A Multidisciplinary Approach toward CMOS Capacitive Sensor Array for Droplet Analysis
Abstract
1. Introduction
2. Related Works
3. Proposed Droplet-on-Chip Sensor
3.1. Multi-Task DoC Platforms
3.1.1. Dielectric Sensing
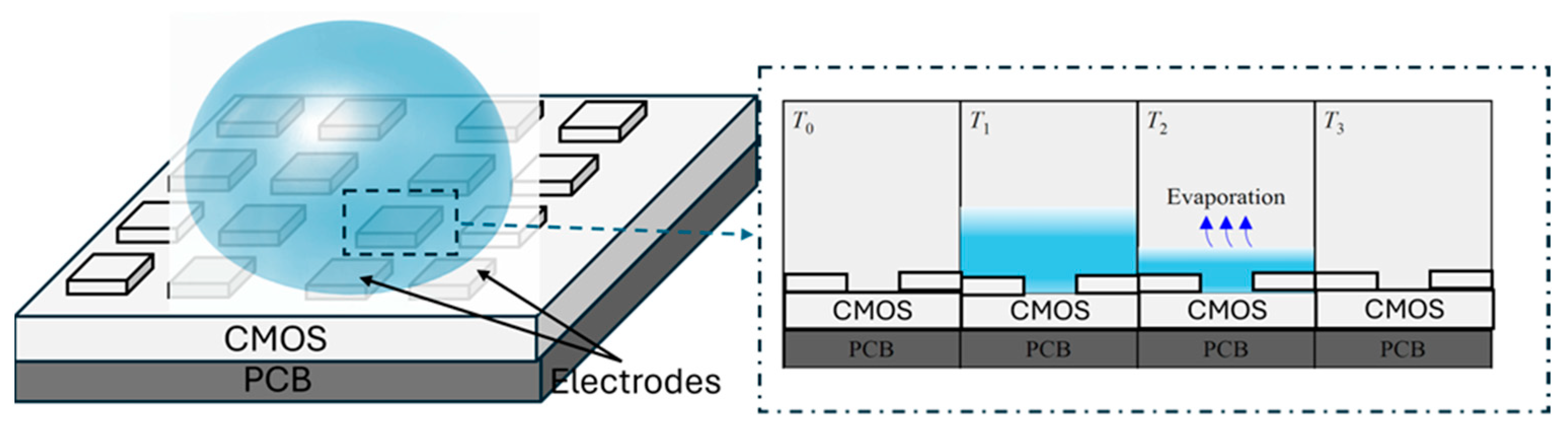
3.1.2. Time of Evaporation Measurement
3.1.3. Monitoring the Shape and Location of Droplets
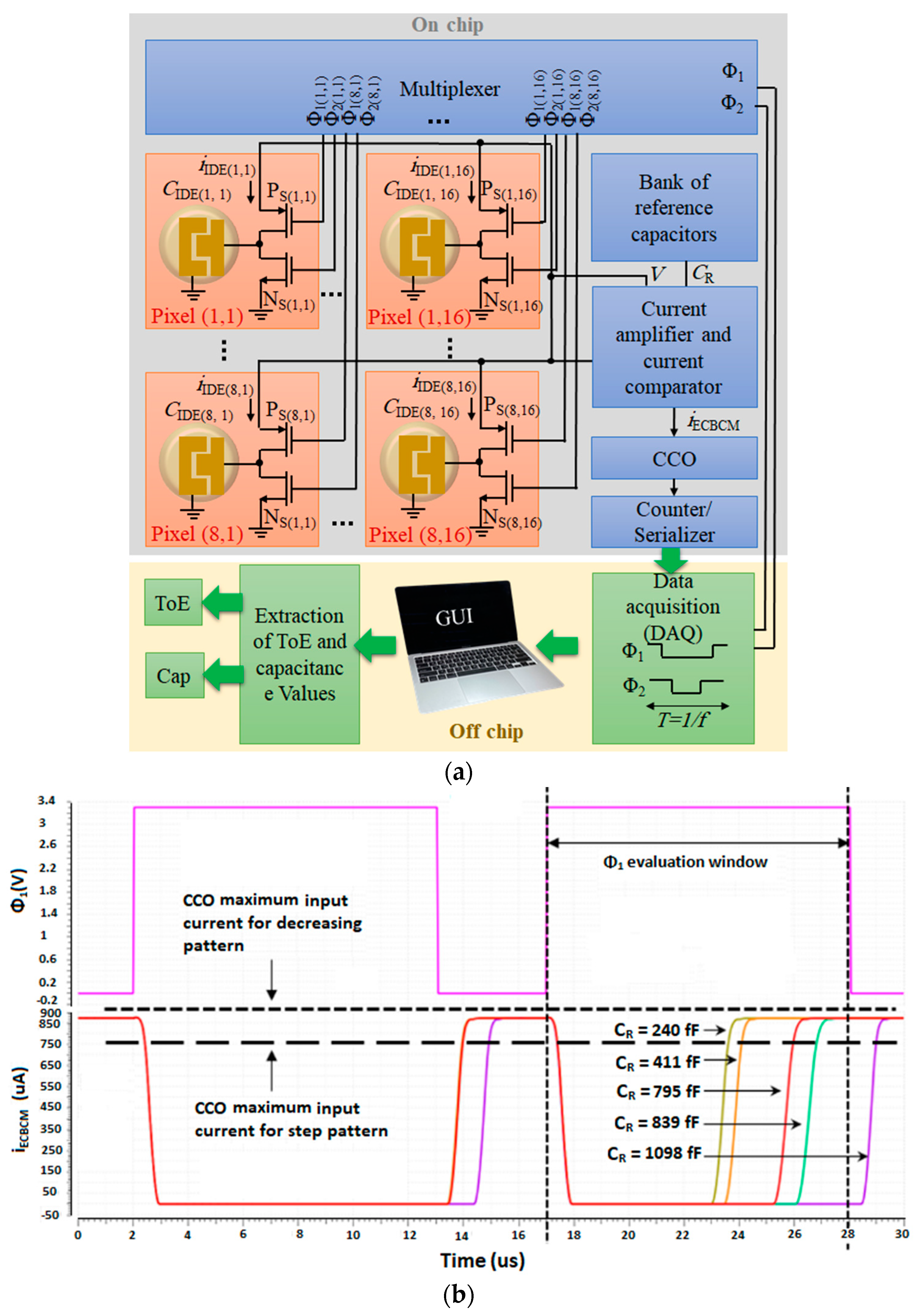
3.2. CMOS Capacitive Sensor Array
4. Experimental Setup and Materials
4.1. Chip Fabrication and Testbench
4.2. Sample Preparation
5. Results
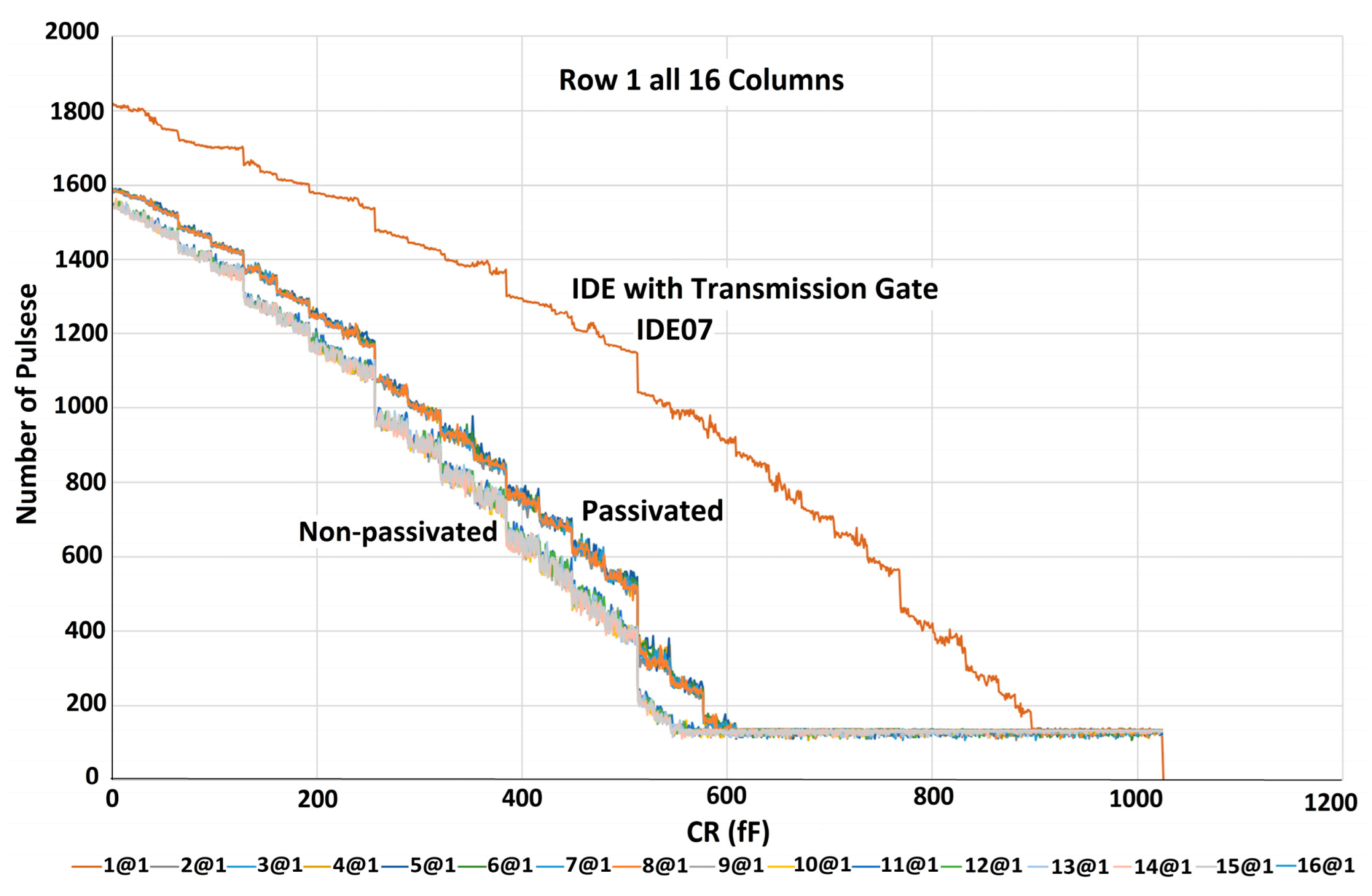
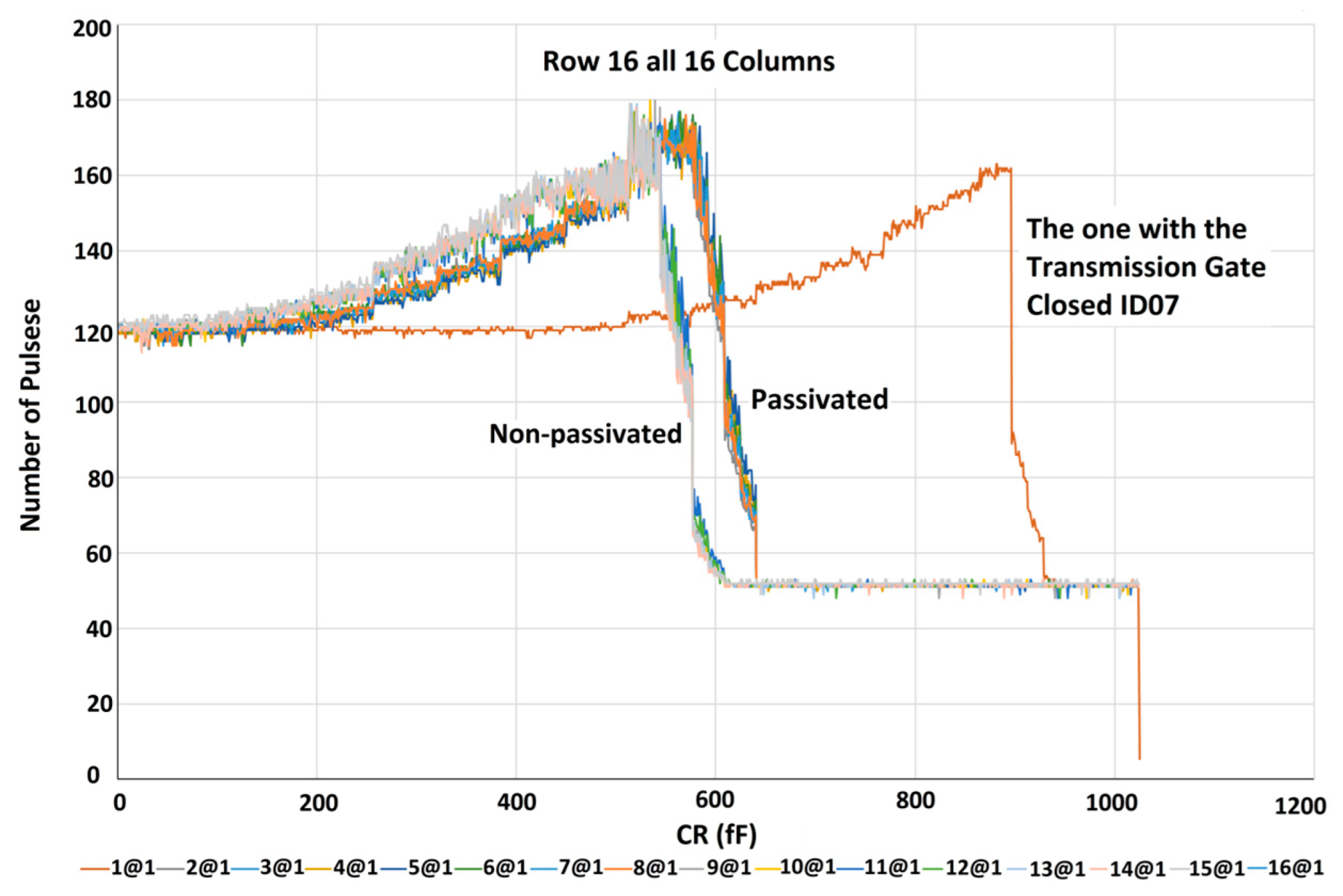
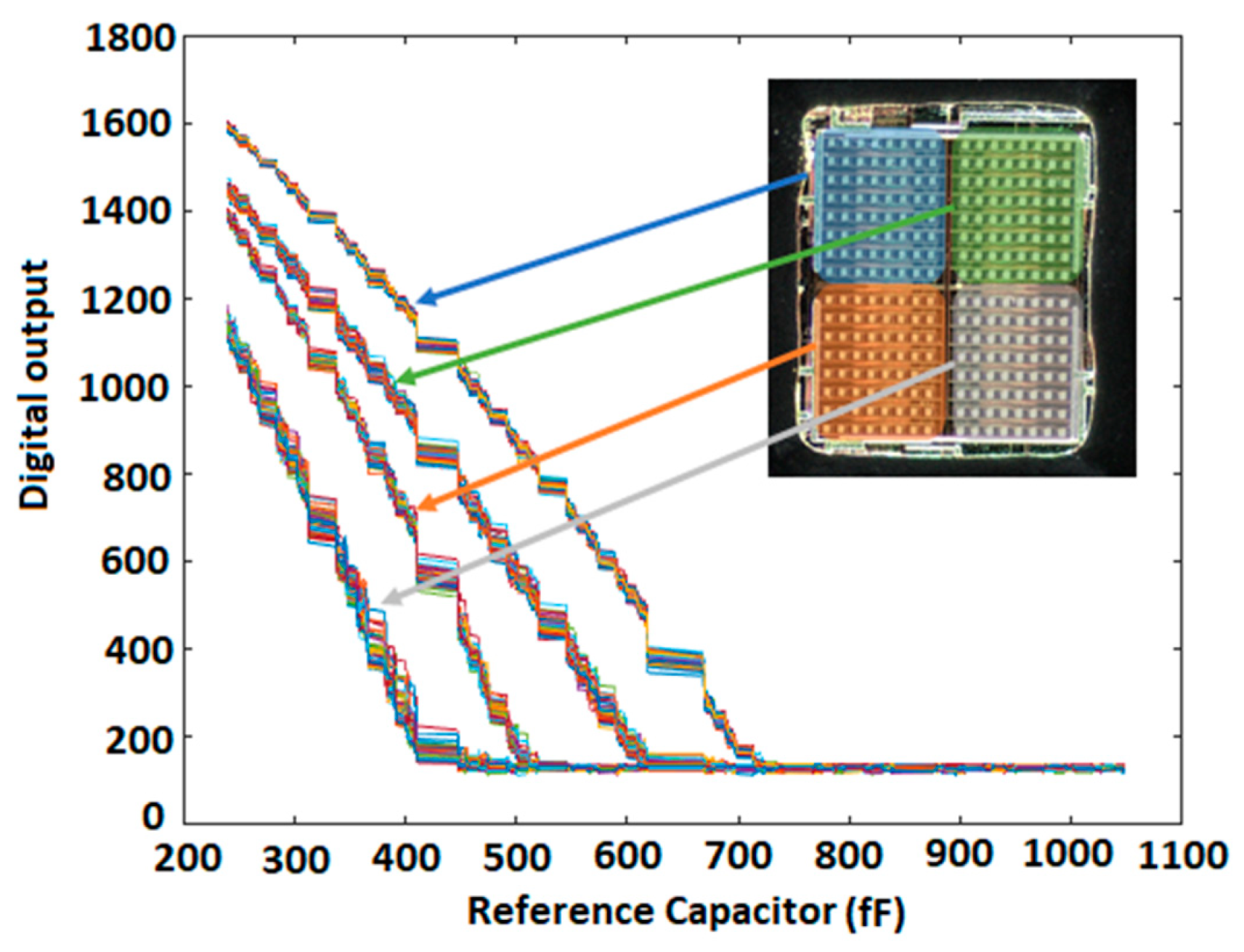
5.1. Characterization of the Sensor Array without Sample
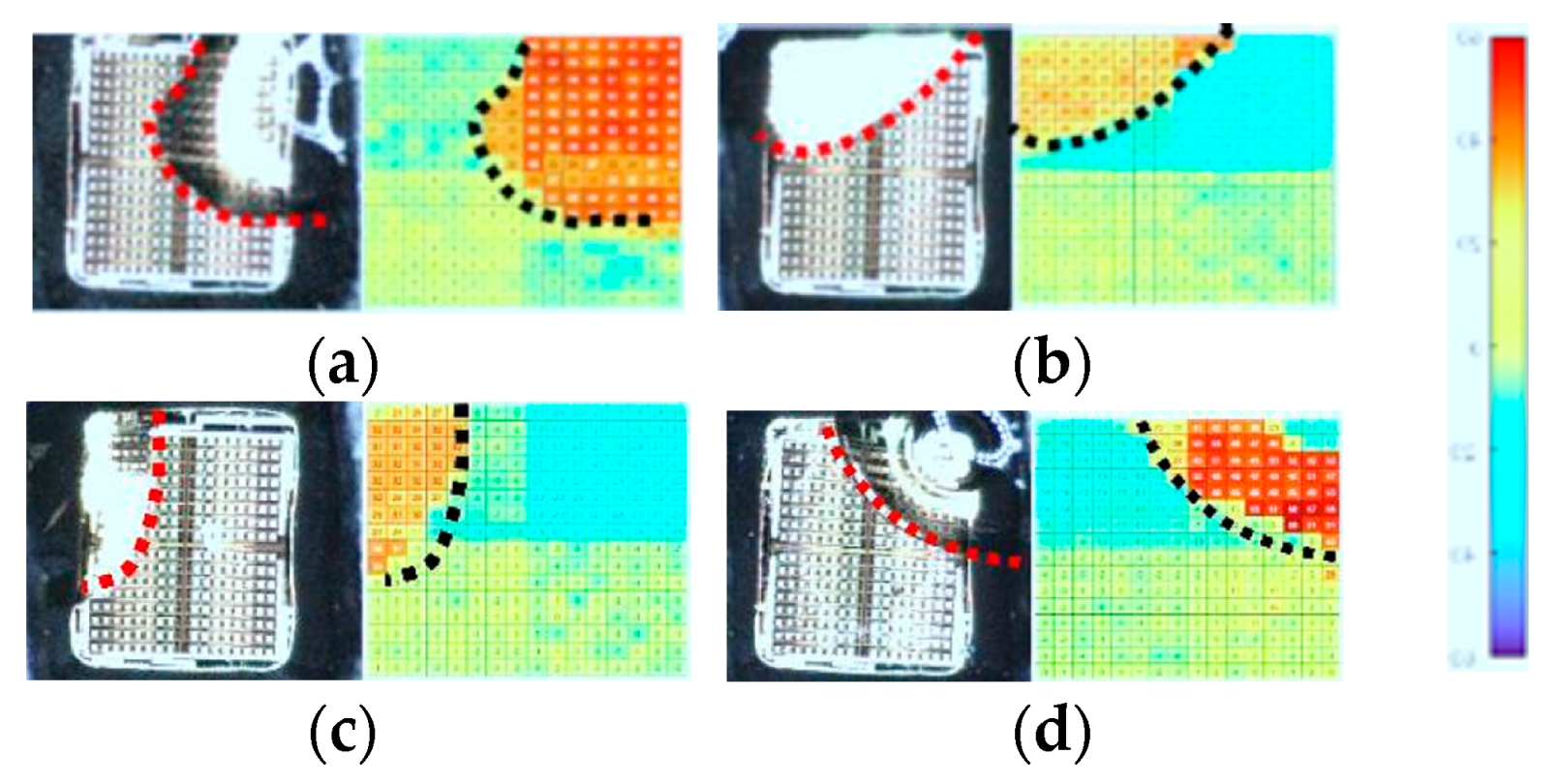
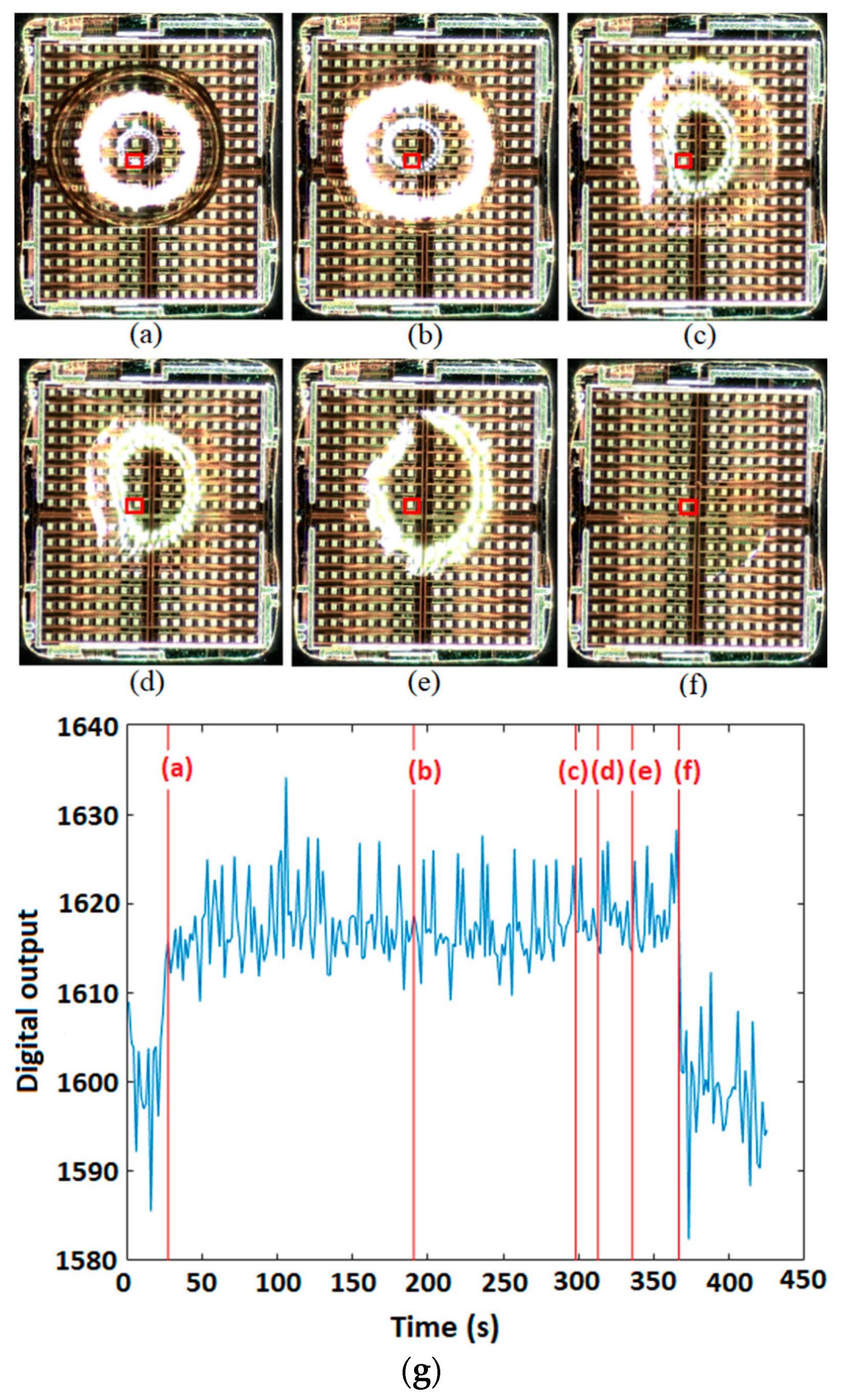
5.2. Monitoring the Shape and Location of the Droplets
5.2.1. Dielectric Sensing
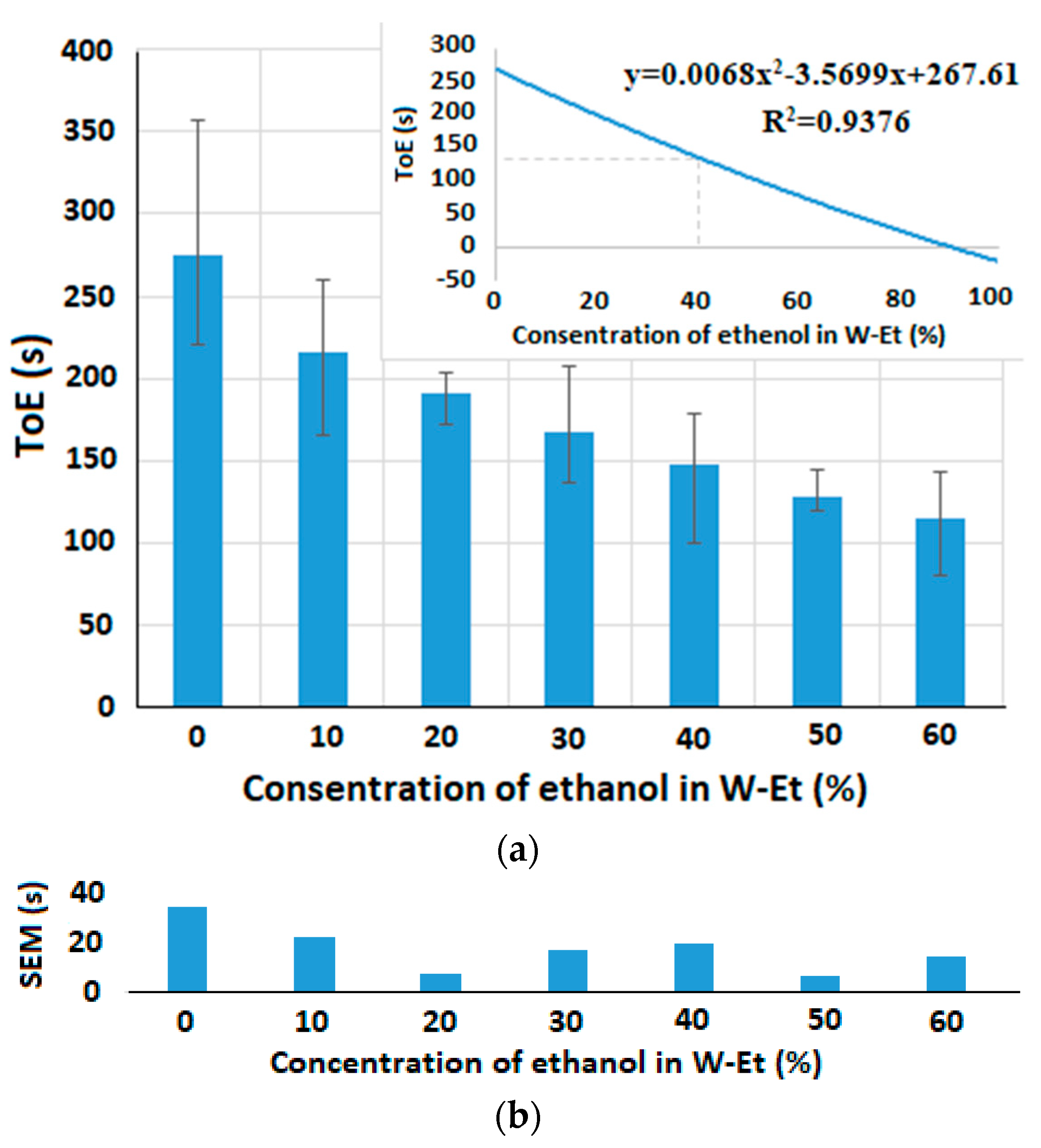
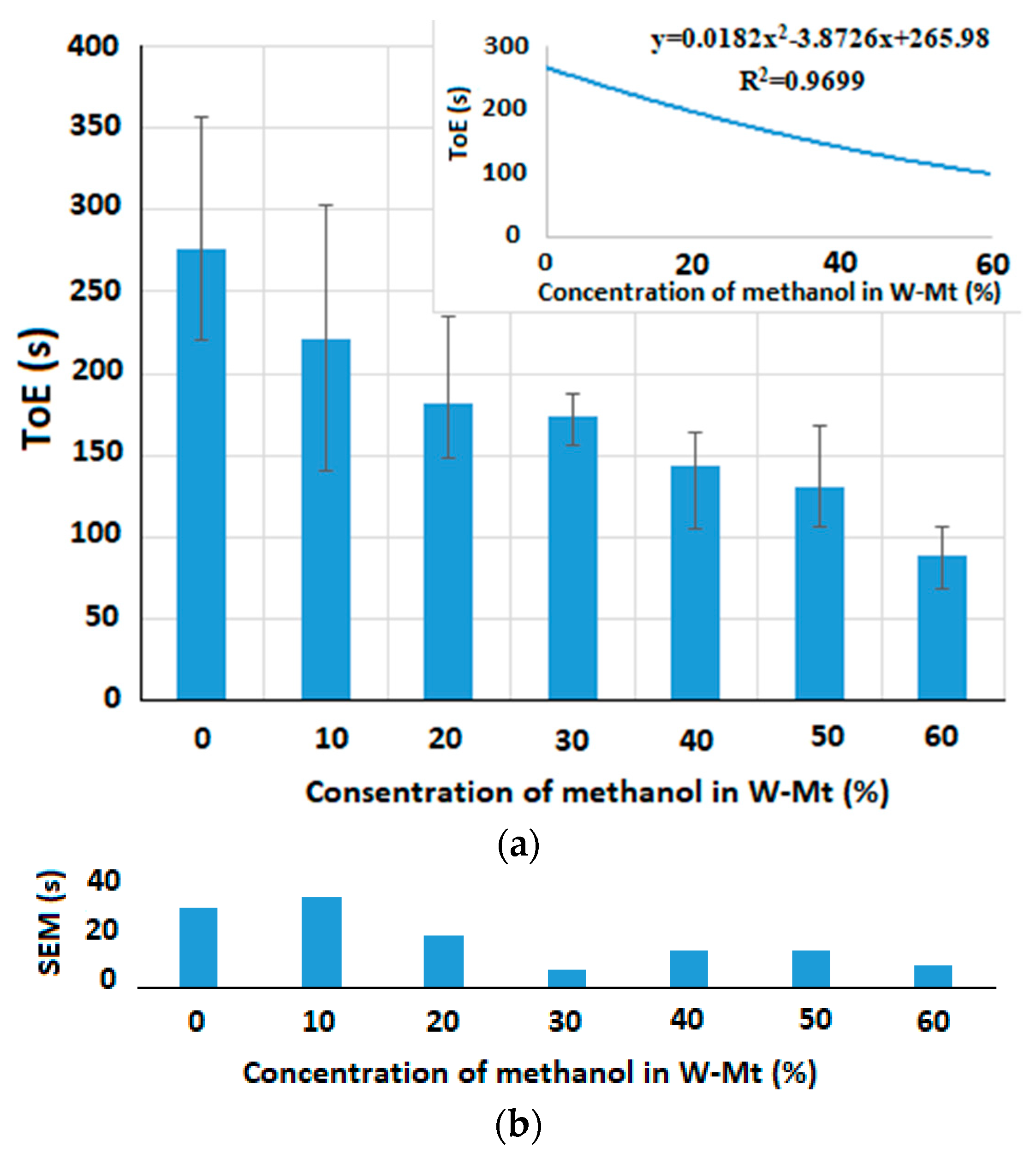
5.2.2. Time of Evaporation Measurement
6. Discussion and Future Work
6.1. Speed of the Readout Circuit
6.2. Mismatches
6.3. Interferences
6.4. Manual Pipetting Errors
6.5. The Non-Flat Sensing Area Inside the Chamber
6.6. Misalignment of the Electrode and Droplet
6.7. Bubble Creation
6.8. Controlling the Environmental Effects
6.9. Complexity of the Fluidic Sample
6.10. Reliability
6.11. Droplet on Chip
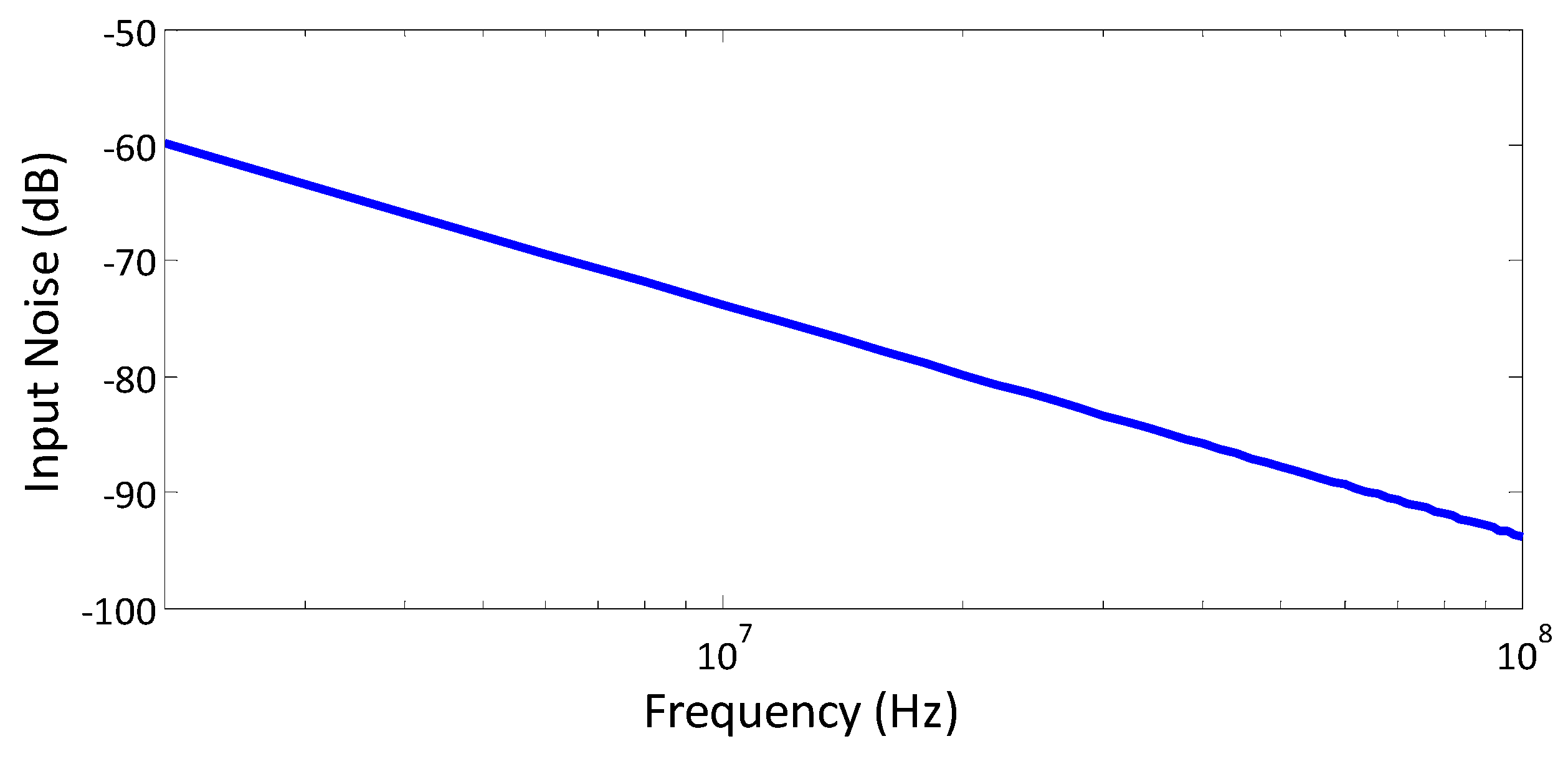
6.12. Noise
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Deng, W.; Wang, L.; Song, S.; Zuo, X. Biosensors in POCT application. Prog. Chem. 2016, 28, 1341. [Google Scholar]
- Reddy, B.; Hassan, U.; Seymour, C.; Angus, D.C.; Isbell, T.S.; White, K.; Weir, W.; Yeh, L.; Vincent, A.; Bashir, R. Point-of-care sensors for the management of sepsis. Nat. Biomed. Eng. 2018, 2, 640–648. [Google Scholar] [CrossRef]
- Jang, B.; Hassibi, A. Biosensor systems in standard CMOS processes: Fact or fiction? IEEE Trans. Ind. Electron. 2009, 56, 979–985. [Google Scholar] [CrossRef]
- Wang, H. Integrated biosensors in CMOS. In Proceedings of the 2011 IEEE 54th International Midwest Symposium on Circuits and Systems (MWSCAS), Seoul, Republic of Korea, 7–10 August 2011; pp. 1–4. [Google Scholar]
- Hosseini, S.N.; Das, P.S.; Lazarjan, V.K.; Gagnon-Turcotte, G.; Bouzid, K.; Gosselin, B. Recent advances in CMOS electrochemical biosensor design for microbial monitoring: Review and design methodology. IEEE Trans. Biomed. Circuits Syst. 2023, 17, 202–228. [Google Scholar] [CrossRef]
- Hassibi, A.; Wood, N.; Manickam, A. CMOS biochips: Challenges and opportunities. In Proceedings of the 2018 IEEE Custom Integrated Circuits Conference (CICC), San Diego, CA, USA, 8–11 April 2018; pp. 1–7. [Google Scholar]
- Khan, S.M.; Gumus, A.; Nassar, J.M.; Hussain, M.M. CMOS enabled microfluidic systems for healthcare based applications. Adv. Mater. 2018, 30, 1705759. [Google Scholar] [CrossRef]
- Arcamone, J.; Ondarçuhu, T.; Dujardin, E.; Rius, G.; Pérez-Murano, F. CMOS integrated nanomechanical mass sensors: Determination of evaporation rate of femtoliter droplets. In Proceedings of the 2007 Digest of papers Microprocesses and Nanotechnology, Kyoto, Japan, 5–8 November 2007; pp. 522–523. [Google Scholar]
- Elhadidy, O.; Shakib, S.; Krenek, K.; Palermo, S.; Entesari, K. A wide-band fully-integrated CMOS ring-oscillator PLL-based complex dielectric spectroscopy system. IEEE Trans. Circuits Syst. I Regul. Pap. 2015, 62, 1940–1949. [Google Scholar] [CrossRef]
- Elkholy, M.; Entesari, K. A Wideband Low-Power LC-DCO-Based Complex Dielectric Spectroscopy System in 0.18-μm CMOS. IEEE Trans. Microw. Theory Tech. 2017, 65, 4461–4474. [Google Scholar] [CrossRef]
- Bajestan, M.M.; Helmy, A.A.; Hedayati, H.; Entesari, K. A 0.62–10 GHz complex dielectric spectroscopy system in CMOS. IEEE Trans. Microw. Theory Tech. 2014, 62, 3522–3537. [Google Scholar] [CrossRef]
- Santra, S.; Hu, G.; Howe, R.C.T.; De Luca, A.; Ali, S.Z.; Udrea, F.; Gardner, J.W.; Ray, S.K.; Guha, P.K.; Hasan, T. CMOS integration of inkjet-printed graphene for humidity sensing. Sci. Rep. 2015, 5, 17374. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-T.; Chang, C.-W.; Chung, Y.-R.; Chien, J.-H.; Kuo, J.-S.; Chen, W.-T.; Chen, P.-H. A novel CMOS sensor for measuring thermal diffusivity of liquids. Sens. Actuators A Phys. 2007, 135, 451–457. [Google Scholar] [CrossRef]
- Adamopoulos, C.; Zarkos, P.; Buchbinder, S.; Bhargava, P.; Niknejad, A.; Anwar, M.; Stojanovic, V. Lab-on-Chip for Everyone: Introducing an Electronic-Photonic Platform for Multiparametric Biosensing Using Standard CMOS Processes. IEEE Open J. Solid-State Circuits Soc. 2021, 1, 198–208. [Google Scholar] [CrossRef]
- Franks, W.; Tosatti, S.; Heer, F.; Seif, P.; Textor, M.; Hierlemann, A. Patterned cell adhesion by self-assembled structures for use with a CMOS cell-based biosensor. Biosens. Bioelectron. 2007, 22, 1426–1433. [Google Scholar] [CrossRef]
- Lin, G.; Makarov, D.; Schmidt, O.G. Magnetic sensing platform technologies for biomedical applications. Lab Chip 2017, 17, 1884–1912. [Google Scholar] [CrossRef] [PubMed]
- Lei, K.-M.; Mak, P.-I.; Law, M.-K.; Martins, R.P. A palm-size μNMR relaxometer using a digital microfluidic device and a semiconductor transceiver for chemical/biological diagnosis. Analyst 2015, 140, 5129–5137. [Google Scholar] [CrossRef][Green Version]
- González-Fernández, A.A.; Hernández-Montero, W.W.; Hernández-Betanzos, J.; Domínguez, C.; Aceves-Mijares, M. Refractive index sensing using a Si-based light source embedded in a fully integrated monolithic transceiver. AIP Adv. 2019, 9, 125215. [Google Scholar] [CrossRef]
- Lai, P.-H.; Chen, W.-F.; Yang, C.-M.; Lu, M.S.-C. CMOS Capacitive Sensor for DNA Detection in Undiluted Ionic Solution. IEEE Sens. Lett. 2023, 7, 4500904. [Google Scholar] [CrossRef]
- Tabrizi, H.O.; Forouhi, S.; Farhanieh, O.; Bozkurt, A.; Magierowski, S.; Ghafar-Zadeh, E. Calibration-free CMOS capacitive sensor for life science applications. IEEE Trans. Instrum. Meas. 2021, 70, 1–12. [Google Scholar] [CrossRef]
- Saeidi, N.; Strutwolf, J.; Maréchal, A.; Demosthenous, A.; Donaldson, N. A capacitive humidity sensor suitable for CMOS integration. IEEE Sens. J. 2013, 13, 4487–4495. [Google Scholar] [CrossRef]
- Annese, V.F.; Hu, C. Integrating Microfluidics and Electronics in Point-of-Care Diagnostics: Current and Future Challenges. Micromachines 2022, 13, 1923. [Google Scholar] [CrossRef]
- Chung, J.; Hwang, H.Y.; Chen, Y.; Lee, T.Y. Microfluidic packaging of high-density CMOS electrode array for lab-on-a-chip applications. Sens. Actuators B Chem. 2018, 254, 542–550. [Google Scholar] [CrossRef]
- Deliorman, M.; Ali, D.S.; Qasaimeh, M.A. Next-Generation Microfluidics for Biomedical Research and Healthcare Applications. Biomed. Eng. Comput. Biol. 2023, 14, 11795972231214388. [Google Scholar] [CrossRef]
- Miled, M.A.; Sawan, M. A new CMOS/microfluidic interface for cells manipulation and separation in LoC devices. In Proceedings of the 2nd Asia Symposium on Quality Electronic Design (ASQED), Penang, Malaysia, 3–4 August 2010; pp. 194–197. [Google Scholar]
- Miled, M.A.; Sawan, M. A new fully integrated cmos interface for a dielectrophoretic lab-on-a-chip device. In Proceedings of the 2011 IEEE International Symposium of Circuits and Systems (ISCAS), Rio de Janeiro, Brazil, 15–18 May 2011; pp. 2349–2352. [Google Scholar]
- Chen, J.C.; Sylvester, D.; Hu, C. An on-chip, interconnect capacitance characterization method with sub-femto-farad resolution. IEEE Trans. Semicond. Manuf. 1998, 11, 204–210. [Google Scholar] [CrossRef]
- Ghafar-Zadeh, E.; Sawan, M. A CMOS-based capacitive sensor for laboratory-on-chips: Design and experimental results. In Proceedings of the 2007 IEEE International Symposium on Circuits and Systems, New Orleans, LA, USA, 27–30 May 2007; pp. 85–88. [Google Scholar]
- Forouhi, S.; Dehghani, R.; Ghafar-Zadeh, E. Toward high throughput core-CBCM CMOS capacitive sensors for life science applications: A novel current-mode for high dynamic range circuitry. Sensors 2018, 18, 3370. [Google Scholar] [CrossRef]
- Raghunandan, K.R.; Viswanathan, T.L.; Viswanathan, T.R. Linear current-controlled oscillator for analog to digital conversion. In Proceedings of the IEEE 2014 Custom Integrated Circuits Conference, San Jose, CA, USA, 15–17 September 2014; pp. 1–4. [Google Scholar]
- Tabrizi, H.O.; Farhanieh, O.; Owen, Q.; Magierowski, S.; Ghafar-Zadeh, E. Wide input dynamic range fully integrated capacitive sensor for life science applications. IEEE Trans. Biomed. Circuits Syst. 2021, 15, 339–350. [Google Scholar] [CrossRef]
- Nabovati, G.; Ghafar-Zadeh, E.; Mirzaei, M.; Ayala-Charca, G.; Awwad, F.; Sawan, M. A New Fully Differential CMOS Capacitance to Digital Converter for Lab-on-Chip Applications. IEEE Trans. Biomed. Circuits Syst. 2015, 9, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Ghafar-Zadeh, E.; Sawan, M. Charge-based capacitive sensor array for CMOS-based laboratory-on-chip applications. IEEE Sens. J. 2008, 8, 325–332. [Google Scholar] [CrossRef]
- Tsouti, V.; Boutopoulos, C.; Zergioti, I.; Chatzandroulis, S. Capacitive microsystems for biological sensing. Biosens. Bioelectron. 2011, 27, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Megriche, A.; Belhadj, A.; Mgaidi, A. Microwave dielectric properties of binary solvent water-alcohol, alcohol-alcohol mixtures at temperatures between −35 °C and +35 °C and dielectric relaxation studies. Mediterr. J. Chem. 2011, 1, 200–209. [Google Scholar] [CrossRef]
- Bozorgmehr, B.; Murray, B.T. Numerical Simulation of Evaporation of Ethanol-Water Mixture Droplets on Isothermal and Heated Substrates. ACS Omega 2021, 6, 12577–12590. [Google Scholar] [CrossRef]
- Jiang, X.; Ward, T.L.; Van Swol, F.; Brinker, C.J. Numerical simulation of ethanol-water-nacl droplet evaporation. Ind. Eng. Chem. Res. 2010, 49, 5631–5643. [Google Scholar] [CrossRef]
- Ye, S.; Mo, D.M.; Zhang, L.; Wu, C.M.; Li, Y.R. Numerical investigation on the coupling effect of sessile ethanol droplet evaporation and the induced thermal flow in its pure vapor environment at low pressure. Int. J. Therm. Sci. 2022, 182, 107836. [Google Scholar] [CrossRef]
- Sterlyagov, A.N.; Letushko, V.N.; Nizovtsev, M.I.; Borodulin, V.Y. Experimental study of evaporation of sessile droplets of the water-ethanol solution with different concentrations. J. Phys. Conf. Ser. 2018, 1105, 012068. [Google Scholar] [CrossRef]
- Starinskaya, E.; Miskiv, N.; Nazarov, A.; Terekhov, V.; Rybdylova, O.; Sazhin, S. Evaporation of water/ethanol droplets in an air flow: Experimental study and modelling. Int. J. Heat Mass Transf. 2021, 177, 121502. [Google Scholar] [CrossRef]
- Yu, Y.S.; Wang, Z.Q.; Zhao, Y.P. Experimental study of evaporation of sessile water droplet on PDMS surfaces. Acta Mech. Sin. Xuebao 2013, 29, 799–805. [Google Scholar] [CrossRef]
- Ozturk, T.; Erbil, H.Y. Evaporation of water-ethanol binary sessile drop on fluoropolymer surfaces: Influence of relative humidity. Colloids Surf. A Physicochem. Eng. Asp. 2018, 553, 327–336. [Google Scholar] [CrossRef]
- Jeong, C.H.; Lee, H.J.; Kim, D.Y.; Ahangar, S.B.; Choi, C.K.; Lee, S.H. Quantitative analysis of contact line behaviors of evaporating binary mixture droplets using surface plasmon resonance imaging. Int. J. Heat Mass Transf. 2021, 165, 120690. [Google Scholar] [CrossRef]
- Tabrizi, H.O.; Forouhi, S.; Ghafar-Zadeh, E. A High Dynamic Range Dual 8 × 16 Capacitive Sensor Array for Life Science Applications. IEEE Trans. Biomed. Circuits Syst. 2022, 16, 1191–1203. [Google Scholar] [CrossRef] [PubMed]
- Avidien. Eliminating Common Pipetting Errors for Repeatable Results. Available online: https://www.avidien.com/blog/eliminating-common-pipetting-errors (accessed on 29 May 2022).
- Artel. Liquid Types and Challenges—Tips for Successful Transfer. Available online: https://www.artel.co/liquid-types-and-challenges-tips-for-successful-transfer (accessed on 29 May 2022).
- Artel. Pipetting Viscous Solutions. Available online: https://www.artel.co/pipetting-viscous-solutions (accessed on 29 May 2022).
- Millet, F.; Barthlen, T. Securing accuracy and precision when pipetting hot and cold liquids with Microman®. Nat. Methods 2007, 4, iii–iv. [Google Scholar] [CrossRef]
- Zafar, S.; Kumar, A.; Gusev, E.; Cartier, E. Threshold voltage instabilities in high-k gate dielectric stacks. IEEE Trans. Device Mater. Reliab. 2005, 5, 45–64. [Google Scholar] [CrossRef]
- Afacan, E.; Yelten, M.B.; Dündar, G. Analog design methodologies for reliability in nanoscale CMOS circuits. In Proceedings of the 2017—14th International Conference on Synthesis Modeling Analysis and Simulation Methods and Applications to Circuit Design (SMACD), Giardini Naxos, Italy, 12–15 June 2017; pp. 1–4. [Google Scholar]
- Kerber, A.; Nigam, T. Bias temperature instability in scaled CMOS technologies: A circuit perspective. Microelectron. Reliab. 2018, 81, 31–40. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osouli Tabrizi, H.; Forouhi, S.; Azadmousavi, T.; Ghafar-Zadeh, E. A Multidisciplinary Approach toward CMOS Capacitive Sensor Array for Droplet Analysis. Micromachines 2024, 15, 232. https://doi.org/10.3390/mi15020232
Osouli Tabrizi H, Forouhi S, Azadmousavi T, Ghafar-Zadeh E. A Multidisciplinary Approach toward CMOS Capacitive Sensor Array for Droplet Analysis. Micromachines. 2024; 15(2):232. https://doi.org/10.3390/mi15020232
Chicago/Turabian StyleOsouli Tabrizi, Hamed, Saghi Forouhi, Tayebeh Azadmousavi, and Ebrahim Ghafar-Zadeh. 2024. "A Multidisciplinary Approach toward CMOS Capacitive Sensor Array for Droplet Analysis" Micromachines 15, no. 2: 232. https://doi.org/10.3390/mi15020232
APA StyleOsouli Tabrizi, H., Forouhi, S., Azadmousavi, T., & Ghafar-Zadeh, E. (2024). A Multidisciplinary Approach toward CMOS Capacitive Sensor Array for Droplet Analysis. Micromachines, 15(2), 232. https://doi.org/10.3390/mi15020232







