A Systematic Study of the Factors Affecting the Surface Quality of Chemically Vapor-Deposited Diamond during Chemical and Mechanical Polishing
Abstract
1. Introduction
2. Materials and Methods
2.1. Diamond Sample Preparation
2.2. Methods of Mechanical Polishing and Grinding
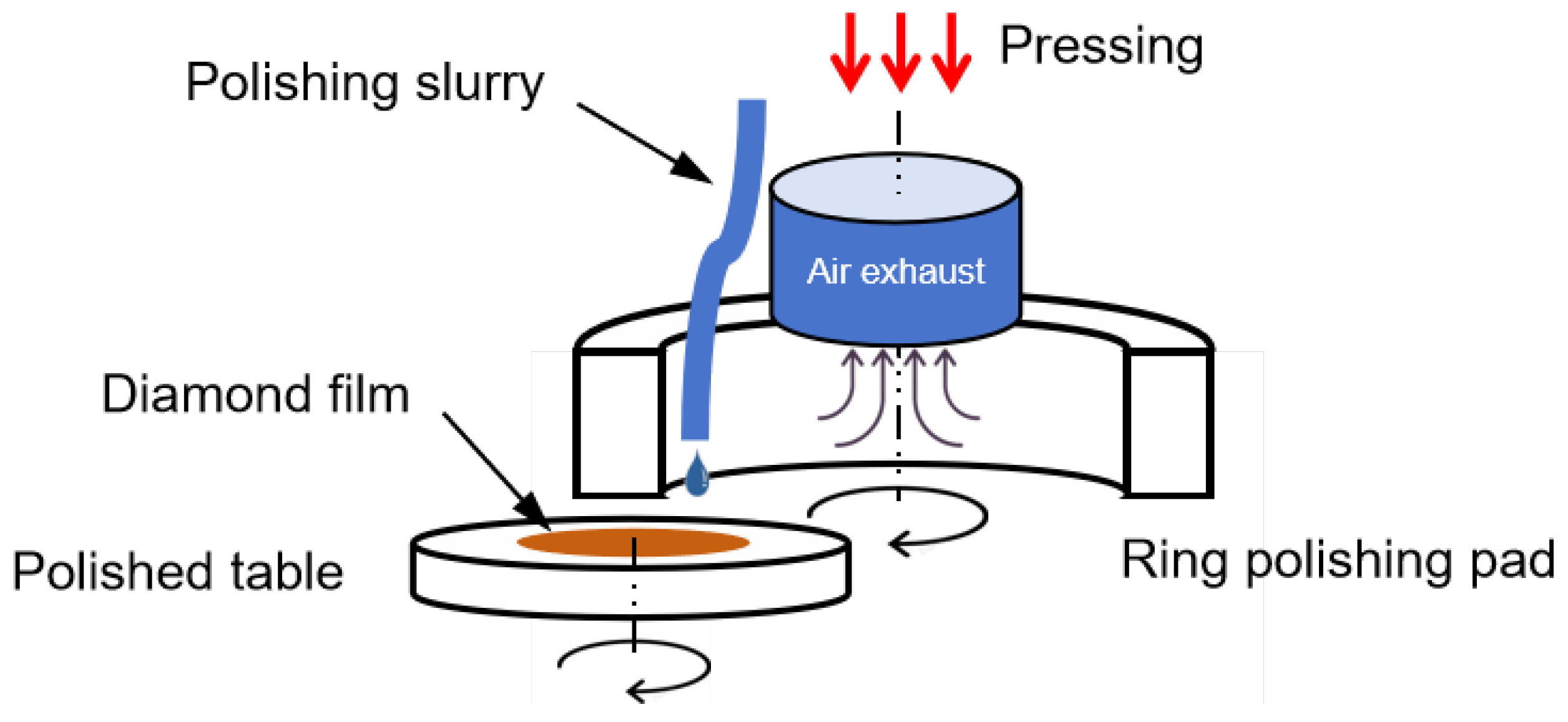
2.3. Method of Chemical Mechanical Polishing
2.4. Characterizations of Surface Quality
3. Results and Discussion
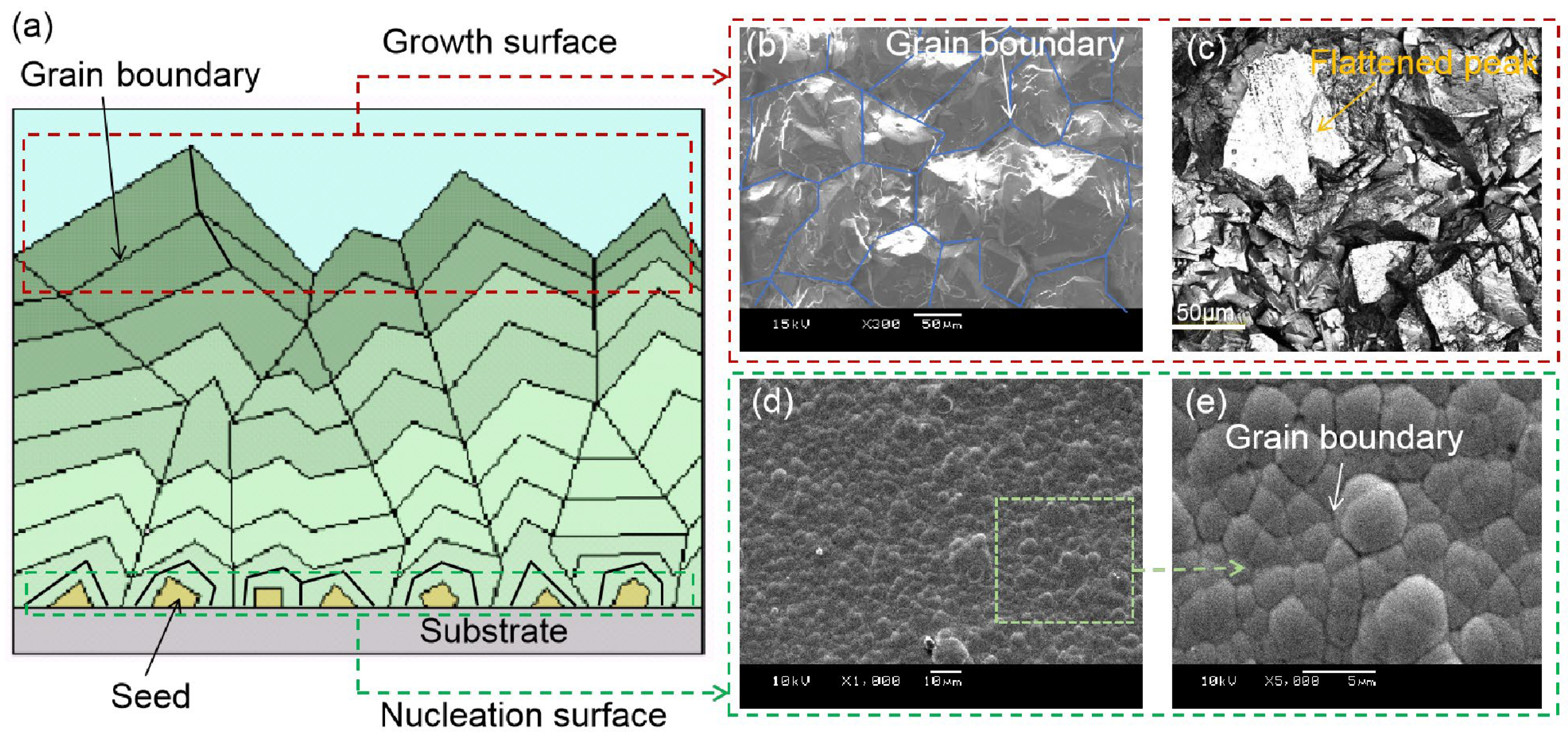
3.1. Influence of CVD Diamond Grain Growth
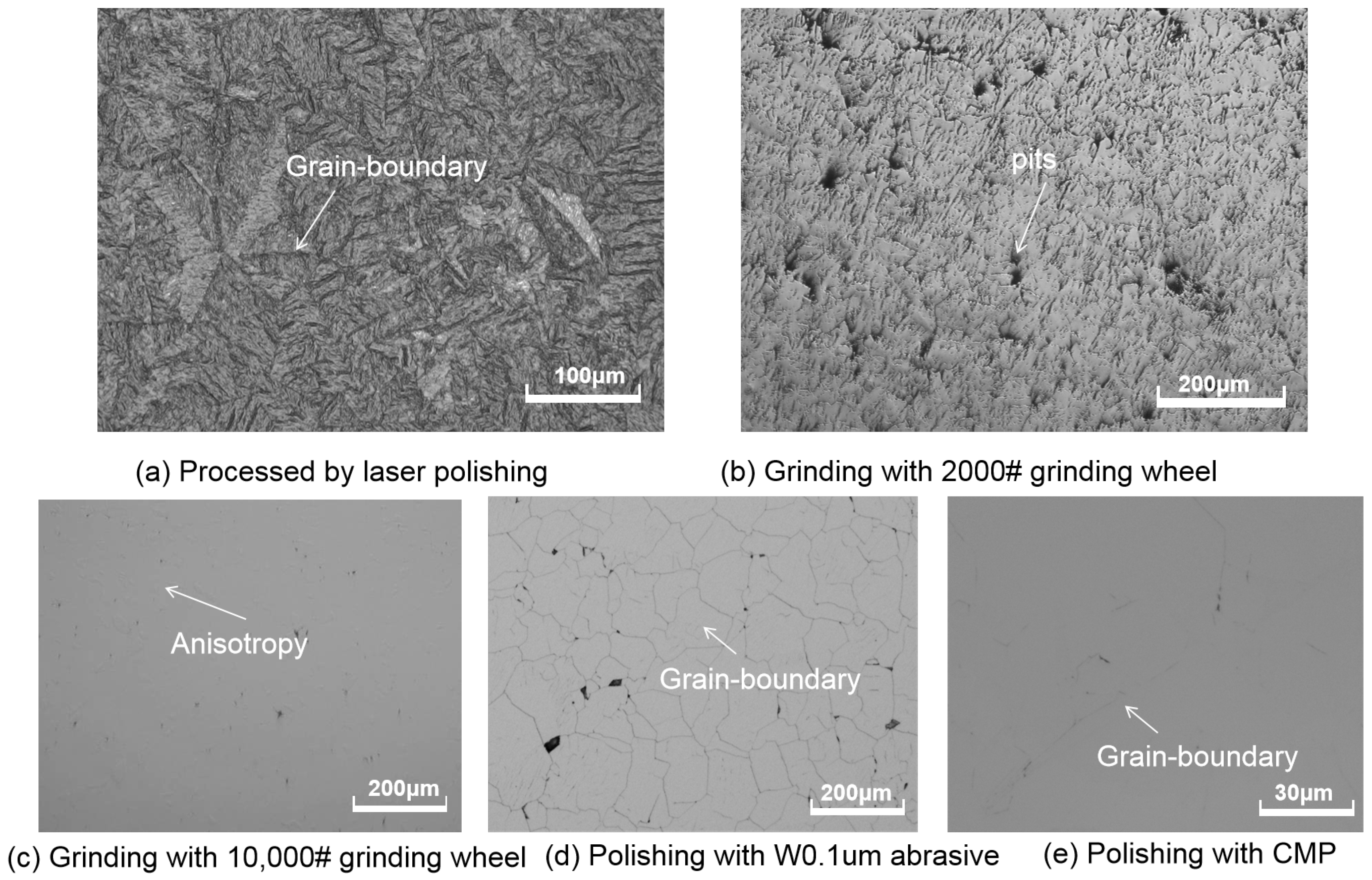
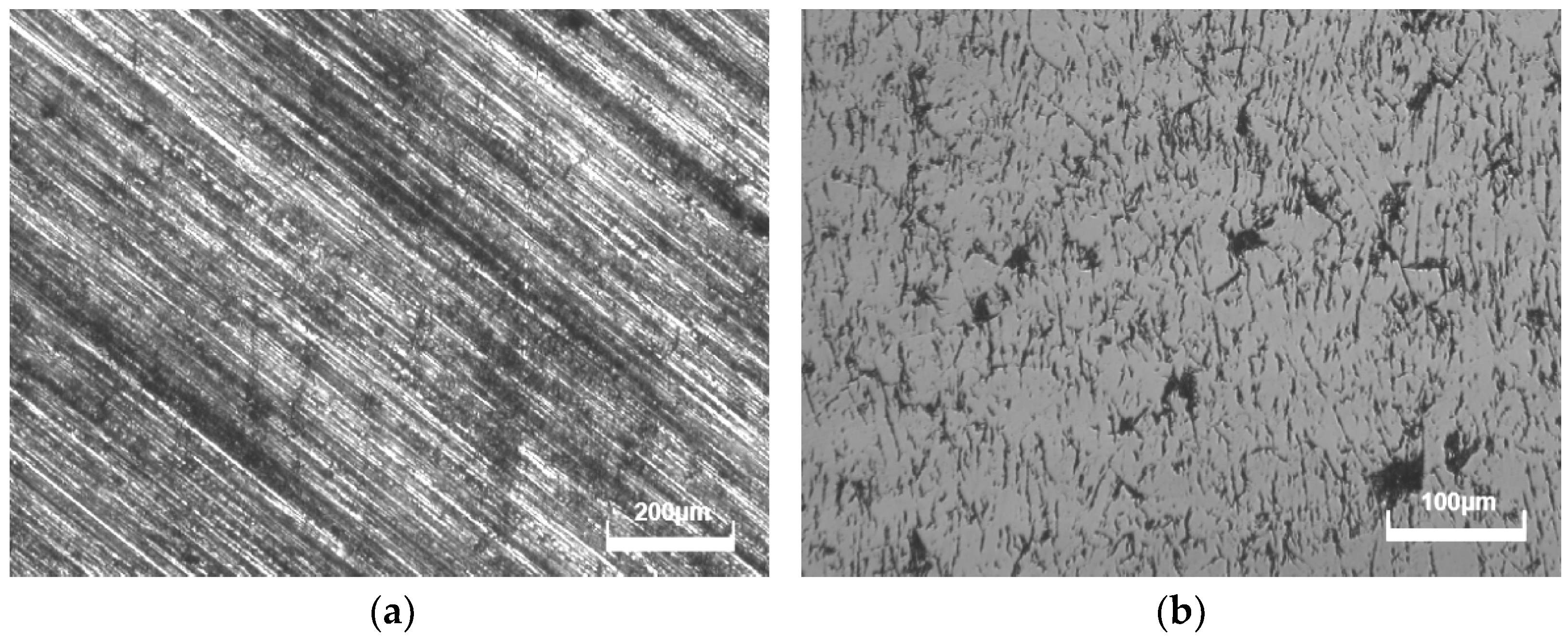
3.2. Influences of Different Polishing Methods
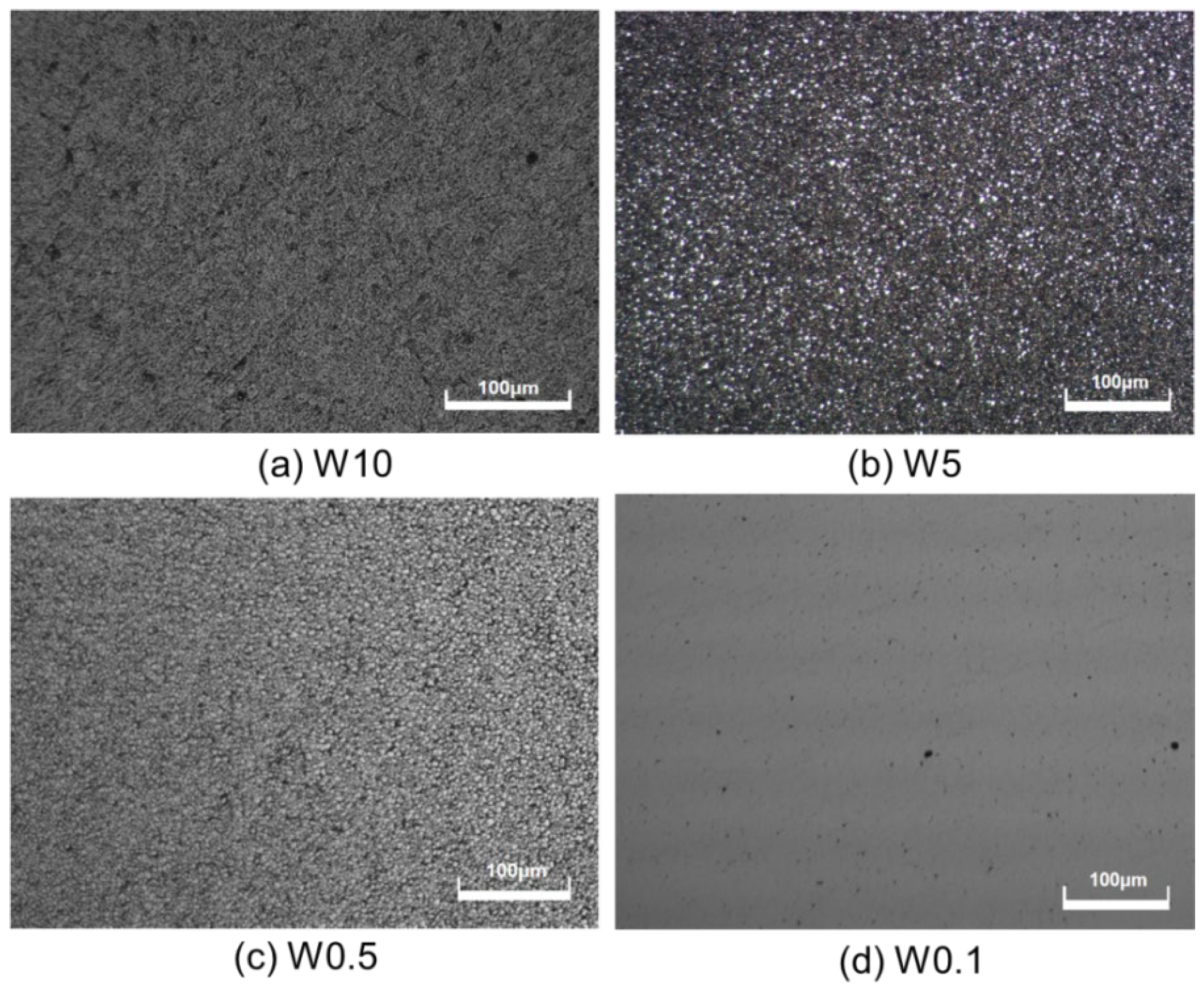
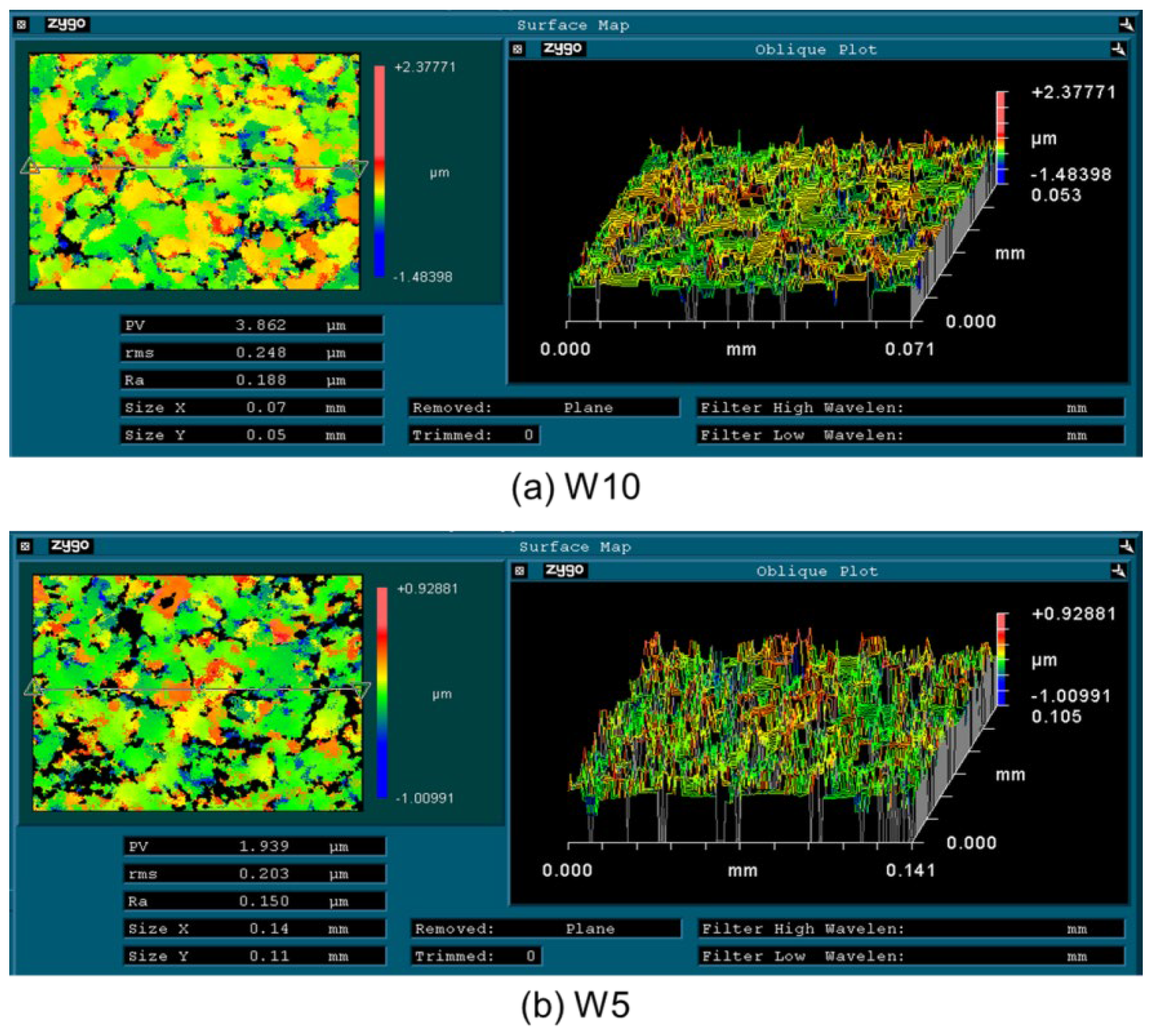
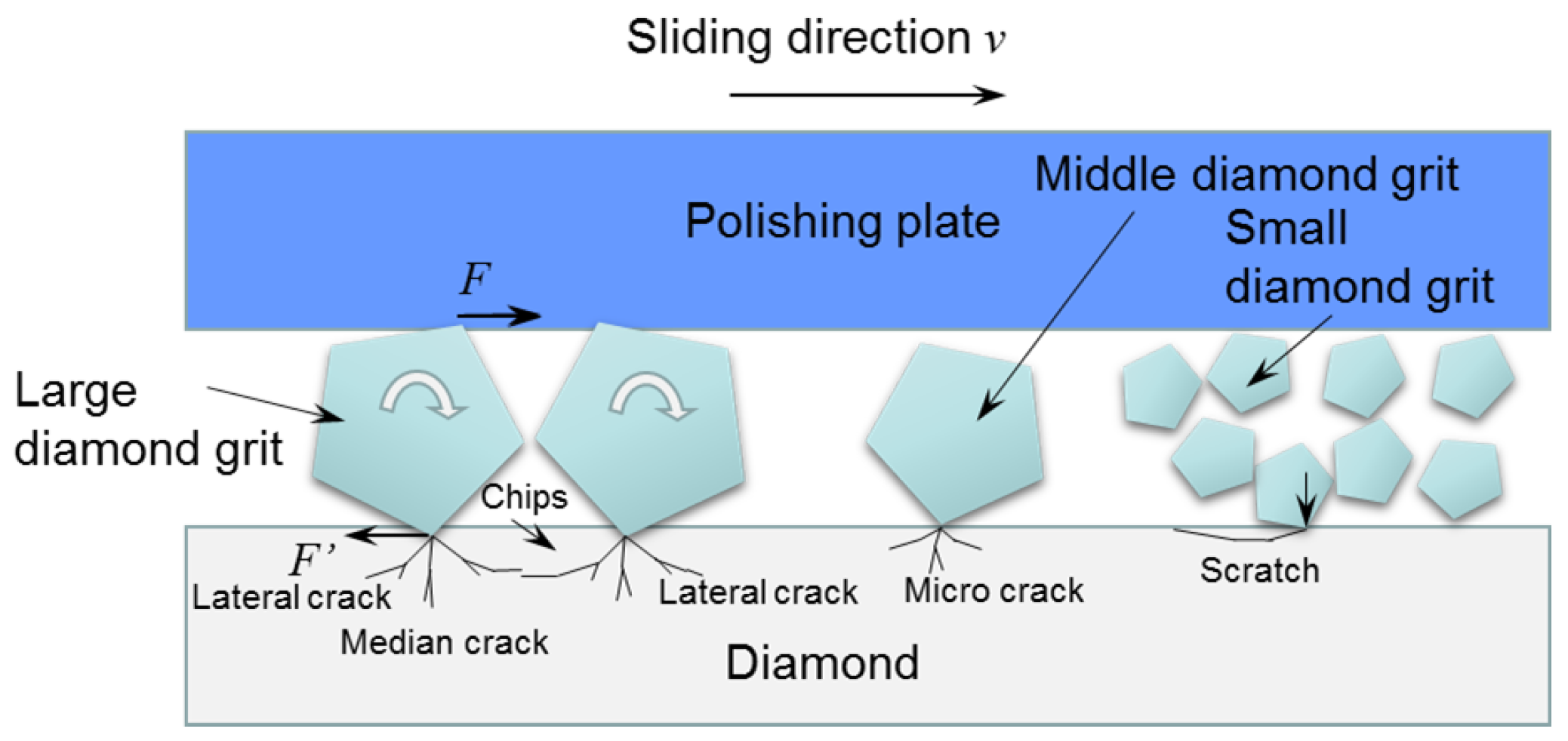
3.3. Influence of Diamond Abrasive Size
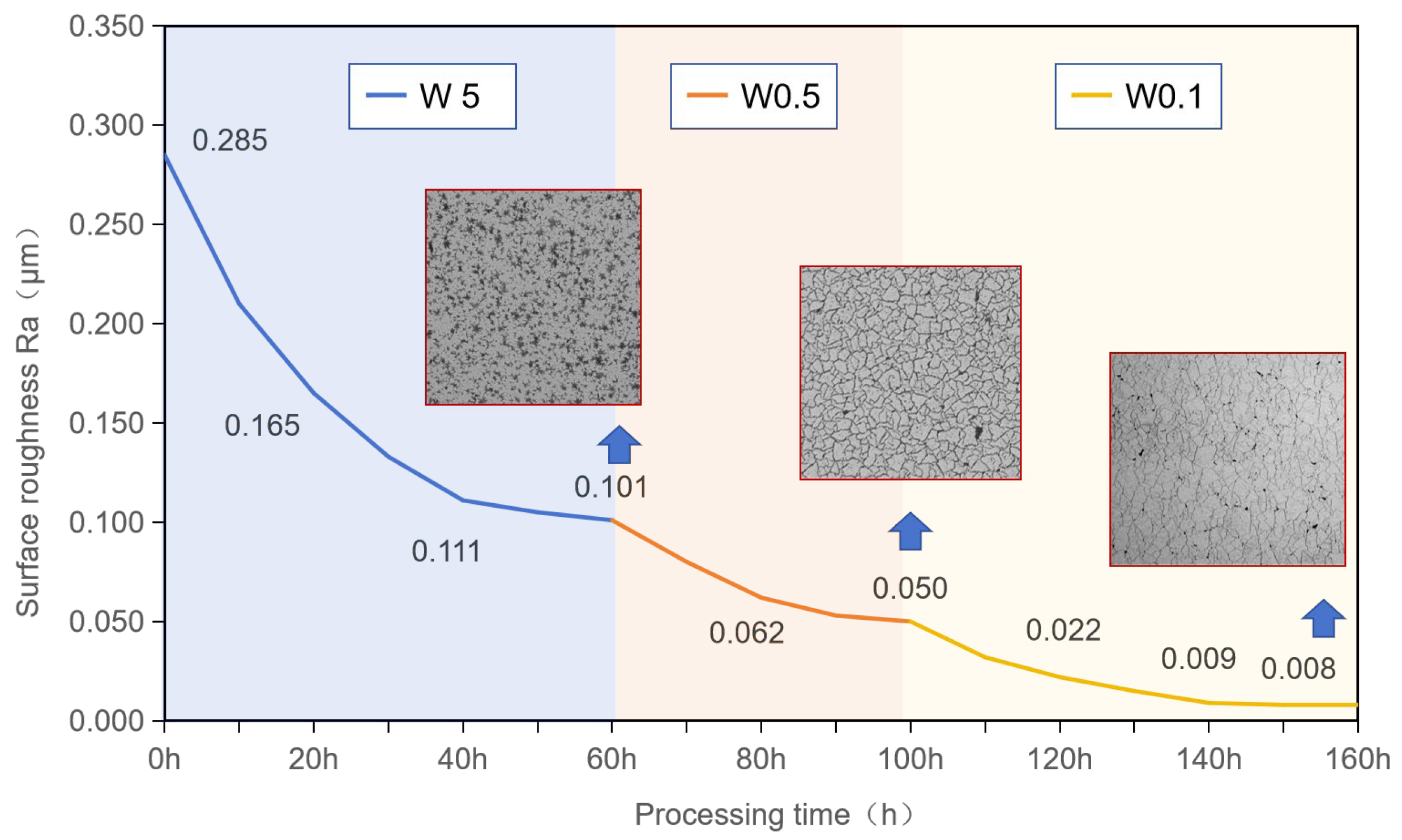
3.4. Influence of Polishing Time
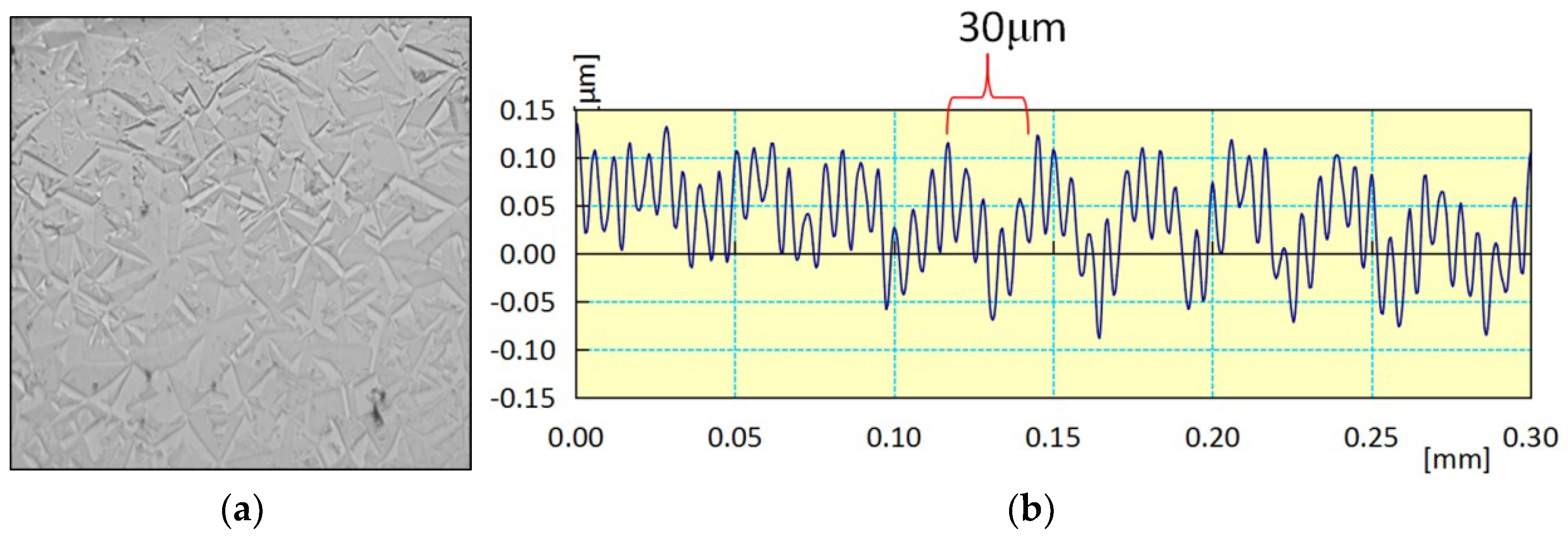
3.5. Influence of Crystal Anisotropy
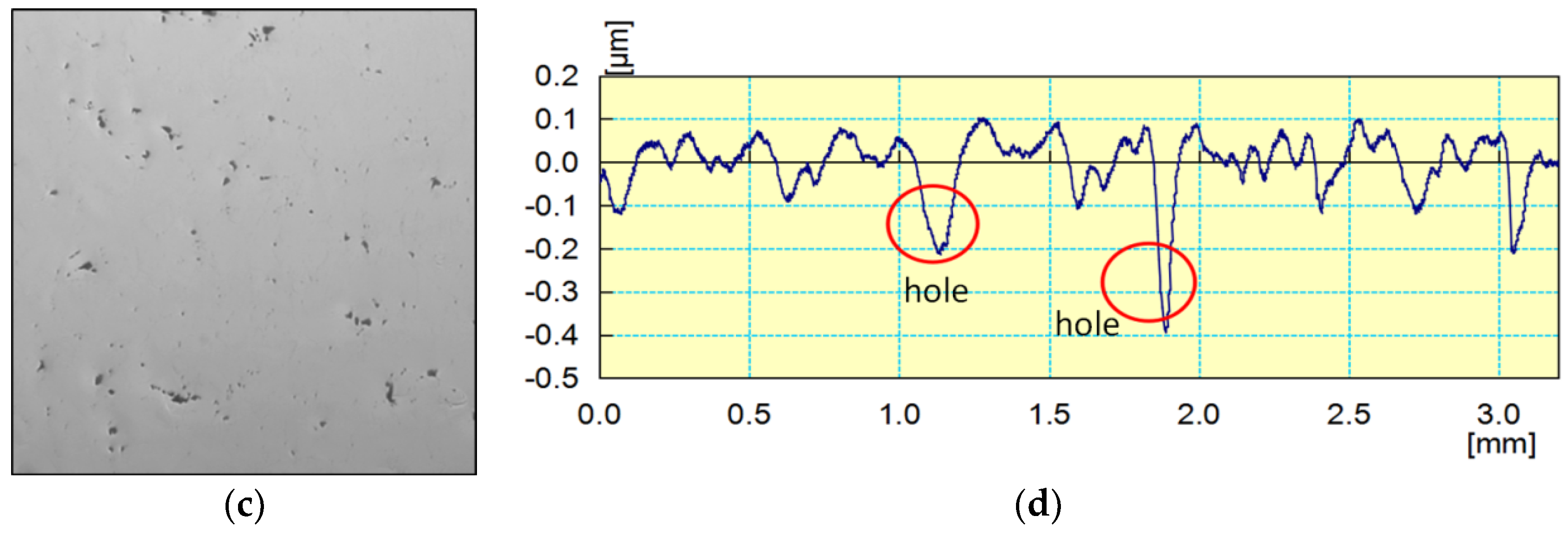
3.6. Influence of Diamond Grain Boundaries and Pores
3.7. Influence of Single-Crystal and Polycrystalline Diamonds
3.8. Influence of Polishing Parameters
4. Conclusions
- (1)
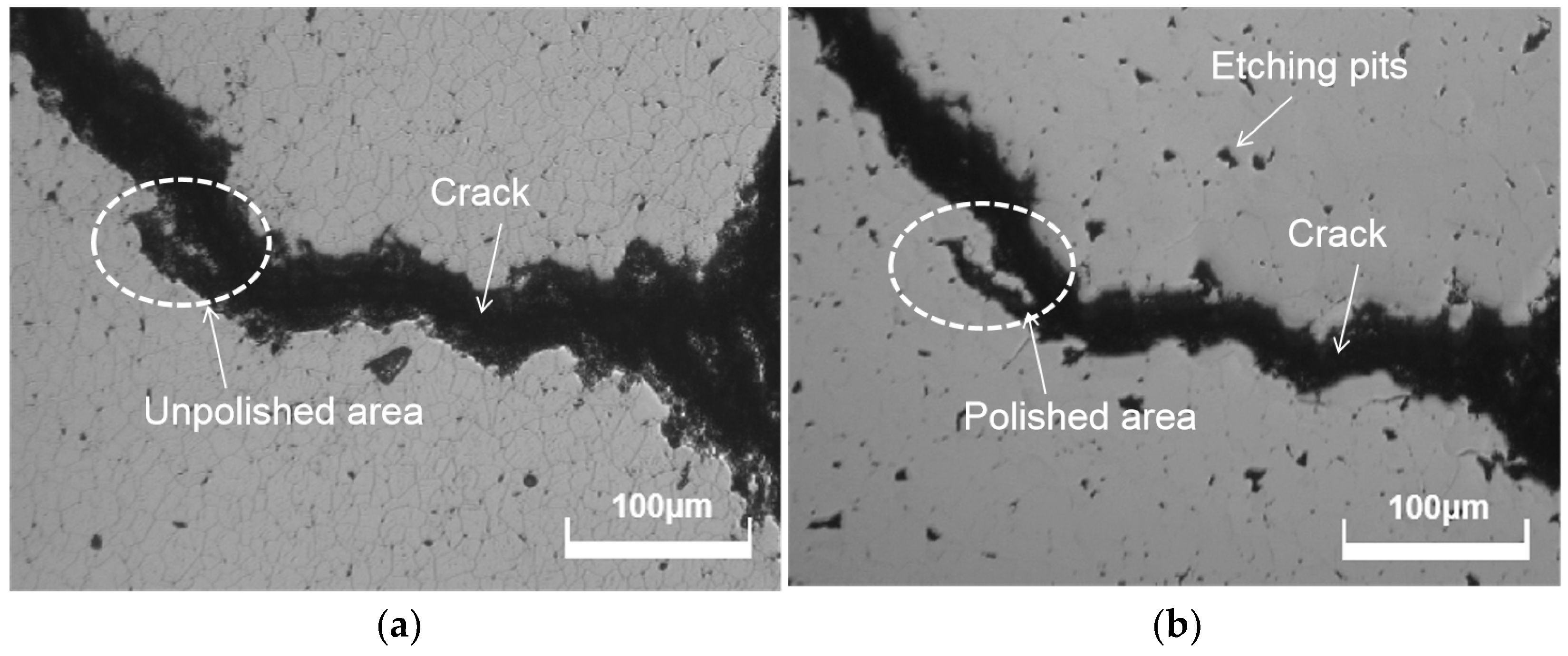
- The crystal growth structure of CVD diamond not only determines its original surface roughness but also has a substantial impact on polishing surface quality. Polycrystalline diamonds’ crystal anisotropy leads to certain steps on the CVD diamond surface due to the varying removal rates in different crystal planes. These steps increase the surface roughness of CVD diamonds during mechanical polishing. Chemical mechanical polishing is effective in removing these steps.
- (2)
- Grain boundaries and holes appear on polycrystalline diamonds when the surface roughness is less than Ra 20 nm. These grain boundaries and holes are the primary variables responsible for increasing CVD diamond surface roughness values during mechanical polishing.
- (3)
- Chemical mechanical polishing is a unique technique to achieve surface quality with a roughness of less than Ra 3 nm, as the anisotropy of the CVD diamond efficiently erases the uneven steps. However, the chemical actions of polishing slurry need to be controlled to prevent the formation of chemical etching pits.
- (4)
- As the anisotropy, grain boundaries, and pores do not interfere with the polishing process, single-crystal diamonds with Ra values less than 3 nm are readily polished.
- (5)
- To obtain a smooth polishing surface, rough and fine-polishing techniques must be carefully planned. A suitable connection should be made between the polishing phases for variable abrasive sizes.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schuelke, T.; Grotjohn, T.A. Diamond polishing. Diam. Relat. Mater. 2013, 32, 17–26. [Google Scholar] [CrossRef]
- Hicks, M.L.; Pakpour-Tabrizi, A.C.; Jackman, R.B. Polishing, preparation and patterning of diamond for device applications. Diam. Relat. Mater. 2019, 97, 107424. [Google Scholar] [CrossRef]
- Mollart, T.P.; Wort, C.J.H.; Pickles, C.S.J. CVD diamond optical components, multispectral properties and performance at elevated temperatures. Proc. SPIE 2001, 4375, 180–198. [Google Scholar]
- Harris, D.C. Properties of diamond for window and dome applications. Proc. SPIE 1994, 2286, 218–228. [Google Scholar]
- Mochalin, V.N.; Shenderova, O.; Ho, D.; Gogotsi, Y. The properties and applications of nanodiamonds. Nat. Nanotechnol. 2012, 7, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Dore, P.; Nucara, A.; Cannavo, D.; De Marzi, G.; Calvani, P.; Marcelli, A.; Sussmann, R.S.; Whitehead, A.J.; Dodge, C.N.; Krehan, A.J.; et al. Infrared properties of chemical-vapor deposition polycrystalline diamond windows. Appl. Opt. 1998, 37, 5731–5736. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.C. Frontiers in infrared window and dome materials. Proc. SPIE 1995, 2552, 325–335. [Google Scholar]
- Yuan, Z. Application and Polishing Technology of Diamond Films; Science Press: Beijing, China, 2023; pp. 38–39. [Google Scholar]
- Gorelov, Y.A.; Lohr, J.; Borchard, P.; Callis, R.W.; Ponce, D. Characteristics of Diamond Windows on the 1 MW, 110 GHz Gyrotron Systems on the DIII-D Tokamak; General Atomics Report GA–A24047. In Proceedings of the Twenty Seventh International Conference on Infrared and Millimeter Waves, San Diego, CA, USA, 22–26 September 2002; pp. 161–162. [Google Scholar]
- Nakahata, H.; Fujii, S.; Higaki, K.; Hachigo, A.; Kitabayashi, H.; Shikata, S.I.; Fujimori, N. Diamond-based surface acoustic wave devices. Semicond. Sci. Technol. 2003, 18, 96–104. [Google Scholar] [CrossRef]
- Fujii, S.; Seki, Y.; Yoshida, K.; Nakahata, H.; Higaki, K.; Kitabayashi, H.; Shikata, S. Diamond wafer for SAW application. In Proceedings of the 1997 IEEE Ultrasonics Symposium Proceedings. An International Symposium, Toronto, ON, Canada, 5–8 October 1997; Volume 1, pp. 183–186. [Google Scholar]
- Shimaoka, T.; Koizumi, S.; Kaneko, J.H. Recent progress in diamond radiation detectors. Funct. Diam. 2022, 1, 205–220. [Google Scholar] [CrossRef]
- Mendoza, F.; Makarov, V.; Weiner, B.R.; Morell, G. Solar-blind field-emission diamond ultraviolet detector. Appl. Phys. Lett. 2015, 107, 201605. [Google Scholar] [CrossRef]
- Yang, N.; Foord, J.S.; Jiang, X. Diamond electrochemistry at the nanoscale: A review. Carbon 2016, 99, 90–110. [Google Scholar] [CrossRef]
- Panizza, M.; Cerisola, G. Application of diamond electrodes to electrochemical processes. Electrochim. Acta 2005, 51, 191–199. [Google Scholar] [CrossRef]
- Yuan, Z.W.; Zheng, P.; Wen, Q.; He, Y. Chemical kinetics mechanism for chemical mechanical polishing diamond and its related hard-inert materials. J. Adv. Manuf. Technol. 2018, 95, 1715–1727. [Google Scholar] [CrossRef]
- Liao, M. Progress in semiconductor diamond photodetectors and MEMS sensors. Funct. Diam. 2022, 1, 29–46. [Google Scholar] [CrossRef]
- Lucca, D.A.; Klopfstein, M.J.; Riemer, O. Ultra-Precision Machining: Cutting with Diamond Tools. ASME J. Manuf. Sci. Eng. 2020, 142, 110817. [Google Scholar] [CrossRef]
- Shao, J.; Zhao, Y.; Zhu, J.; Yuan, Z.; Du, H.; Wen, Q. A new slurry for photocatalysis-assisted chemical mechanical polishing of monocrystal diamond. Machines 2023, 11, 664. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, Z.; Song, Z.; Zhou, S.; Zhang, Z.; Chen, G.; Liao, M.; Chen, G. The polishing methods for large area CVD diamond wafer. Funct. Diam. 2023, 3, 2246495. [Google Scholar] [CrossRef]
- Luo, H.; Ajmal, K.M.; Liu, W.; Yamamura, K.; Deng, H. Polishing and planarization of single crystal diamonds: State-of-the-art and perspectives. Int. J. Extrem. Manuf. 2021, 3, 022003. [Google Scholar] [CrossRef]
- Malshe, A.P.; Park, B.S.; Brown, W.D.; Naseem, H.A. A review of techniques for polishing and planarizing chemically vapor-deposited (CVD) diamond films and substrates. Diam. Relat. Mater. 1999, 8, 1198–1213. [Google Scholar] [CrossRef]
- Kubota, A.; Nagae, S.; Motoyama, S. High-precision mechanical polishing method for diamond substrate using micron-sized diamond abrasive grains. Diam. Relat. Mater. 2020, 101, 107644. [Google Scholar] [CrossRef]
- Zaitsev, A.M.; Kosaca, G.; Richarz, B.; Raiko, V.; Job, R.; Fries, T.; Fahrner, W.R. Thermochemical polishing of CVD diamond films. Diam. Relat. Mater. 1998, 7, 1108–1117. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.C.; Arsecularatne, J.A. Polishing of polycrystalline diamond by the technique of dynamic friction. Part 2: Material removal mechanism. Int. J. Mach. Tool Manuf. 2007, 47, 1615–1624. [Google Scholar] [CrossRef]
- Liu, H.G.; Xie, L.R.; Lin, W.; Hong, M.H. Optical quality laser polishing of CVD diamond by UV pulsed laser irradiation. Adv. Opt. Mater. 2021, 9, 2100537. [Google Scholar] [CrossRef]
- Liu, N.; Sugimoto, K.; Yoshitaka, N.; Yamada, H.; Sun, R.; Arima, K.; Yamamura, K. Highly efficient finishing of large-sized single crystal diamond substrates by combining nanosecond pulsed laser trimming and plasma-assisted polishing. Ceram. Int. 2023, 49, 19109–19123. [Google Scholar] [CrossRef]
- Mi, S.; Toros, A.; Graziosi, T.; Quack, N. Non-contact polishing of single crystal diamond by ion beam etching. Diam. Relat. Mater. 2019, 92, 248–252. [Google Scholar] [CrossRef]
- Yuan, Z.; Jin, Z.; Zhang, Y.; Wen, Q. Chemical mechanical polishing slurries for chemically vapor-deposited diamond films. J. Manuf. Sci. Eng. 2013, 135, 041006. [Google Scholar] [CrossRef]
- Wang, C.Y.; Zhang, F.L.; Kuang, T.C.; Chen, C.L. Chemical/mechanical polishing of diamond films assisted by molten mixture of LiNO3 and KNO3. Thin Solid Films. 2006, 496, 698–702. [Google Scholar] [CrossRef]
- Kuhnle, J.; Weis, O. Mechanochemical superpolishing of diamond using NaNO3 or KNO3 as oxidizing agents. Surf. Sci. 1995, 340, 16–22. [Google Scholar] [CrossRef]
- Bouwelen, F.M.V.; Enckevort, W.J.P.V. A simple model to describe the anisotropy of diamond polishing. Diam. Relat. Mater. 1999, 8, 840–844. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Z.; Cheng, Z.; Feng, Y. A Systematic Study of the Factors Affecting the Surface Quality of Chemically Vapor-Deposited Diamond during Chemical and Mechanical Polishing. Micromachines 2024, 15, 459. https://doi.org/10.3390/mi15040459
Yuan Z, Cheng Z, Feng Y. A Systematic Study of the Factors Affecting the Surface Quality of Chemically Vapor-Deposited Diamond during Chemical and Mechanical Polishing. Micromachines. 2024; 15(4):459. https://doi.org/10.3390/mi15040459
Chicago/Turabian StyleYuan, Zewei, Zhihui Cheng, and Yusen Feng. 2024. "A Systematic Study of the Factors Affecting the Surface Quality of Chemically Vapor-Deposited Diamond during Chemical and Mechanical Polishing" Micromachines 15, no. 4: 459. https://doi.org/10.3390/mi15040459
APA StyleYuan, Z., Cheng, Z., & Feng, Y. (2024). A Systematic Study of the Factors Affecting the Surface Quality of Chemically Vapor-Deposited Diamond during Chemical and Mechanical Polishing. Micromachines, 15(4), 459. https://doi.org/10.3390/mi15040459






