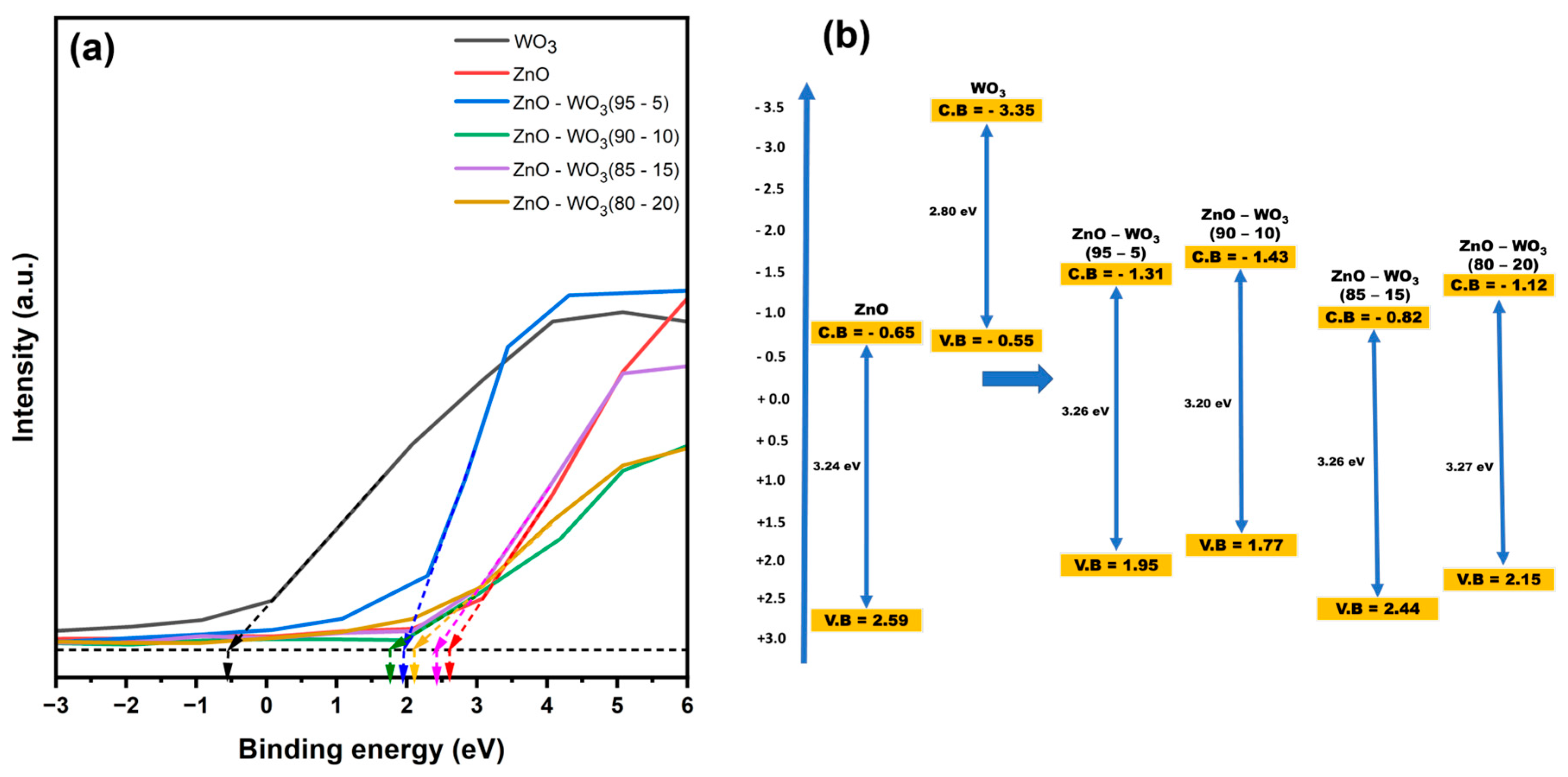
3.5. Analysis of the Optical Bandgap of ZnO–WO3 Hybrid NCs
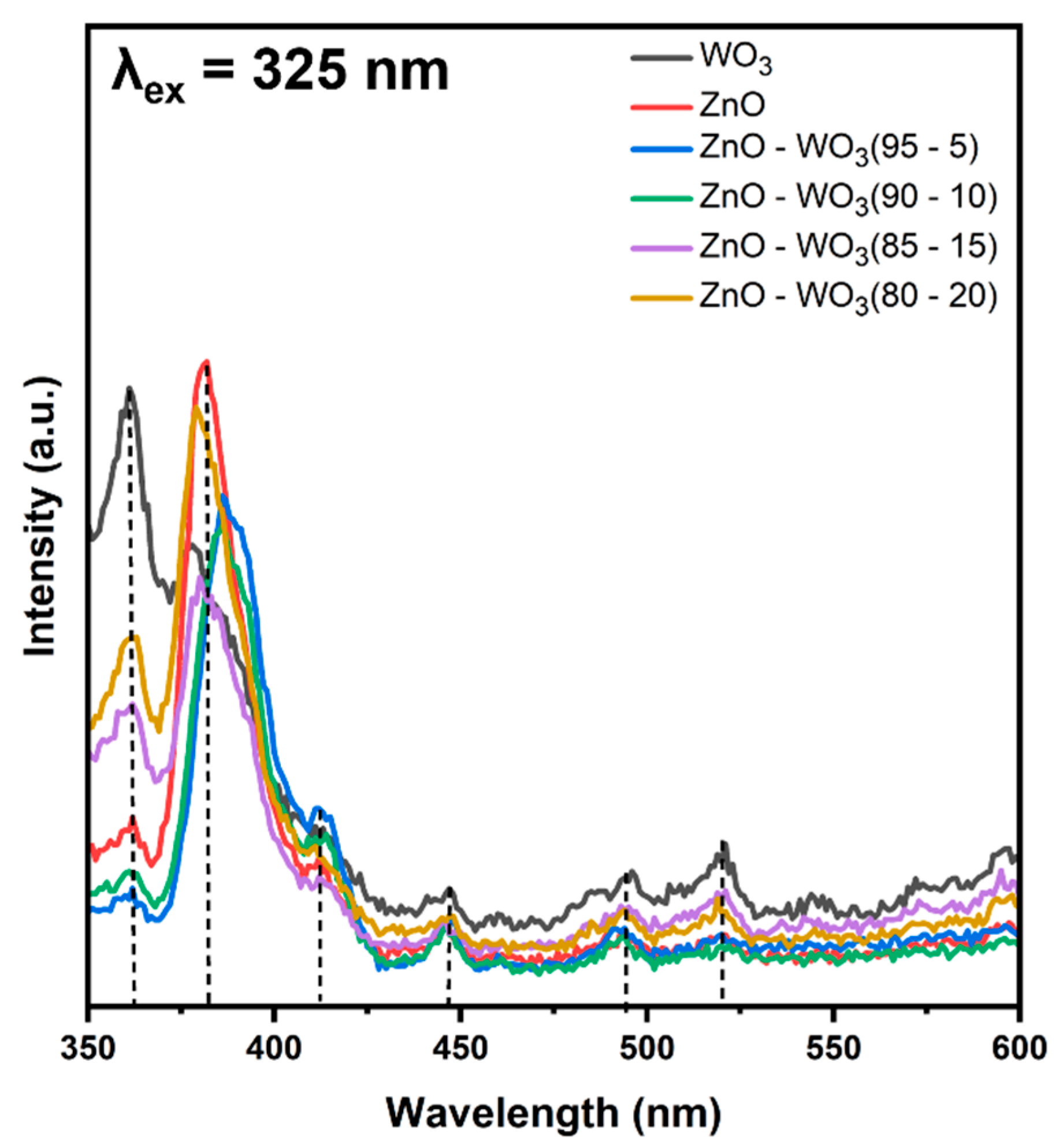
3.6. Photoluminescence Emission Behavior of ZnO–WO3 Hybrid NCs
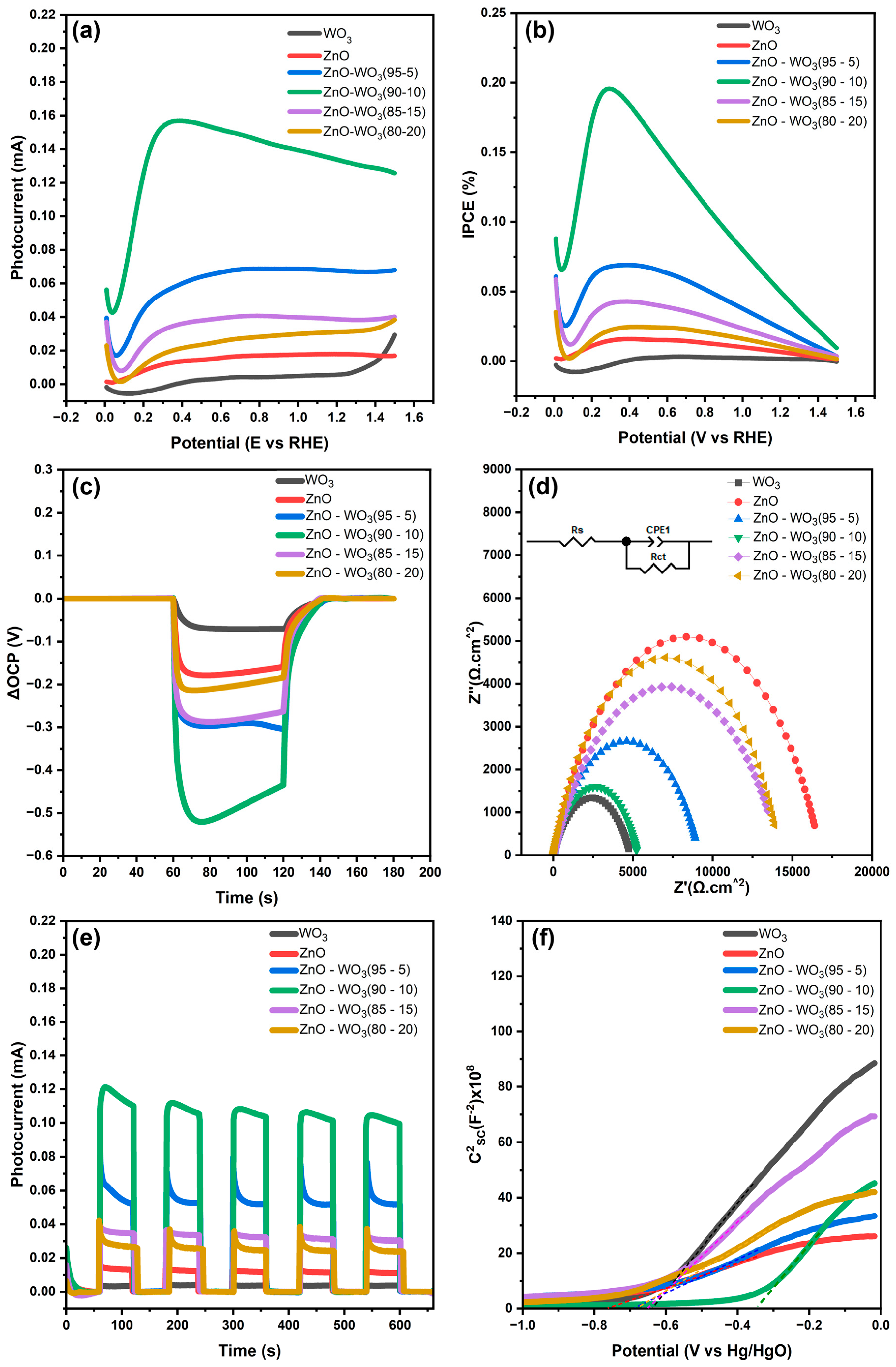
3.7. Photoelectrochemical Water Splitting Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bard, A.J.; Fox, M.A. Artificial Photosynthesis: Solar Splitting of Water to Hydrogen and Oxygen. Accounts Chem. Res. 1995, 28, 141–145. [Google Scholar] [CrossRef]
- Yuan, K.; Cao, Q.; Li, X.; Chen, H.-Y.; Deng, Y.; Wang, Y.-Y.; Luo, W.; Lu, H.-L.; Zhang, D.W. Synthesis of WO3@ZnWO4@ZnO-ZnO hierarchical nanocactus arrays for efficient photoelectrochemical water splitting. Nano Energy 2017, 41, 543–551. [Google Scholar] [CrossRef]
- Nguyen, T.; Thuy, T.; Cho, S.K.; Amangeldinova, Y. WO3–ZnO and CuO–ZnO nanocomposites as highly efficient photoanodes under visible light. Nanotechnology 2020, 31, 255702. [Google Scholar]
- Kalanur, S.S.; Duy, L.T.; Seo, H. Recent Progress in Photoelectrochemical Water Splitting Activity of WO3 Photoanodes. Top. Catal. 2018, 61, 1043–1076. [Google Scholar] [CrossRef]
- Palmstrom, A.F.; Santra, P.K.; Bent, S.F. Atomic layer deposition in nanostructured photovoltaics: Tuning optical, electronic and surface properties. Nanoscale 2015, 7, 12266–12283. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Carraro, G.; Gasparotto, A.; Maccato, C.; Altantzis, T.; Sada, C.; Kaunisto, K.; Ruoko, T.; Bals, S. Vapor Phase Fabrication of Nanoheterostructures Based on ZnO for Photoelectrochemical Water Splitting. Adv. Mater. Interfaces 2017, 4, 1700161. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, L.; Gao, R.; Zhang, Y.-C.; Pan, L.; Huang, C.; Liu, K.; Chang, X.-Y.; Zhang, X.; Zou, J.-J. Polarization-Enhanced direct Z-scheme ZnO-WO3−x nanorod arrays for efficient piezoelectric-photoelectrochemical Water splitting. Appl. Catal. B Environ. 2019, 259, 118079. [Google Scholar] [CrossRef]
- Wang, G.; Wang, H.; Ling, Y.; Tang, Y.; Yang, X.; Fitzmorris, R.C.; Wang, C.; Zhang, J.Z.; Li, Y. Hydrogen-treated TiO2 nanowire arrays for photoelectrochemical water splitting. Nano Lett. 2011, 11, 3026–3033. [Google Scholar] [CrossRef]
- Mishra, M.; Chun, D.-M. α-Fe2O3 as a photocatalytic material: A review. Appl. Catal. A Gen. 2015, 498, 126–141. [Google Scholar] [CrossRef]
- Sivula, K.; Le Formal, F.; Grätzel, M. Solar water splitting: Progress using hematite (α-Fe2O3) photoelectrodes. ChemSusChem 2011, 4, 432–449. [Google Scholar] [CrossRef]
- Su, J.; Guo, L.; Bao, N.; Grimes, C.A. Nanostructured WO3/BiVO4 heterojunction films for efficient photoelectrochemical water splitting. Nano Lett. 2011, 11, 1928–1933. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Chong, M.N.; Chan, E.S. Nanostructured tungsten trioxide thin films synthesized for photoelectrocatalytic water oxidation: A review. ChemSusChem 2014, 7, 2974–2997. [Google Scholar] [CrossRef] [PubMed]
- Palmas, S.; Castresana, P.A.; Mais, L.; Vacca, A.; Mascia, M.; Ricci, P.C. TiO2–WO3 nanostructured systems for photoelectrochemical applications. RSC Adv. 2016, 6, 101671–101682. [Google Scholar] [CrossRef]
- Wudil, Y.; Ahmad, U.; Gondal, M.; Al-Osta, M.A.; Almohammedi, A.; Sa’Id, R.; Hrahsheh, F.; Haruna, K.; Mohamed, M. Tuning of graphitic carbon nitride (g-C3N4) for photocatalysis: A critical review. Arab. J. Chem. 2023, 16. [Google Scholar] [CrossRef]
- Abd-Elrahim, A.; Chun, D.-M. Facile one-step deposition of ZnO-graphene nanosheets hybrid photoanodes for enhanced photoelectrochemical water splitting. J. Alloys Compd. 2021, 870, 159430. [Google Scholar] [CrossRef]
- Rao, P.M.; Cai, L.; Liu, C.; Cho, I.S.; Lee, C.H.; Weisse, J.M.; Yang, P.; Zheng, X. Simultaneously efficient light absorption and charge separation in WO3/BiVO4 Core/shell nanowire photoanode for photoelectrochemical water oxidation. Nano Lett. 2014, 14, 1099–1105. [Google Scholar] [CrossRef]
- Kim, M.-S.; Chun, D.-M.; Choi, J.-O.; Lee, J.-C.; Kim, K.-S.; Kim, Y.H.; Lee, C.S.; Ahn, S.-H. Room temperature deposition of TiO2 using nano particle deposition system (NPDS): Application to dye-sensitized solar cell (DSSC). Int. J. Precis. Eng. Manuf. 2011, 12, 749–752. [Google Scholar] [CrossRef]
- Galán-González, A.; Sivan, A.K.; Hernández-Ferrer, J.; Bowen, L.; Di Mario, L.; Martelli, F.; Benito, A.M.; Maser, W.K.; Chaudhry, M.U.; Gallant, A.; et al. Cobalt-Doped ZnO Nanorods Coated with Nanoscale Metal–Organic Framework Shells for Water-Splitting Photoanodes. ACS Appl. Nano Mater. 2020, 3, 7781–7788. [Google Scholar] [CrossRef]
- Han, J.; Liu, Z. Optimization and Modulation Strategies of Zinc Oxide-based Photoanodes for Highly Efficient Photoelectrochemical Water Splitting. ACS Appl. Energy Mater. 2021, 4, 1004–1013. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, G.; Liu, W.; Xi, Y.; Golosov, D.; Zavadski, S.; Melnikov, S. 3D core-shell WO3@α-Fe2O3 photoanode modified by ultrathin FeOOH layer for enhanced photoelectrochemical performances. J. Alloys Compd. 2020, 834, 154992. [Google Scholar] [CrossRef]
- Xu, Y.; Cao, Q.; Yi, Z.; Wu, P.; Cai, S. Adjusting the energy bands of WO3@ZnO nanocomposite heterojunction through the combination of WO3 thin film to improve its photoelectric performance. IEEE Access 2020, 8, 171350–171358. [Google Scholar] [CrossRef]
- Ji, J.; Sang, P.; Kim, J.H. Improving the photoelectrochemical performance of spin-coated WO3/BiVO4/ZnO photoanodes by maximizing charge transfer using an optimized ZnO decoration layer. Ceram. Int. 2021, 47, 26260–26270. [Google Scholar] [CrossRef]
- Chun, D.M.; Kim, M.H.; Lee, J.C.; Ahn, S.H. A nano-particle deposition system for ceramic and metal coating at room temperature and low vacuum conditions. Int. J. Precis. Eng. Manuf. 2008, 9, 51–53. [Google Scholar]
- Chun, D.-M.; Ahn, S.-H. Deposition mechanism of dry sprayed ceramic particles at room temperature using a nano-particle deposition system. Acta Mater. 2011, 59, 2693–2703. [Google Scholar] [CrossRef]
- Chun, D.-M.; Choi, J.-O.; Lee, C.S.; Ahn, S.-H. Effect of stand-off distance for cold gas spraying of fine ceramic particles (<5 μm) under low vacuum and room temperature using nano-particle deposition system (NPDS). Surf. Coatings Technol. 2012, 206, 2125–2132. [Google Scholar] [CrossRef]
- Abd-Elrahim, A.; Chun, D.-M. Fabrication of efficient nanostructured Co3O4-Graphene bifunctional catalysts: Oxygen evolution, hydrogen evolution, and H2O2 sensing. Ceram. Int. 2020, 46, 23479–23498. [Google Scholar] [CrossRef]
- Yogamalar, R.; Srinivasan, R.; Vinu, A.; Ariga, K.; Bose, A.C. X-ray peak broadening analysis in ZnO nanoparticles. Solid State Commun. 2009, 149, 1919–1923. [Google Scholar] [CrossRef]
- Kalanur, S.S.; Hwang, Y.J.; Chae, S.Y.; Joo, O.S. Facile growth of aligned WO3 nanorods on FTO substrate for enhanced photoanodic water oxidation activity. J. Mater. Chem. A 2013, 1, 3479–3488. [Google Scholar] [CrossRef]
- Abd-Elrahim, A.; Chun, D.-M. Nanosized Co3O4–MoS2 heterostructure electrodes for improving the oxygen evolution reaction in an alkaline medium. J. Alloys Compd. 2020, 853, 156946. [Google Scholar] [CrossRef]
- Kim, H.; Choi, D.; Kim, K.; Chu, W.; Chun, D.-M.; Lee, C.S. Effect of particle size and amorphous phase on the electrochromic properties of kinetically deposited WO3 films. Sol. Energy Mater. Sol. Cells 2018, 177, 44–50. [Google Scholar] [CrossRef]
- Mohammed, M.M.; Abd-Elrahim, A.; Chun, D.-M. One-step deposition of a Ni(OH)2-graphene hybrid prepared by vacuum kinetic spray for high energy density hybrid supercapacitor. Mater. Chem. Phys. 2020, 244, 122701. [Google Scholar] [CrossRef]
- Daniel, M.; Desbat, B.; Lassegues, J.; Gerand, B.; Figlarz, M. Infrared and Raman study of WO3 tungsten trioxides and WO3, xH2O tungsten trioxide tydrates. J. Solid State Chem. 1987, 67, 235–247. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Song, G.; Hong, J.; Van, T.K.; Pawar, A.U.; Kim, D.Y.; Kim, C.W.; Haider, Z.; Kang, Y.S. Facile fabrication of WO3 nanoplates thin films with dominant crystal facet of (002) for water splitting. Cryst. Growth Des. 2014, 14, 6057–6066. [Google Scholar] [CrossRef]
- Ou, J.Z.; Yaacob, M.H.; Breedon, M.; Zheng, H.D.; Campbell, J.L.; Latham, K.; Plessis, J.D.; Wlodarski, W.; Kalantar-Zadeh, K. In situ Raman spectroscopy of H2 interaction with WO3 films. Phys. Chem. Chem. Phys. 2011, 13, 7330–7339. [Google Scholar] [CrossRef] [PubMed]
- Wolcott, A.; Kuykendall, T.R.; Chen, W.; Chen, S.; Zhang, J.Z. Synthesis and characterization of ultrathin WO3 nanodisks utilizing long-chain Poly(ethylene glycol). J. Phys. Chem. B 2006, 110, 25288–25296. [Google Scholar] [CrossRef] [PubMed]
- Spectroscopies, Y.; Shigesato, Y.; Murayama, A.; Kamimori, T.; Matsuhiro, K. Characterization of Evaporated Amorphous WO3 Films. Appl. Surf. Sci. 1988, 33, 811. [Google Scholar]
- Díaz-Reyes, J.; Castillo-Ojeda, R.; Galván-Arellano, M.; Zaca-Moran, O. Characterization of WO3 thin films grown on silicon by HFMOD. Adv. Condens. Matter Phys. 2013, 2013, 591787. [Google Scholar] [CrossRef]
- Zhuo, R.F.; Feng, H.T.; Liang, Q.; Liu, J.Z.; Chen, J.T.; Yan, D.; Feng, J.J.; Li, H.J.; Cheng, S.; Geng, B.S.; et al. Morphology-controlled synthesis, growth mechanism, optical and microwave absorption properties of ZnO nanocombs. J. Phys. D Appl. Phys. 2008, 41, 185405. [Google Scholar] [CrossRef]
- Zhang, R.; Yin, P.-G.; Wang, N.; Guo, L. Photoluminescence and Raman scattering of ZnO nanorods. Solid State Sci. 2009, 11, 865–869. [Google Scholar] [CrossRef]
- Venkatesh, P.S.; Ramakrishnan, V.; Jeganathan, K. Raman silent modes in vertically aligned undoped ZnO nanorods. Phys. B Condens. Matter 2016, 481, 204–208. [Google Scholar] [CrossRef]
- Souissi, A.; Marzouki, A.; Sayari, A.; Sallet, V.; Lusson, A.; Oueslati, M. Origin of the Raman mode at 379 cm−1 observed in ZnO thin films grown on sapphire. J. Raman Spectrosc. 2011, 42, 1574–1577. [Google Scholar] [CrossRef]
- Georgekutty, R.; Seery, M.K.; Pillai, S.C. A highly efficient Ag-ZnO photocatalyst: Synthesis, properties, and mechanism. J. Phys. Chem. C 2008, 112, 13563–13570. [Google Scholar] [CrossRef]
- Decremps, F.; Pellicer-Porres, J.; Saitta, A.M.; Chervin, J.-C.; Polian, A. High-pressure Raman spectroscopy study of wurtzite ZnO. Phys. Rev. B 2002, 65, 092101. [Google Scholar] [CrossRef]
- Khan, A. Raman Spectroscopic Study of the ZnO Nanostructures. J. Pakistan Mater. Soc. 2010, 4, 5–9. [Google Scholar]
- Tayebi, M.; Tayyebi, A.; Masoumi, Z.; Lee, B.-K. Photocorrosion suppression and photoelectrochemical (PEC) enhancement of ZnO via hybridization with graphene nanosheets. Appl. Surf. Sci. 2019, 502, 144189. [Google Scholar] [CrossRef]
- Liu, F.; Chen, X.; Xia, Q.; Tian, L.; Chen, X. Ultrathin tungsten oxide nanowires: Oleylamine assisted nonhydrolytic growth, oxygen vacancies and good photocatalytic properties. RSC Adv. 2015, 5, 77423–77428. [Google Scholar] [CrossRef]
- Rettie, A.J.E.; Klavetter, K.C.; Lin, J.-F.; Dolocan, A.; Celio, H.; Ishiekwene, A.; Bolton, H.L.; Pearson, K.N.; Hahn, N.T.; Mullins, C.B. Improved visible light harvesting of WO3 by incorporation of sulfur or iodine: A tale of two impurities. Chem. Mater. 2014, 26, 1670–1677. [Google Scholar] [CrossRef]
- Thiyagarajan, T.; Deivasigamani, V.; Raj, M.; Joseph, C.; Dheivasigamani, T.; Palanivel, B.; Hamdy, M.S.; Shkir, M. Facile synthesis and characterization of WO3/CuWO4 nanocomposites for the removal of toxic methylene blue dye. Korean J. Chem. Eng. 2021, 38, 952–965. [Google Scholar] [CrossRef]
- Ilican, S.; Caglar, M.; Aksoy, S.; Caglar, Y. XPS studies of electrodeposited grown f-doped ZnO rods and electrical properties of p-Si/n-FZN heterojunctions. J. Nanomater. 2016, 2016, 6729032. [Google Scholar] [CrossRef]
- Limwichean, S.; Kasayapanand, N.; Ponchio, C.; Nakajima, H.; Patthanasettakul, V.; Eiamchai, P.; Meng, G.; Horprathum, M. Morphology-controlled fabrication of nanostructured WO3 thin films by magnetron sputtering with glancing angle deposition for enhanced efficiency photo-electrochemical water splitting. Ceram. Int. 2021, 47, 34455–34462. [Google Scholar] [CrossRef]
- Xu, S.; Fu, D.; Song, K.; Wang, L.; Yang, Z.; Yang, W.; Hou, H. One-dimensional WO3/BiVO4 heterojunction photoanodes for efficient photoelectrochemical water splitting. Chem. Eng. J. 2018, 349, 368–375. [Google Scholar] [CrossRef]
- He, L.; Zhang, S.; Zhang, J.; Chen, G.; Meng, S.; Fan, Y.; Zheng, X.; Chen, S. Investigation on the mechanism and inner impetus of photogenerated charge transfer in WO3/ZnO heterojunction photocatalysts. J. Phys. Chem. C 2020, 124, 27916–27929. [Google Scholar] [CrossRef]
- Leonard, K.C.; Nam, K.M.; Lee, H.C.; Kang, S.H.; Park, H.S.; Bard, A.J. ZnWO4/WO3 composite for improving photoelectrochemical water oxidation. J. Phys. Chem. C 2013, 117, 15901–15910. [Google Scholar] [CrossRef]
- Tahir, M.B.; Riaz, K.N.; Asiri, A.M. Boosting the performance of visible light-driven WO3/g-C3N4 anchored with BiVO4 nanoparticles for photocatalytic hydrogen evolution. Int. J. Energy Res. 2019, 43, 5747–5758. [Google Scholar] [CrossRef]
- Othman, A.; Ali, M.A.; Ibrahim, E.; Osman, M. Influence of Cu doping on structural, morphological, photoluminescence, and electrical properties of ZnO nanostructures synthesized by ice-bath assisted sonochemical method. J. Alloys Compd. 2016, 683, 399–411. [Google Scholar] [CrossRef]
- Saikia, L.; Bhuyan, D.; Saikia, M.; Malakar, B.; Dutta, D.K.; Sengupta, P. Photocatalytic performance of ZnO nanomaterials for self sensitized degradation of malachite green dye under solar light. Appl. Catal. A Gen. 2015, 490, 42–49. [Google Scholar] [CrossRef]
- Othman, A.; Osman, M.; Ibrahim, E.; Ali, M.A. Sonochemically synthesized ZnO nanosheets and nanorods: Annealing temperature effects on the structure, and optical properties. Ceram. Int. 2016, 43, 527–533. [Google Scholar] [CrossRef]
- Mazhdi, M.; Saydi, J.; Karimi, M.; Seidi, J.; Mazhdi, F. A study on optical, photoluminescence and thermoluminescence properties of ZnO and Mn doped-ZnO nanocrystalline particles. Optik 2013, 124, 4128–4133. [Google Scholar] [CrossRef]
- Othman, A.; Osman, M.; Ibrahim, E.; Ali, M.A.; Abd-Elrahim, A. Mn-doped ZnO nanocrystals synthesized by sonochemical method: Structural, photoluminescence, and magnetic properties. Mater. Sci. Eng. B 2017, 219, 1–9. [Google Scholar] [CrossRef]
- Zeng, H.; Duan, G.; Li, Y.; Yang, S.; Xu, X.; Cai, W. Blue luminescence of ZnO nanoparticles based on non-equilibrium processes: Defect origins and emission controls, Adv. Funct. Mater. 2010, 20, 561–572. [Google Scholar] [CrossRef]
- Reddy, A.J.; Kokila, M.; Nagabhushana, H.; Chakradhar, R.; Shivakumara, C.; Rao, J.; Nagabhushana, B. Structural, optical and EPR studies on ZnO:Cu nanopowders prepared via low temperature solution combustion synthesis. J. Alloys Compd. 2011, 509, 5349–5355. [Google Scholar] [CrossRef]
- Pancielejko, A.; Rzepnikowska, M.; Zaleska-Medynska, A.; Łuczak, J.; Mazierski, P. Enhanced visible light active WO3 thin films toward air purification: Effect of the synthesis conditions. Materials 2020, 13, 3506. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhao, Y.; Liu, Z.; Dunnill, C.W.; Gregory, D.H.; Zhu, Y. Nanostructural Evolution: From One-Dimensional Tungsten Oxide Nanowires to Three-Dimensional Ferberite Flowers. Chem. Mater. 2008, 20, 5657–5665. [Google Scholar] [CrossRef]
- Kato, K.; Uemura, Y.; Asakura, K.; Yamakata, A. Role of Oxygen Vacancy in the Photocarrier Dynamics of WO3Photocatalysts: The Case of Recombination Centers. J. Phys. Chem. C 2022, 126, 9257–9263. [Google Scholar] [CrossRef]
- Szilágyi, I.M.; Fórizs, B.; Rosseler, O.; Szegedi, Á.; Németh, P.; Király, P.; Tárkányi, G.; Vajna, B.; Varga-Josepovits, K.; László, K.; et al. WO3 photocatalysts: Influence of structure and composition. J. Catal. 2012, 294, 119–127. [Google Scholar] [CrossRef]
- Ha, J.-H.; Muralidharan, P.; Kim, D.K. Hydrothermal synthesis and characterization of self-assembled h-WO3 nanowires/nanorods using EDTA salts. J. Alloys Compd. 2009, 475, 446–451. [Google Scholar] [CrossRef]
- Wang, M.; Kafizas, A.; Sathasivam, S.; Blunt, M.O.; Moss, B.; Gonzalez-Carrero, S.; Carmalt, C.J. ZnO/BiOI heterojunction photoanodes with enhanced photoelectrochemical water oxidation activity. Appl. Catal. B Environ. 2023, 331, 122657. [Google Scholar] [CrossRef]
- Abd-Elrahim, A.; Chun, D.-M. Room-temperature deposition of ZnO-graphene nanocomposite hybrid photocatalysts for improved visible-light-driven degradation of methylene blue. Ceram. Int. 2021, 47, 12812–12825. [Google Scholar] [CrossRef]
- Chen, H.M.; Chen, C.K.; Liu, R.-S.; Zhang, L.; Zhang, J.; Wilkinson, D.P. Nano-architecture and material designs for water splitting photoelectrodes. Chem. Soc. Rev. 2012, 41, 5654–5671. [Google Scholar] [CrossRef]
- Jung, J.-Y.; Yu, J.-Y.; Lee, J.-H. Dynamic Photoelectrochemical Device with Open-Circuit Potential Insensitive to Thermodynamic Voltage Loss. J. Phys. Chem. Lett. 2018, 9, 5412–5418. [Google Scholar] [CrossRef]
- Finger, F.; Welter, K.; Urbain, F.; Smirnov, V.; Kaiser, B.; Jaegermann, W. Photoelectrochemical Water Splitting using Adapted Silicon Based Multi-Junction Solar Cell Structures: Development of Solar Cells and Catalysts, Upscaling of Combined Photovoltaic-Electrochemical Devices and Performance Stability. Z. Phys. Chem. 2020, 234, 1055–1095. [Google Scholar] [CrossRef]
- Guo, S.; Zhao, X.; Zhang, W.; Wang, W. Optimization of electrolyte to significantly improve photoelectrochemical water splitting performance of ZnO nanowire arrays. Mater. Sci. Eng. B 2018, 227, 129–135. [Google Scholar] [CrossRef]
- Yagi, M.; Maruyama, S.; Sone, K.; Nagai, K.; Norimatsu, T. Preparation and photoelectrocatalytic activity of a nano-structured WO3 platelet film. J. Solid State Chem. 2008, 181, 175–182. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Li, W.; Han, S.; Liu, C. Photoelectrochemical properties and photocatalytic activity of nitrogen-doped nanoporous WO3 photoelectrodes under visible light. Appl. Surf. Sci. 2012, 258, 5038–5045. [Google Scholar] [CrossRef]
- Li, W.; Li, J.; Wang, X.; Ma, J.; Chen, Q. Photoelectrochemical and physical properties of WO3 films obtained by the polymeric precursor method. Int. J. Hydrogen Energy 2010, 35, 13137–13145. [Google Scholar] [CrossRef]
- Gelderman, K.; Lee, L.; Donne, S.W.; Hermans, Y.; Klein, A.; Ellmer, K.; van de Krol, R.; Toupance, T.; Jaegermann, W.; Kang, H.-Y.; et al. Flat-band potential of a semiconductor: Using the Mott-Schottky equation. J. Chem. Educ. 2007, 84, 685–688. [Google Scholar] [CrossRef]
- Schwarzburg, K.; Willig, F. Modeling of electrical transients in the semiconductor/electrolyte cell for photogeneration of charge carriers in the bulk. J. Phys. Chem. B 1997, 101, 2451–2458. [Google Scholar] [CrossRef]
- Zhang, W.; Yan, D.; Appavoo, K.; Cen, J.; Wu, Q.; Orlov, A.; Sfeir, M.Y.; Liu, M. Unravelling Photocarrier Dynamics beyond the Space Charge Region for Photoelectrochemical Water Splitting. Chem. Mater. 2017, 29, 4036–4043. [Google Scholar] [CrossRef]
- Ghorbani, M.; Abdizadeh, H.; Taheri, M.; Golobostanfard, M.R. Enhanced photoelectrochemical water splitting in hierarchical porous ZnO/Reduced graphene oxide nanocomposite synthesized by sol-gel method. Int. J. Hydrogen Energy 2018, 43, 7754–7763. [Google Scholar] [CrossRef]
- Lv, R.; Wang, T.; Su, F.; Zhang, P.; Li, C.; Gong, J. Facile synthesis of ZnO nanopencil arrays for photoelectrochemical water splitting. Nano Energy 2014, 7, 143–150. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Wang, Y.; Liu, Z.; Han, J.; Ya, J. ZnO/CuInS2 core/shell heterojunction nanoarray for photoelectrochemical water splitting. Int. J. Hydrogen Energy 2012, 37, 15029–15037. [Google Scholar] [CrossRef]

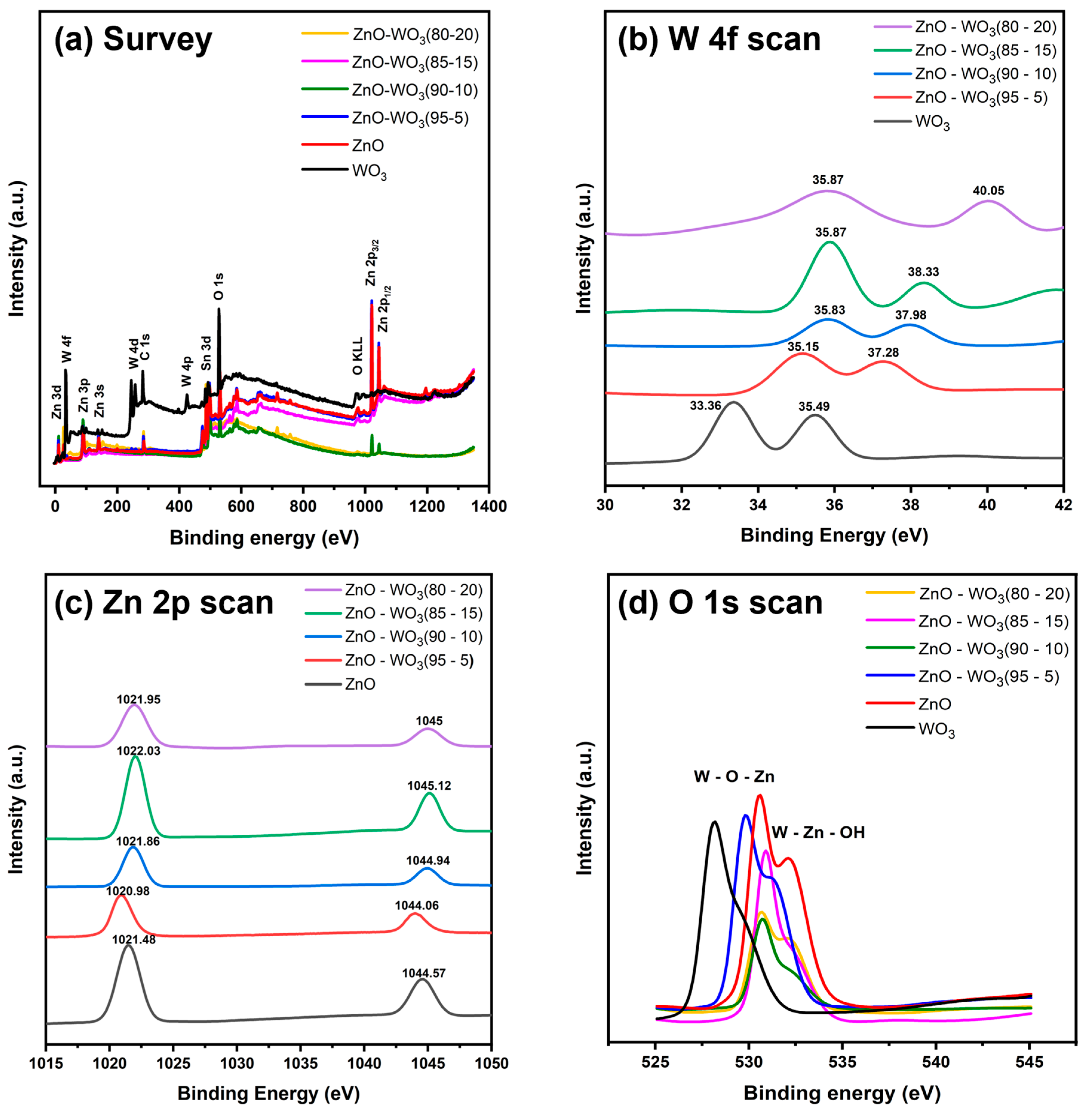


| Band | Binding Energy (eV) | |||||
|---|---|---|---|---|---|---|
| WO3 | ZnO–WO3 (80–20%) | ZnO–WO3 (85–15%) | ZnO–WO3 (90–10%) | ZnO–WO3 (95–5%) | ZnO | |
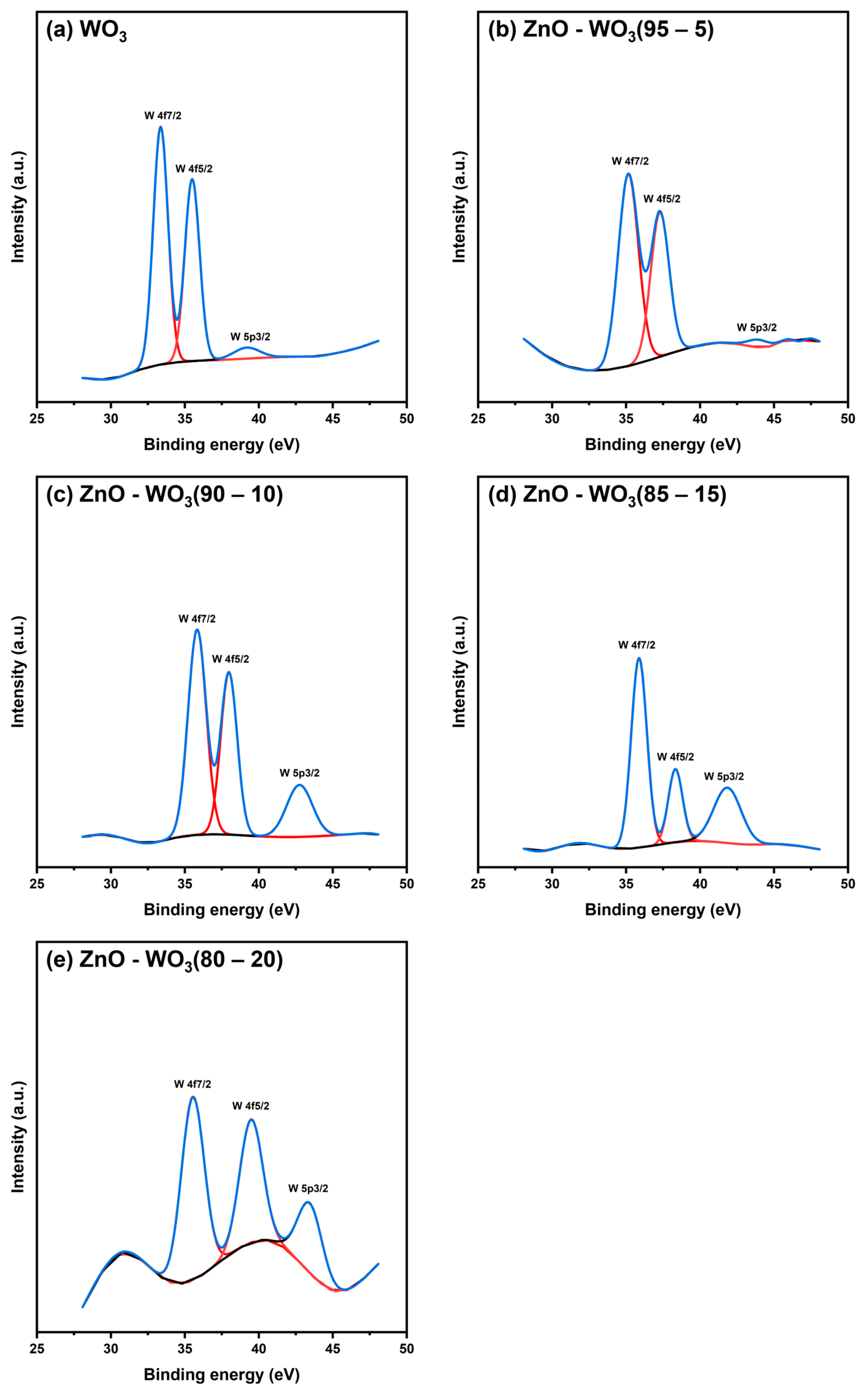
| W 4f | 33.36 | 35.87 | 35.87 | 35.83 | 35.15 | - |
| 35.49 | 40.05 | 38.33 | 37.98 | 37.28 | - | |
| O 1s | 528.04 | 530.56 | 530.83 | 530.66 | 529.72 | 530.49 |
| 529.24 | 532.14 | 531.90 | 531.90 | 531.27 | 532.16 | |
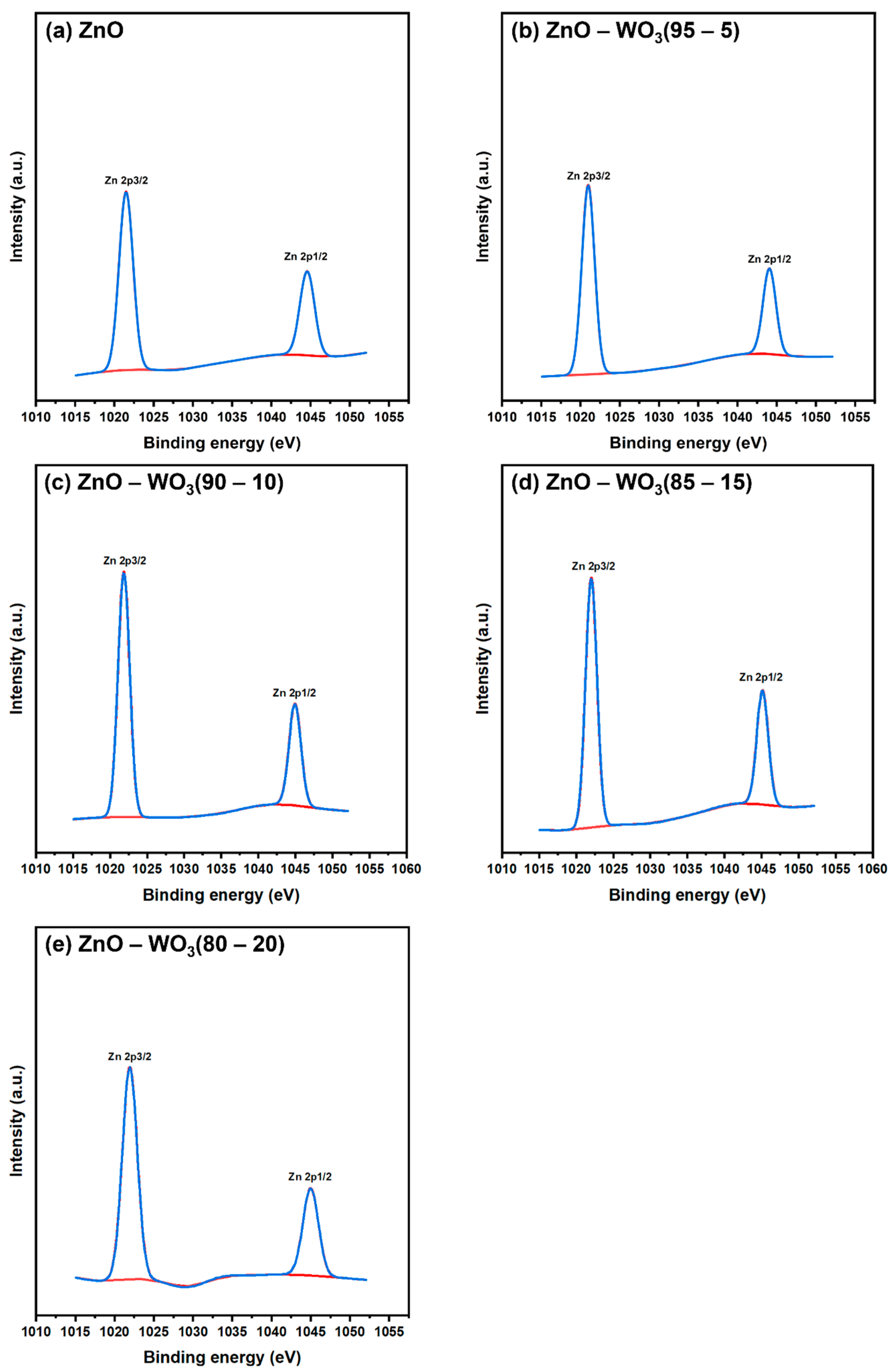
| Zn 2p | - | 1021.95 | 1022.03 | 1021.86 | 1020.98 | 1021.48 |
| - | 1045 | 1045.12 | 1044.94 | 1044.06 | 1044.57 | |
| Electrodes | Photocurrent (mA·cm−2) | IPCE (%) |
|---|---|---|
| WO3 | 0.006 | 0.003 |
| ZnO | 0.018 | 0.016 |
| ZnO–WO3 NCs (5 wt.% WO3) | 0.066 | 0.069 |
| ZnO–WO3 NCs (10 wt.% WO3) | 0.133 | 0.196 |
| ZnO–WO3 NCs (15 wt.% WO3) | 0.038 | 0.043 |
| ZnO–WO3 NCs (20 wt.% WO3) | 0.031 | 0.025 |
| Electrodes | Rs (Ω) | Rct (kΩ) | CPE (F) |
|---|---|---|---|
| WO3 | 8.34 | 4.82 | 0.00021 |
| ZnO | 10.97 | 16.76 | 0.000099 |
| ZnO–WO3 NCs (5 wt.% WO3) NCs | 9.76 | 9.13 | 0.00017 |
| ZnO–WO3 NCs (10 wt.% WO3) NCs | 11.43 | 5.27 | 0.00016 |
| ZnO–WO3 NCs (15 wt.% WO3) NCs | 8.88 | 14.22 | 0.00017823 |
| ZnO–WO3 NCs (20 wt.% WO3) NCs | 13.09 | 14.56 | 0.00016 |
| Electrode | Vfb | ND × 1026 | Wscl (nm) |
|---|---|---|---|
| WO3 | −0.64 | 0.46 | 12.84 |
| ZnO | −0.78 | 9.24 | 1.24 |
| ZnO–WO3 NCs (5 wt.% WO3) | −0.69 | 6.70 | 1.42 |
| ZnO–WO3 NCs (10 wt.% WO3) | −0.36 | 2.69 | 1.96 |
| ZnO–WO3 NCs (15 wt.% WO3) | −0.65 | 4.06 | 1.79 |
| ZnO–WO3 NCs (20 wt.% WO3) | −0.69 | 5.70 | 1.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malik, M.S.; Roy, D.; Chun, D.-M.; Abd-Elrahim, A.G. One-Step Dry Coating of Hybrid ZnO–WO3 Nanosheet Photoanodes for Photoelectrochemical Water Splitting with Composition-Dependent Performance. Micromachines 2023, 14, 2189. https://doi.org/10.3390/mi14122189
Malik MS, Roy D, Chun D-M, Abd-Elrahim AG. One-Step Dry Coating of Hybrid ZnO–WO3 Nanosheet Photoanodes for Photoelectrochemical Water Splitting with Composition-Dependent Performance. Micromachines. 2023; 14(12):2189. https://doi.org/10.3390/mi14122189
Chicago/Turabian StyleMalik, Muhammad Shehroze, Deepto Roy, Doo-Man Chun, and A. G. Abd-Elrahim. 2023. "One-Step Dry Coating of Hybrid ZnO–WO3 Nanosheet Photoanodes for Photoelectrochemical Water Splitting with Composition-Dependent Performance" Micromachines 14, no. 12: 2189. https://doi.org/10.3390/mi14122189
APA StyleMalik, M. S., Roy, D., Chun, D.-M., & Abd-Elrahim, A. G. (2023). One-Step Dry Coating of Hybrid ZnO–WO3 Nanosheet Photoanodes for Photoelectrochemical Water Splitting with Composition-Dependent Performance. Micromachines, 14(12), 2189. https://doi.org/10.3390/mi14122189








