Using Compound Neural Action Potentials for Functional Validation of a High-Density Intraneural Interface: A Preliminary Study
Abstract
1. Introduction
1.1. Peripheral Nerve Interfaces
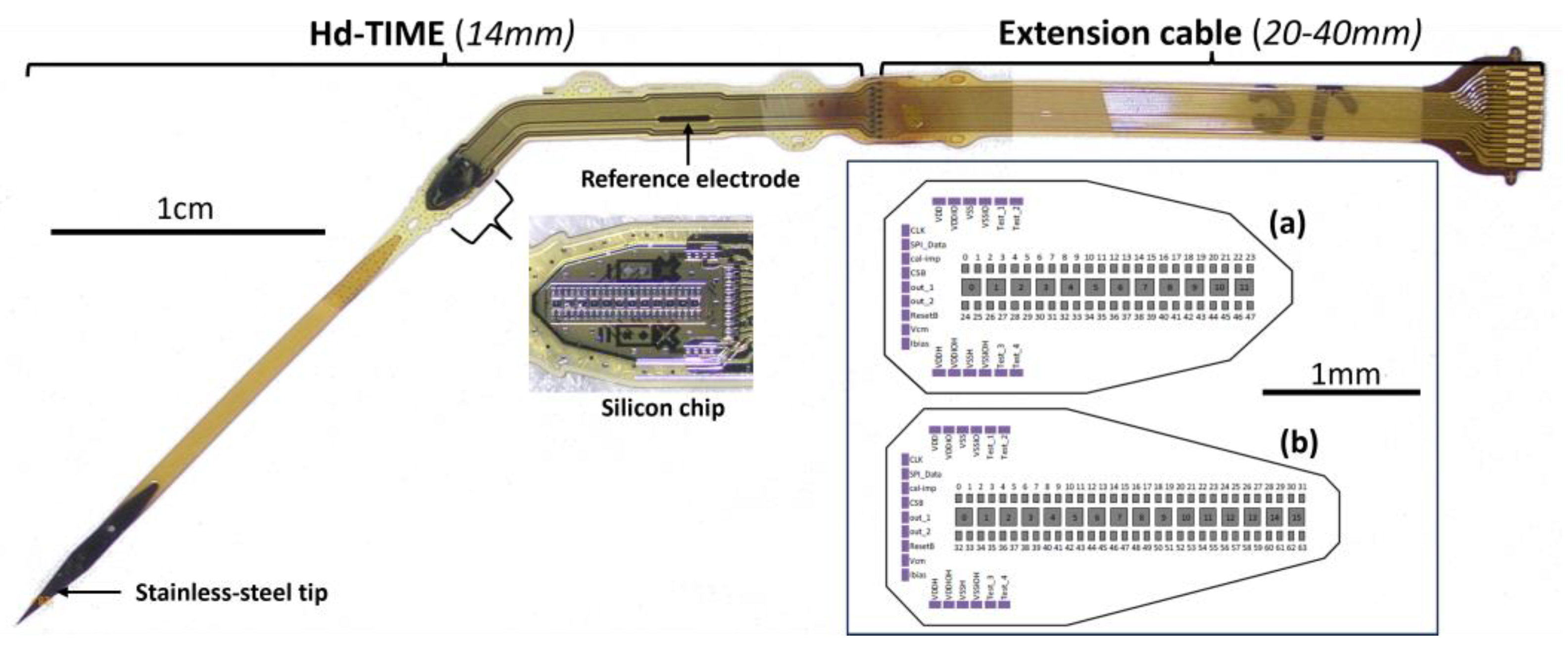
1.2. High-Density Transverse Intrafascicular Multichannel Electrode and Integrated Electronics
1.3. Metrics for Functional Assessment
2. Materials and Methods
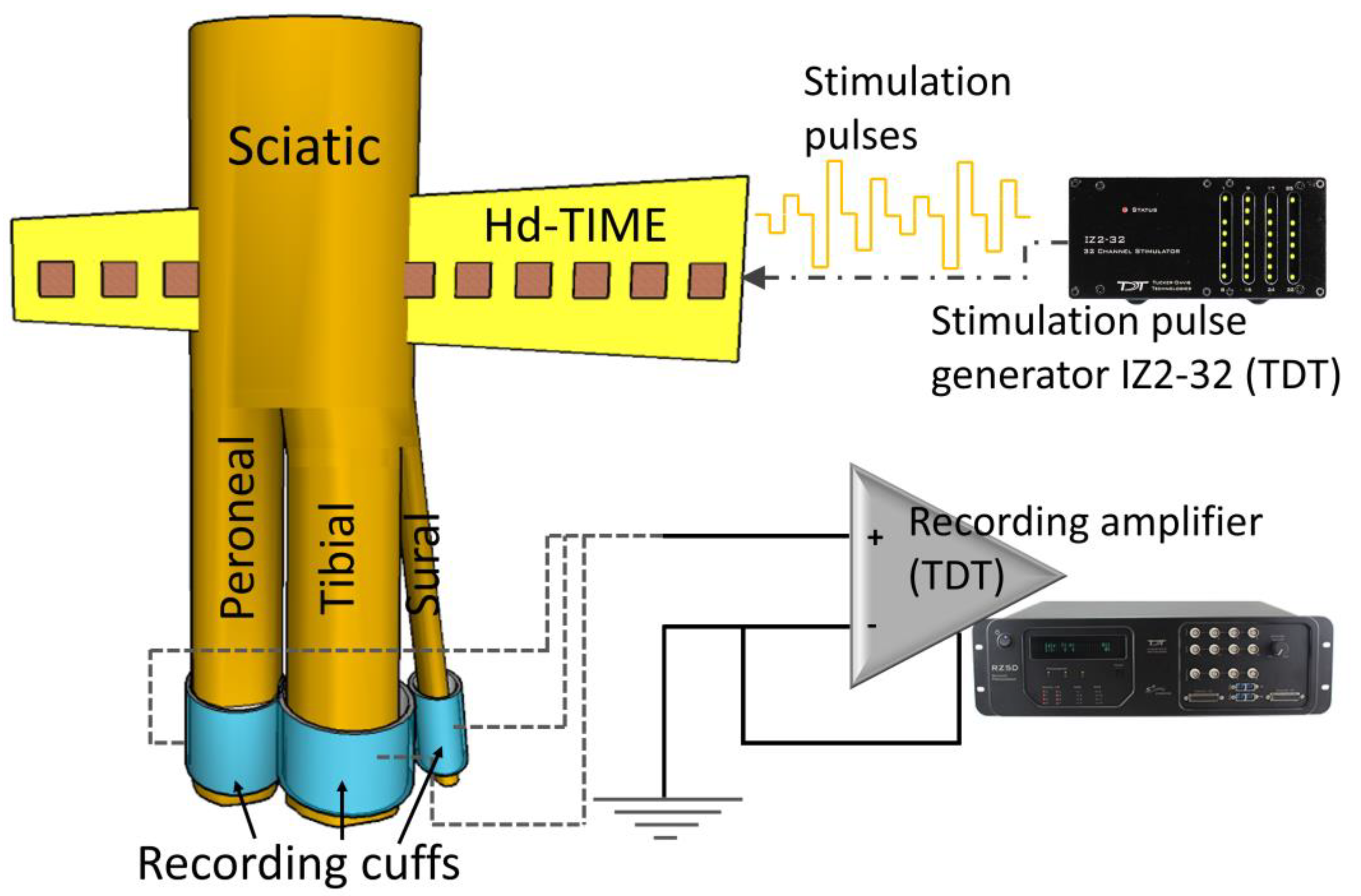
2.1. Animal Preparation
2.2. The Electrodes Implanted
2.3. The Stimulation Paradigm
2.4. Data Collection and Analysis
2.5. Analysis of the Selective Recruitment of Nerve Fascicles
2.6. Predicting the Electrode Location Inside the Nerve
3. Results and Discussion
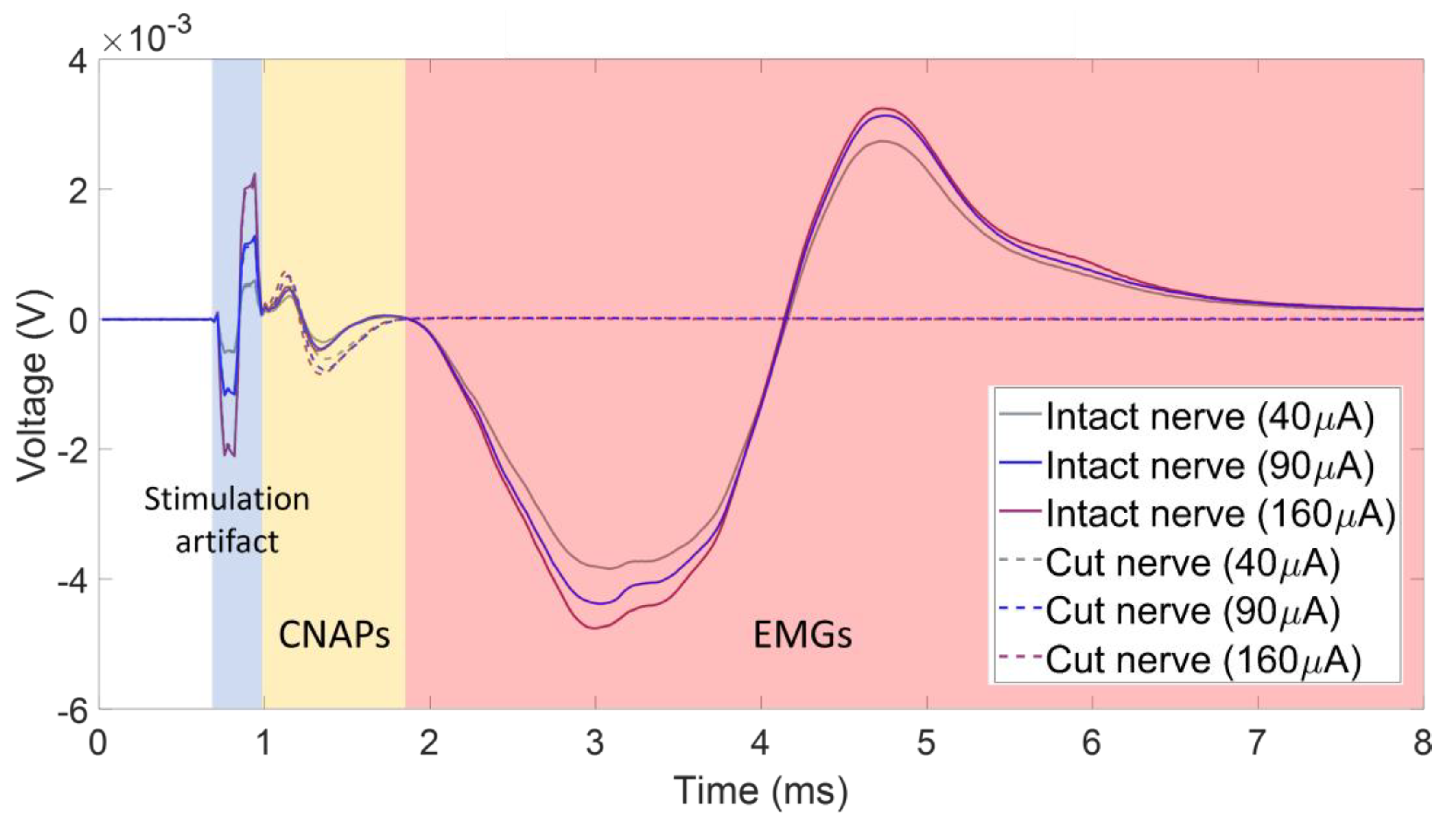
3.1. CNAPs with Respect to Stimulation Artifacts and Parasitic EMG Signals
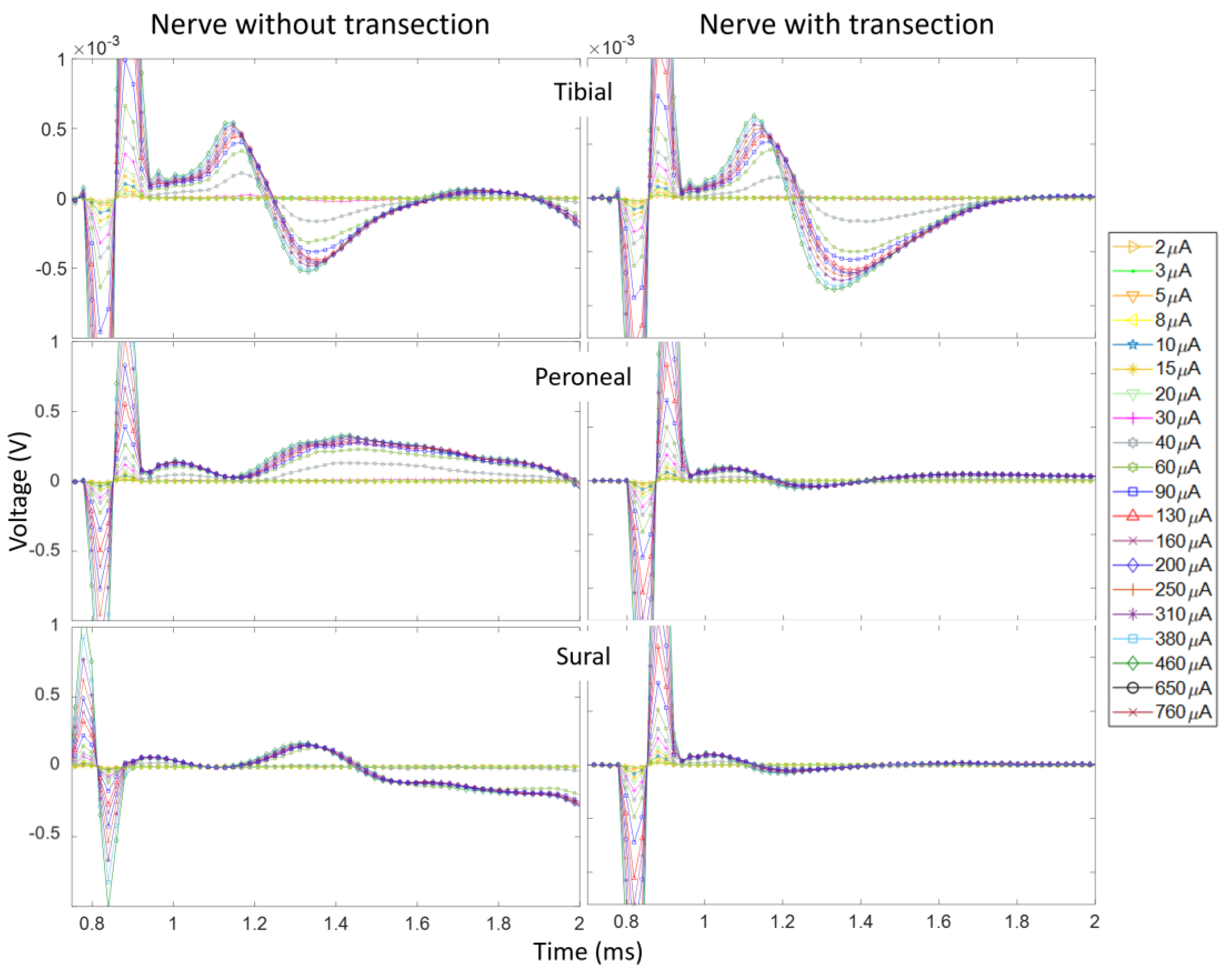
3.2. CNAPs and Recruitment Plots: Intact vs. Transected Nerves
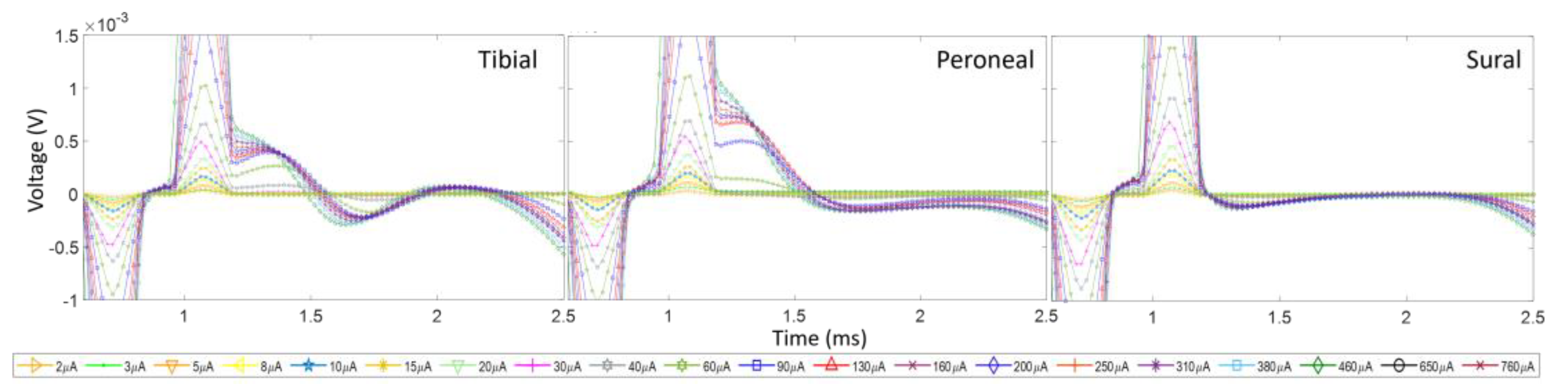
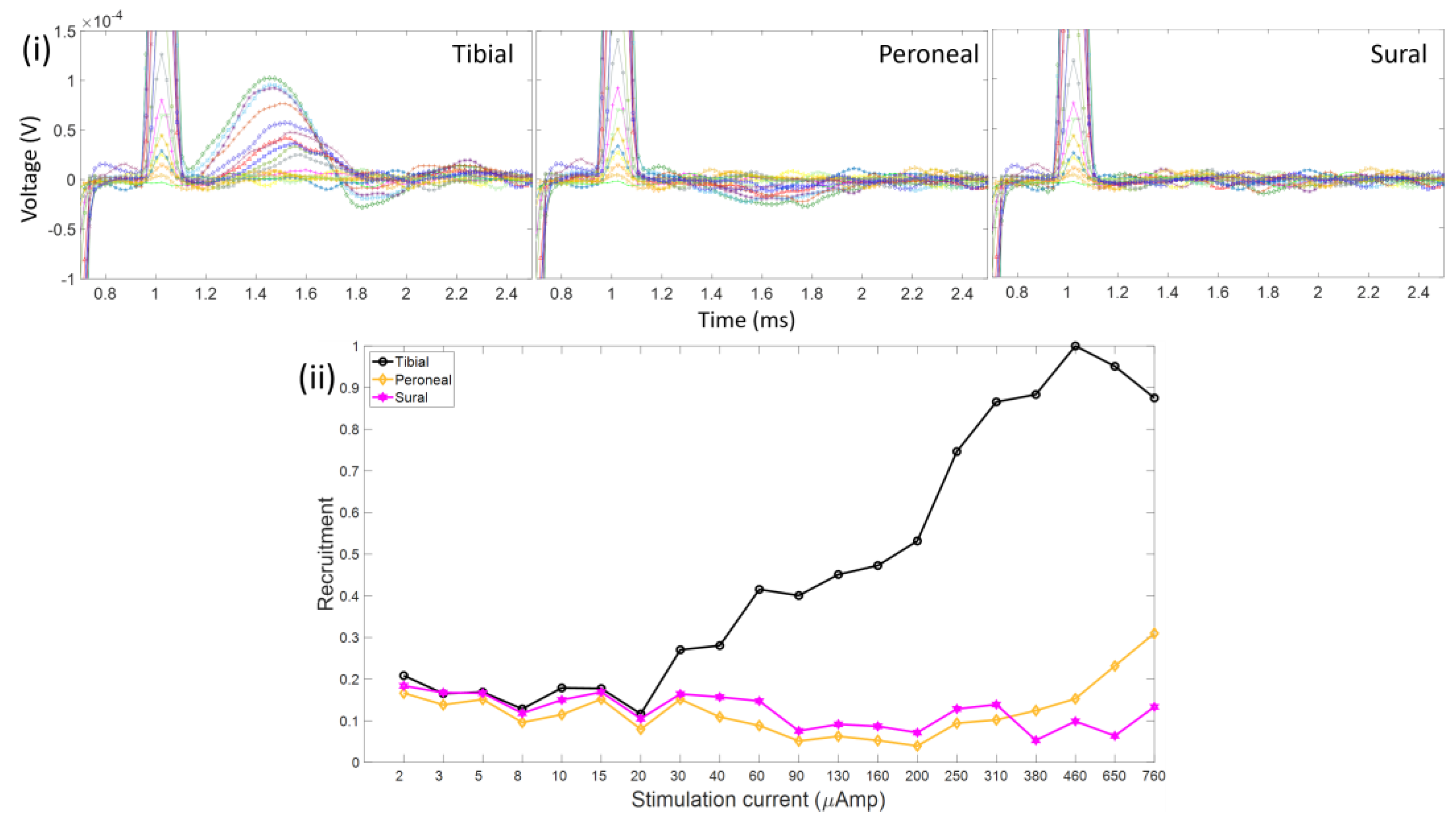
3.3. Activation of the Sciatic Nerve Branches and Placement of the Electrode
3.4. Surgery and Implantation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horch, K.W.; Kipke, D.R. Neuroprosthetics: Theory And Practice, 2nd ed.; World Scientific: Singapore, 2017. [Google Scholar]
- Jung, R.; Abbas, J.J.; Kuntaegowdanahalli, S.; Thota, A.K. Bionic intrafascicular interfaces for recording and stimulating peripheral nerve fibers. Bioelectron. Med. 2018, 1, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Navarro, X.; Krueger, T.B.; Lago, N.; Micera, S.; Stieglitz, T.; Dario, P. A critical review of interfaces with the peripheral nervous system for the control of neuroprostheses and hybrid bionic systems. J. Peripher. Nerv. Syst. 2005, 10, 229–258. [Google Scholar] [CrossRef] [PubMed]
- Rijnbeek, E.H.; Eleveld, N.; Olthuis, W. Update on Peripheral Nerve Electrodes for Closed-Loop Neuroprosthetics. Front. Neurosci. 2018, 12, 350. [Google Scholar] [CrossRef]
- Russell, C.; Roche, A.D.; Chakrabarty, S. Peripheral nerve bionic interface: A review of electrodes. Int. J. Intell. Robot. Appl. 2019, 3, 11–18. [Google Scholar] [CrossRef]
- Grill, W.M.; Norman, S.E.; Bellamkonda, R.V. Implanted Neural Interfaces: Biochallenges and Engineered Solutions. Annu. Rev. Biomed. Eng. 2009, 11, 1–24. [Google Scholar] [CrossRef]
- Grill, W.M.; Mortimer, J.T. Quantification of recruitment properties of multiple contact cuff electrodes. IEEE Trans. Rehabil. Eng. 1996, 4, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Grill, W.M.; Mortimer, J.T. Stability of the input-output properties of chronically implanted multiple contact nerve cuff stimulating electrodes. IEEE Trans. Rehabil. Eng. 1998, 6, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.N.; Kurstjens, G.A.M.; Struijk, J.J. Transverse Versus Longitudinal Tripolar Configuration for Selective Stimulation With Multipolar Cuff Electrodes. IEEE Trans. Biomed. Eng. 2011, 58, 913–919. [Google Scholar] [CrossRef]
- Kurstjens, M.; Jensen, W. Selectivity of longitudinal versus transverse tripolar stimulation of median nerve in pigs using a multicontact nerve cuff electrode. In Proceedings of the 10th Vienna International Workshop on Functional Electrical Stimulation, Vienna, Austria, 8–12 September 2010. [Google Scholar]
- Veraart, C.; Grill, W.M.; Mortimer, J.T. Selective control of muscle activation with a multipolar nerve cuff electrode. IEEE Trans. Biomed. Eng. 1993, 40, 640–653. [Google Scholar] [CrossRef]
- Polasek, K.H.; Hoyen, H.A.; Keith, M.W.; Kirsch, R.F.; Tyler, D.J. Stimulation Stability and Selectivity of Chronically Implanted Multicontact Nerve Cuff Electrodes in the Human Upper Extremity. IEEE Trans. Neural Syst. Rehabil. Eng. 2009, 17, 428–437. [Google Scholar] [CrossRef]
- Polasek, K.H.; Hoyen, H.A.; Keith, M.W.; Tyler, D.J. Human Nerve Stimulation Thresholds and Selectivity Using a Multi-contact Nerve Cuff Electrode. IEEE Trans. Neural Syst. Rehabil. Eng. 2007, 15, 76–82. [Google Scholar] [CrossRef]
- Schiefer, M.A.; Polasek, K.H.; Triolo, R.J.; Pinault, G.C.J.; Tyler, D.J. Selective stimulation of the human femoral nerve with a flat interface nerve electrode. J. Neural Eng. 2010, 7, 026006. [Google Scholar] [CrossRef] [PubMed]
- Tyler, D.J.; Durand, D.M. Functionally selective peripheral nerve stimulation with a flat interface nerve electrode. IEEE Trans. Neural Syst. Rehabil. Eng. 2002, 10, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Leventhal, D.K.; Durand, D.M. Chronic measurement of the stimulation selectivity of the flat interface nerve electrode. IEEE Trans. Biomed. Eng. 2004, 51, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
- Tyler, D.J.; Durand, D.M. Chronic Response of the Rat Sciatic Nerve to the Flat Interface Nerve Electrode. Ann. Biomed. Eng. 2003, 31, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Thoma, H.; Grisch, W.; Holle, J.; Mayr, W. Technology and Long-term Application of an Epineural Electrode. ASAIO J. 1989, 35, 490–494. [Google Scholar] [CrossRef]
- Yoshida, K.; Horch, K. Selective stimulation of peripheral nerve fibers using dual intrafascicular electrodes. IEEE Trans. Biomed. Eng. 1993, 40, 492–494. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Jovanović, K.; Stein, R.B. Intrafascicular electrodes for stimulation and recording from mudpuppy spinal roots. J. Neurosci. Methods 2000, 96, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Jensen, W.; Yoshida, K. Long-term recording properties of longitudinal intra-fascicular electrodes. In Proceedings of the 7th Annual Conference of the International Functional Electrical Stimulation Society, IFESS 2002, Ljubljana, Slovenia, 25–29 June 2002; pp. 138–140. [Google Scholar]
- Lefurge, T.; Goodall, E.; Horch, K.; Stensaas, L.; Schoenberg, A. Chronically implanted intrafascicular recording electrodes. Ann. Biomed. Eng. 1991, 19, 197–207. [Google Scholar] [CrossRef]
- Yoshida, K.; Horch, K. Closed-loop control of ankle position using muscle afferent feedback with functional neuromuscular stimulation. IEEE Trans. Biomed. Eng. 1996, 43, 167–176. [Google Scholar] [CrossRef]
- Horch, K.; Meek, S.; Taylor, T.G.; Hutchinson, D.T. Object Discrimination With an Artificial Hand Using Electrical Stimulation of Peripheral Tactile and Proprioceptive Pathways With Intrafascicular Electrodes. IEEE Trans. Neural Syst. Rehabil. Eng. 2011, 19, 483–489. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Lawrence, S.M.; Hutchinson, D.T.; Horch, K.W. Residual function in peripheral nerve stumps of amputees: Implications for neural control of artificial limbs1 1No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article. J. Hand Surg. 2004, 29, 605–615. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Horch, K.W. Direct neural sensory feedback and control of a prosthetic arm. IEEE Trans. Neural Syst. Rehabil. Eng. 2005, 13, 468–472. [Google Scholar] [CrossRef]
- Citi, L.; Carpaneto, J.; Yoshida, K.; Hoffmann, K.; Koch, K.; Dario, P.; Micera, S. Characterization of tfLIFE Neural Response for the Control of a Cybernetic Hand. In Proceedings of the First IEEE/RAS-EMBS International Conference on Biomedical Robotics and Biomechatronics, 2006. BioRob 2006, Pisa, Italy, 20–22 February 2006; pp. 477–482. [Google Scholar] [CrossRef]
- Yoshida, K.; Kurstjens, M.; Citi, L.; Koch, K.P.; Micera, S. Recording experience with the thin-film Longitudinal Intra-Fascicular Electrode, a multichannel peripheral nerve interface. In Proceedings of the 2007 IEEE 10th International Conference on Rehabilitation Robotics, Noordwijk, Netherlands, 13–15 June 2007; pp. 862–867. [Google Scholar] [CrossRef]
- Kurstjens, M.; Jensen, W.; Yoshida, K. Selective Activation of Pig Forearm Muscles using Thin-Film Intrafascicular Electrodes Implanted in the Median Nerve. In Proceedings of the 13th Annual Conference of the International Functional Electrical Stimulation Society, Freiburg, Germany, 21–25 September 2008. [Google Scholar]
- Lago, N.; Yoshida, K.; Koch, K.P.; Navarro, X. Assessment of Biocompatibility of Chronically Implanted Polyimide and Platinum Intrafascicular Electrodes. IEEE Trans. Biomed. Eng. 2007, 54, 281–290. [Google Scholar] [CrossRef]
- Navarro, X.; Lago, N.; Vivo, M.; Yoshida, K.; Koch, K.P.; Poppendieck, W.; Micera, S. Neurobiological evaluation of thin-film longitudinal intrafascicular electrodes as a peripheral nerve interface. In Proceedings of the 2007 IEEE 10th International Conference on Rehabilitation Robotics, Noordwijk, Netherlands, 13–15 June 2007; pp. 643–649. [Google Scholar] [CrossRef]
- Badia, J.; Boretius, T.; Pascual-Font, A.; Udina, E.; Stieglitz, T.; Navarro, X. Biocompatibility of Chronically Implanted Transverse Intrafascicular Multichannel Electrode (TIME) in the Rat Sciatic Nerve. IEEE Trans. Biomed. Eng. 2011, 58, 2324–2332. [Google Scholar] [CrossRef]
- Badia, J.; Boretius, T.; Andreu, D.; Azevedo-Coste, C.; Stieglitz, T.; Navarro, X. Comparative analysis of transverse intrafascicular multichannel, longitudinal intrafascicular and multipolar cuff electrodes for the selective stimulation of nerve fascicles. J. Neural Eng. 2011, 8, 036023. [Google Scholar] [CrossRef]
- Kundu, A.; Harreby, K.R.; Yoshida, K.; Boretius, T.; Stieglitz, T.; Jensen, W.; Selectivity of the, S. “Thin-Film Longitudinal Intrafascicular Electrode” (tfLIFE) and the “Transverse Intrafascicular Multi-Channel Electrode” (TIME) in the Large Nerve Animal Model. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 400–410. [Google Scholar] [CrossRef]
- Harreby, K.R.; Kundu, A.; Yoshida, K.; Boretius, T.; Stieglitz, T.; Jensen, W. Subchronic Stimulation Performance of Transverse Intrafascicular Multichannel Electrodes in the Median Nerve of the Göttingen Minipig. Artif. Organs 2015, 39, E36–E48. [Google Scholar] [CrossRef] [PubMed]
- Raspopovic, S.; Capogrosso, M.; Petrini, F.M.; Bonizzato, M.; Rigosa, J.; Di Pino, G.; Carpaneto, J.; Controzzi, M.; Boretius, T.; Fernandez, E.; et al. Restoring natural sensory feedback in real-time bidirectional hand prostheses. Sci. Transl. Med. 2014, 6, 222ra19. [Google Scholar] [CrossRef] [PubMed]
- Branner, A.; Stein, R.B.; Normann, R.A. Selective Stimulation of Cat Sciatic Nerve Using an Array of Varying-Length Microelectrodes. J. Neurophysiol. 2001, 85, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- Branner, A.; Stein, R.B.; Fernandez, E.; Aoyagi, Y.; Normann, R.A. Long-term stimulation and recording with a penetrating microelectrode array in cat sciatic nerve. IEEE Trans. Biomed. Eng. 2004, 51, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Branner, A.; Normann, R.A. A multielectrode array for intrafascicular recording and stimulation in sciatic nerve of cats. Brain Res. Bull. 2000, 51, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, K.A.; Shin, A.Y.; Kaufman, K.R. Interfaces with the peripheral nervous system for the control of a neuroprosthetic limb: A review. J. NeuroEng. Rehabil. 2020, 17, 43. [Google Scholar] [CrossRef] [PubMed]
- Boretius, T.; Badia, J.; Pascual-Font, A.; Schuettler, M.; Navarro, X.; Yoshida, K.; Stieglitz, T. A transverse intrafascicular multichannel electrode (TIME) to interface with the peripheral nerve. Biosens. Bioelectron. 2010, 26, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Martínez, I.; Badia, J.; Pascual-Font, A.; Rodríguez-Baeza, A.; Navarro, X. Fascicular Topography of the Human Median Nerve for Neuroprosthetic Surgery. Front. Neurosci. 2016, 10, 286. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, K.J.; Pinault, G.C.; Neville, J.J.; Syed, I.; Davis, J.A., Jr.; Jean-Claude, J.; Triolo, R.J. Fascicular anatomy of human femoral nerve: Implications for neural prostheses using nerve cuff electrodes. J. Rehabil. Res. Dev. 2009, 46, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Verplancke, R.; Cauwe, M.; Schaubroeck, D.; Cuypers, D.; Vandecasteele, B.; Mader, L.; Vanhaverbeke, C.; Ballini, M.; O’callaghan, J.; Goikoetxea, E.; et al. Development of an active high-density transverse intrafascicular micro-electrode probe. J. Micromechanics Microeng. 2019, 30, 015010. [Google Scholar] [CrossRef]
- Ballini, M.; Bae, J.; Marrocco, N.; Verplancke, R.; Schaubroeck, D.; Cuypers, D.; Cauwe, M.; O’Callaghan, J.; Fahmy, A.; Maghari, N.; et al. traneural active probe for bidirectional peripheral nerve interface. In Proceedings of the 2017 Symposium on VLSI Circuits, Kyoto, Japan, 5–8 June 2017; pp. C50–C51. [Google Scholar] [CrossRef]
- Ortiz-Catalan, M.; Brånemark, R.; Håkansson, B.; Delbeke, J. On the viability of implantable electrodes for the natural control of artificial limbs: Review and discussion. BioMedical Eng. OnLine 2012, 11, 33. [Google Scholar] [CrossRef]
- Heiduschka, P.; Thanos, S. Implantable bioelectronic interfaces for lost nerve functions. Prog. Neurobiol. 1998, 55, 433–461. [Google Scholar] [CrossRef]
- Wurth, S.; Capogrosso, M.; Raspopovic, S.; Gandar, J.; Federici, G.; Kinany, N.; Cutrone, A.; Piersigilli, A.; Pavlova, N.; Guiet, R.; et al. Long-term usability and bio-integration of polyimide-based intra-neural stimulating electrodes. Biomaterials 2017, 122, 114–129. [Google Scholar] [CrossRef]
- Farina, D.; Merletti, R.; Enoka, R.M. The extraction of neural strategies from the surface EMG: An update. J. Appl. Physiol. 2014, 117, 1215–1230. [Google Scholar] [CrossRef] [PubMed]
- Vieira, T.M.; Botter, A.; Muceli, S.; Farina, D. Specificity of surface EMG recordings for gastrocnemius during upright standing. Sci. Rep. 2017, 7, 13300. [Google Scholar] [CrossRef] [PubMed]
- Badia, J.; Pascual-Font, A.; Vivó, M.; Udina, E.; Navarro, X. Topographical distribution of motor fascicles in the sciatic-tibial nerve of the rat. Muscle Nerve 2010, 42, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Farina, D.; Akay, M.; Jensen, W. Multichannel Intraneural and Intramuscular Techniques for Multiunit Recording and Use in Active Prostheses. Proc. IEEE 2010, 98, 432–449. [Google Scholar] [CrossRef]
- Kundu, A.; Harreby, K.R.; Jensen, W. Comparison of median and ulnar nerve morphology of Danish landrace pigs and Göttingen mini pigs. In Proceedings of the Annual Conference of the International Functional Electrical Stimulation Society, IFESS, Banff, AB, Canada, 9–12 September 2012. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kundu, A.; Patrick, E.; Currlin, S.; Madler, R.; Delgado, F.; Fahmy, A.; Verplancke, R.; Ballini, M.; Braeken, D.; de Beeck, M.O.; et al. Using Compound Neural Action Potentials for Functional Validation of a High-Density Intraneural Interface: A Preliminary Study. Micromachines 2024, 15, 280. https://doi.org/10.3390/mi15020280
Kundu A, Patrick E, Currlin S, Madler R, Delgado F, Fahmy A, Verplancke R, Ballini M, Braeken D, de Beeck MO, et al. Using Compound Neural Action Potentials for Functional Validation of a High-Density Intraneural Interface: A Preliminary Study. Micromachines. 2024; 15(2):280. https://doi.org/10.3390/mi15020280
Chicago/Turabian StyleKundu, Aritra, Erin Patrick, Seth Currlin, Ryan Madler, Francisco Delgado, Ahmed Fahmy, Rik Verplancke, Marco Ballini, Dries Braeken, Maaike Op de Beeck, and et al. 2024. "Using Compound Neural Action Potentials for Functional Validation of a High-Density Intraneural Interface: A Preliminary Study" Micromachines 15, no. 2: 280. https://doi.org/10.3390/mi15020280
APA StyleKundu, A., Patrick, E., Currlin, S., Madler, R., Delgado, F., Fahmy, A., Verplancke, R., Ballini, M., Braeken, D., de Beeck, M. O., Maghari, N., Otto, K. J., & Bashirullah, R. (2024). Using Compound Neural Action Potentials for Functional Validation of a High-Density Intraneural Interface: A Preliminary Study. Micromachines, 15(2), 280. https://doi.org/10.3390/mi15020280







