Optimization of Glucose Dehydrogenase Immobilization Strategies in a 3D-Printed Millireactor
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
Chemicals
2.2. Methods
2.2.1. Glucose Dehydrogenase Assay
2.2.2. Determination of the Enzyme Concentration Using the Linearized Bradford Assay
2.2.3. Determination of Glucose Concentration Using the Enzymatic GOD-PAP Method
2.2.4. Spectrophotometric Measurement of the NADH Concentration
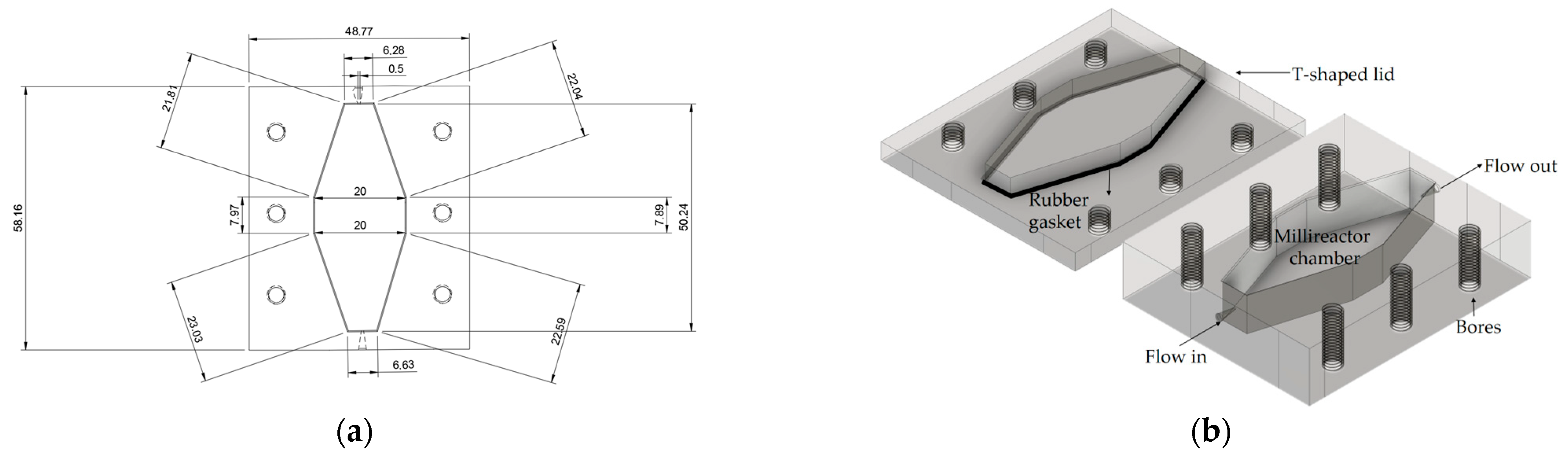
2.2.5. Millireactor Design and Fabrication
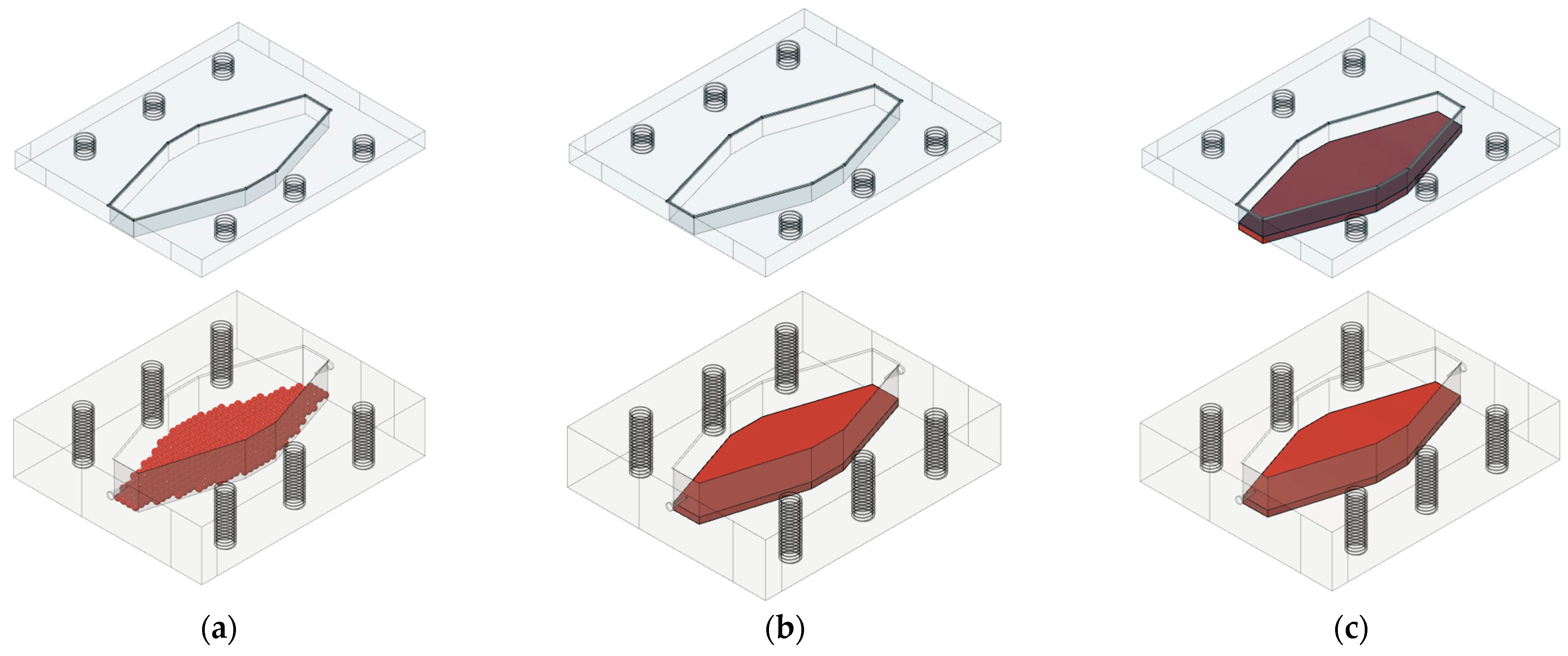
2.2.6. GDH Immobilization in an Alginate Gel
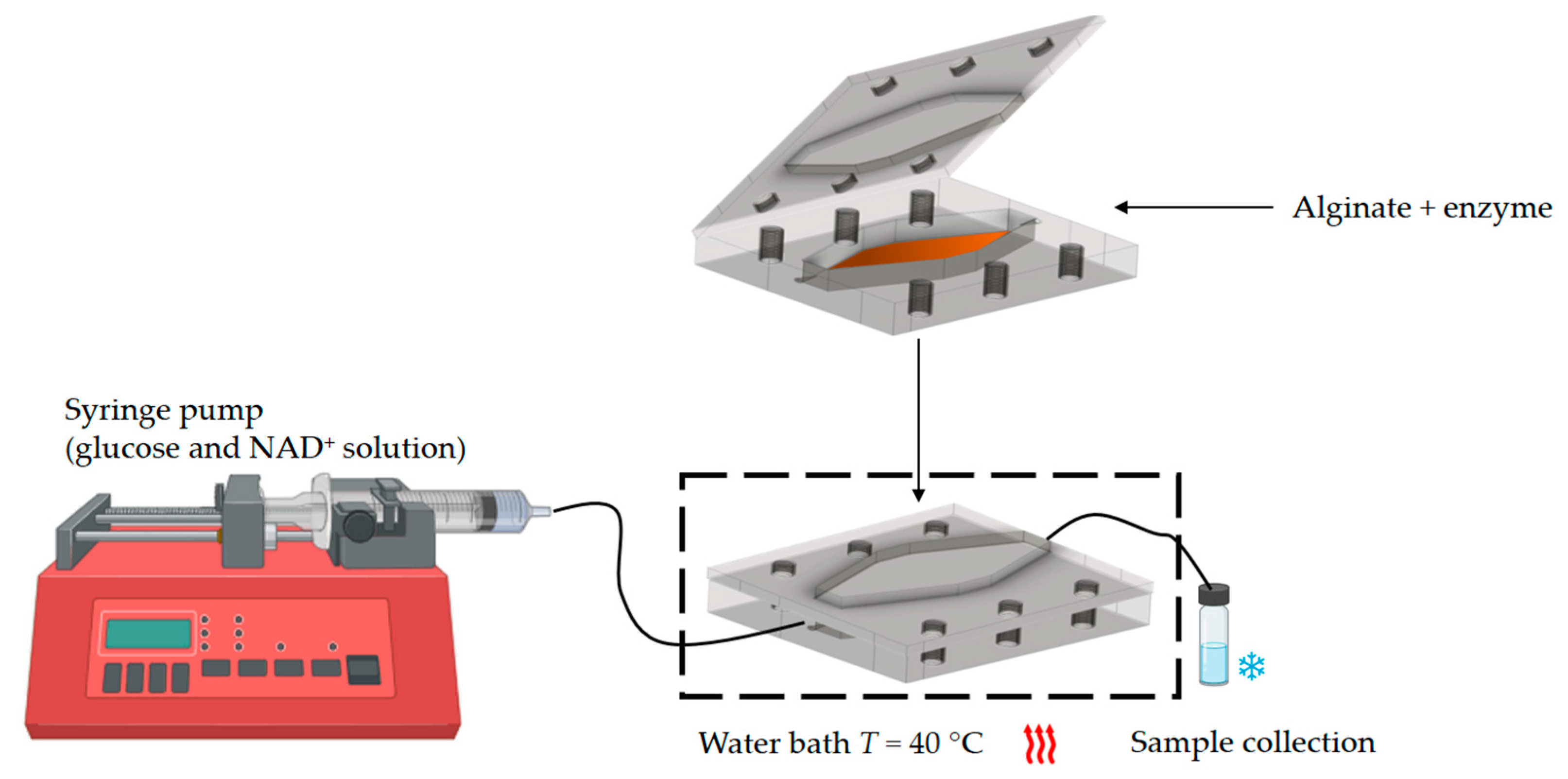
2.2.7. Glucose Oxidation in a Millireactor
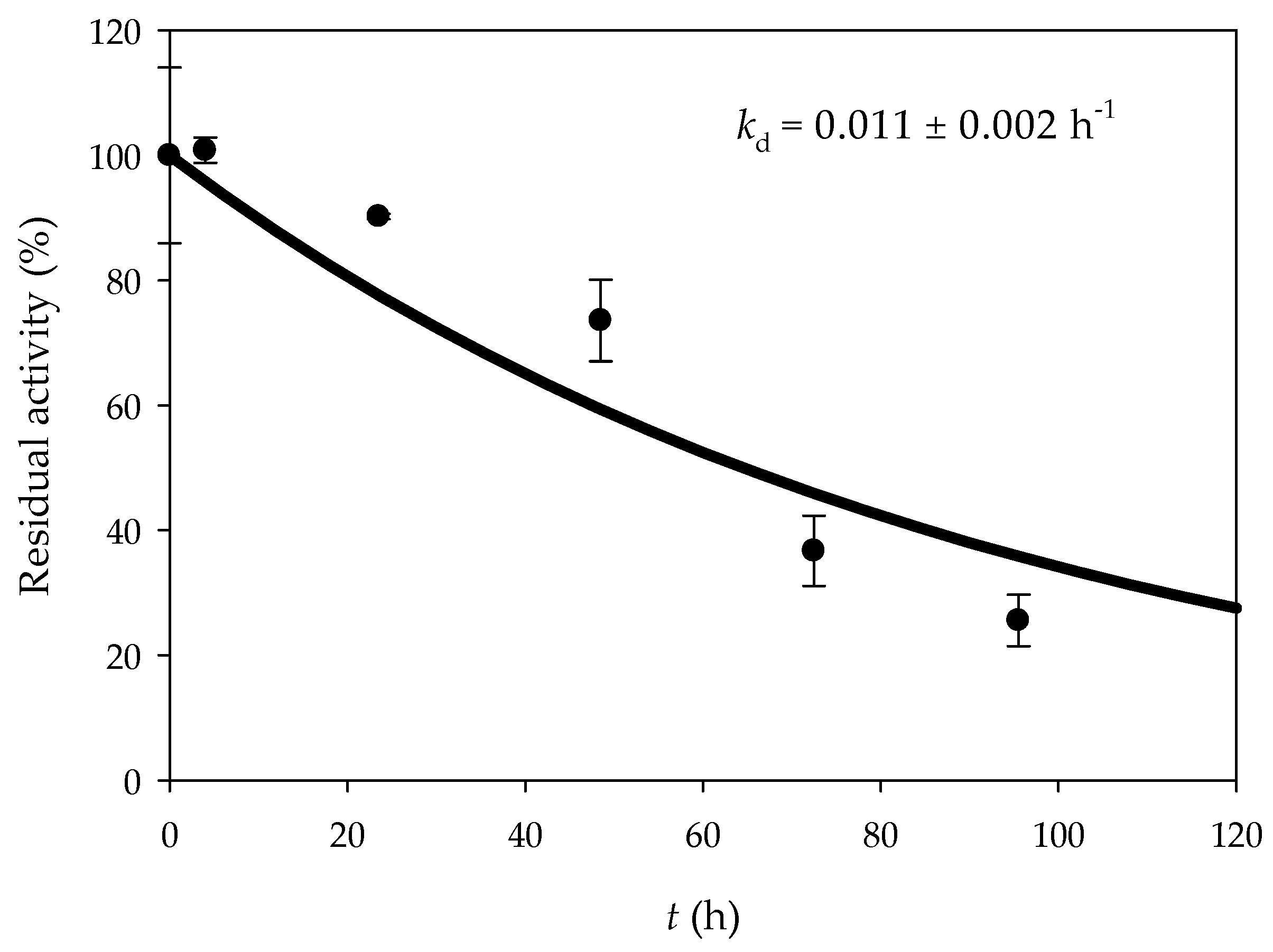
2.2.8. Operational Stability
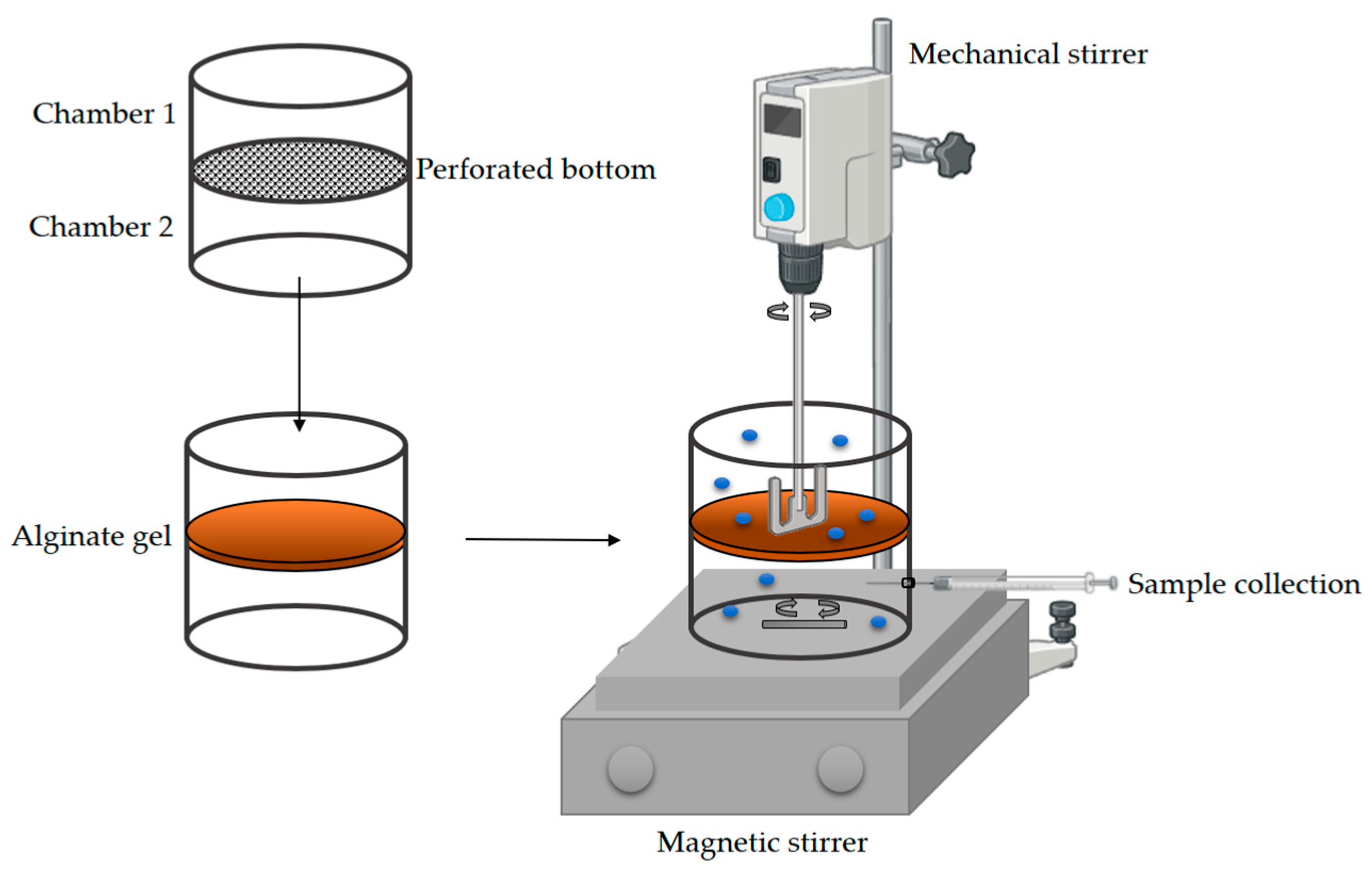
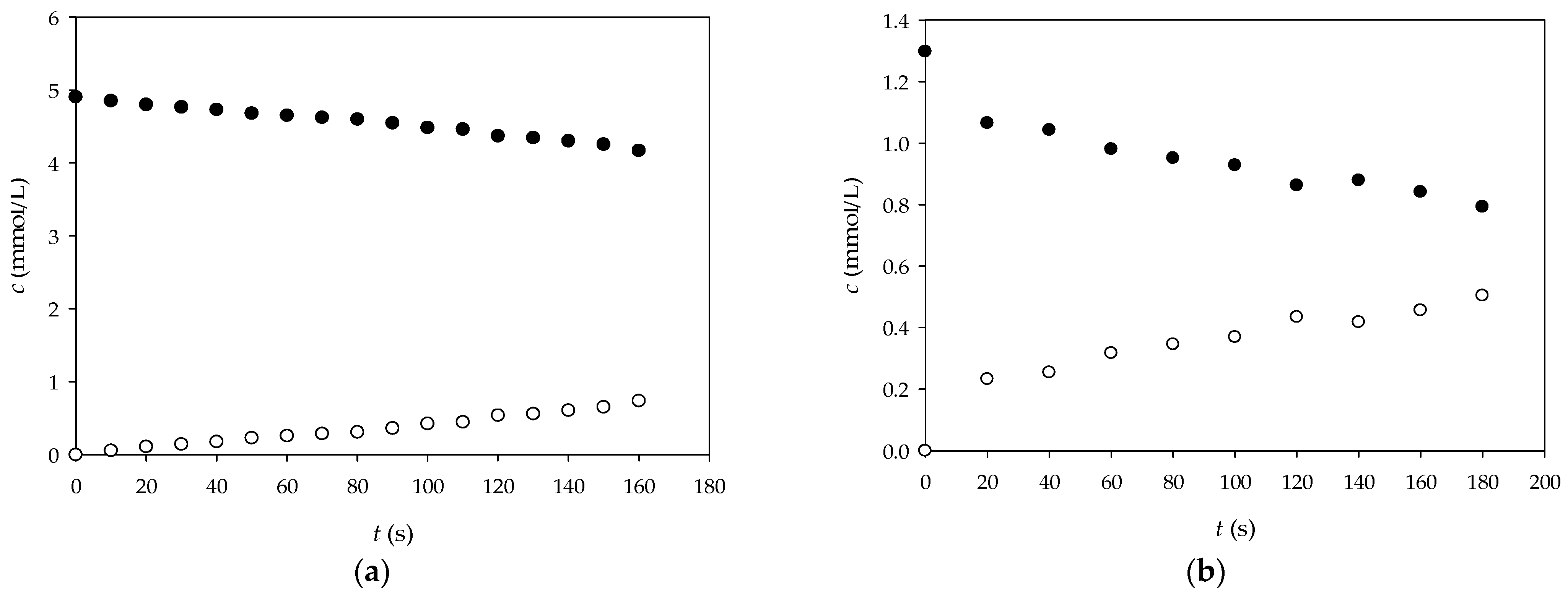
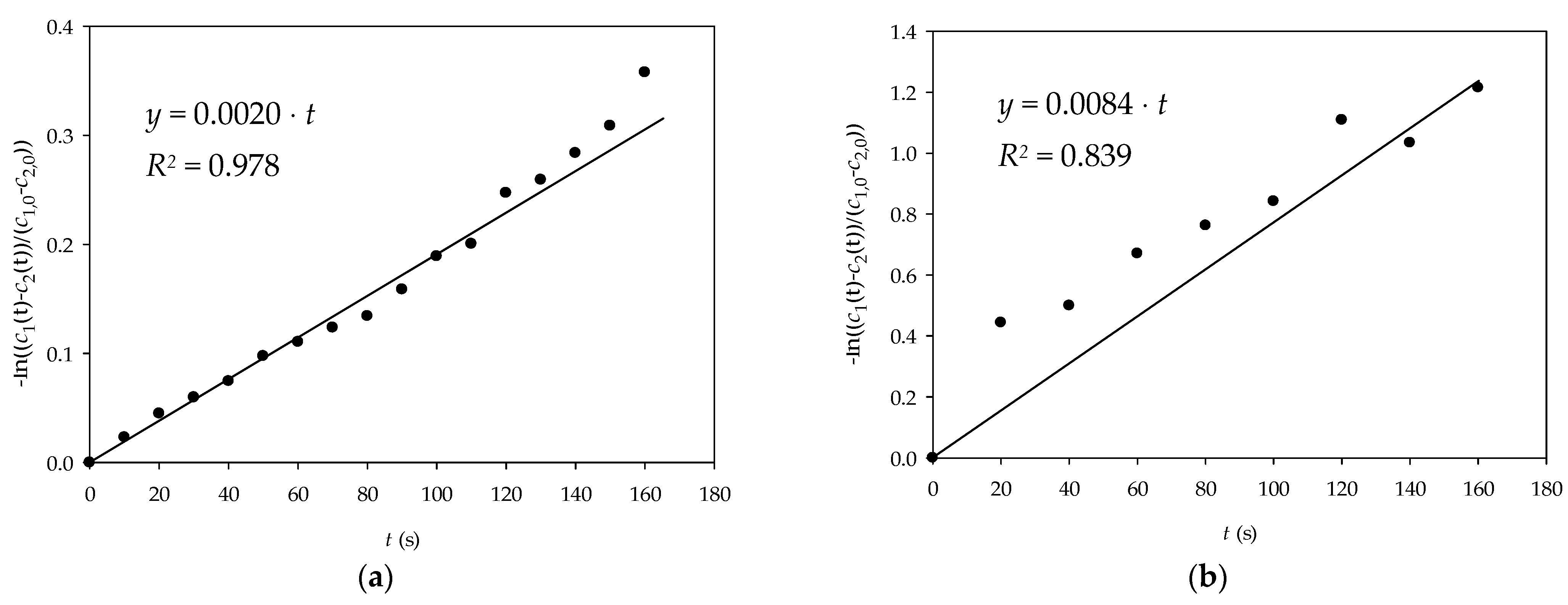
2.2.9. Diffusion Measurement of Glucose and NADH Through Alginate Gel
2.2.10. Computational Fluid Dynamics (CFD) Modelling
3. Results
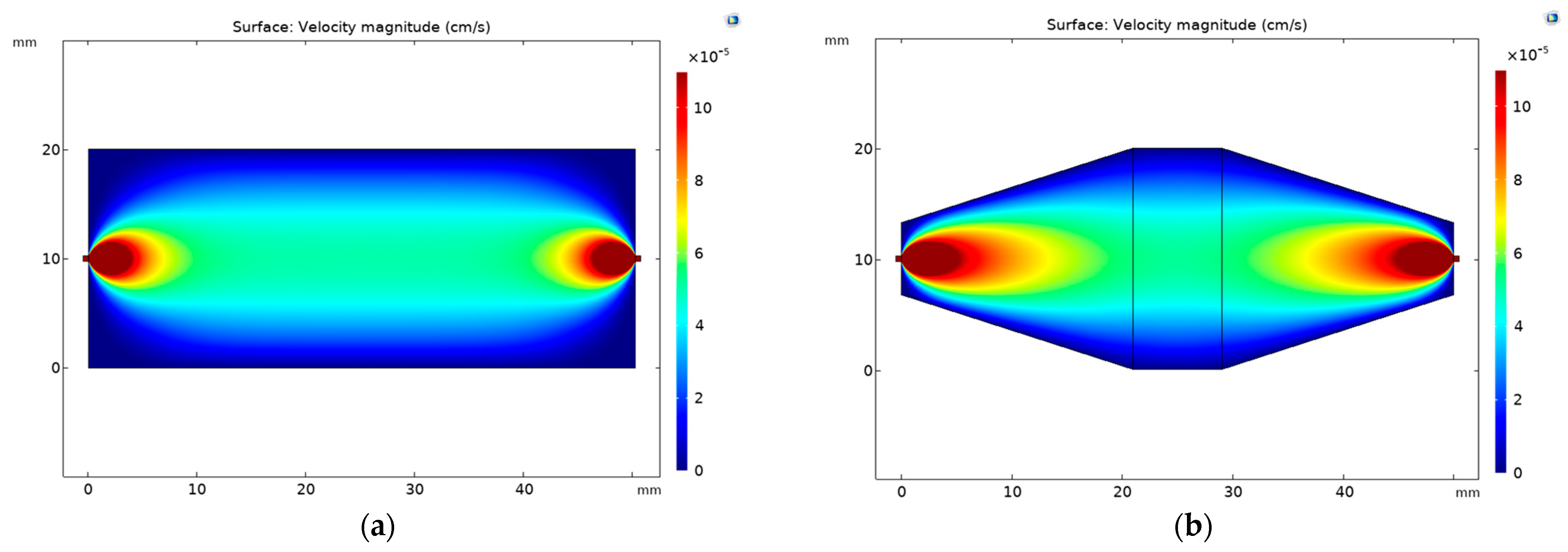
3.1. Millireactor Design
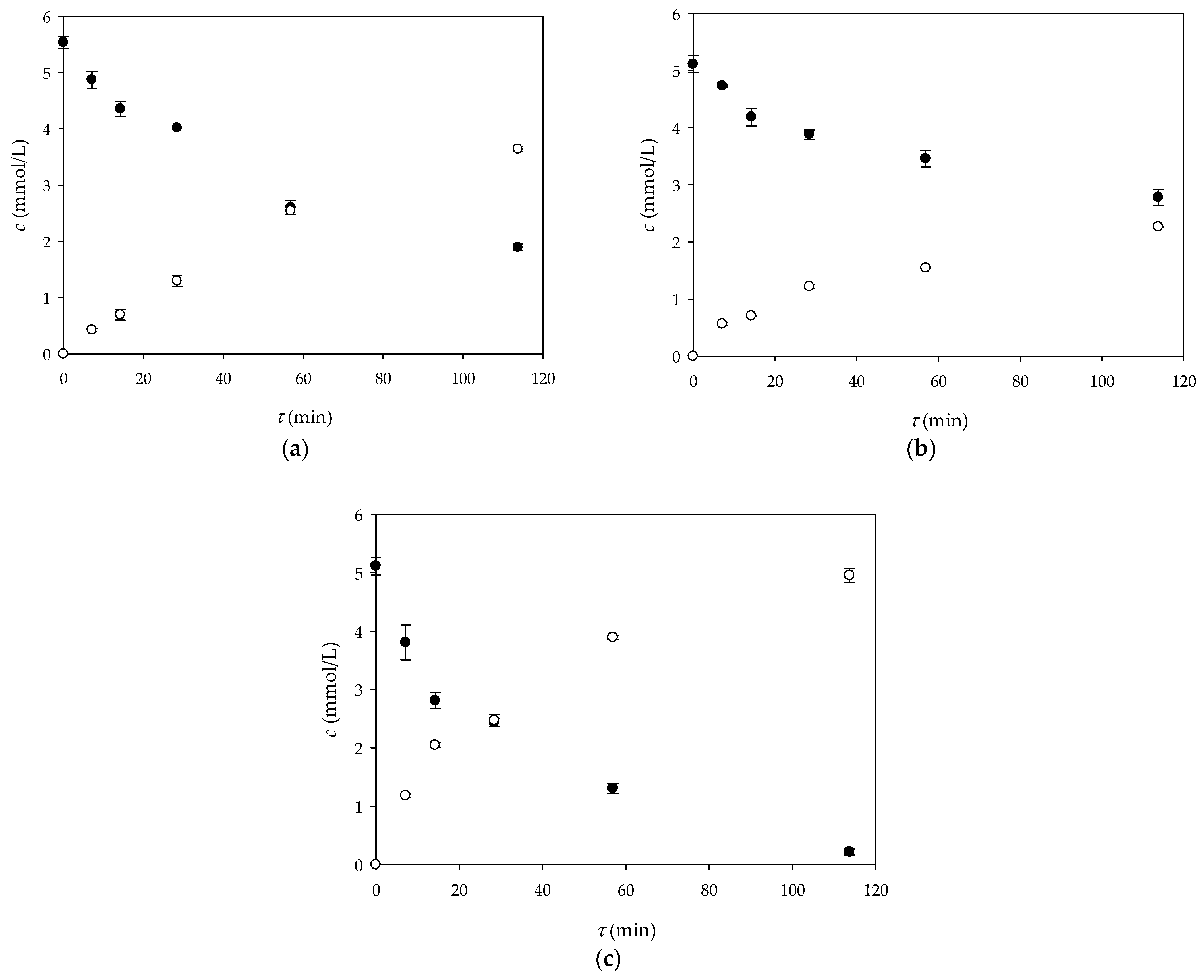
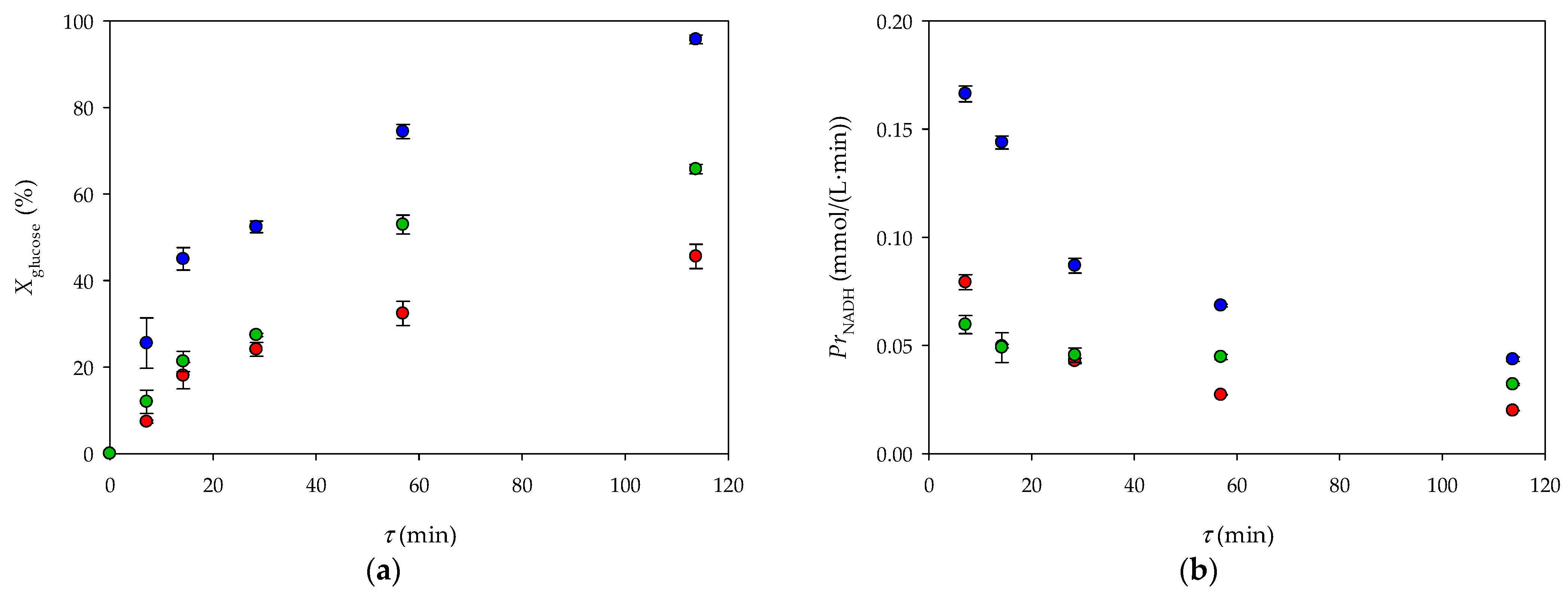
3.2. Glucose Oxidation in a 3D-Printed Millireactor
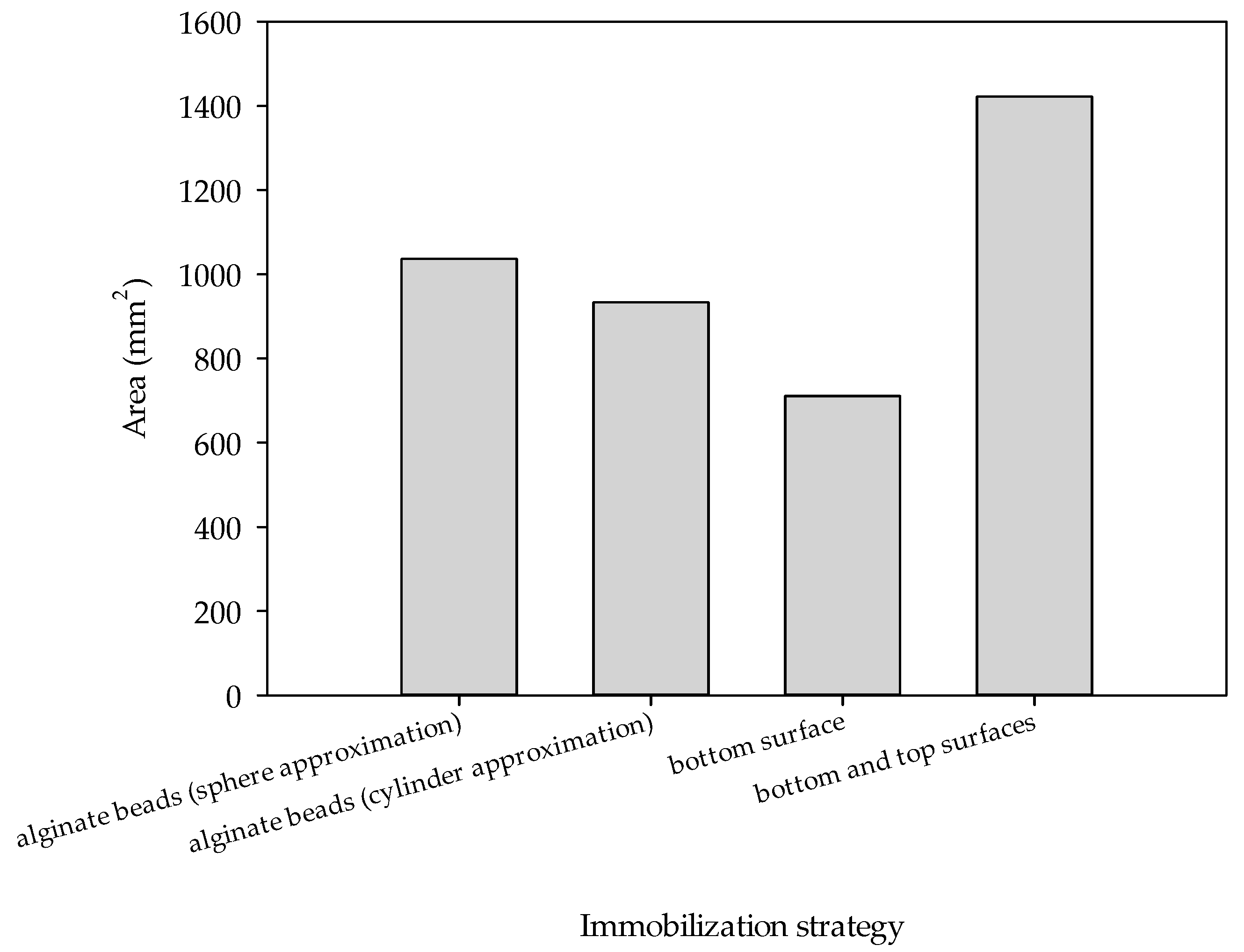
3.3. Diffusion of Glucose and NADH Through Alginate Gel
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palmer, T.; Bonner, P.L. An introduction to enzymes. In Enzymes, 2nd ed.; Palmer, T., Bonner, P.L., Eds.; Woodhead Publishing: Cambridge, UK, 2011; pp. 2–13. [Google Scholar] [CrossRef]
- Mokrani, S.; Nabti, E.H. Recent status in production, biotechnological applications, commercial aspects, and future prospects of microbial enzymes: A comprehensive review. Int. J. Agric. Sc. Food Technol. 2024, 10, 006–020. [Google Scholar] [CrossRef]
- Gouseti, O.; Larsen, M.E.; Amin, A.; Bakalis, S.; Petersen, I.L.; Lametsch, R.; Jensen, P.E. Applications of Enzyme Technology to Enhance Transition to Plant Proteins: A Review. Foods 2023, 12, 2518. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Dhiman, S.; Krishan, B.; Samtiya, M.; Kumari, A.; Pathak, N.; Kumari, A.; Aluko, R.E.; Dhewa, T. Microbial enzymes and major applications in the food industry: A concise review. Food Prod. Process Nutr. 2024, 6, 85. [Google Scholar] [CrossRef]
- Dahiru, M.M.; Abdulhamid, A.A.; Abaka, A.M. Review: Current perspectives on enzyme applications in medicine, agriculture, and industries. Asian J. Trop. Biotechnol. 2024, 21, 10–25. [Google Scholar]
- Patil, P.D.; Gargate, N.; Dongarsane, K.; Jagtap, H.; Phirke, A.N.; Tiwari, M.S.; Nadar, S.S. Revolutionizing biocatalysis: A review on innovative design and applications of enzyme-immobilized microfluidic devices. Int. J. Biol. Macromol. 2024, 281, 136193. [Google Scholar] [CrossRef]
- Mustafa, M.G.; Khan, M.G.M.; Nguyen, D.; Iqbal, S. Techniques in Biotechnology. In Omics Technologies and Bio-Engineering; Academic Press: Cambridge, MA, USA, 2018; pp. 233–249. [Google Scholar] [CrossRef]
- Chapman, J.; Ismail, A.E.; Dinu, C.Z. Industrial Applications of Enzymes: Recent Advances, Techniques, and Outlooks. Catalysts 2018, 8, 238. [Google Scholar] [CrossRef]
- Mohidem, N.A.; Mohamad, M.; Rashid, M.U.; Norizan, M.N.; Hamzah, F.; Mat, H.B. Recent Advances in Enzyme Immobilisation Strategies: An Overview of Techniques and Composite Carriers. J. Compos. Sci. 2023, 7, 488. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Hussain, C.M. Sustainable textile finishing processes and pollution control based on enzyme technology. In Green Chemistry for Sustainable Textiles, 1st ed.; Ibrahim, N.A., Eid, B.M., Amin, H.A., Eds.; Woodhead Publishing: Cambridge, UK, 2021; pp. 385–415. [Google Scholar] [CrossRef]
- Marques, S.M.; Planas-Iglesias, J. Web-based tools for computational enzyme design. Curr. Opin. Struct. Biol. 2021, 69, 19–34. [Google Scholar] [CrossRef]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef]
- Prabhakar, T.; Giaretta, J.; Zulli, R.; Rath, R.J.; Farajikhah, S.; Talebian, S.; Dehjhani, F. Covalent immobilization: A review from an enzyme perspective. Chem. Eng. J. 2024, 503, 158054. [Google Scholar] [CrossRef]
- Tang, Z.; Oku, Y.; Matsuda, T. Application of Immobilized Enzymes in Flow Biocatalysis for Efficient Synthesis. Org. Process Res. Dev. 2024, 28, 1308–1326. [Google Scholar] [CrossRef]
- Mirsalami, S.M.; Mirsalami, M.; Ghodousian, A. Techniques for immobilizing enzymes to create durable and effective biocatalysts. Results Chem. 2024, 7, 101486. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of Different Enzyme Immobilization Strategies to Improve Enzyme Performance. Adv. Synth. Catal. 2011, 16, 2885–2904. [Google Scholar] [CrossRef]
- Pyne, M.; Abedi, D.; Zhang, L.; Chou, C.P. Enzyme Biocatalysis. In Comprehensive Biotechnology: Principles, Applications and regulations in Industry, Agriculture, Medicine and the Environment, 2nd ed.; Murray, M.Y., Ed.; Elsevier: Amsterdan, The Netherlands, 2011; Volume 3, pp. 15–24. [Google Scholar] [CrossRef]
- Mahgraby, Y.R.; El-Shabasy, R.M.; Ibrahim, A.H.; Azzazy, H.M.S. Enzyme Immobilization Technologies and Industrial Applications. ACS Omega 2023, 8, 5184–5196. [Google Scholar] [CrossRef]
- Romero, G.; Contreras, L.M.; Aguirre Céspedes, C.; Wilkesman, J.; Clemente-Jiménez, J.M.; Rodríguez-Vico, F.; Las Heras-Vázquez, F.J. Efficiency Assessment between Entrapment and Covalent Bond Immobilization of Mutant β-Xylosidase onto Chitosan Support. Polymers 2023, 15, 3170. [Google Scholar] [CrossRef]
- Begall, M.J.; Herbstritt, F.; Sengen, A.L.; Mhamdi, A.; Heck, J.; Mitsos, A. Hierarchical heat transfer modeling of a continuous millireactor. Comput. Chem. Eng. 2024, 183, 108621. [Google Scholar] [CrossRef]
- Dong, Z.; Wen, Z.; Zhao, F.; Kuhn, S.; Noël, T. Scale-up of micro- and milli-reactors: An overview of strategies, design principles and applications. Chem. Eng. Sci. 2021, 10, 100097. [Google Scholar] [CrossRef]
- Kitson, P.J.; Rosnes, M.H.; Sans, V.; Dragone, V.; Cronin, L. Configurable 3D-Printed millifluidic and microfluidic ’lab on a chip’ reactionware devices. Lab. Chip. 2012, 12, 3267–3268. [Google Scholar] [CrossRef]
- Santana, H.S.; Rodrigues, A.C.; Lopes, M.G.M.; Russo, F.N.; Silva, J.L., Jr.; Taranto, O.P. 3D printed millireactors for process intensification. Chin. J. Chem. Eng. 2018, 28, 180–190. [Google Scholar] [CrossRef]
- Laguna, O.H.; Lietor, P.F.; Iglesias Godino, F.J.; Corpas-Iglesias, F.A. A review on additive manufacturing and materials for catalytic applications: Milestones, key concepts, advances and perspectives. Mater. Des. 2021, 208, 109927. [Google Scholar] [CrossRef]
- Ćevid, I.; Cingesar, I.K.; Marković, M.-P.; Vrsaljko, D. Development of Static Mixers for Millireactors and Their Production by Vat Photopolymerization. Micromachines 2024, 15, 682. [Google Scholar] [CrossRef] [PubMed]
- Šercer, M.; Rezic, T.; Godec, D.; Oros, D.; Pilipovic, A.; Ivušic, F.; Rezic, I.; Andlar, M.; Ludwig, R.; Šantek, B. Microreactor production by PolyJet Matrix 3D-printing technology: Hydrodynamic characterization. Food Technol. Biotechnol. 2019, 57, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Ernst, O.; Zor, T. Linearization of the Bradford protein assay. J. Vis. Exp. 2010, 38, e1918. [Google Scholar] [CrossRef]
- Falk, B.; Garramone, S.; Shivkumar, S. Diffusion coefficient of paracetamol in a chitosan hydrogel. Mater. Lett. 2004, 58, 3261–3265. [Google Scholar] [CrossRef]
- Gojun, M.; Pustahija, L.; Jurinjak Tušek, A.; Šalić, A.; Valinger, D.; Zelić, B. Kinetic Parameter Estimation and Mathematical Modelling of Lipase Catalysed Biodiesel Synthesis in a Microreactor. Micromachines 2019, 10, 759. [Google Scholar] [CrossRef]
- Menegatti, T.; Žnidaršič-Plazl, P. Copolymeric Hydrogel-Based Immobilization of Yeast Cells for Continuous Biotransformation of Fumaric Acid in a Microreactor. Micromachines 2019, 10, 867. [Google Scholar] [CrossRef]
- Bajić, M.; Khiawjan, S.; Hilton, S.T.; Lye, G.J.; Marques, M.P.C.; Szita, N. A paradigm shift for biocatalytic microreactors: Decoupling application from reactor design. Biochem. Eng. J. 2024, 205, 109260. [Google Scholar] [CrossRef]
- Macown, R.J.; Veraitch, F.S.; Szita, N. Robust, microfabricated culture devices with improved control over the soluble microenvironment for the culture of embryonic stem cells. Biotechnol. J. 2014, 9, 805–813. [Google Scholar] [CrossRef]
- Urrea, D.A.M.; Gimenez, A.V.F.; Rodriguez, Y.E.; Contreras, E.M. Immobilization of horseradish peroxidase in Ca-alginate beads: Evaluation of the enzyme leakage on the overall removal of an azo-dye and mathematical modelling. Process Saf. Environ. Prot. 2021, 156, 134–143. [Google Scholar] [CrossRef]
- Oktaviani, M.; Damin, B.C.S.; Suryanegara, L.; Yanto, D.H.Y.; Watanabe, T. Immobilization of fungal mycelial and laccase from Trametes hirsuta EDN082 in alginate-cellulose beads and its use in Remazol Brilliant Blue R dye decolorization. Bioresour. Technol. Rep. 2024, 26, 101828. [Google Scholar] [CrossRef]
- Gao, H.; Khera, E.; Lee, J.K.; Wen, F. Immobilization of Multi-biocatalysts in Alginate Beads for Cofactor Regeneration and Improved Reusability. J. Vis. Exp. 2016, 110, 53944. [Google Scholar] [CrossRef]
- Abka-Khajouei, R.; Tounsi, L.; Shahabi, N.; Patel, A.K.; Abdelkafi, S.; Michaud, P. Structures, Properties and Applications of Alginates. Mar. Drugs. 2022, 20, 364. [Google Scholar] [CrossRef] [PubMed]
- Lavrentev, F.V.; Shilovskikh, V.V.; Alabusheva, V.S.; Yurova, V.Y.; Nikitina, A.A.; Ulasevich, S.A.; Skorb, E.V. Diffusion-Limited Processes in Hydrogels with Chosen Applications from Drug Delivery to Electronic Components. Molecules 2023, 28, 5931. [Google Scholar] [CrossRef]
- Chan, T.C.; Li, H.T.; Li, K.Y. Effects of Shapes of Solute Molecules on Diffusion: A Study of Dependences on Solute Size, Solvent, and Temperature. J. Phys. Chem. B 2015, 119, 15718–15728. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boroša, V.M.; Koštan, K.; Vičević, R.; Cingesar, I.K.; Vrsaljko, D.; Zelić, B.; Jurinjak Tušek, A.; Šalić, A. Optimization of Glucose Dehydrogenase Immobilization Strategies in a 3D-Printed Millireactor. Micromachines 2024, 15, 1514. https://doi.org/10.3390/mi15121514
Boroša VM, Koštan K, Vičević R, Cingesar IK, Vrsaljko D, Zelić B, Jurinjak Tušek A, Šalić A. Optimization of Glucose Dehydrogenase Immobilization Strategies in a 3D-Printed Millireactor. Micromachines. 2024; 15(12):1514. https://doi.org/10.3390/mi15121514
Chicago/Turabian StyleBoroša, Vilim Marijan, Kristian Koštan, Renata Vičević, Ivan Karlo Cingesar, Domagoj Vrsaljko, Bruno Zelić, Ana Jurinjak Tušek, and Anita Šalić. 2024. "Optimization of Glucose Dehydrogenase Immobilization Strategies in a 3D-Printed Millireactor" Micromachines 15, no. 12: 1514. https://doi.org/10.3390/mi15121514
APA StyleBoroša, V. M., Koštan, K., Vičević, R., Cingesar, I. K., Vrsaljko, D., Zelić, B., Jurinjak Tušek, A., & Šalić, A. (2024). Optimization of Glucose Dehydrogenase Immobilization Strategies in a 3D-Printed Millireactor. Micromachines, 15(12), 1514. https://doi.org/10.3390/mi15121514









