Effects of Oxidized Metal Powders on Pore Defects in Powder-Fed Direct Energy Deposition
Abstract
1. Introduction
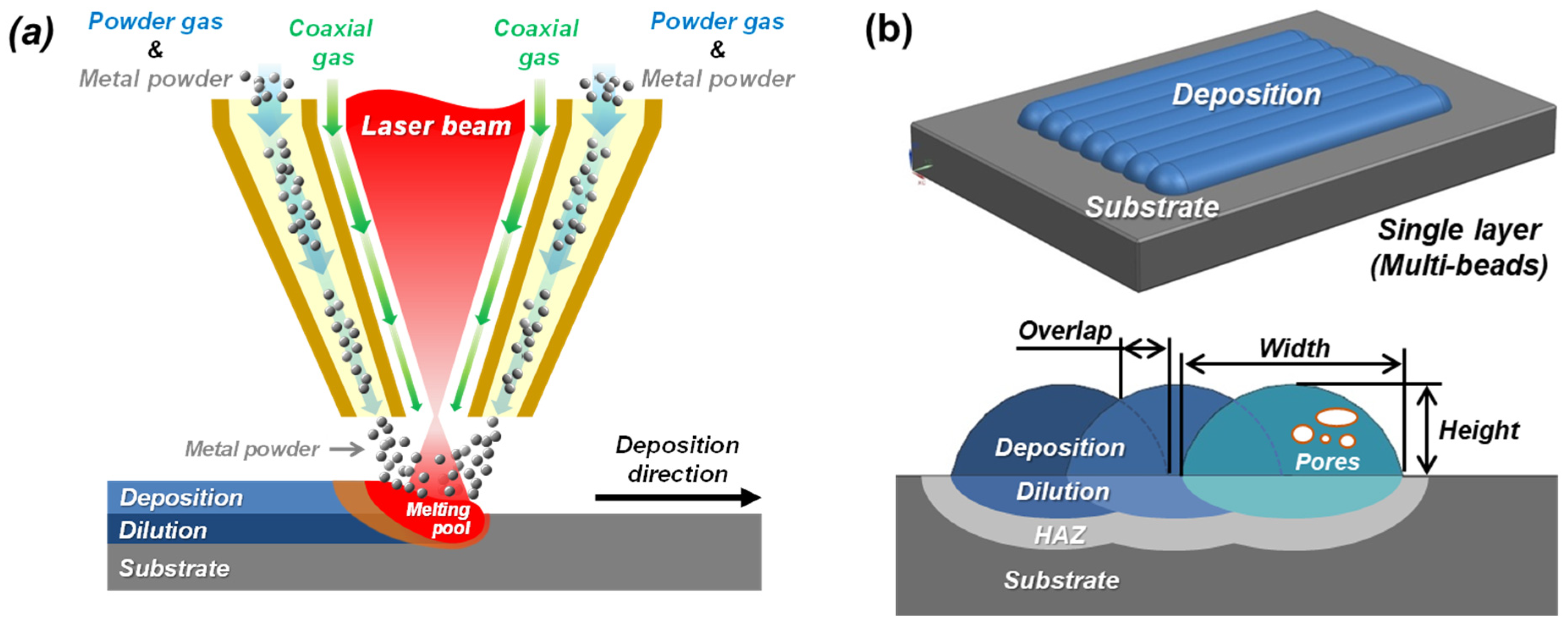
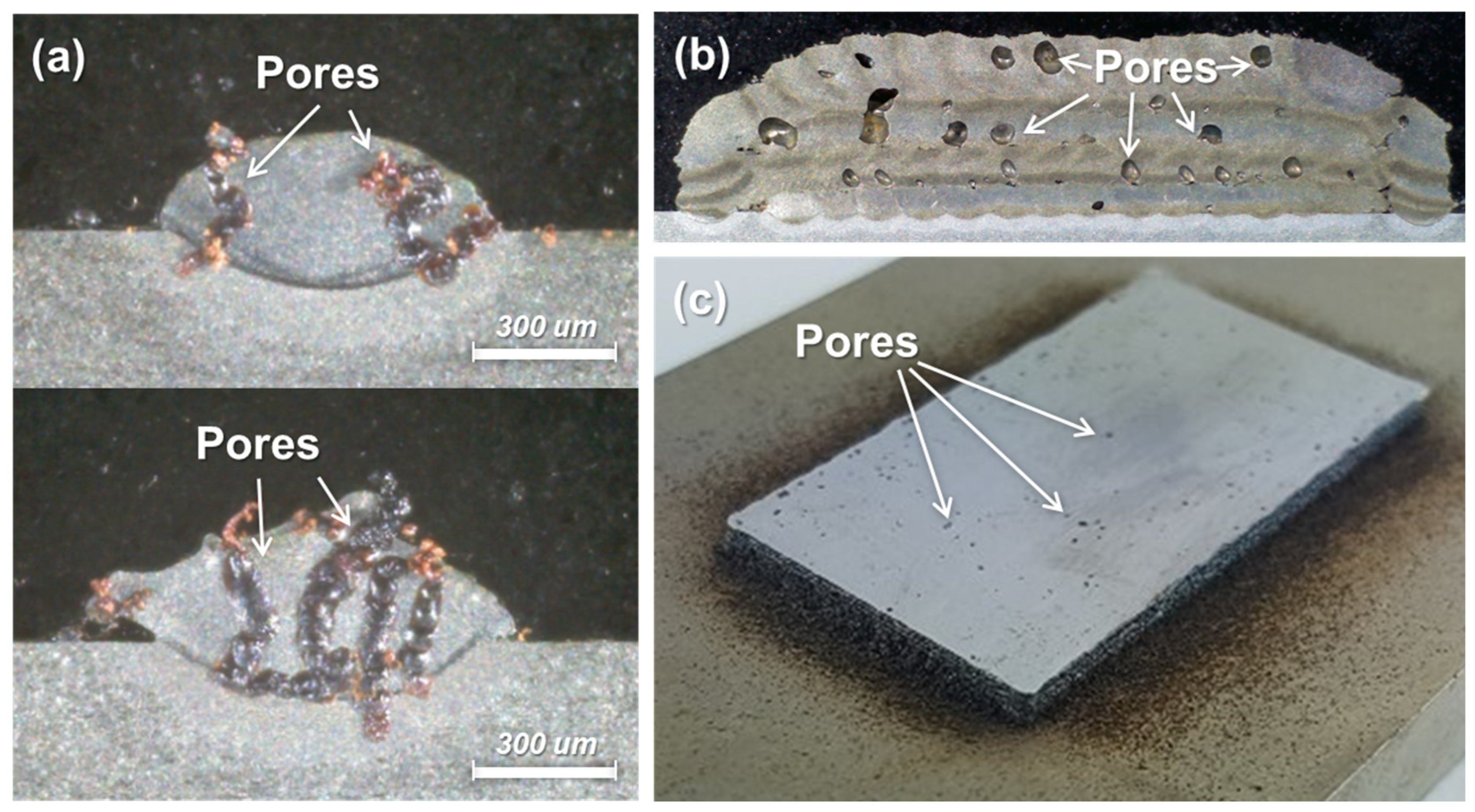
2. Mechanisms of the Formation of Pores in DED Processes
3. Materials and Methods
3.1. Materials
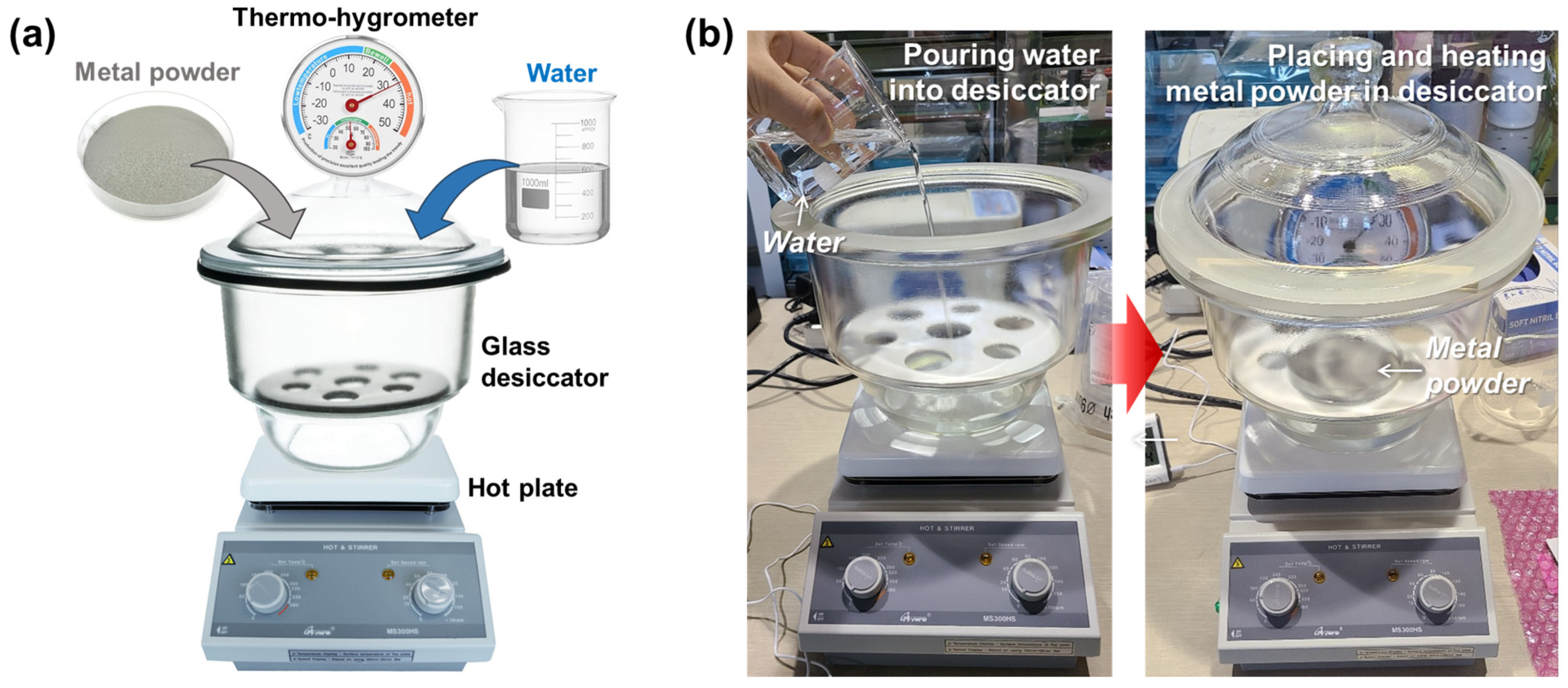
3.2. Oxidation of Metal Powders
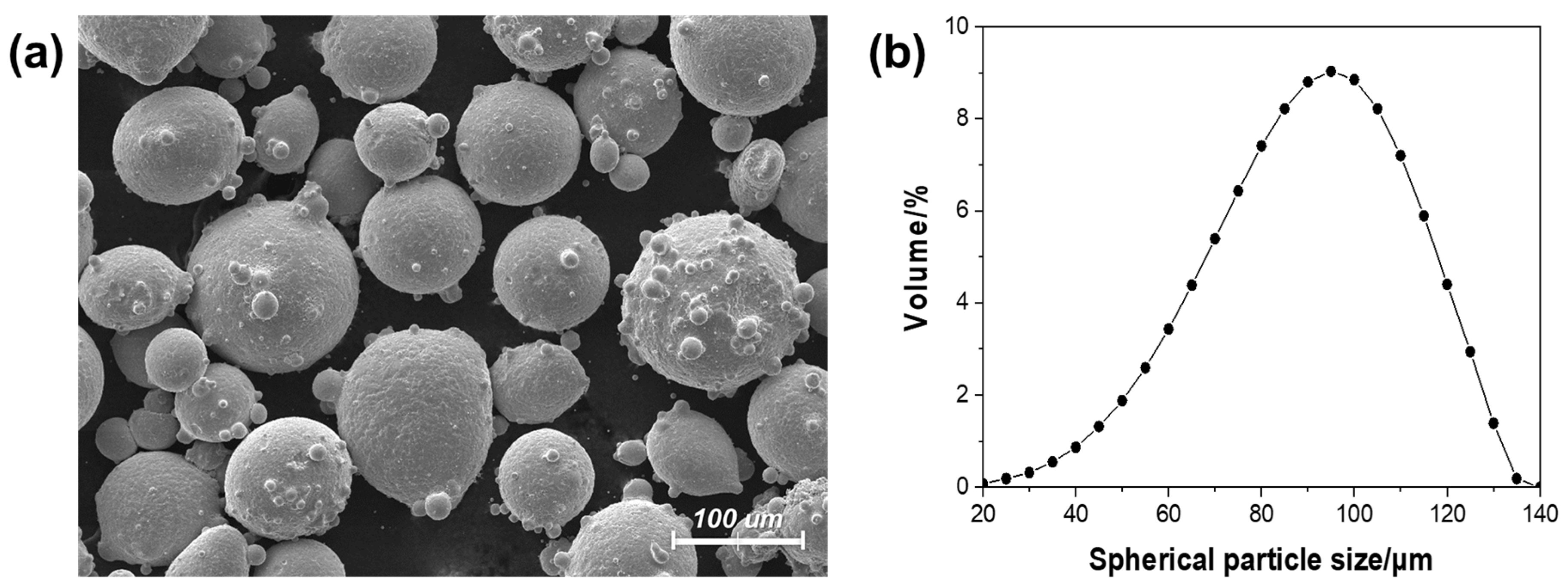
3.3. Characterizations of Metal Powders
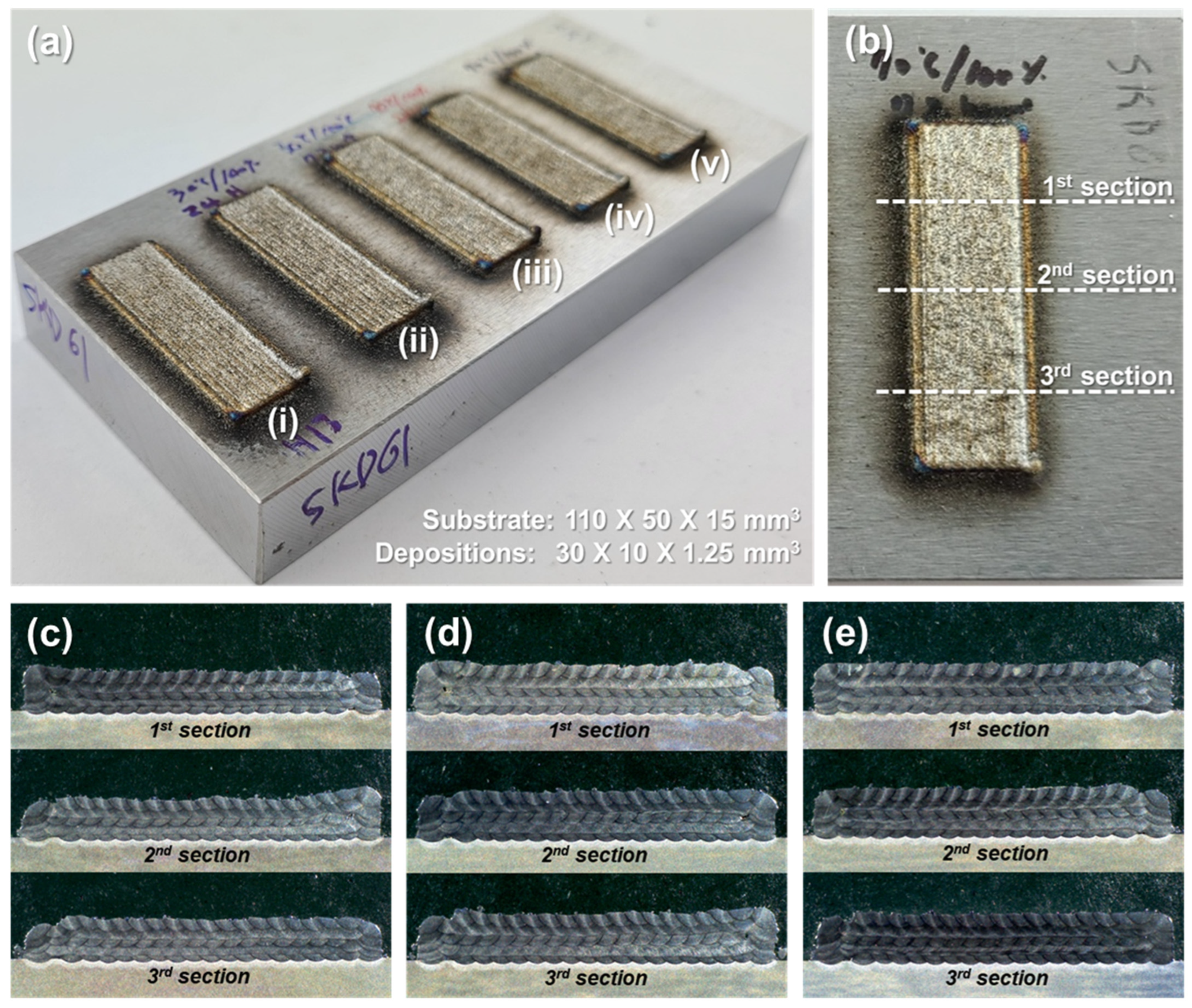
3.4. Deposition of Metal Powders
3.5. Characterizations of Deposited Microstructures
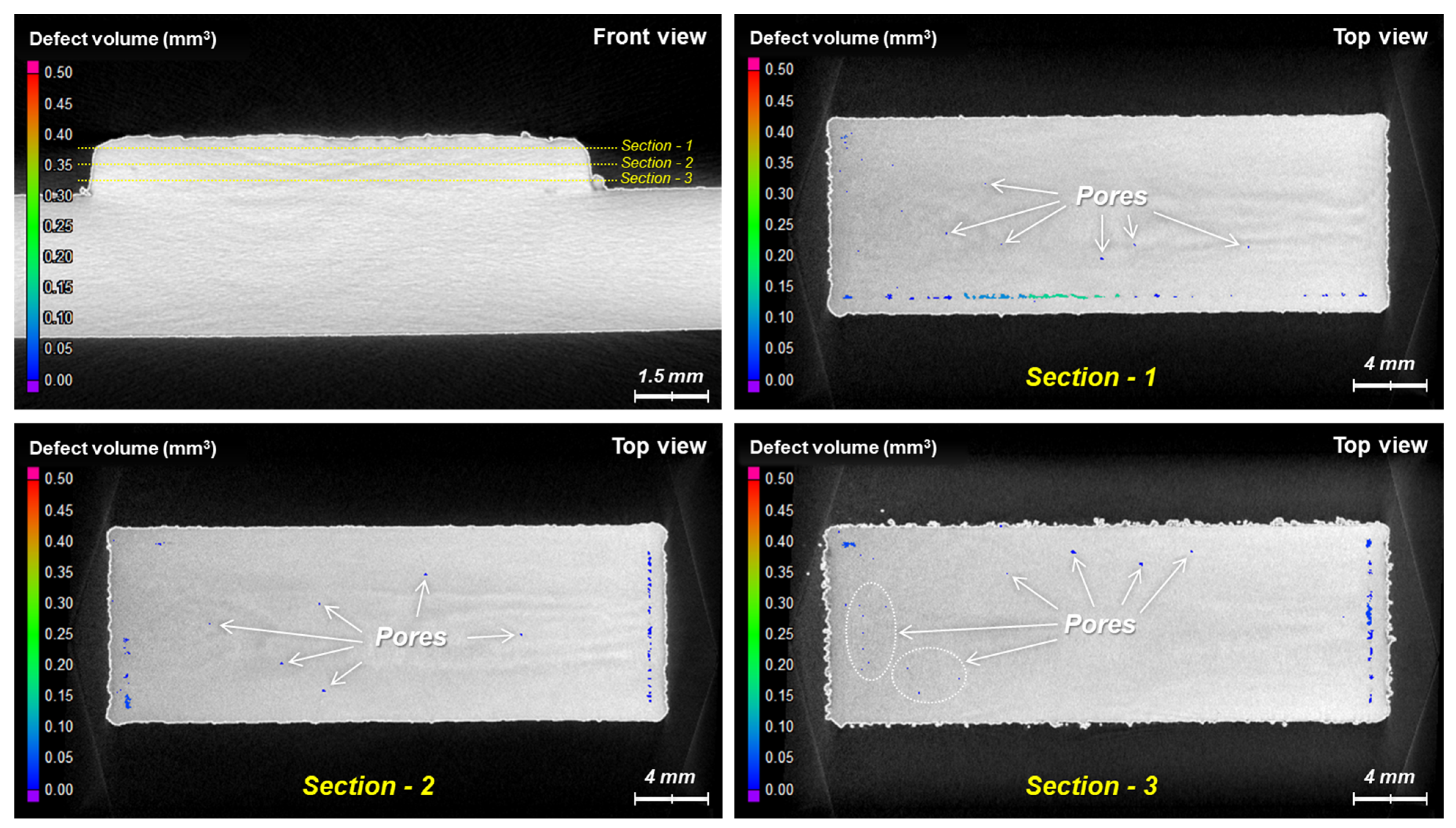
4. Results and Discussion
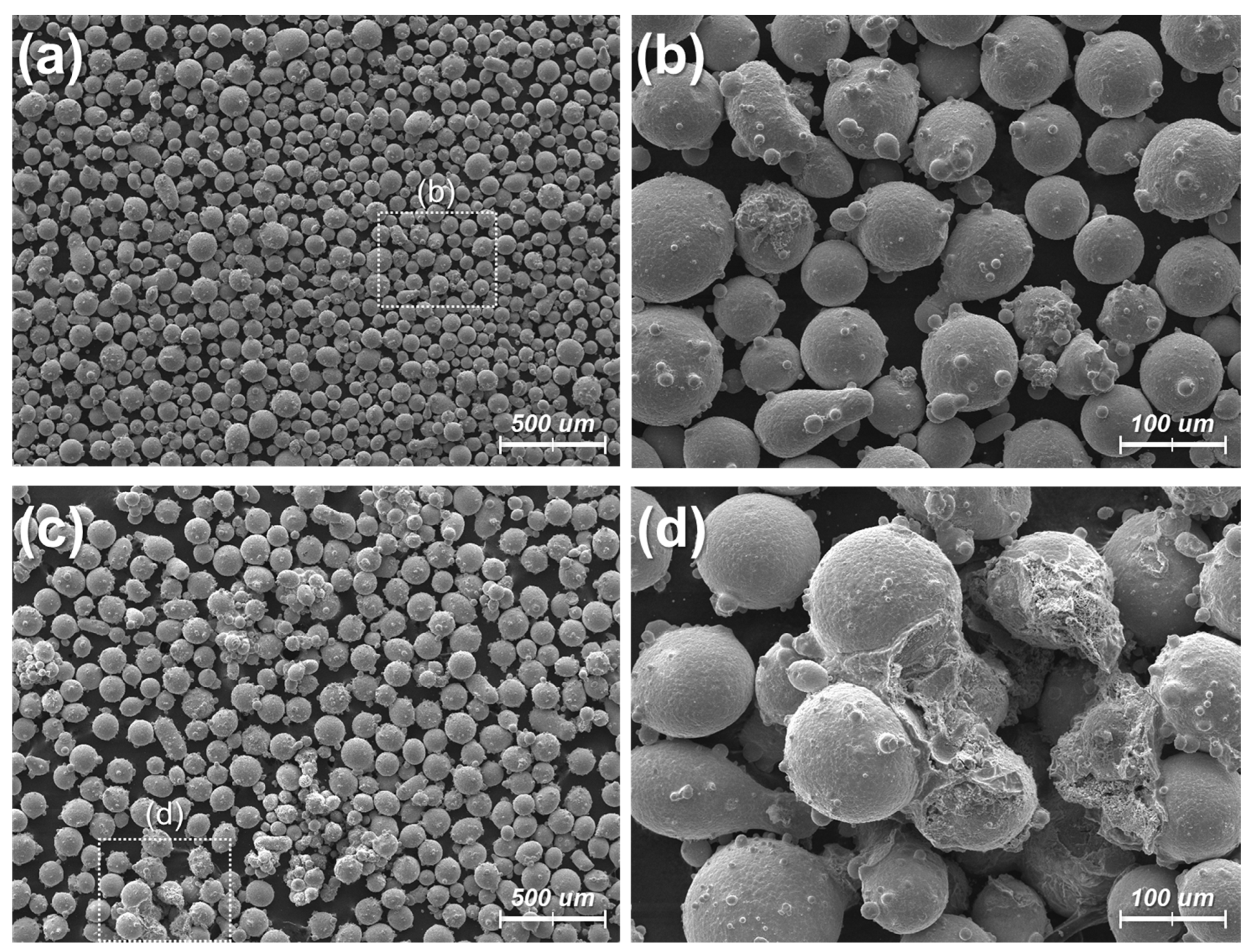
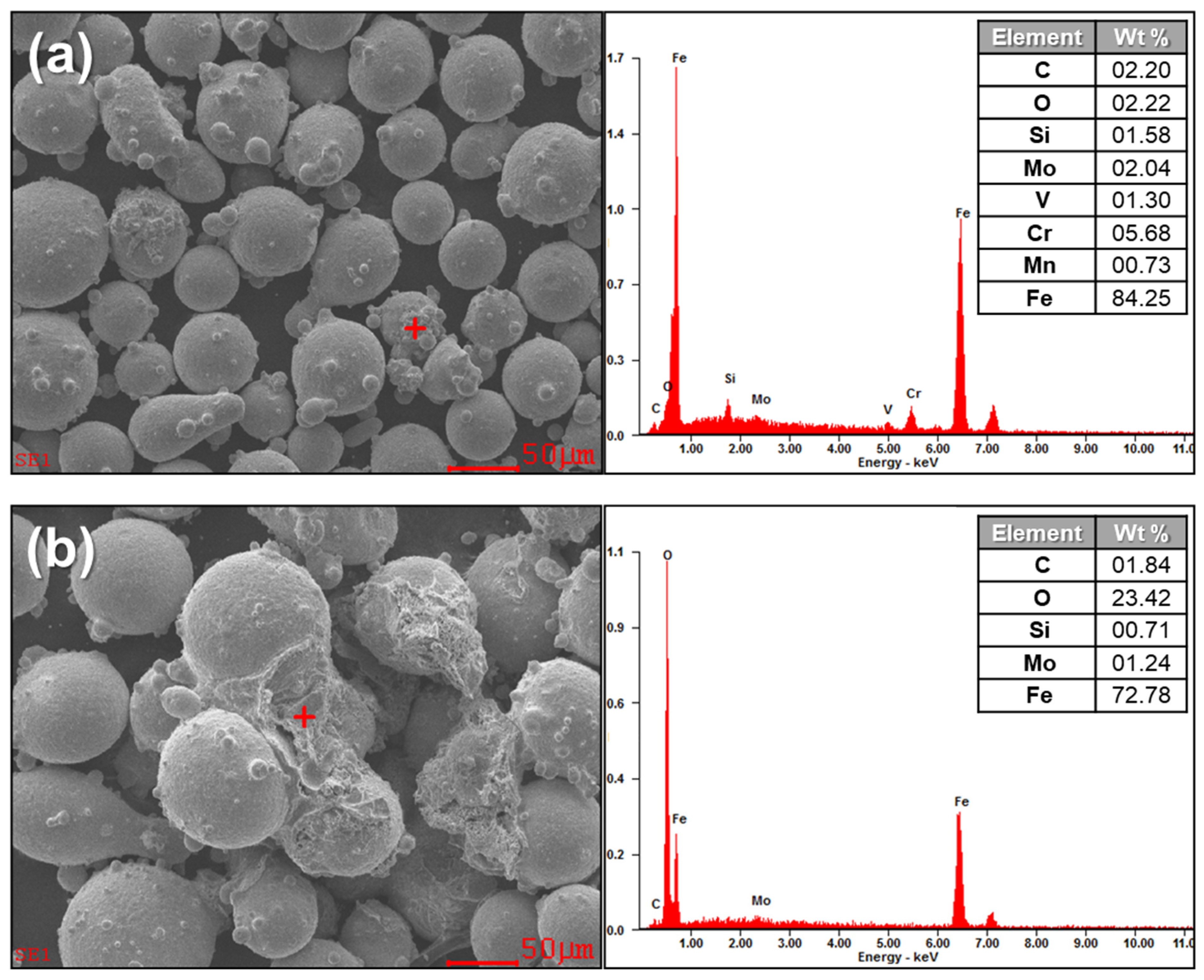
4.1. Oxidized Metal Powders
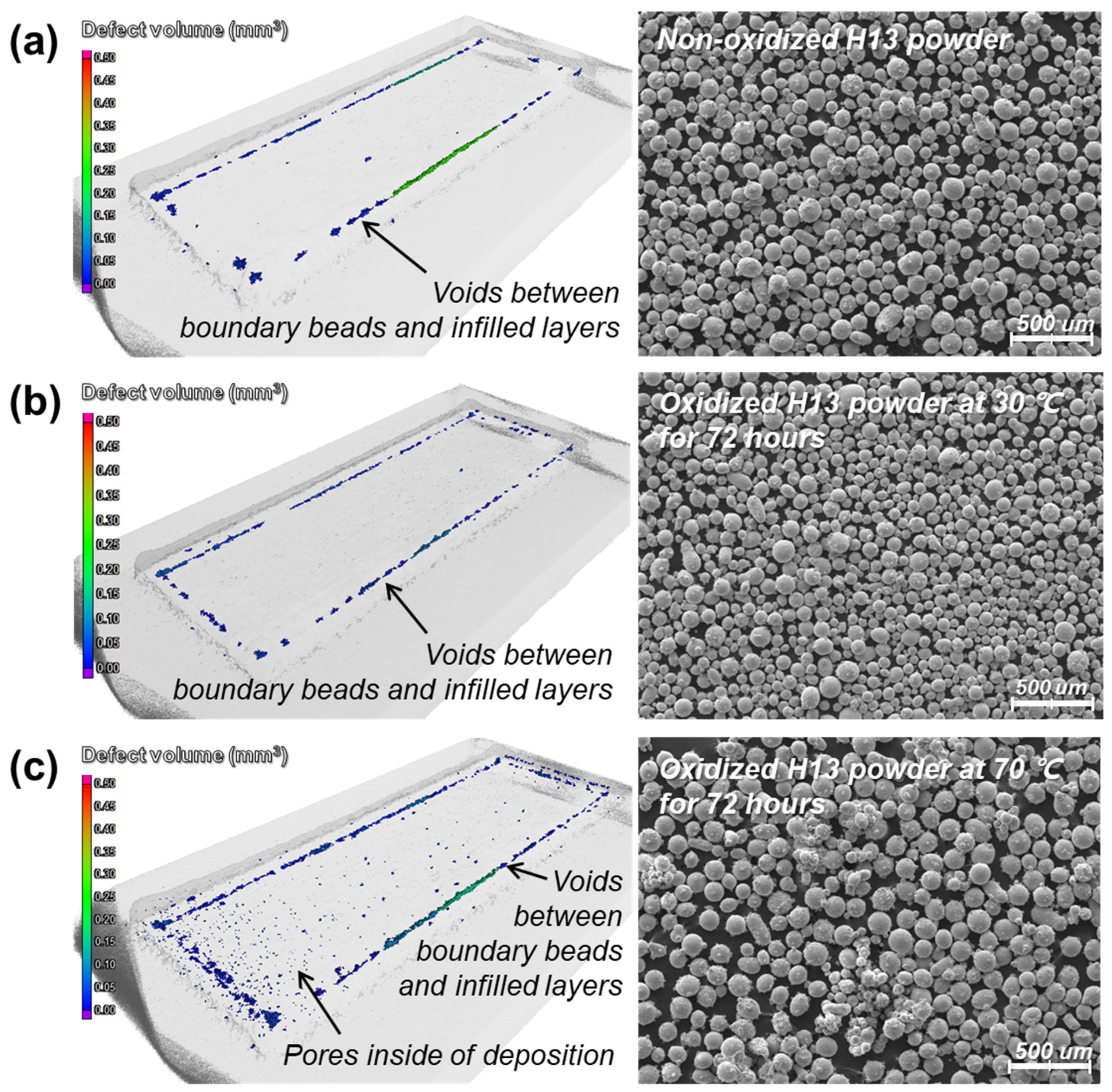
4.2. Deposition of Oxidized Powders
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Thompson, S.M.; Bian, L.; Shamsaei, N.; Yadollahi, A. An overview of direct laser deposition for additive manufacturing; Part I: Transport phenomena, modeling and diagnostics. Addit. Manuf. 2015, 8, 36–62. [Google Scholar] [CrossRef]
- Shamsaei, N.; Yadollahi, A.; Bian, L.; Thompsona, S.M. An overview of direct laser deposition for additive manufacturing; Part II: Mechanical behavior, process parameter optimization and control. Addit. Manuf. 2015, 8, 12–35. [Google Scholar] [CrossRef]
- Shahrubudina, N.; Leea, T.C.; Ramlana, R. An overview on 3D printing technology: Technological, materials, and applications. Procedia Manuf. 2019, 35, 1286–1296. [Google Scholar] [CrossRef]
- Choi, J.; Chang, Y. Characteristics of laser aided direct metal/material deposition process for tool steel. Int. J. Mach. Tools Manuf. 2005, 45, 597–607. [Google Scholar] [CrossRef]
- Capello, E.; Colombo, D.; Previtali, B. Repairing of sintered tools using laser cladding by wire. J. Mater. Process. Technol. 2005, 164–165, 990–1000. [Google Scholar] [CrossRef]
- Sanaei, N.; Fatemi, A. Defects in additive manufactured metals and their effect on fatigue performance: A state-of-the-art review. Prog. Mater. Sci. 2021, 117, 100724. [Google Scholar] [CrossRef]
- Meng, G.; Zhang, J.; Li, J.; Jiang, Z.; Gong, Y.; Zhao, J. Impact of pore defects on laser additive manufacturing of Inconel 718 alloy based on a novel finite element model: Thermal and stress evaluation. Opt. Laser Technol. 2023, 167, 109782. [Google Scholar] [CrossRef]
- Luo, Y.W.; Zhang, B.; Feng, X.; Song, Z.M.; Qi, X.B.; Li, C.P.; Chen, G.F.; Zhang, G.P. Pore-affected fatigue life scattering and prediction of additively manufactured Inconel 718: An investigation based on miniature specimen testing and machine learning approach. Mater. Sci. Eng. A 2021, 802, 140693. [Google Scholar] [CrossRef]
- Dang, L.; He, X.; Tang, D.; Li, Y.; Wang, T. A fatigue life prediction approach for laser-directed energy deposition titanium alloys by using support vector regression based on pore-induced failures. Int. J. Fatigue 2022, 159, 106748. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, L.; Yao, J.; Xia, H.; Li, J.; Liu, R. Effects of electromagnetic compound field on the escape behavior of pores in molten pool during laser cladding. Surf. Coat. Technol. 2020, 383, 125198. [Google Scholar] [CrossRef]
- Wang, L.; Pratt, P.; Felicelli, S.D.; El Kadiri, H.; Berry, J.T.; Wang, P.T.; Horstemeyer, M.F. Pore formation in laser-assisted powder deposition process. J. Manuf. Sci. Eng. 2009, 131, 051008. [Google Scholar] [CrossRef]
- Son, J.-Y.; Lee, K.-Y.; Shin, G.-Y.; Choi, C.-H.; Shim, D.-S. Mechanical and thermal properties of the high thermal conductivity steel (HTCS) additively manufactured via powder-fed direct energy deposition. Micromachines 2023, 14, 872. [Google Scholar] [CrossRef] [PubMed]
- Ning, F.; Jiang, D.; Liu, Z.; Wang, H.; Cong, W. Ultrasonic frequency effects on the melt pool formation, porosity, and thermal-dependent property of Inconel 718 fabricated by ultrasonic vibration-assisted directed energy deposition. J. Manuf. Sci. Eng. 2021, 143, 051009. [Google Scholar] [CrossRef]
- Ren, Z.; Gao, L.; Clark, S.J.; Fezzaa, K.; Shevchenko, P.; Choi, A.; Everhart, W.; Rollett, A.D.; Chen, L.; Sun, T. Machine learning–aided real-time detection of keyhole pore generation in laser powder bed fusion. Science 2023, 379, 89–94. [Google Scholar] [CrossRef]
- Yang, Q.; Yang, S.; Ma, S.; Zhou, J.; Zhou, Y.; Huang, R.; Wei, K.; Qu, Z.; Yang, X. In-situ X-ray computed tomography tensile tests and analysis of damage mechanism and mechanical properties in laser powder bed fused Invar 36 alloy. J. Mater. Sci. Technol. 2024, 175, 29–46. [Google Scholar] [CrossRef]
- Chen, L.; Yao, X.; Tan, C.; He, W.; Su, J.; Weng, F.; Chew, Y.; Ng, N.P.H.; Moon, S.K. In-situ crack and keyhole pore detection in laser directed energy deposition through acoustic signal and deep learning. Addit. Manuf. 2023, 69, 103547. [Google Scholar] [CrossRef]
- Wang, H.; Pfefferkorn, F.E.; Wolff, S.J. Investigation of pore formation mechanisms induced by spherical-powder delivery in directed energy deposition using in situ high-speed X-ray imaging. Addit. Manuf. Lett. 2022, 3, 100050. [Google Scholar] [CrossRef]
- Zhou, C.; Zhao, S.; Wang, Y.; Liu, F.; Gao, W.; Lin, X. Mitigation of pores generation at overlapping zone during laser cladding. J. Mater. Process. Technol. 2015, 216, 369–374. [Google Scholar] [CrossRef]
- Zhong, C.; Gasser, A.; Kittel, J.; Wissenbach, K.; Poprawe, R. Improvement of material performance of Inconel 718 formed by high deposition-rate laser metal deposition. Mater. Des. 2016, 98, 128–134. [Google Scholar] [CrossRef]
- Zhong, C.; Gasser, A.; Schopphoven, T.; Poprawe, R. Experimental study of porosity reduction in high deposition-rate laser material deposition. Opt. Laser Technol. 2015, 75, 87–92. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, A.; Weng, Z.; Xiong, D.; Ye, B.; Qi, X. Porosity elimination and heat treatment of diode laser-clad homogeneous coating on cast aluminum-copper alloy. Surf. Coat. Technol. 2017, 321, 26–35. [Google Scholar] [CrossRef]
- Yan, J.; Zhou, Y.; Gu, R.; Zhang, X.; Quach, W.M.; Yan, M. A Comprehensive study of steel powders (316L, H13, P20 and 18Ni300) for their selective laser melting additive manufacturing. Metals 2019, 9, 86. [Google Scholar] [CrossRef]
- Oro, R.; Campos, M.; Hryha, E.; Torralba, J.M.; Nyborg, L. Surface phenomena during the early stages of sintering in steels modified with Fe–Mn–Si–C master alloys. Mater. Charact. 2013, 86, 80–91. [Google Scholar] [CrossRef]
- Min, Y.A.; Wu, X.C.; Wang, R.; Li, L.; Xu, L.P. Prediction and analysis on oxidation of H13 hot work steel. J. Iron Steel Res. Int. 2006, 13, 44–49. [Google Scholar] [CrossRef]
- Chena, G.; Xuea, L.; Wanga, J.; Tangb, Z.; Lic, X.; Dong, H. Investigation of surface modifications for combating the molten aluminum corrosion of AISI H13 steel. Corros. Sci. 2020, 174, 108836. [Google Scholar] [CrossRef]
- Leung, C.L.A.; Marussi, S.; Atwood, R.C.; Towrie, M.; Withers, P.J.; Lee, P.D. In situ X-ray imaging of defect and molten pool dynamics in laser additive manufacturing. Nat. Commun. 2018, 9, 1355. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.L.A.; Marussi, S.; Towrie, M.; Atwood, R.C.; Withers, P.J.; Lee, P.D. The effect of powder oxidation on defect formation in laser additive manufacturing. Acta Mater. 2019, 166, 294–305. [Google Scholar] [CrossRef]

| Material | C | Si | Mn | P | S | Mo | Cr | Ni | N | V | O |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AISI H13 | 0.41 | 1.0 | 0.45 | <0.015 | 0.02 | 1.5 | 5.33 | 0.25 | 0.1 | 1.05 | 0.1 |
| KS STD61 | 0.32 | 0.8 | <0.5 | <0.03 | 0.03 | 1.0 | 4.5 | <0.25 | 0.03 | 0.8 | - |
| Powder Weight | Temperature | Humidity | Time |
|---|---|---|---|
| 60 g | 30, 70 °C | 100% | 12 h |
| 24 h | |||
| 72 h |
| Process Parameters | Value |
|---|---|
| Laser power | 800 W |
| Powder feeding rate | 3.3 g/min |
| Bead spacing | 0.52 mm |
| Laser scanning speed | 800 mm/min |
| Laser beam diameter | 1 mm |
| Powder gas | 2.5 L/min |
| Coaxial gas | 8.0 L/min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, J.-Y.; Lee, K.-Y.; Lee, S.H.; Choi, C.-H. Effects of Oxidized Metal Powders on Pore Defects in Powder-Fed Direct Energy Deposition. Micromachines 2024, 15, 243. https://doi.org/10.3390/mi15020243
Son J-Y, Lee K-Y, Lee SH, Choi C-H. Effects of Oxidized Metal Powders on Pore Defects in Powder-Fed Direct Energy Deposition. Micromachines. 2024; 15(2):243. https://doi.org/10.3390/mi15020243
Chicago/Turabian StyleSon, Jong-Youn, Ki-Yong Lee, Seung Hwan Lee, and Chang-Hwan Choi. 2024. "Effects of Oxidized Metal Powders on Pore Defects in Powder-Fed Direct Energy Deposition" Micromachines 15, no. 2: 243. https://doi.org/10.3390/mi15020243
APA StyleSon, J.-Y., Lee, K.-Y., Lee, S. H., & Choi, C.-H. (2024). Effects of Oxidized Metal Powders on Pore Defects in Powder-Fed Direct Energy Deposition. Micromachines, 15(2), 243. https://doi.org/10.3390/mi15020243







