Fabrication of a Microfluidic Test Device with a 3D Printer and Its Combination with the Loop Mediated Isothermal Amplification Method to Detect Streptococcus pyogenes
Abstract
1. Introduction
2. Materials and Methods
2.1. Microfluidic Test Device Design with 3D Printer
2.2. Production and Identification of S. pyogenes ATCC Strain
2.3. DNA Isolation from S. pyogenes Colonies
2.4. LAMP PCR Primer Set Design
2.5. Conducting LAMP Study on the Microfluidic Device and on a Microcentrifuge Tube
2.6. The Test Performance Indicators Calculations
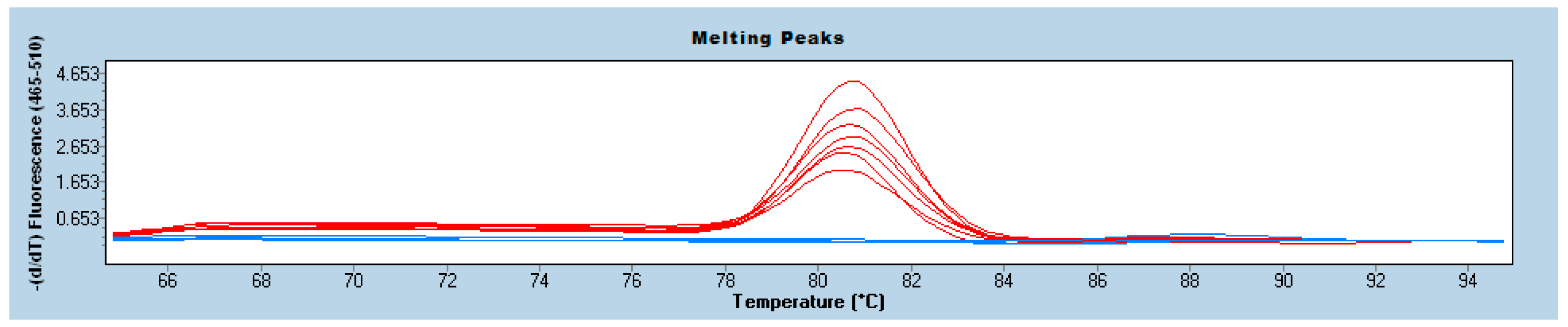
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kebede, D.; Admas, A.; Mekonnen, D. Prevalence and antibiotics susceptibility profiles of Streptococcus pyogenes among pediatric patients with acute pharyngitis at Felege Hiwot Comprehensive Specialized Hospital, Northwest Ethiopia. BMC Microbiol. 2021, 21, 135. [Google Scholar] [CrossRef]
- Cao, C.; Zhang, F.; Ji, M.; Pei, F.; Fan, X.; Shen, H.; Wang, Q.; Yang, W.; Wang, Y. Development of a loop-mediated isothermal amplification method for rapid detection of Streptococcal pyrogenic exotoxin B. Toxicon 2016, 117, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Avire, N.J.; Whiley, H.; Ross, K. A Review of Streptococcus pyogenes: Public Health Risk Factors, Prevention and Control. Pathogens 2021, 10, 248. [Google Scholar] [CrossRef]
- Elf, S.; Olli, J.; Hirvonen, S.; Auvinen, P.; Eboigbodin, K.E. Molecular Detection of Streptococcus pyogenes by Strand Invasion Based Amplification Assay. Mol. Diagn. Ther. 2018, 22, 595–602. [Google Scholar] [CrossRef]
- Othman, A.M.; Assayaghi, R.M.; Al-Shami, H.Z.; Saif-Ali, R. Asymptomatic carriage of Streptococcus pyogenes among school children in Sana’a city, Yemen. BMC Res. Notes 2019, 12, 339. [Google Scholar] [CrossRef]
- Nayiga, I.; Okello, E.; Lwabi, P.; Ndeezi, G. Prevalence of group a streptococcus pharyngeal carriage and clinical manifestations in school children aged 5–15 yrs in Wakiso District, Uganda. BMC Infect. Dis. 2017, 17, 248. [Google Scholar] [CrossRef]
- Khademi, F.; Vaez, H.; Sahebkar, A.; Taheri, R.A. Group A Streptococcus Antibiotic Resistance in Iranian Children: A Meta-analysis. Oman Med. J. 2021, 36, e222. [Google Scholar] [CrossRef]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, E63. [Google Scholar] [CrossRef] [PubMed]
- Njiru, Z.K. Loop-mediated isothermal amplification technology: Towards point of care diagnostics. PLoS Negl. Trop. Dis. 2012, 6, e1572. [Google Scholar] [CrossRef] [PubMed]
- Mejía-Salazar, J.R.; Rodrigues Cruz, K.; Materón Vásques, E.M.; Novais de Oliveira, O.J. Microfluidic Point-of-Care Devices: New Trends and Future Prospects for eHealth Diagnostics. Sensors 2020, 20, 1951. [Google Scholar] [CrossRef] [PubMed]
- Kadimisetty, K.; Song, J.; Doto, A.M.; Hwang, Y.; Peng, J.; Mauk, M.G.; Bushman, F.D.; Gross, R.; Jarvis, J.N.; Liu, C. Fully 3D printed integrated reactor array for point-of-care molecular diagnostics. Biosens. Bioelectron. 2018, 109, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Quyen, T.L.; Hung, T.Q.; Chin, W.H.; Wolff, A.; Bang, D.D. A lab-on-a-chip system with integrated sample preparation and loop-mediated isothermal amplification for rapid and quantitative detection of Salmonella spp. in food samples. Lab Chip 2015, 15, 1898–1904. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Jing, W.; Sun, X.; Liu, Q.; Yang, C.; Liu, S.; Qin, K.; Sui, G. High-throughput microfluidic device for LAMP analysis of airborne bacteria. ACS Sens. 2016, 1, 958–962. [Google Scholar] [CrossRef]
- Ruiz, C.; Kadimisetty, K.; Yin, K.; Mauk, M.G.; Zhao, H.; Liu, C. Fabrication of Hard-Soft Microfluidic Devices Using Hybrid 3D Printing. Micromachines 2020, 11, 567. [Google Scholar] [CrossRef] [PubMed]
- Yin, B.; Qian, C.; Wan, X.; Muhtasim Fuad Sohan, A.S.M.; Lin, X. Tape integrated self-designed microfluidic chip for point-of-care immunoassays simultaneous detection of disease biomarkers with tunable detection range. Biosens. Bioelectron. 2022, 212, 114429. [Google Scholar] [CrossRef]
- Lin, X.; Wu, H.; Zeng, S.; Peng, T.; Zhang, P.; Wan, X.; Lang, Y.; Zhang, B.; Jia, Y.; Shen, R.; et al. A self-designed device integrated with a Fermat spiral microfluidic chip for ratiometric and automated point-of-care testing of anthrax biomarker in real samples. Biosens. Bioelectron. 2023, 230, 115283. [Google Scholar] [CrossRef]
- Ong, L.J.Y.; Islam, A.; DasGupta, R.; Iyer, N.G.; Leo, H.L.; Toh, Y.C. A 3D printed microfluidic perfusion device for multicellular spheroid cultures. Biofabrication 2017, 9, 045005. [Google Scholar] [CrossRef]
- Salmon, I.; Grebenyuk, S.; Abdel Fattah, A.R.; Rustandi, G.; Pilkington, T.; Verfaillie, C.; Ranga, A. Engineering neurovascular organoids with 3D printed microfluidic chips. Lab Chip 2022, 22, 1615–1629. [Google Scholar] [CrossRef]
- Coelho, B.J.; Veigas, B.; Águas, H.; Fortunato, E.; Martins, R.; Baptista, P.V.; Igreja, R. A Digital Microfluidics Platform for Loop-Mediated Isothermal Amplification Detection. Sensors 2017, 17, 2616. [Google Scholar] [CrossRef]
- Razavi Bazaz, S.; Rouhi, O.; Raoufi, M.A.; Ejeian, F.; Asadnia, M.; Jin, D.; Ebrahimi Warkiani, M. 3D Printing of Inertial Microfluidic Devices. Sci. Rep. 2020, 10, 5929. [Google Scholar] [CrossRef]
- Ongaro, A.E.; Di Giuseppe, D.; Kermanizadeh, A.; Crespo, A.M.; Mencattini, A.; Ghibelli, L.; Mancini, V.; Wlodarczyk, K.L.; Hand, D.P.; Martinelli, E.; et al. Polylactic is a Sustainable, Low Absorption, Low Autofluorescence Alternative to Other Plastics for Microfluidic and Organ-on-Chip Applications. Anal. Chem. 2020, 92, 6693–6701. [Google Scholar] [CrossRef]
- Eryıldız, M.; Bilgic, V.; Ekici, S.; Yığın, A.; Demirci, M. Salmonella spp. tespiti için ilmiğe dayalı izotermal amplifikasyon (LAMP) ile kombine üç boyutlu (3B) yazıcıda mikroakışkan çip imalatı. Etlik Vet. Mikrobiyoloji Derg. 2022, 33, 64–69. [Google Scholar] [CrossRef]
- Forootan, A.; Sjöback, R.; Björkman, J.; Sjögreen, B.; Linz, L.; Kubista, M. Methods to determine limit of detection and limit of quantification in quantitative real-time PCR (qPCR). Biomol. Detect. Quantif. 2017, 12, 1–6. [Google Scholar] [CrossRef]
- Inaba, M.; Higashimoto, Y.; Toyama, Y.; Horiguchi, T.; Hibino, M.; Iwata, M.; Imaizumi, K.; Doi, Y. Diagnostic accuracy of LAMP versus PCR over the course of SARS-CoV-2 infection. Int. J. Infect. Dis. 2021, 107, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Simon Simon, A.; Kumar Subbiah, S.; Thian Lung Than, L.; Osman, M.; Awang Hamat, R. Improved Visual Detection of speB Gene in Streptococcus pyogenes Isolates by Real-time Loop-Mediated Isothermal Amplification Turbidimetry Method. Jundishapur J. Microbiol. 2021, 14, e108540. [Google Scholar] [CrossRef]
- Henson, A.M.; Carter, D.; Todd, K.; Shulman, S.T.; Zheng, X. Detection of Streptococcus pyogenes by use of Illumigene group A Streptococcus assay. J. Clin. Microbiol. 2013, 51, 4207–4209. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; He, X.; Li, H.; Zhao, J.; Huang, S.; Liu, W.; Wei, X.; Ding, Y.; Wang, Z.; Zou, D.; et al. Detection of Streptococcus pyogenes using rapid visual molecular assay. FEMS Microbiol. Lett. 2015, 362, fnv148. [Google Scholar] [CrossRef][Green Version]
- Hytönen, J.; Haataja, S.; Gerlach, D.; Podbielski, A.; Finne, J. The SpeB virulence factor of Streptococcus pyogenes, a multifunctional secreted and cell surface molecule with strepadhesin, laminin-binding and cysteine protease activity. Mol. Microbiol. 2001, 39, 512–519. [Google Scholar] [CrossRef]
- Huang, E.; Wang, Y.; Yang, N.; Shu, B.; Zhang, G.; Liu, D. A fully automated microfluidic PCR-array system for rapid detection of multiple respiratory tract infection pathogens. Anal. Bioanal. Chem. 2021, 413, 1787–1798. [Google Scholar] [CrossRef]
- Mohajeri, S.; Moayedi, S.; Azimi, L.; Akrami, M.; Rad-Malekshahi, M.; Fazeli, M.R.; Fallah, F.; Haririan, I. Nanobiosensor Based on Sugar Code-AuNPs Aggregation: A Key to Opening New Gates in Rapid Diagnosis of Streptococcal pharyngitis. Front. Bioeng. Biotechnol. 2022, 10, 957271. [Google Scholar] [CrossRef]
- Lee, W.; Kwon, D.; Choi, W.; Jung, G.Y.; Jeon, S. 3D-printed microfluidic device for the detection of pathogenic bacteria using size-based separation in helical channel with trapezoid cross-section. Sci. Rep. 2015, 5, 7717, Erratum in Sci. Rep. 2015, 5, 9701. [Google Scholar] [CrossRef]
- El-Tholoth, M.; Bai, H.; Mauk, M.G.; Saif, L.; Bau, H.H. A portable, 3D printed, microfluidic device for multiplexed, real time, molecular detection of the porcine epidemic diarrhea virus, transmissible gastroenteritis virus, and porcine deltacoronavirus at the point of need. Lab Chip 2021, 21, 1118–1130. [Google Scholar] [CrossRef]
- Wan, L.; Chen, T.; Gao, J.; Dong, C.; Wong, A.H.-H.; Jia, Y.; Mak, P.-I.; Deng, C.-X.; Martins, R.P. A digital microfluidic system for loop-mediated isothermal amplification and sequence specific pathogen detection. Sci. Rep. 2017, 7, 14586. [Google Scholar] [CrossRef]
- Shulman, S.T.; Bisno, A.L.; Clegg, H.W.; Gerber, M.A.; Kaplan, E.L.; Lee, G.; Martin, J.M.; Van Beneden, C. Clinical practice guideline for the diagnosis and management of group A Streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2012, 55, e86–e102, Erratum in Clin. Infect. Dis. 2014, 58, 1496. [Google Scholar] [CrossRef]
- Banerjee, D.; Michael, J.; Schmitt, B.; Salimnia, H.; Mhaissen, N.; Goldfarb, D.M.; Lachance, P.; Faron, M.L.; Aufderheide, T.; Ledeboer, N.; et al. Multicenter Clinical Evaluation of the Revogene Strep A Molecular Assay for Detection of Streptococcus pyogenes from Throat Swab Specimens. J. Clin. Microbiol. 2020, 58, e01775-19. [Google Scholar] [CrossRef]
- Reijtman, V.; García, M.E.; Mastroianni, A.; Isasmendi, A.; Pinheiro, J.L.; Pérez, G.; Hernandez, C. Evaluation of a rapid diagnostic test for the detection of Streptococcus pyogenes in invasive infections. Rev. Argent. Microbiol. 2020, 52, 261–265. [Google Scholar] [CrossRef]
- Cohen, R.; Varon, E.; Bidet, P.; Cohen, J.F.; Béchet, S.M.; Couloigner, V.; Michot, A.S.; Guiheneuf, C.; Bonacorsi, S.; Levy, C. Diagnostic Accuracy of Group A Streptococcus Rapid Antigen Detection Test on Middle Ear Fluid in Children with Acute Otitis Media with Spontaneous Perforation: A Prospective Multicenter Evaluation. Pediatr. Infect. Dis. J. 2023, 42, 816–818. [Google Scholar] [CrossRef] [PubMed]
- Sølvik, U.Ø.; Boija, E.E.; Ekvall, S.; Jabbour, A.; Breivik, A.C.; Nordin, G.; Sandberg, S. Performance and user-friendliness of the rapid antigen detection tests QuickVue Dipstick Strep A test and DIAQUICK Strep A Blue Dipstick for pharyngotonsillitis caused by Streptococcus pyogenes in primary health care. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.F.; Bertille, N.; Cohen, R.; Chalumeau, M. Rapid antigen detection test for group A streptococcus in children with pharyngitis. Cochrane Database Syst. Rev. 2016, 7, CD010502. [Google Scholar] [CrossRef] [PubMed]
- Guarner, J.; Sumner, J.; Paddock, C.D.; Shieh, W.J.; Greer, P.W.; Reagan, S.; Fischer, M.; Van Beneden, C.A.; Zaki, S.R. Diagnosis of invasive group a streptococcal infections by using immunohistochemical and molecular assays. Am. J. Clin. Pathol. 2006, 126, 148–155. [Google Scholar] [CrossRef]
- Rivas-Macho, A.; Eletxigerra, U.; Diez-Ahedo, R.; Merino, S.; Sanjuan, A.; Bou-Ali, M.M.; Ruiz-Rubio, L.; Del Campo, J.; Vilas-Vilela, J.L.; Goñi-de-Cerio, F.; et al. Design and 3D printing of an electrochemical sensor for Listeria monocytogenes detection based on loop mediated isothermal amplification. Heliyon 2022, 9, e12637. [Google Scholar] [CrossRef] [PubMed]
- Papadakis, G.; Pantazis, A.K.; Fikas, N.; Chatziioannidou, S.; Tsiakalou, V.; Michaelidou, K.; Pogka, V.; Megariti, M.; Vardaki, M.; Giarentis, K.; et al. Portable real-time colorimetric LAMP-device for rapid quantitative detection of nucleic acids in crude samples. Sci. Rep. 2022, 12, 3775. [Google Scholar] [CrossRef] [PubMed]
- Glatzel, S.; Hezwani, M.; Kitson Philip, J.; Gromski Piotr, S.; Schürer, S.; Cronin, L. A porTable 3D printer system for the diagnosis and treatment of multidrug-resistant bacteria. Chem 2016, 1, 494–504. [Google Scholar] [CrossRef]
- Amoia, S.S.; Loconsole, G.; Ligorio, A.; Pantazis, A.K.; Papadakis, G.; Gizeli, E.; Minafra, A. A Colorimetric LAMP Detection of Xylella fastidiosa in Crude Alkaline Sap of Olive Trees in Apulia as a Field-Based Tool for Disease Containment. Agriculture 2023, 13, 448. [Google Scholar] [CrossRef]
- Zhou, Q.; Pan, J.; Mo, L.; Luo, Z.; Qin, Z.; Dai, Z.; Yi, C. Fluorescent on-site detection of multiple pathogens using smartphone-based portable device with paper-based isothermal amplification chip. Mikrochim. Acta 2022, 189, 333. [Google Scholar] [CrossRef] [PubMed]
- Kotz, F.; Mader, M.; Dellen, N.; Risch, P.; Kick, A.; Helmer, D.; Rapp, B.E. Fused Deposition Modeling of Microfluidic Chips in Polymethylmethacrylate. Micromachines 2020, 11, 873. [Google Scholar] [CrossRef]
- Jin, Y.; Xiong, P.; Xu, T.; Wang, J. Time-efficient fabrication method for 3D-printed microfluidic devices. Sci. Rep. 2022, 12, 1233. [Google Scholar] [CrossRef]
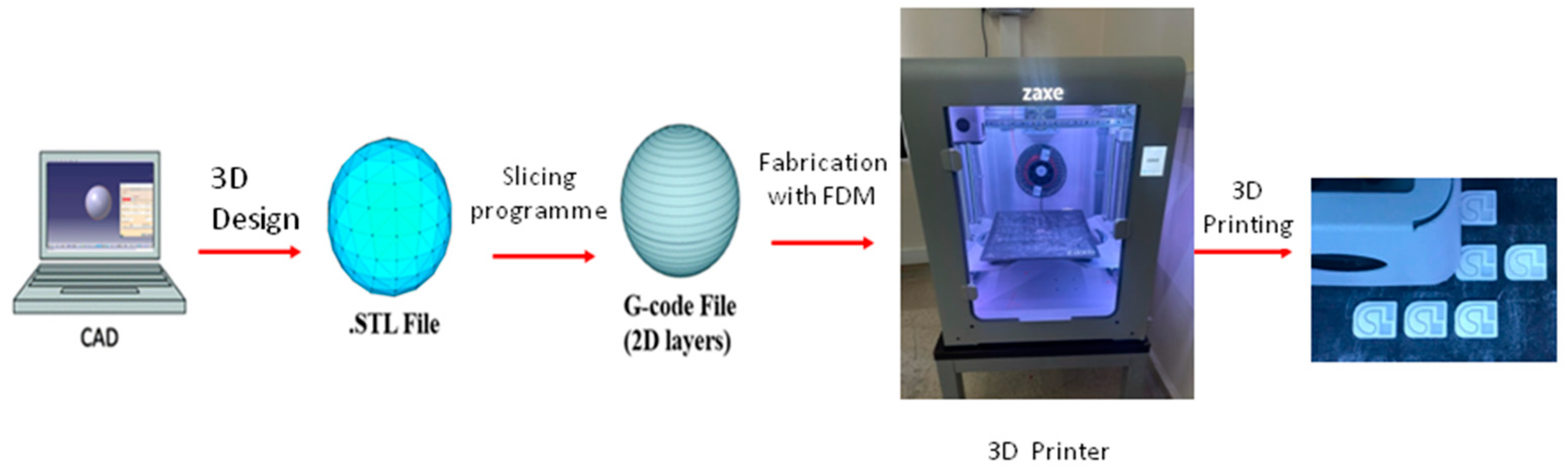
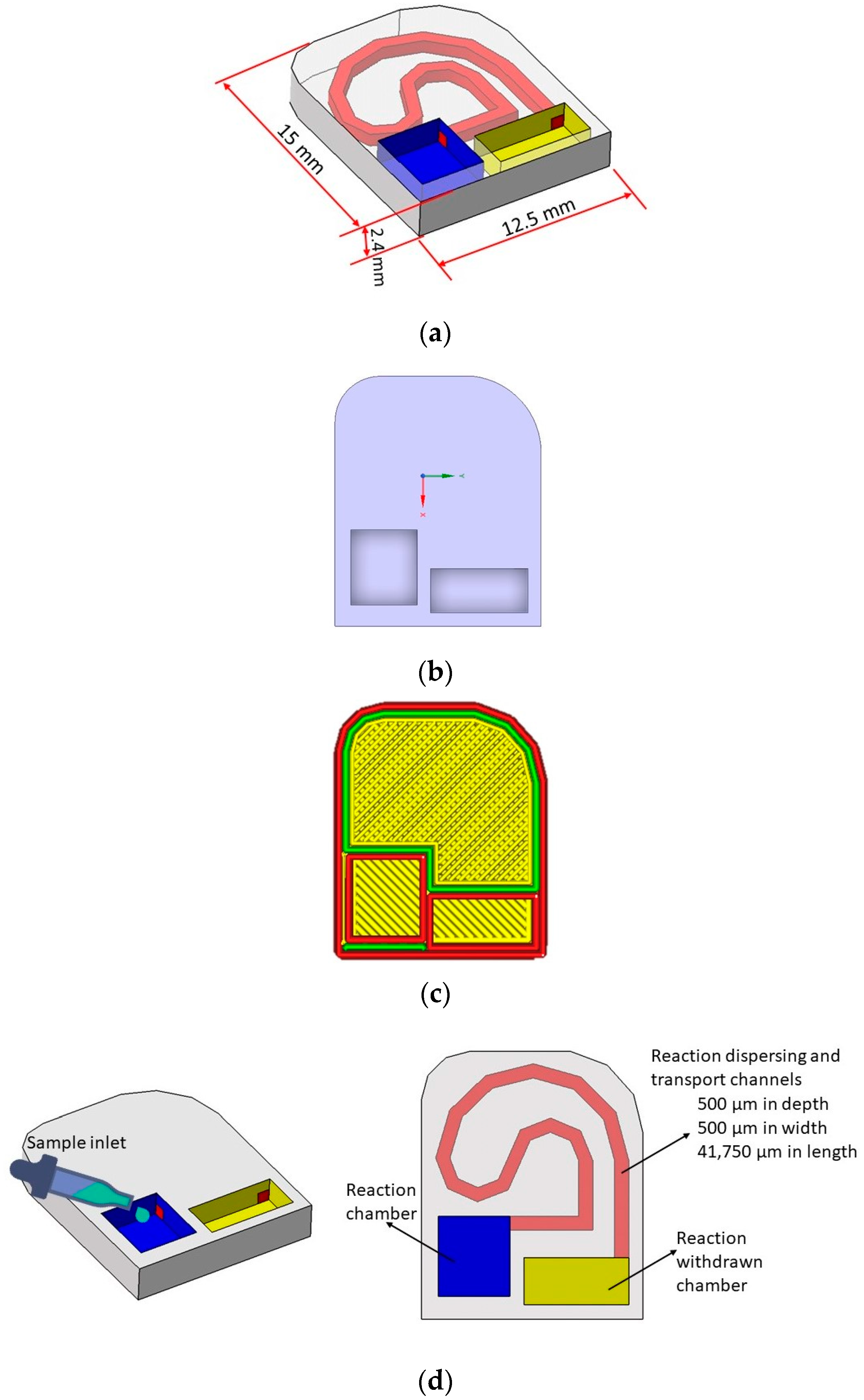
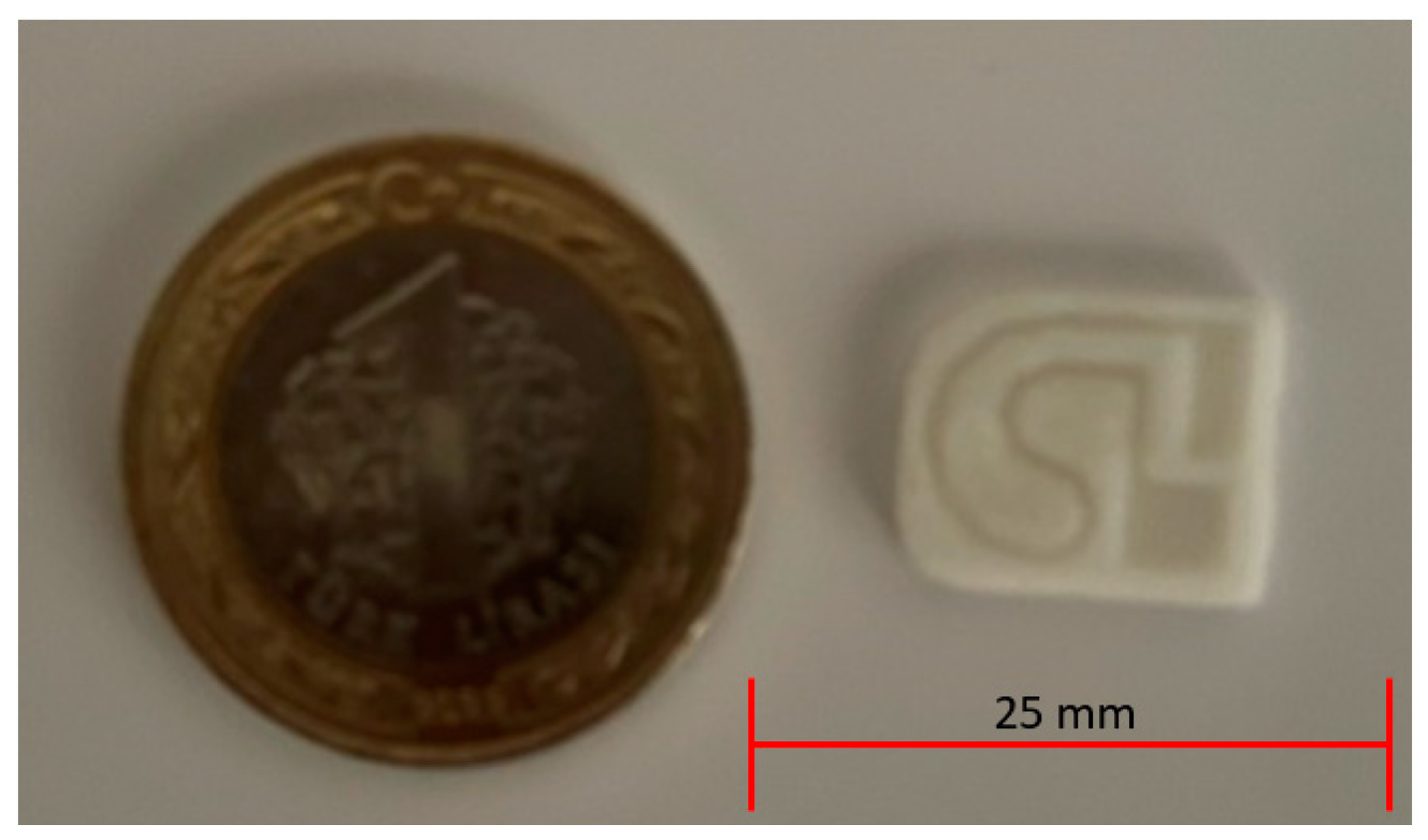
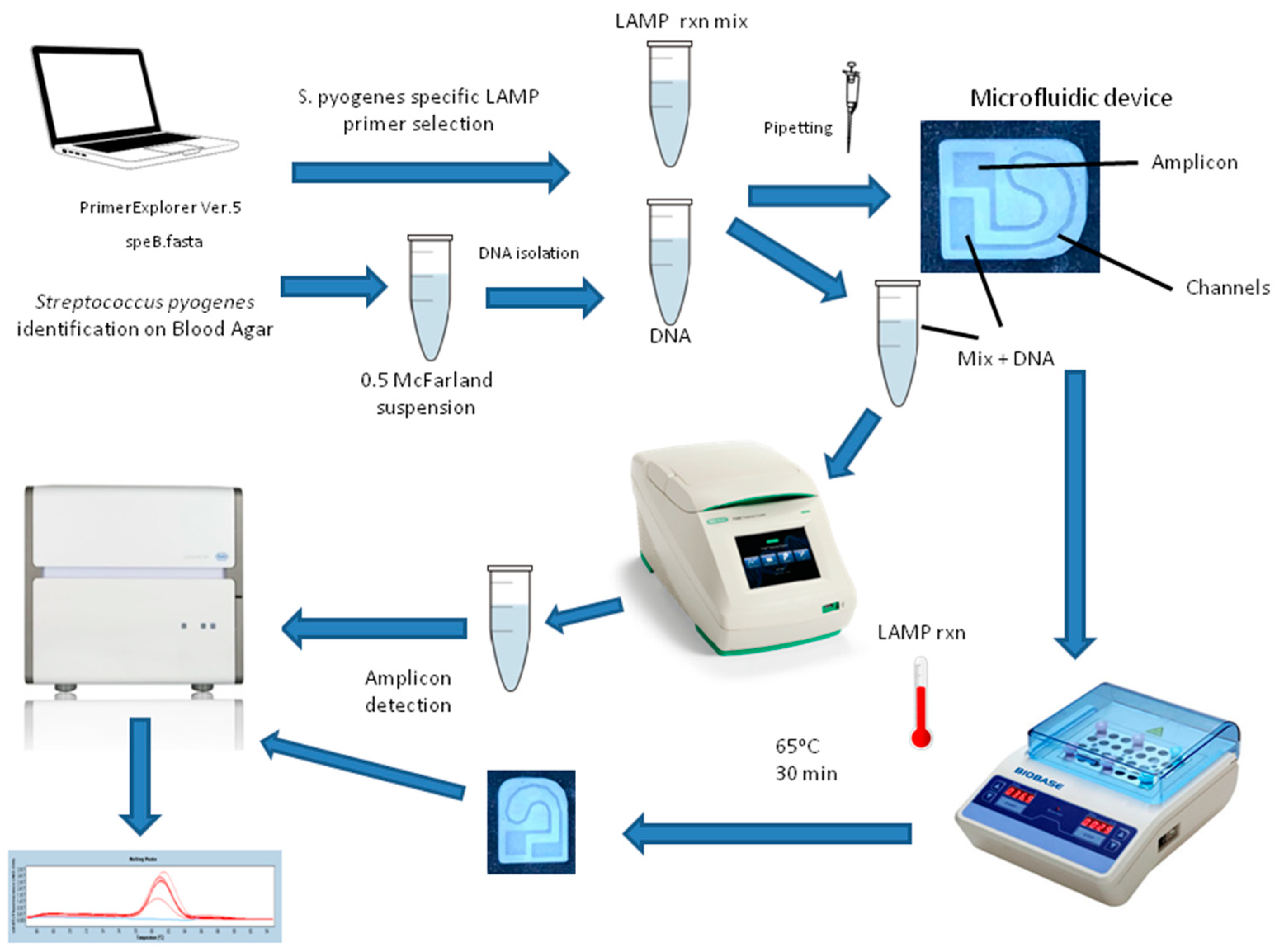
| Nozzle Diameter | Layer Thickness | Filling Rate | Nozzle Temperature | Heated Bed Temperature | Oppression Speed |
|---|---|---|---|---|---|
| 0.4 mm | 0.2 mm | 100% | 190 °C | 60 °C | 50 mm/s |
| Label | 5′pos | 3′pos | len | Sequence |
|---|---|---|---|---|
| F3 | 896 | 916 | 21 | CTACCTACTTATAGCGGAAGA |
| B3 | 1089 | 1109 | 21 | CTTCCCAATCTTGTTTGCTAA |
| FIP | 917 | 993 | 48 | GGACCATAATCCATGTCTACTGAAA-GAATCTAACGTTCAAAAAATGGC |
| BIP | 1008 | 1087 | 41 | CAGGTAGCTCTCGTGTTCAAAG-GTCGCTACGGTTAATTTGG |
| LF | 948 | 968 | 21 | AATTGATGGCTGATGTTGGTA |
| LB | 1045 | 1064 | 20 | CTTTGGCTACAACCAATCTG |
| Strain (CFU/mL) | Control Type |
|---|---|
| S. pyogenes ATCC 19615 (1.5 × 106) | Positive Control |
| S. pyogenes ATCC 19615 (1.5 × 105) | Positive Control |
| S. pyogenes ATCC 19615 (1.5 × 104) | Positive Control |
| S. pyogenes ATCC 19615 (1.5 × 103) | Positive Control |
| S. pyogenes ATCC 19615 (1.5 × 102) | Positive Control |
| Streptococcus agalactiae ATCC 12386 (1.5 × 106) | Negative Control |
| Streptococcus mutans ATCC 25175 (1.5 × 106) | Negative Control |
| Haemophilus influenzae ATCC 49247 (1.5 × 106) | Negative Control |
| Staphylococcus epidermidis ATCC 12228 (1.5 × 106) | Negative Control |
| Lactobacillus acidophilus ATCC 4356 (1.5 × 106) | Negative Control |
| C. albicans ATCC 10231 (1.5 × 106) | Negative Control |
| Klebsiella pneumoniae ATCC 13883 (1.5 × 106) | Negative Control |
| Staphylococcus aureus ATCC 29213 (1.5 × 106) | Negative Control |
| Escherichia coli ATCC 25922 (1.5 × 106) | Negative Control |
| Pseudomonas aeruginosa ATCC 27853 (1.5 × 106) | Negative Control |
| (a) | ||||
| S. pyogenes | Culture | |||
| Positive | Negative | |||
| LAMP rxn on the microfluidic device | Positive | 42 | 10 | 52 |
| Negative | 8 | 90 | 98 | |
| 50 | 100 | |||
| (b) | ||||
| S. pyogenes | Culture | |||
| Positive | Negative | |||
| LAMP rxn on microcentrifuge tube | Positive | 46 | 6 | 52 |
| Negative | 4 | 94 | 98 | |
| 50 | 100 | |||
| Target | Test Performance Indicator | LAMP Reaction Performed on the Microfluidic Device | LAMP Reaction Performed on the Microcentrifuge Tube |
|---|---|---|---|
| S. pyogenes | Sensitivity | 84.00% | 92.00% |
| Specificity | 90.00% | 94.00% | |
| Positive Predictive Value (PPV) | 80.77% | 88.46% | |
| Negative Predictive Value (NPV) | 91.84% | 95.92% | |
| Limit of Detection (LOD) | 1.5 × 102 | 1.5 × 102 | |
| Limit of Quantification (LOQ) | 7.4 × 102 | 2.46 × 102 | |
| Cohen’s Kappa (κ) value | 0.705 | 0.620 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirkoyun Uysal, H.; Eryildiz, M.; Demirci, M. Fabrication of a Microfluidic Test Device with a 3D Printer and Its Combination with the Loop Mediated Isothermal Amplification Method to Detect Streptococcus pyogenes. Micromachines 2024, 15, 365. https://doi.org/10.3390/mi15030365
Kirkoyun Uysal H, Eryildiz M, Demirci M. Fabrication of a Microfluidic Test Device with a 3D Printer and Its Combination with the Loop Mediated Isothermal Amplification Method to Detect Streptococcus pyogenes. Micromachines. 2024; 15(3):365. https://doi.org/10.3390/mi15030365
Chicago/Turabian StyleKirkoyun Uysal, Hayriye, Meltem Eryildiz, and Mehmet Demirci. 2024. "Fabrication of a Microfluidic Test Device with a 3D Printer and Its Combination with the Loop Mediated Isothermal Amplification Method to Detect Streptococcus pyogenes" Micromachines 15, no. 3: 365. https://doi.org/10.3390/mi15030365
APA StyleKirkoyun Uysal, H., Eryildiz, M., & Demirci, M. (2024). Fabrication of a Microfluidic Test Device with a 3D Printer and Its Combination with the Loop Mediated Isothermal Amplification Method to Detect Streptococcus pyogenes. Micromachines, 15(3), 365. https://doi.org/10.3390/mi15030365








