Three-Dimensional-Printed Instrument for Isothermal Nucleic Acid Amplification with Real-Time Colorimetric Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Body Fluid Sample Collection
2.2. RNA Isolation with DNase Treatment
2.3. Primer Information for Messenger RNA Targets
2.4. Colorimetric LAMP
2.5. isoTAAC System
2.6. Mock Study
3. Results and Discussion
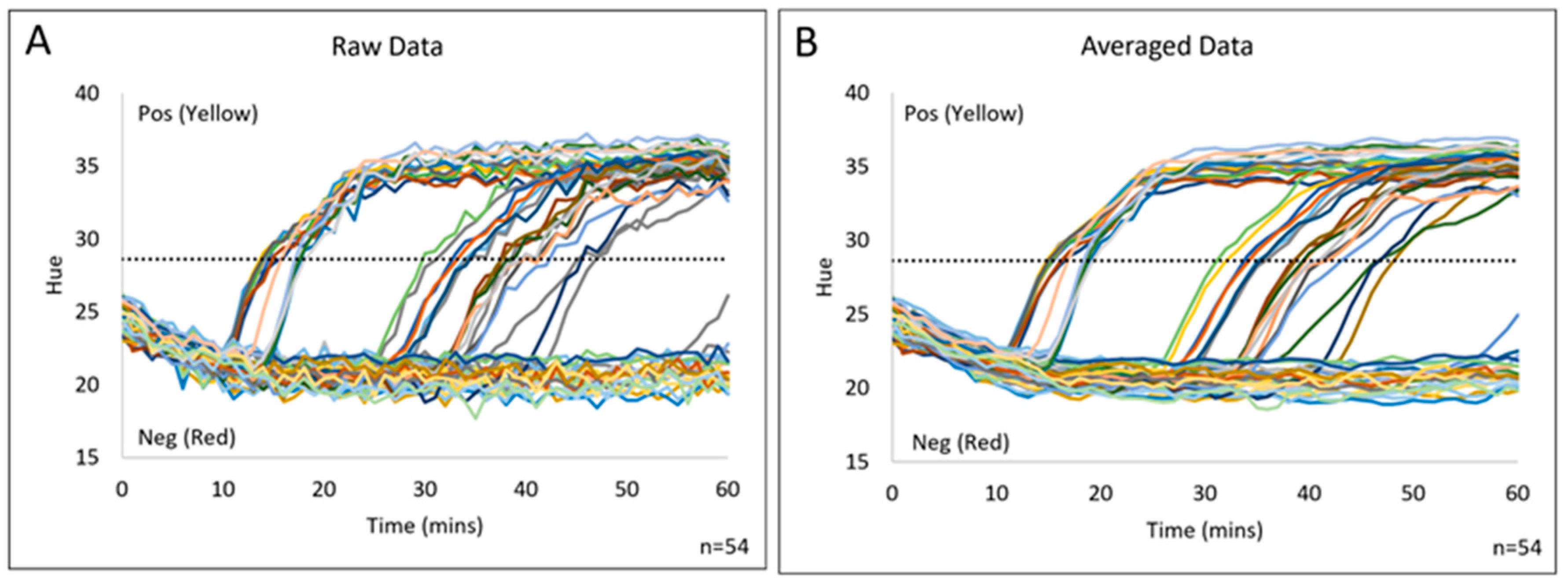
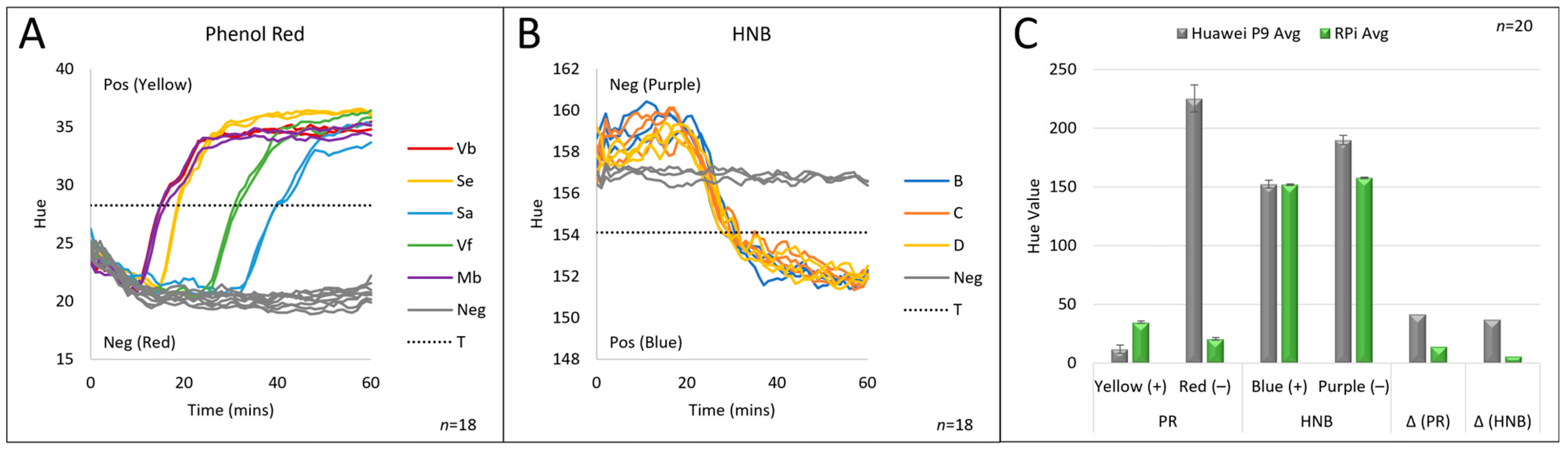
3.1. Colorimetric Analysis
3.2. The IsoTAAC System
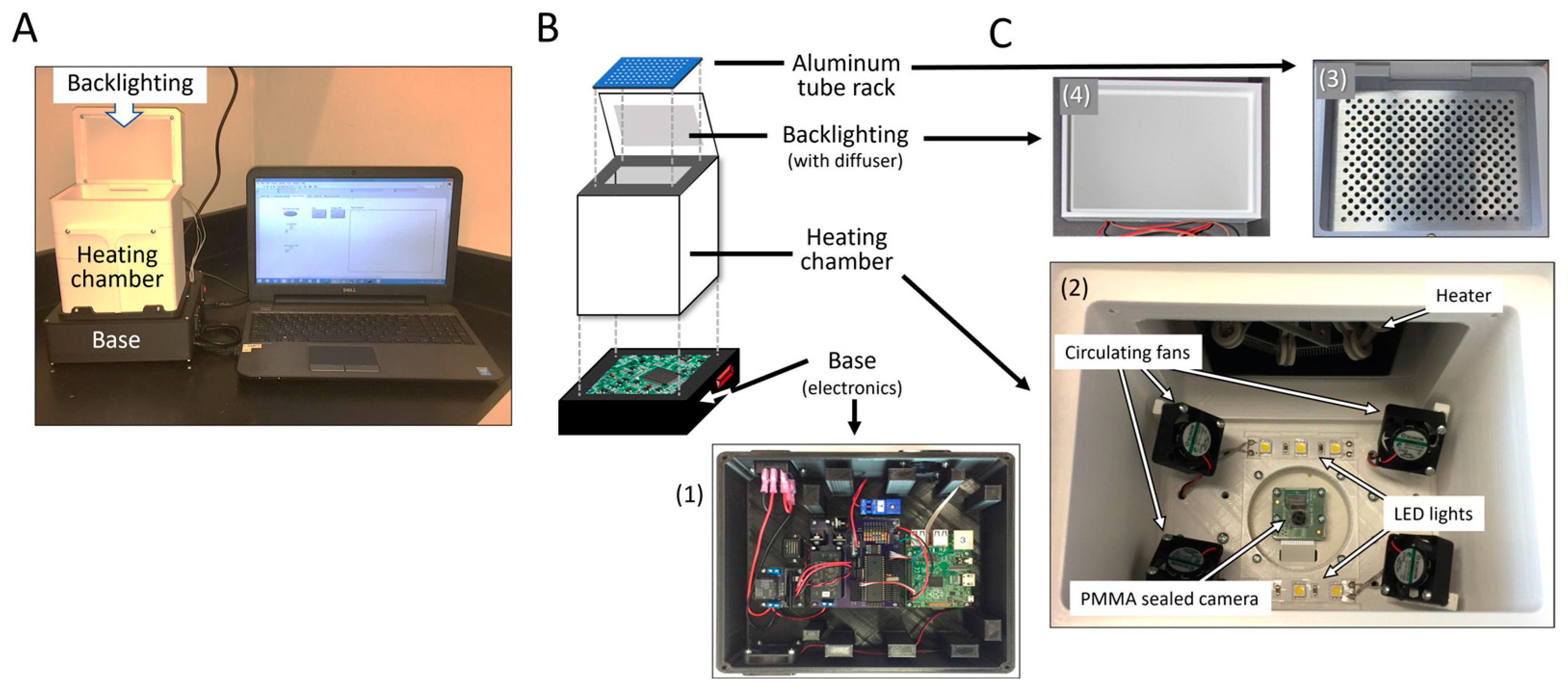
3.2.1. Hardware
3.2.2. Isothermal Heating
3.2.3. System Software Control
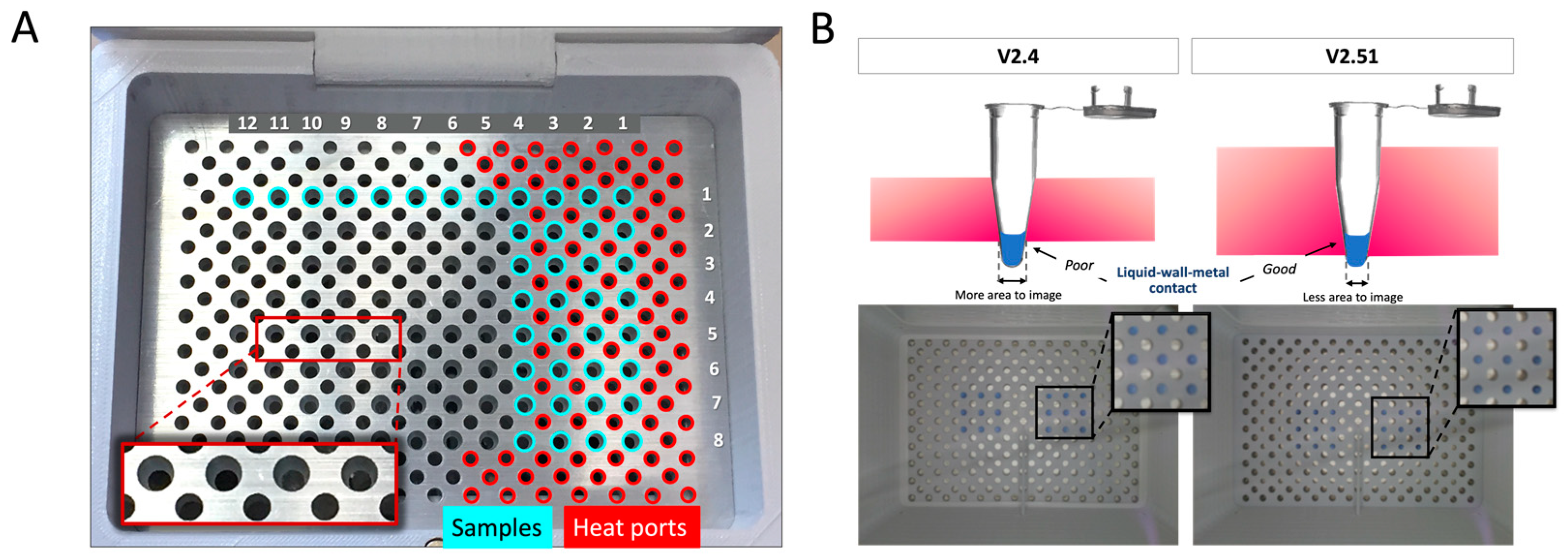
3.2.4. Optimizing the Heating Rate and Stabilization
3.3. Colorimetric Image Analysis
3.4. Mock Study Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Notomi, T. Loop-Mediated Isothermal Amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef]
- Huang, W.E.; Lim, B.; Hsu, C.; Xiong, D.; Wu, W.; Yu, Y.; Jia, H.; Wang, Y.; Zeng, Y.; Ji, M.; et al. RT-LAMP for Rapid Diagnosis of Coronavirus SARS-CoV-2. Microb. Biotechnol. 2020, 13, 950–961. [Google Scholar] [CrossRef]
- Bartosik, M.; Jirakova, L.; Anton, M.; Vojtesek, B.; Hrstka, R. Genomagnetic LAMP-Based Electrochemical Test for Determination of High-Risk HPV16 and HPV18 in Clinical Samples. Anal. Chim. Acta 2018, 1042, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Tsai, L.-C.; Su, C.-W.; Lee, J.C.-I.; Lu, Y.-S.; Chen, H.-C.; Lin, Y.-C.; Linacre, A.; Hsieh, H.-M. The Detection and Identification of Saliva in Forensic Samples by RT-LAMP. Forensic Sci. Med. Pathol. 2018, 14, 469–477. [Google Scholar] [CrossRef]
- Satoh, T.; Kouroki, S.; Ogawa, K.; Tanaka, Y.; Matsumura, K.; Iwase, S. Development of mRNA-Based Body Fluid Identification Using Reverse Transcription Loop-Mediated Isothermal Amplification. Anal. Bioanal. Chem. 2018, 410, 4371–4378. [Google Scholar] [CrossRef] [PubMed]
- Tanner, N.A.; Zhang, Y.; Evans, T.C. Simultaneous Multiple Target Detection in Real-Time Loop-Mediated Isothermal Amplification. BioTechniques 2012, 53, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Nagamine, K.; Hase, T.; Notomi, T. Accelerated Reaction by Loop-Mediated Isothermal Amplification Using Loop Primers. Mol. Cell. Probes 2002, 16, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Parida, M.; Horioke, K.; Ishida, H.; Dash, P.K.; Saxena, P.; Jana, A.M.; Islam, M.A.; Inoue, S.; Hosaka, N.; Morita, K. Rapid Detection and Differentiation of Dengue Virus Serotypes by a Real-Time Reverse Transcription-Loop-Mediated Isothermal Amplification Assay. J. Clin. Microbiol. 2005, 43, 2895–2903. [Google Scholar] [CrossRef] [PubMed]
- Shirato, K.; Semba, S.; El-Kafrawy, S.A.; Hassan, A.M.; Tolah, A.M.; Takayama, I.; Kageyama, T.; Notomi, T.; Kamitani, W.; Matsuyama, S.; et al. Development of Fluorescent Reverse Transcription Loop-Mediated Isothermal Amplification (RT-LAMP) Using Quenching Probes for the Detection of the Middle East Respiratory Syndrome Coronavirus. J. Virol. Methods 2018, 258, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, H.; Wang, Y.; Zhang, L.; Xu, J.; Ye, C. Loop-Mediated Isothermal Amplification Label-Based Gold Nanoparticles Lateral Flow Biosensor for Detection of Enterococcus faecalis and Staphylococcus aureus. Front. Microbiol. 2017, 8, 192. [Google Scholar] [CrossRef]
- Goto, M.; Honda, E.; Ogura, A.; Nomoto, A.; Hanaki, K.-I. Colorimetric Detection of Loop-Mediated Isothermal Amplification Reaction by Using Hydroxy Naphthol Blue. BioTechniques 2009, 46, 167–172. [Google Scholar] [CrossRef]
- Miyamoto, S.; Sano, S.; Takahashi, K.; Jikihara, T. Method for Colorimetric Detection of Double-Stranded Nucleic Acid Using Leuco Triphenylmethane Dyes. Anal. Biochem. 2015, 473, 28–33. [Google Scholar] [CrossRef]
- Nogami, H.; Tsutsumi, H.; Komuro, T.; Mukoyama, R. Rapid and Simple Sex Determination Method from Dental Pulp by Loop-Mediated Isothermal Amplification. Forensic Sci. Int. Genet. 2008, 2, 349–353. [Google Scholar] [CrossRef]
- Scott, A.T.; Layne, T.R.; O’Connell, K.C.; Tanner, N.A.; Landers, J.P. Comparative Evaluation and Quantitative Analysis of Loop-Mediated Isothermal Amplification Indicators. Anal. Chem. 2020, 92, 13343–13353. [Google Scholar] [CrossRef]
- Fischbach, J.; Xander, N.C.; Frohme, M.; Glökler, J.F. Shining a Light on LAMP Assays’ A Comparison of LAMP Visualization Methods Including the Novel Use of Berberine. BioTechniques 2015, 58, 189–194. [Google Scholar] [CrossRef]
- Woolf, M.S.; Dignan, L.M.; Scott, A.T.; Landers, J.P. Digital Postprocessing and Image Segmentation for Objective Analysis of Colorimetric Reactions. Nat. Protoc. 2021, 16, 218–238. [Google Scholar] [CrossRef] [PubMed]
- Priye, A.; Ball, C.S.; Meagher, R.J. Colorimetric-Luminance Readout for Quantitative Analysis of Fluorescence Signals with a Smartphone CMOS Sensor. Anal. Chem. 2018, 90, 12385–12389. [Google Scholar] [CrossRef] [PubMed]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next Generation of Scientific Image Data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Choi, S.; Yang, J.-S.; Song, J.; Choi, J.-S.; Jung, H.-I. Smart Forensic Phone: Colorimetric Analysis of a Bloodstain for Age Estimation Using a Smartphone. Sens. Actuators B Chem. 2017, 243, 221–225. [Google Scholar] [CrossRef]
- Krauss, S.T.; Nauman, A.Q.; Garner, G.T.; Landers, J.P. Color Manipulation through Microchip Tinting for Colorimetric Detection Using Hue Image Analysis. Lab. Chip 2017, 17, 4089–4096. [Google Scholar] [CrossRef] [PubMed]
- Layne, T.; Jackson, K.; Scott, A.; Tanner, N.A.; Piland, A.; Haverstick, D.M.; Landers, J.P. Optimization of Novel Loop-mediated Isothermal Amplification with Colorimetric Image Analysis for Forensic Body Fluid Identification. J. Forensic Sci. 2021, 66, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.; Birch, C.; Mills, D.; Nouwairi, R.L.; Turiello, R.; Panesar, S.; Li, J.; Le Roux, D.; Nelson, D.; Thompson, B.; et al. Rapid Swab-to-Genotype for Human Identification on a Portable, Ultra-Rapid Microfluidic Disc Analysis System. 2024; in preparation. [Google Scholar]
- De Wolff, T.R.; Kal, A.J.; Berger, C.E.H.; Kokshoorn, B. A Probabilistic Approach to Body Fluid Typing Interpretation: An Exploratory Study on Forensic Saliva Testing. Law Probab. Risk 2015, 14, mgv014. [Google Scholar] [CrossRef]
- Jackson, K.R.; Layne, T.; Dent, D.A.; Tsuei, A.; Li, J.; Haverstick, D.M.; Landers, J.P. A Novel Loop-Mediated Isothermal Amplification Method for Identification of Four Body Fluids with Smartphone Detection. Forensic Sci. Int. Genet. 2020, 45, 102195. [Google Scholar] [CrossRef] [PubMed]
- Norris, J.V.; Manning, K.; Linke, S.J.; Ferrance, J.P.; Landers, J.P. Expedited, Chemically Enhanced Sperm Cell Recovery from Cotton Swabs for Rape Kit Analysis. J. Forensic Sci. 2007, 52, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, P.; Schürenkamp, M.; Schütte, U. DNA Extraction from Mixtures of Body Fluid Using Mild Preferential Lysis. Int. J. Leg. Med. 1992, 104, 359–360. [Google Scholar] [CrossRef]
- Shen, H.; Wang, S.; Huang, J.; Lin, Q.; Zhang, C.; Liu, Z.; Zhang, J.; Liao, M. A Novel, Cleaved Probe-Based Reverse Transcription Loop-Mediated Isothermal Amplification Method for Specific and Sensitive Detection of Porcine Deltacoronavirus. Front. Vet. Sci. 2022, 9, 896416. [Google Scholar] [CrossRef]
- Papadakis, G.; Pantazis, A.K.; Fikas, N.; Chatziioannidou, S.; Tsiakalou, V.; Michaelidou, K.; Pogka, V.; Megariti, M.; Vardaki, M.; Giarentis, K.; et al. Portable Real-Time Colorimetric LAMP-Device for Rapid Quantitative Detection of Nucleic Acids in Crude Samples. Sci. Rep. 2022, 12, 3775. [Google Scholar] [CrossRef]
- Myers, F.B.; Moffatt, B.; El Khaja, R.; Chatterjee, T.; Marwaha, G.; McGee, M.; Mitra, D. A Robust, Low-Cost Instrument for Real-Time Colorimetric Isothermal Nucleic Acid Amplification. PLoS ONE 2022, 17, e0256789. [Google Scholar] [CrossRef]
- Chang, J.; Kang, H.; Kang, M.G. Correction of Axial and Lateral Chromatic Aberration with False Color Filtering. IEEE Trans. Image Process. 2013, 22, 1186–1198. [Google Scholar] [CrossRef]
- Scott, A.; Jackson, K.; Carter, M.; Comeau, R.; Layne, T.; Landers, J. Rapid Sperm Lysis and Novel Screening Approach for Human Male DNA via Colorimetric Loop-Mediated Isothermal Amplification. Forensic Sci. Int. Genet. 2019, 43, 102139. [Google Scholar] [CrossRef] [PubMed]
- Giardina, E. DNA Fingerprinting. In Brenner’s Encyclopedia of Genetics; Elsevier: Amsterdam, The Netherlands, 2013; pp. 356–359. ISBN 978-0-08-096156-9. [Google Scholar]
- Sijen, T.; Harbison, S. On the Identification of Body Fluids and Tissues: A Crucial Link in the Investigation and Solution of Crime. Genes. 2021, 12, 1728. [Google Scholar] [CrossRef] [PubMed]
- Haas, C.; Klesser, B.; Maake, C.; Bär, W.; Kratzer, A. mRNA Profiling for Body Fluid Identification by Reverse Transcription Endpoint PCR and Realtime PCR. Forensic Sci. Int. Genet. 2009, 3, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.; Fleming, R. RNA-based Approaches for Body Fluid Identification in Forensic Science. WIREs Forensic Sci. 2021, 3, e1407. [Google Scholar] [CrossRef]
- Sakurada, K.; Akutsu, T.; Watanabe, K.; Fujinami, Y.; Yoshino, M. Expression of Statherin mRNA and Protein in Nasal and Vaginal Secretions. Leg. Med. 2011, 13, 309–313. [Google Scholar] [CrossRef]




| Sample Number | Mock Sample | Vb | Se | Sa | Vf | ||||
|---|---|---|---|---|---|---|---|---|---|
| E | A | E | A | E | A | E | A | ||
| 1 | VF swab spiked with semen (100 µL 1:100) | − | − | + | − | − | + | + | + |
| 2 | MB swab spiked with semen (50 µL 1:100) | + | + | + | − | − | + | + | + |
| 3 | MB swab | + | + | − | − | − | − | + | + |
| 4 | Nasal swab spiked with saliva (100 µL) | − | + | − | − | + | + | − | − |
| 5 | VF smear on cloth, spiked with male saliva (100 µL) | − | − | − | − | + | + | + | + |
| 6 | Blood smear on jeans | + | + | − | − | − | − | − | − |
| 7 | VF smear on jeans, spiked with semen (100 µL 1:100) | − | + | + | − | − | − | + | + |
| 8 | 4 yr old blood on filter paper | + | + | − | − | − | − | − | − |
| 9 | 4 yr old semen on filter paper | − | − | + | − | − | + | − | − |
| 10 | Nasal swab | − | − | − | − | − | − | − | − |
| 11 | Liquid breast milk | − | − | − | − | − | − | − | − |
| 12 | Breast milk on swab, spiked with saliva (100 µL) | − | − | − | − | + | + | − | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Layne, T.R.; Scott, A.; Cunha, L.L.; Turiello, R.; Landers, J.P. Three-Dimensional-Printed Instrument for Isothermal Nucleic Acid Amplification with Real-Time Colorimetric Imaging. Micromachines 2024, 15, 271. https://doi.org/10.3390/mi15020271
Layne TR, Scott A, Cunha LL, Turiello R, Landers JP. Three-Dimensional-Printed Instrument for Isothermal Nucleic Acid Amplification with Real-Time Colorimetric Imaging. Micromachines. 2024; 15(2):271. https://doi.org/10.3390/mi15020271
Chicago/Turabian StyleLayne, Tiffany R., Anchi Scott, Larissa L. Cunha, Rachelle Turiello, and James P. Landers. 2024. "Three-Dimensional-Printed Instrument for Isothermal Nucleic Acid Amplification with Real-Time Colorimetric Imaging" Micromachines 15, no. 2: 271. https://doi.org/10.3390/mi15020271
APA StyleLayne, T. R., Scott, A., Cunha, L. L., Turiello, R., & Landers, J. P. (2024). Three-Dimensional-Printed Instrument for Isothermal Nucleic Acid Amplification with Real-Time Colorimetric Imaging. Micromachines, 15(2), 271. https://doi.org/10.3390/mi15020271







