Recent Advancements in Co3O4-Based Composites for Enhanced Electrocatalytic Water Splitting
Abstract
1. Introduction
2. Promising Features of Co3O4 Beneficial for Electrocatalytic Applications
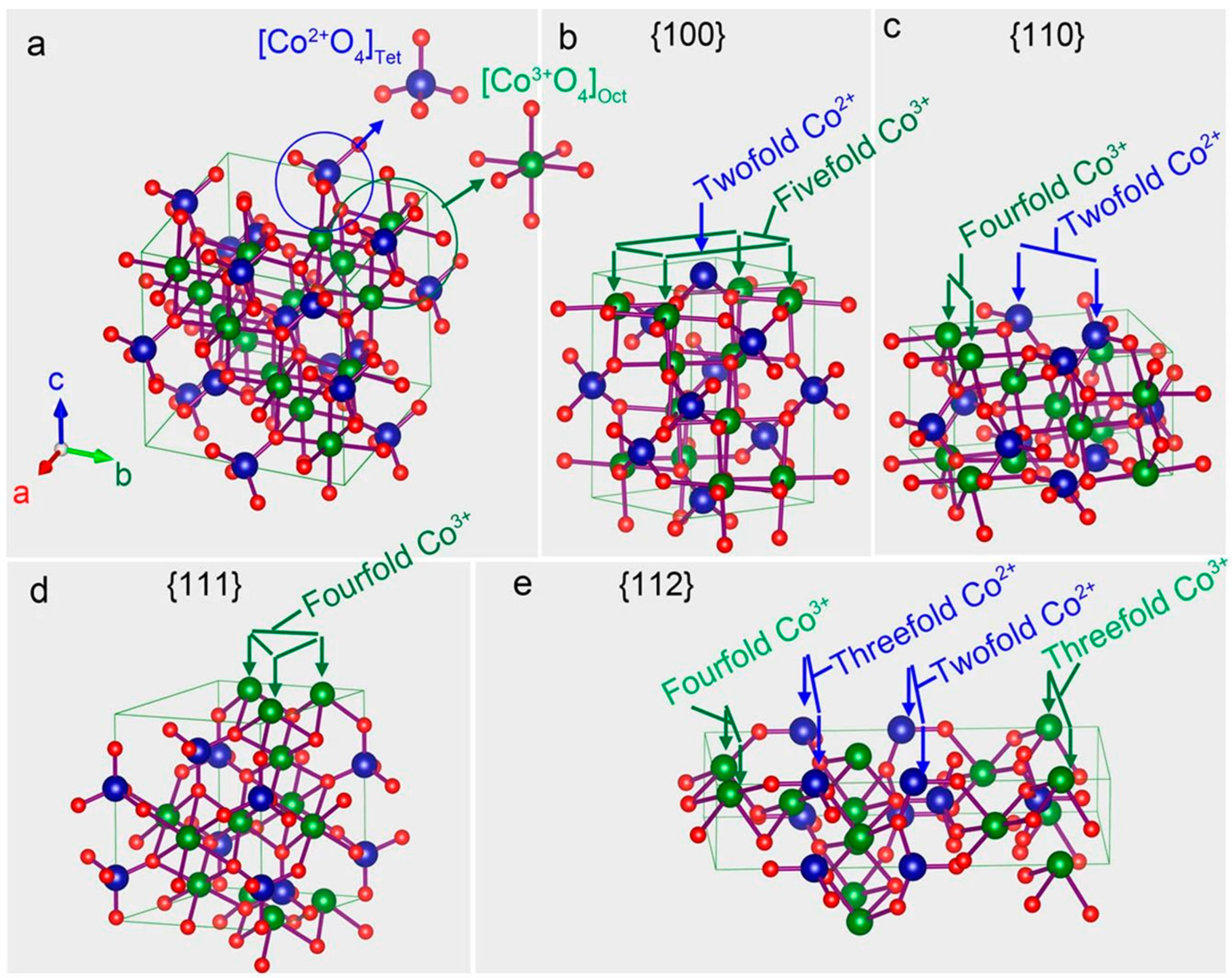
2.1. Mixed Valence States (Co2+/Co3+)
2.2. Chemical Stability in Alkaline Electrolytes
2.3. Structural and Morphological Flexibility
2.4. Magnetic and Electronic Properties
3. Co3O4-Based Composite Electrocatalysts for the HER and OER
3.1. Composites of Co3O4 with Carbon Derivatives
3.2. MOF-Derived Composites of Co3O4
3.3. Composites of Co3O4 with Other Materials
4. Conclusions
5. Limitations
- Stability and Durability: the prolonged operation of Co3O4-based catalysts in harsh electrochemical environments often leads to degradation, posing a major obstacle to ensuring long-term stability.
- Optimization of Catalytic Performance: although improvements have been made, the catalytic performance of Co3O4 composites still falls short compared to other best-performing precious metal-based catalysts.
- Scalability: developing scalable synthesis methods for Co3O4-based composites remains a critical challenge, particularly in producing catalysts with consistent and reliable properties for industrial applications.
- Mechanistic Understanding: while research on Co3O4 composites is expanding, the exact mechanisms driving their enhanced catalytic activity remain unclear. Further investigations are needed to clarify these processes at the atomic level.
6. Future Prospects
- Advanced Material Design: the exploration of novel composite structures, such as heterostructures and core–shell architectures, could unlock new levels of catalytic performance.
- Integration with Other Technologies: integrating Co3O4-based composites with emerging technologies, such as solar-driven water splitting or hybrid energy systems, could pave the way for integrated renewable energy solutions.
- In situ Characterization Techniques: the development of advanced characterization methods, particularly in-situ and operando studies, can provide deeper insights into catalytic mechanisms, enabling the rational design of more efficient and durable catalysts.
- Sustainability and Cost Reduction: efforts to optimize low-cost, scalable synthesis methods will be critical to ensuring the economic viability of Co3O4-based composites for industrial-scale applications.
- Computational Modeling and Machine Learning: harnessing computational tools and machine learning techniques could substantially accelerate the discovery and optimization of Co3O4-based materials with enhanced properties.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hu, Z.; Hao, L.; Quan, F.; Guo, R. Recent Developments of Co3O4-Based Materials as Catalysts for the Oxygen Evolution Reaction. Catal. Sci. Technol. 2022, 12, 436–461. [Google Scholar] [CrossRef]
- Ang, T.Z.; Salem, M.; Kamarol, M.; Das, H.S.; Nazari, M.A.; Prabaharan, N. A Comprehensive Study of Renewable Energy Sources: Classifications, Challenges and Suggestions. Energy Strategy Rev. 2022, 43, 100939. [Google Scholar] [CrossRef]
- Vine, E. Breaking down the Silos: The Integration of Energy Efficiency, Renewable Energy, Demand Response and Climate Change. Energy Effic. 2008, 1, 49–63. [Google Scholar] [CrossRef]
- Midilli, A.; Ay, M.; Dincer, I.; Rosen, M.A. On Hydrogen and Hydrogen Energy Strategies I : Current Status and Needs. Renew. Sustain. Energy Rev. 2005, 9, 255–271. [Google Scholar] [CrossRef]
- Rosen, M.A.; Scottf, D.S. Comparative Efficiency Assessments for A Range of Hydrogen Production Processes. Int. J. Hydrogen Energy 1998, 23, 653–659. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhong, C.J. Hydrogen Production from Water Electrolysis: Role of Catalysts. Nano Converg. 2021, 8, 4. [Google Scholar] [CrossRef]
- Ma, J.; Wei, H.; Liu, Y.; Ren, X.; Li, Y.; Wang, F.; Han, X.; Xu, E.; Cao, X.; Wang, G.; et al. Application of Co3O4-based materials in electrocatalytic hydrogen evolution reaction: A review. Int. J. Hydrogen Energy 2020, 45, 21205–21220. [Google Scholar] [CrossRef]
- Ashik, U.P.M.; Viswan, A.; Kudo, S.; Hayashi, J. Nanomaterials as Catalysts. In Applications of Nanomaterials: Advances and Key Technologies; Elsevier: Amsterdam, The Netherlands, 2018; pp. 45–82. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Y.; Wang, H.; Dai, H. Strongly Coupled Inorganic/Nanocarbon Hybrid Materials for Advanced Electrocatalysis. J. Am. Chem. Soc. 2013, 135, 2013–2036. [Google Scholar] [CrossRef]
- Manjunatha, R.; Karajić, A.; Liu, M.; Zhai, Z.; Dong, L.; Yan, W.; Wilkinson, D.P.; Zhang, J. A Review of Composite/Hybrid Electrocatalysts and Photocatalysts for Nitrogen Reduction Reactions: Advanced Materials, Mechanisms, Challenges and Perspectives. Electrochem. Energy Rev. 2020, 3, 506–540. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, Z.; Fang, L.; Xu, H.; Zhang, H.; Gu, X.; Wang, Y. Probing the Crystal Plane Effect of Co3O4 for Enhanced Electrocatalytic Performance toward Efficient Overall Water Splitting. ACS Appl. Mater. Interfaces 2017, 9, 27736–27744. [Google Scholar] [CrossRef]
- Zhu, B.; Yang, G.; Gao, D.; Wu, Y.; Zhao, J.; Fu, Y.; Ma, S. Special Atmosphere Annealed Co3O4 Porous Nanoclusters with Oxygen Defects and High Proportion of Co2+ for Oxygen Evolution Reaction. J. Alloys Compd. 2019, 806, 163–169. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.; Li, K.; Li, L.; Wei, J.; Feng, L.; Fu, Q. Morphology-Controlled Fabrication of Co3O4 Nanostructures and Their Comparative Catalytic Activity for Oxygen Evolution Reaction. J. Alloys Compd. 2016, 680, 146–154. [Google Scholar] [CrossRef]
- Li, D.; Xu, D.; Pei, Y.; Zhang, Q.; Lu, Y.; Zhang, B. Isolated Octahedral Pt-Induced Electron Transfer to Ultralow-Content Ruthenium-Doped Spinel Co3O4 for Enhanced Acidic Overall Water Splitting. J. Am. Chem. Soc. 2024, 146, 28728–28738. [Google Scholar] [CrossRef] [PubMed]
- Tahira, A.; Ibupoto, Z.H.; Willander, M.; Nur, O. Advanced Co3O4–CuO Nano-Composite Based Electrocatalyst for Efficient Hydrogen Evolution Reaction in Alkaline Media. Int. J. Hydrogen Energy 2019, 44, 26148–26157. [Google Scholar] [CrossRef]
- Zhong, W.; Yang, C.; Wu, J.; Xu, W.; Zhao, R.; Xiang, H.; Shen, K.; Zhang, Q.; Li, X. Oxygen Vacancies Induced by Charge Compensation Tailoring Ni-Doped Co3O4 Nanoflakes for Efficient Hydrogen Evolution. Chem. Eng. J. 2022, 436, 134813. [Google Scholar] [CrossRef]
- Lu, B.; Zang, J.; Li, W.; Li, J.; Zou, Q.; Zhou, Y.; Wang, Y. Co-Doped NixPy Loading on Co3O4 Embedded in Ni Foam as a Hierarchically Porous Self-Supported Electrode for Overall Water Splitting. Chem. Eng. J. 2021, 422, 130062. [Google Scholar] [CrossRef]
- Feng, Z.; Pu, J.; Zhang, X.; Zhang, W.; Liu, M.; Cui, L.; Liu, J. Heterostructured CoO/Co3O4 Nanowire Array on Titanium Mesh as Efficient Electrocatalysts for Hydrogen Evolution Reaction. J. Alloys Compd. 2021, 881, 160603. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Zhang, B.; Ruan, Y.; Wan, H.; Ji, X.; Xu, K.; Zha, D.; Miao, L.; Jiang, J. Mutually beneficial Co3O4@MoS2 heterostructures as a highly efficient bifunctional catalyst for electrochemical overall water splitting. J. Mater. Chem. A 2018, 6, 2067–2072. [Google Scholar] [CrossRef]
- Aftab, U.; Tahira, A.; Gradone, A.; Morandi, V.; Abro, M.I.; Baloch, M.M.; Bhatti, A.L.; Nafady, A.; Vomiero, A.; Ibupoto, Z.H. Two Step Synthesis of TiO2–Co3O4 Composite for Efficient Oxygen Evolution Reaction. Int. J. Hydrogen Energy 2021, 46, 9110–9122. [Google Scholar] [CrossRef]
- Du, H.; Pu, W.; Yang, C. Morphology Control of Co3O4 with Nickel Incorporation for Highly Efficient Oxygen Evolution Reaction. Appl. Surf. Sci. 2021, 541, 148221. [Google Scholar] [CrossRef]
- Gujjula, S.R.; Karingula, S.; Shajahan, S.; Siliveri, S.; Goskula, S.; Chirra, S.; Gobi, K.V.; Narayanan, V. Rational Design of Metal Organic Framework Derived Porous Au@Co3O4/C Nanocomposite Materials for the Electrochemical Overall Water Splitting. J. Mater. Sci. 2023, 58, 9130–9147. [Google Scholar] [CrossRef]
- Yang, X.; Chen, J.; Chen, Y.; Feng, P.; Lai, H.; Li, J.; Luo, X. Novel CO3O4 Nanoparticles/Nitrogen-Doped Carbon Composites with Extraordinary Catalytic Activity for Oxygen Evolution Reaction (OER). Nanomicro Lett. 2018, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, S.; Sun, B.; Su, D.; Huang, X.; Liu, H.; Yan, Y.; Sun, K.; Wang, G. Graphene-Co3O4 Nanocomposite as Electrocatalyst with High Performance for Oxygen Evolution Reaction. Sci. Rep. 2015, 5, 7629. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Canaff, C.; Rousseau, J.; Arrii-Clacens, S.; Napporn, T.W.; Habrioux, A.; Kokoh, K.B. Effect of the Oxide-Carbon Heterointerface on the Activity of Co3O4/NRGO Nanocomposites toward ORR and OER. J. Phys. Chem. C 2016, 120, 7949–7958. [Google Scholar] [CrossRef]
- Vignesh, S.; Nam, S.; Kim, H. Interfacial Engineering of α-Fe2O3 Coupled Co3O4 Heterostructures Anchored on g-C3N4 Structure for Enhanced Electrocatalytic Performance in Alkaline Oxygen Evolution Reaction. Int. J. Hydrogen Energy 2024, 53, 1445–1456. [Google Scholar] [CrossRef]
- Huynh, N.D.; Jana, J.; Nivetha, R.; Van Phuc, T.; Chung, J.S.; Hur, S.H. 2D Siloxene Supported NiO/Co3O4 Electrocatalyst for the Stable and Efficient Hydrogen Evolution Reaction. Curr. Appl. Phys. 2022, 44, 102–109. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Addad, A.; Wang, Q.; Roussel, P.; Amin, M.A.; Szunerits, S.; Boukherroub, R. 0D/2D Co3O4/Ti3C2MXene Composite: A Dual-Functional Electrocatalyst for Energy-Saving Hydrogen Production and Urea Oxidation. ACS Appl. Energy Mater. 2022, 5, 15471–15482. [Google Scholar] [CrossRef]
- Alhashmialameer, D.; Shariq, M.; Fani, I.A.; Alharbi, A.F.; Althikrallah, H.A.; Almashnowi, M.Y.A.; Azooz, R.E.; Ahmed, I. A Facile Synthesis Strategy of Supported g-C3N4 and Molybdenum Disulfide over Co3O4 Spinal Composite Nanostructure: An Excellent Catalyst for HER. Energy Fuels 2024, 38, 15771–15779. [Google Scholar] [CrossRef]
- Liu, H.; Xia, G.; Zhang, R.; Jiang, P.; Chen, J.; Chen, Q. MOF-Derived RuO2/Co3O4 Heterojunctions as Highly Efficient Bifunctional Electrocatalysts for HER and OER in Alkaline Solutions. RSC Adv. 2017, 7, 3686–3694. [Google Scholar] [CrossRef]
- Aftab, U.; Tahira, A.; Samo, A.H.; Abro, M.I.; Baloch, M.M.; Kumar, M.; Sirajuddin; Ibupoto, Z.H. Mixed CoS2@Co3O4 Composite Material: An Efficient Nonprecious Electrocatalyst for Hydrogen Evolution Reaction. Int. J. Hydrogen Energy 2020, 45, 13805–13813. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, H.; Ge, R.; Ren, X.; Ren, J.; Yang, D.; Zhang, L.; Sun, X. Phosphorus-Doped Co3O4 Nanowire Array: A Highly Efficient Bifunctional Electrocatalyst for Overall Water Splitting. ACS Catal. 2018, 8, 2236–2241. [Google Scholar] [CrossRef]
- Duan, W.; Han, S.; Fang, Z.; Xiao, Z.; Lin, S. In Situ Filling of the Oxygen Vacancies with Dual Heteroatoms in Co3O4 for Efficient Overall Water Splitting. Molecules 2023, 28, 4134. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, H.; Jiang, Y.; Mao, Y.; Shen, W.; Li, M.; He, R. Adjustable Heterointerface-Vacancy Enhancement Effect in RuO2@Co3O4 Electrocatalysts for Efficient Overall Water Splitting. Appl. Catal. B 2023, 324, 122294. [Google Scholar] [CrossRef]
- Tian, G.; Liu, X.; Song, S.; Zhang, Q.; Wang, Z.; Liu, Y.; Zheng, Z.; Cheng, H.; Dai, Y.; Huang, B.; et al. In Situ Formation of CoP/Co3O4 Heterojunction for Efficient Overall Water Splitting. Chem.-A Eur. J. 2023, 29, e202301478. [Google Scholar] [CrossRef]
- Kim, K.H.; Choi, Y.H. Surface Oxidation of Cobalt Carbonate and Oxide Nanowires by Electrocatalytic Oxygen Evolution Reaction in Alkaline Solution. Mater. Res. Express 2022, 9, 034001. [Google Scholar] [CrossRef]
- Chen, T.; Li, S.; Ma, L.; Zhao, X.; Fang, G. Aldehyde Reduced Co3O4 to Form Oxygen Vacancy and Enhance the Electrochemical Performance for Oxygen Evolution Reaction and Supercapacitors. Nanotechnology 2019, 30, 395403. [Google Scholar] [CrossRef]
- Arciga-Duran, E.; Meas, Y.; Pérez-Bueno, J.J.; Ballesteros, J.C.; Trejo, G. Electrochemical Synthesis of Co3O4−x Films for Their Application as Oxygen Evolution Reaction Electrocatalysts: Role of Oxygen Vacancies. J. Electrochem. Soc. 2018, 165, H3178–H3186. [Google Scholar] [CrossRef]
- Soodi, S.; Zhang, J.J.; Zhang, J.; Liu, Y.; Lashgari, M.; Zafeiratos, S.; Züttel, A.; Zhao, K.; Luo, W. Selective electroreduction of CO2 to C2+ products on cobalt decorated copper catalysts. Chem. Synth. 2024, 4, 44. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, H.; Qian, X.; He, G.; Chen, H. Oxygen and Sulfur Dual Vacancy Engineering on a 3D Co3O4/Co3S4 Heterostructure to Improve Overall Water Splitting Activity. Green Chem. 2022, 24, 9220–9232. [Google Scholar] [CrossRef]
- Guo, X.; Liu, X.; Wang, M.; Yan, J.; Chen, Y.; Liu, S. Unveiling the Origin of Co3O4 Quantum Dots for Photocatalytic Overall Water Splitting. Small 2023, 19, 2206695. [Google Scholar] [CrossRef]
- Zhang, W.; Cui, L.; Liu, J. Recent Advances in Cobalt-Based Electrocatalysts for Hydrogen and Oxygen Evolution Reactions. J. Alloys Compd. 2020, 821, 153542. [Google Scholar] [CrossRef]
- Li, S.; Hao, X.; Abudula, A.; Guan, G. Nanostructured Co-Based Bifunctional Electrocatalysts for Energy Conversion and Storage: Current Status and Perspectives. J. Mater. Chem. A Mater. 2019, 7, 18674–18707. [Google Scholar] [CrossRef]
- Wei, R.; Fang, M.; Dong, G.; Lan, C.; Shu, L.; Zhang, H.; Bu, X.; Ho, J.C. High-Index Faceted Porous Co3O4 Nanosheets with Oxygen Vacancies for Highly Efficient Water Oxidation. ACS Appl. Mater. Interfaces 2018, 10, 7079–7086. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ding, F.; Deng, C.; Zhen, S.; Li, X.; Xue, Y.; Yan, Y.M.; Sun, K. Crystal Plane-Dependent Electrocatalytic Activity of Co3O4 toward Oxygen Evolution Reaction. Catal. Commun. 2015, 67, 78–82. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, B.; Kim, H.; Kang, K. Recent Progress on Multimetal Oxide Catalysts for the Oxygen Evolution Reaction. Adv. Energy Mater. 2018, 8, 1702774. [Google Scholar] [CrossRef]
- Su, D.; Dou, S.; Wang, G. Single Crystalline Co3O4 Nanocrystals Exposed with Different Crystal Planes for Li-O2 Batteries. Sci. Rep. 2014, 4, 5767. [Google Scholar] [CrossRef]
- David, M.; Ocampo-Martínez, C.; Sánchez-Peña, R. Advances in Alkaline Water Electrolyzers: A Review. J. Energy Storage 2019, 23, 392–403. [Google Scholar] [CrossRef]
- Zuo, Y.; Bellani, S.; Ferri, M.; Saleh, G.; Shinde, D.V.; Zappia, M.I.; Brescia, R.; Prato, M.; De Trizio, L.; Infante, I.; et al. High-Performance Alkaline Water Electrolyzers Based on Ru-Perturbed Cu Nanoplatelets Cathode. Nat. Commun. 2023, 14, 4680. [Google Scholar] [CrossRef]
- Ehlers, J.C.; Feidenhans’l, A.A.; Therkildsen, K.T.; Larrazábal, G.O. Affordable Green Hydrogen from Alkaline Water Electrolysis: Key Research Needs from an Industrial Perspective. ACS Energy Lett. 2023, 8, 1502–1509. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, T.; An, T.; Zong, Y.; Lee, J.Y. Unraveling the Electrocatalytically Active Sites and Stability of Co & Co Oxides on Nanocarbon for Oxygen Evolution Reaction in Acid Solution. J. Energy Chem. 2020, 49, 8–13. [Google Scholar] [CrossRef]
- Yao, L.; Zhong, H.; Deng, C.; Li, X.; Zhang, H. Template-Assisted Synthesis of Hierarchically Porous Co3O4 with Enhanced Oxygen Evolution Activity. J. Energy Chem. 2016, 25, 153–157. [Google Scholar] [CrossRef]
- Chen, Z.; Kronawitter, C.X.; Koel, B.E. Facet-Dependent Activity and Stability of Co3O4 Nanocrystals towards the Oxygen Evolution Reaction. Phys. Chem. Chem. Phys. 2015, 17, 29387–29393. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.G.; Choi, Y.H. Electrocatalytic Properties of Co3O4 Prepared on Carbon Fibers by Thermal Metal–Organic Deposition for the Oxygen Evolution Reaction in Alkaline Water Electrolysis. Nanomaterials 2023, 13, 1021. [Google Scholar] [CrossRef] [PubMed]
- Etzi Coller Pascuzzi, M.; van Velzen, M.; Hofmann, J.P.; Hensen, E.J.M. On the Stability of Co3O4 Oxygen Evolution Electrocatalysts in Acid. ChemCatChem 2021, 13, 459–467. [Google Scholar] [CrossRef]
- Phan La, H.P.; Thi Tran, K.T.; Hoang Nguyen, L.B.; Van Tran, M.; Van Pham, V. Development of Co3O4 Nanomaterials on Flexible Carbon Cloth Substrates for Hydrogen and Oxygen Evolution Reactions. Int. J. Hydrogen Energy 2024, 52, 482–493. [Google Scholar] [CrossRef]
- Li, R.; Zhou, D.; Luo, J.; Xu, W.; Li, J.; Li, S.; Cheng, P.; Yuan, D. The Urchin-like Sphere Arrays Co3O4 as a Bifunctional Catalyst for Hydrogen Evolution Reaction and Oxygen Evolution Reaction. J. Power Sources 2017, 341, 250–256. [Google Scholar] [CrossRef]
- Wu, K.; Shen, D.; Meng, Q.; Wang, J. Octahedral Co3O4 Particles with High Electrochemical Surface Area as Electrocatalyst for Water Splitting. Electrochim. Acta 2018, 288, 82–90. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Peng, J.; Zhang, W.; Peng, K. Magnetic Field Enhancing Electrocatalysis of Co3O4/NF for Oxygen Evolution Reaction. J. Power Sources 2019, 433, 226704. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Ren, J.; Hong, H.; Liu, D.; Liu, L.; Wang, D. Electric-Field-Treated Ni/Co3O4 Film as High-Performance Bifunctional Electrocatalysts for Efficient Overall Water Splitting. Nanomicro Lett. 2022, 14, 148. [Google Scholar] [CrossRef]
- Begum, H.; Jeon, S. Highly Efficient and Stable Bifunctional Electrocatalyst for Water Splitting on Fe–Co3O4/Carbon Nanotubes. Int. J. Hydrogen Energy 2018, 43, 5522–5529. [Google Scholar] [CrossRef]
- Mohana, P.; Swathi, S.; Yuvakkumar, R.; Ravi, G.; Thambidurai, M.; Nguyen, H.D. Co3O4/g-C3N4 Nanocomposite for Enriched Electrochemical Water Splitting. Int. J. Hydrogen Energy 2024, 49, 376–389. [Google Scholar] [CrossRef]
- Yan, D.; Chen, R.; Xiao, Z.; Wang, S. Engineering the Electronic Structure of Co3O4 by Carbon-Doping for Efficient Overall Water Splitting. Electrochim. Acta 2019, 303, 316–322. [Google Scholar] [CrossRef]
- Jayaseelan, S.S.; Bhuvanendran, N.; Xu, Q.; Su, H. Co3O4 Nanoparticles Decorated Polypyrrole/Carbon Nanocomposite as Efficient Bi-Functional Electrocatalyst for Electrochemical Water Splitting. Int. J. Hydrogen Energy 2020, 45, 4587–4595. [Google Scholar] [CrossRef]
- Ahmed, I.; Dastider, S.G.; Biswas, R.; Roy, A.; Mondal, K.; Haldar, K.K. Co3O4/WO3/C Nanorods with Porous Structures as High-Performance Electrocatalysts for Water Splitting. ACS Appl. Nano Mater. 2024, 7, 4035–4050. [Google Scholar] [CrossRef]
- Ahmed, I.; Biswas, R.; Patil, R.A.; Halder, K.K.; Singh, H.; Banerjee, B.; Kumar, B.; Ma, Y.R.; Haldar, K.K. Graphitic Carbon Nitride Composites with MoO3-Decorated Co3O4 Nanorods as Catalysts for Oxygen and Hydrogen Evolution. ACS Appl. Nano Mater. 2021, 4, 12672–12681. [Google Scholar] [CrossRef]
- Du, S.; Ren, Z.; Zhang, J.; Wu, J.; Xi, W.; Zhu, J.; Fu, H. Co3O4 Nanocrystal Ink Printed on Carbon Fiber Paper as a Large-Area Electrode for Electrochemical Water Splitting. Chem. Commun. 2015, 51, 8066–8069. [Google Scholar] [CrossRef]
- Han, H.; Kim, I.; Park, S. Thermally Templated Cobalt Oxide Nanobubbles on Crumpled Graphene Sheets: A Promising Non-Precious Metal Catalysts for Acidic Oxygen Evolution. Electrochim. Acta 2021, 382, 138277. [Google Scholar] [CrossRef]
- El Jaouhari, A.; Slassi, A.; Zhang, X.; Pershin, A.; Ahsaine, H.A.; Li, C.; Lin, Y. High Electrochemical Stability and Low-Agglomeration of Defective Co3O4 Nanoparticles Supported on N-Doped Graphitic Carbon Nano-Spheres for Oxygen Evolution Reaction. Int. J. Hydrogen Energy 2023, 48, 21723–21734. [Google Scholar] [CrossRef]
- Abd-Elrahim, A.G.; Chun, D.M. Fabrication of Efficient Nanostructured Co3O4-Graphene Bifunctional Catalysts: Oxygen Evolution, Hydrogen Evolution, and H2O2 Sensing. Ceram. Int. 2020, 46, 23479–23498. [Google Scholar] [CrossRef]
- Wang, B.; Xu, L.; Liu, G.; Zhang, P.; Zhu, W.; Xia, J.; Li, H. Biomass Willow Catkin-Derived Co3O4/N-Doped Hollow Hierarchical Porous Carbon Microtubes as an Effective Tri-Functional Electrocatalyst. J. Mater. Chem. A Mater. 2017, 5, 20170–20179. [Google Scholar] [CrossRef]
- Ha, Y.; Shi, L.; Chen, Z.; Wu, R. Phase-Transited Lysozyme-Driven Formation of Self-Supported Co3O4@C Nanomeshes for Overall Water Splitting. Adv. Sci. 2019, 6, 1900272. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Fang, X.; Zhang, X.; Fernandes, G.; Yan, Y.; Yan, D.; Xiang, X.; He, J. Space-Confined Earth-Abundant Bifunctional Electrocatalyst for High-Efficiency Water Splitting. ACS Appl. Mater. Interfaces 2017, 9, 36762–36771. [Google Scholar] [CrossRef] [PubMed]
- Dung, D.T.; Lam, D.V.; Roh, E.; Ji, S.; Yuk, J.M.; Kim, J.H.; Kim, H.; Lee, S.M. Ni/Co/Co3O4@C Nanorods Derived from a MOF@MOF Hybrid for Efficient Overall Water Splitting. Nanoscale 2022, 15, 1794–1805. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Liu, H.; Dai, M.; Xiao, L.; Wu, X. Metal-Organic Framework Derived Co3O4/PPy Bifunctional Electrocatalysts for Efficient Overall Water Splitting. Chin. Chem. Lett. 2020, 31, 2295–2299. [Google Scholar] [CrossRef]
- Singh, T.; Das, C.; Bothra, N.; Sikdar, N.; Das, S.; Pati, S.K.; Maji, T.K. MOF Derived Co3O4@Co/NCNT Nanocomposite for Electrochemical Hydrogen Evolution, Flexible Zinc-Air Batteries, and Overall Water Splitting. Inorg. Chem. 2020, 59, 3160–3170. [Google Scholar] [CrossRef]
- Zhu, J.; Tu, W.; Bai, Z.; Pan, H.; Ji, P.; Zhang, H.; Deng, Z.; Zhang, H. Zeolitic-Imidazolate-Framework-Derived Co@Co3O4 Embedded into Iron, Nitrogen, Sulfur Co-Doped Reduced Graphene Oxide as Efficient Electrocatalysts for Overall Water Splitting and Zinc-Air Batteries. Electrochim. Acta 2019, 323, 134821. [Google Scholar] [CrossRef]
- Muthurasu, A.; Maruthapandian, V.; Kim, H.Y. Metal-Organic Framework Derived Co3O4/MoS2 Heterostructure for Efficient Bifunctional Electrocatalysts for Oxygen Evolution Reaction and Hydrogen Evolution Reaction. Appl. Catal. B 2019, 248, 202–210. [Google Scholar] [CrossRef]
- Wu, Q.; Dong, A.; Yang, C.; Ye, L.; Zhao, L.; Jiang, Q. Metal-Organic Framework Derived Co3O4@Mo-Co3S4-Ni3S2 Heterostructure Supported on Ni Foam for Overall Water Splitting. Chem. Eng. J. 2021, 413, 127482. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, X.; Miao, X.; Yang, W.; Wang, C.; Pan, Q. ZIF-L-Co@carbon Fiber Paper Composite Derived Co/Co3O4@C Electrocatalyst for ORR in Alkali/Acidic Media and Overall Seawater Splitting. Int. J. Hydrogen Energy 2020, 45, 33028–33036. [Google Scholar] [CrossRef]
- Lu, K.; Gu, T.; Zhang, L.; Wu, Z.; Wang, R.; Li, X. POM-Assisted Coating of MOF-Derived Mo-Doped Co3O4 Nanoparticles on Carbon Nanotubes for Upgrading Oxygen Evolution Reaction. Chem. Eng. J. 2021, 408, 127352. [Google Scholar] [CrossRef]
- Madhu, R.; Karmakar, A.; Bera, K.; Nagappan, S.; Dhandapani, H.N.; De, A.; Roy, S.S.; Kundu, S. Recent Developments in Transition Metal-Based MOFs for Electrocatalytic Water Splitting Emphasizing Fundamental and Structural Aspects. Mater. Chem. Front. 2023, 7, 2120–2152. [Google Scholar] [CrossRef]
- Ran, J.; Zhang, Z.; Feng, H.; Zhao, H.; Chai, D.F.; Huang, X.; Zhang, W.; Zhao, M.; Dong, G.; Zang, Y.; et al. P-Doped Co-Based Nanoarray Heterojunction with Multi-Interfaces for Complementary HER/OER Electrocatalysts towards High-Efficiency Overall Water Splitting in Alkaline. Int. J. Hydrogen Energy 2024, 64, 935–946. [Google Scholar] [CrossRef]
- Gu, L.; Zhu, H.; Yu, D.N.; Zhang, S.G.; Chen, J.; Wang, J.; Wan, M.; Zhang, M.; Du, M.L. A Facile Strategy to Synthesize Cobalt-Based Self-Supported Material for Electrocatalytic Water Splitting. Part. Part. Syst. Charact. 2017, 34, 1700189. [Google Scholar] [CrossRef]
- Xiao, Z.; Wang, Y.; Huang, Y.C.; Wei, Z.; Dong, C.L.; Ma, J.; Shen, S.; Li, Y.; Wang, S. Filling the Oxygen Vacancies in Co3O4 with Phosphorus: An Ultra-Efficient Electrocatalyst for Overall Water Splitting. Energy Environ. Sci. 2017, 10, 2563–2569. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, X.; Lin, H.; Xia, Q.; Jing, M.; Yuan, W.; Ming Li, C. Three-Dimensional Ni Foam Supported NiCoO2@Co3O4 Nanowire-on-Nanosheet Arrays with Rich Oxygen Vacancies as Superior Bifunctional Catalytic Electrodes for Overall Water Splitting. Nanoscale 2023, 15, 14068–14080. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Shi, Y.; Fei, J.; Zhu, J.; Song, L.; Li, C.; Zhan, T.; Lai, J.; Wang, L. Three-Dimensional Interconnected Nanofibers Consisting of Ultra-Small Ni-Doped Co3O4 Nanoparticles for Acidic Overall Water Splitting at High Current Density. Appl. Catal. B 2024, 358, 124367. [Google Scholar] [CrossRef]
- Balaji, R.; Nguyen, T.T.; Harish, K.; Kim, N.H.; Lee, J.H. Modulating Heterointerfaces of Tungsten Incorporated CoSe/Co3O4 as a Highly Efficient Electrocatalyst for Overall Water Splitting. J. Mater. Chem. A Mater. 2022, 10, 3782–3792. [Google Scholar] [CrossRef]
- Wang, C.; Du, X.; Zhang, X. Controlled Synthesis of W–Co3S4@Co3O4 as an Environmentally Friendly and Low Cost Electrocatalyst for Overall Water Splitting. Int. J. Hydrogen Energy 2023, 48, 12739–12752. [Google Scholar] [CrossRef]
- Yu, W.; Zhang, S.; Gao, K.; Lin, X.; Han, Y.; Zhang, Z. Co3O4-MoSe2@C Nanocomposite as a Multi-Functional Catalyst for Electrochemical Water Splitting and Lithium-Oxygen Battery. Nanotechnology 2022, 33, 505402. [Google Scholar] [CrossRef]
- QayoomMugheri, A.; AneelaTahira; Aftab, U.; IshaqAbro, M.; Chaudhry, S.R.; Amaral, L.; Ibupoto, Z.H. Co3O4/NiO Bifunctional Electrocatalyst for Water Splitting. Electrochim. Acta 2019, 306, 9–17. [Google Scholar] [CrossRef]
- Zhao, X.; Yin, F.; He, X.; Chen, B.; Li, G. Efficient Overall Water Splitting over a Mo(IV)-Doped Co3O4/NC Electrocatalyst. Int. J. Hydrogen Energy 2021, 46, 20905–20918. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, M.; Yao, M.; Zhu, J.; Yang, S.; Chen, L.; Sun, B.; Zhang, J.; Hu, W.; Zhao, P. Interfacial Engineering of an FeOOH@Co3O4 Heterojunction for Efficient Overall Water Splitting and Electrocatalytic Urea Oxidation. J. Colloid. Interface Sci. 2022, 623, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Yuan, Y.; Yuan, W.; Peng, K. Vacancies and Phosphorus Atoms Assembled in Amorphous Urchin-like Co3O4 for Highly Efficient Overall Water Splitting. Int. J. Hydrogen Energy 2021, 46, 24117–24127. [Google Scholar] [CrossRef]
- Xu, X.; Su, L.; Yu, X.; Sun, J.; Miao, X. Spin Configuration Modulation of Co3O4 by Ru Doping for Boosting the Overall Water Splitting and Hydrazine Oxidation Reactions. Inorg. Chem. Front. 2024, 11, 1381–1393. [Google Scholar] [CrossRef]
- Feng, Y.; Li, Z.; Cheng, C.Q.; Kang, W.J.; Mao, J.; Shen, G.R.; Yang, J.; Dong, C.K.; Liu, H.; Du, X.W. Strawberry-like Co3O4-Ag Bifunctional Catalyst for Overall Water Splitting. Appl. Catal. B 2021, 299, 120658. [Google Scholar] [CrossRef]
- Tian, W.; Xie, X.; Zhang, X.; Li, J.; Waterhouse, G.I.N.; Ding, J.; Liu, Y.; Lu, S. Synergistic Interfacial Effect of Ru/Co3O4 Heterojunctions for Boosting Overall Water Splitting. Small 2024, 20, 2309633. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Xu, J.; Feng, L. An Effective Bimetallic Oxide Catalyst of RuO2-Co3O4 for Alkaline Overall Water Splitting. Nano Res. 2024, 17, 3785–3793. [Google Scholar] [CrossRef]


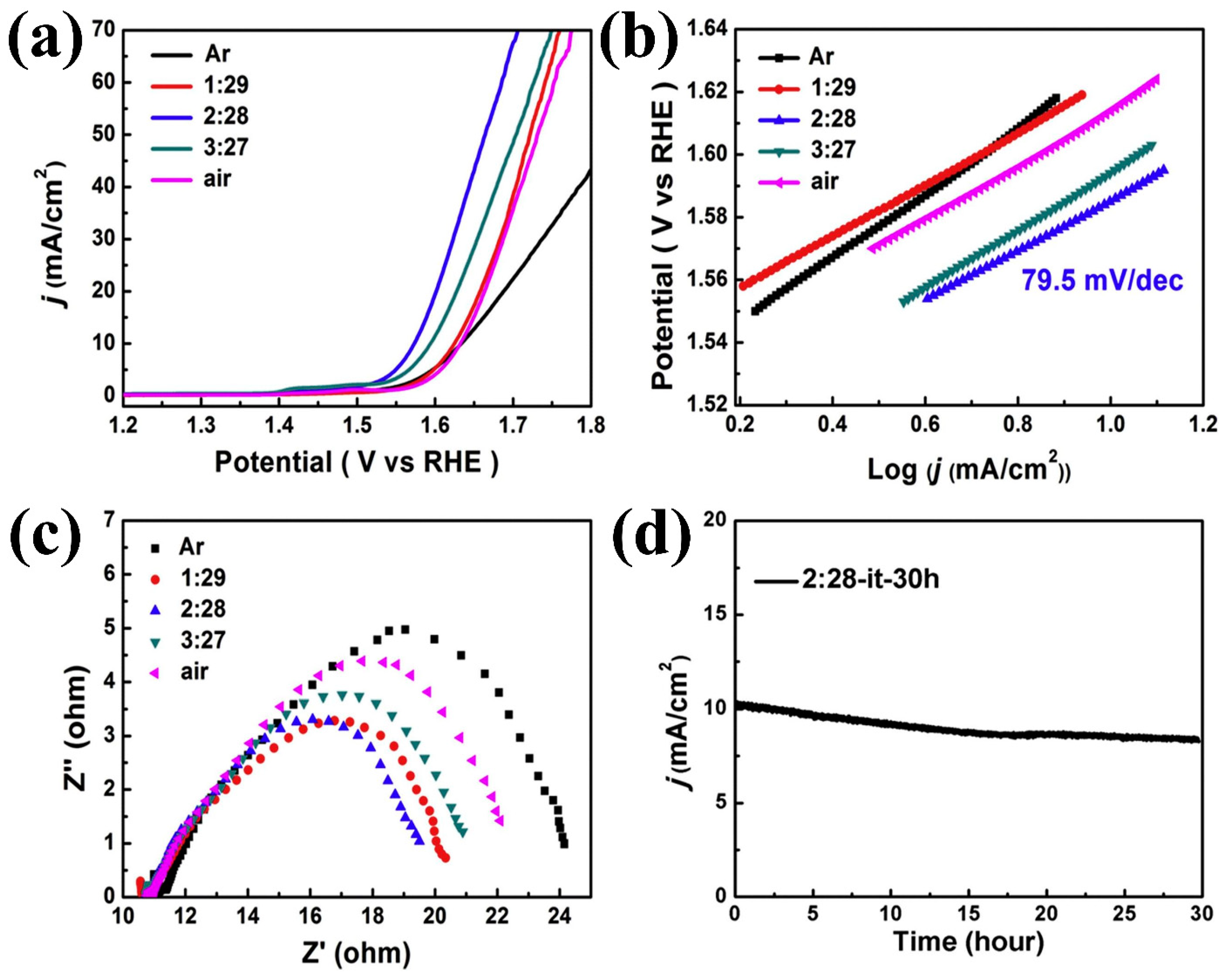

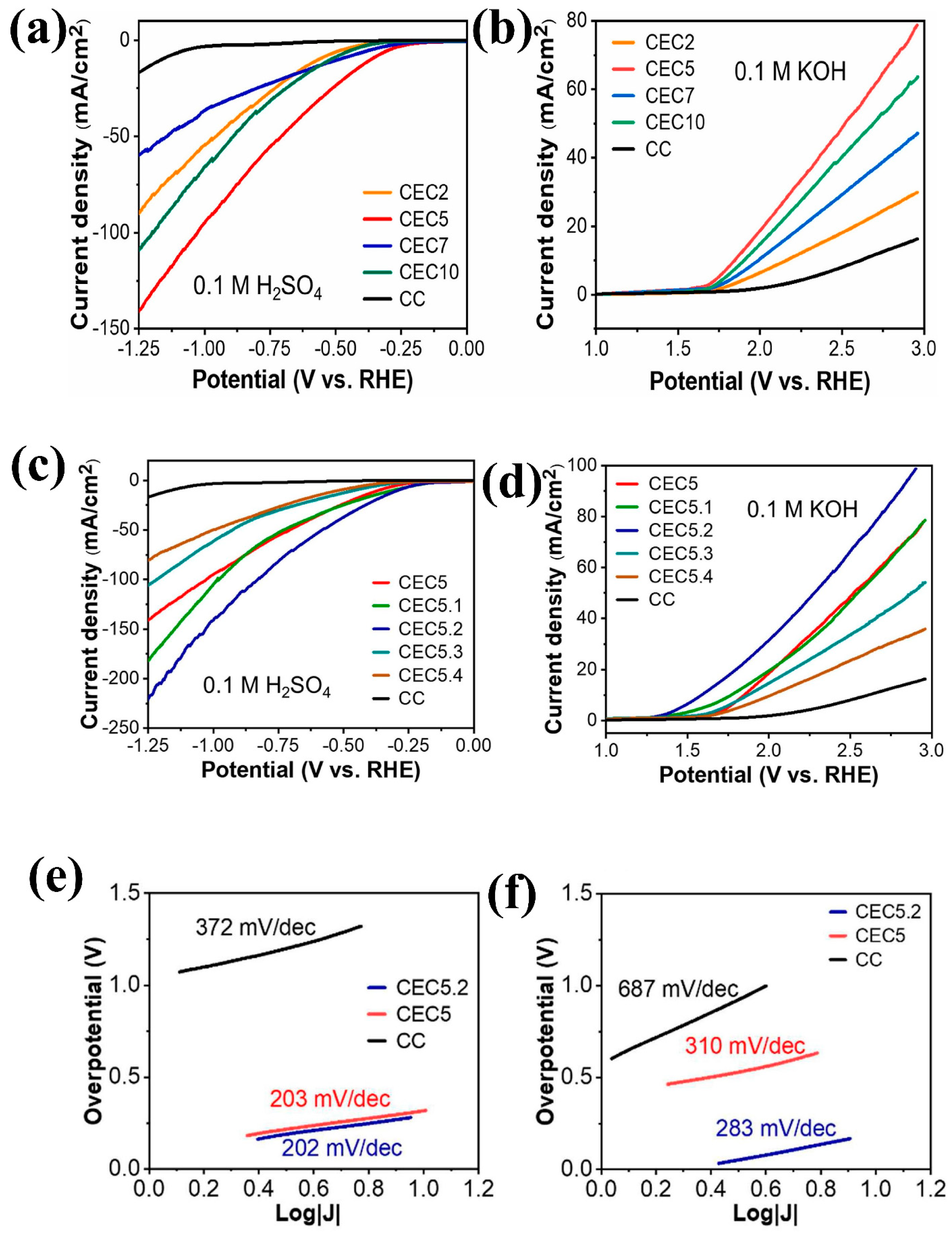

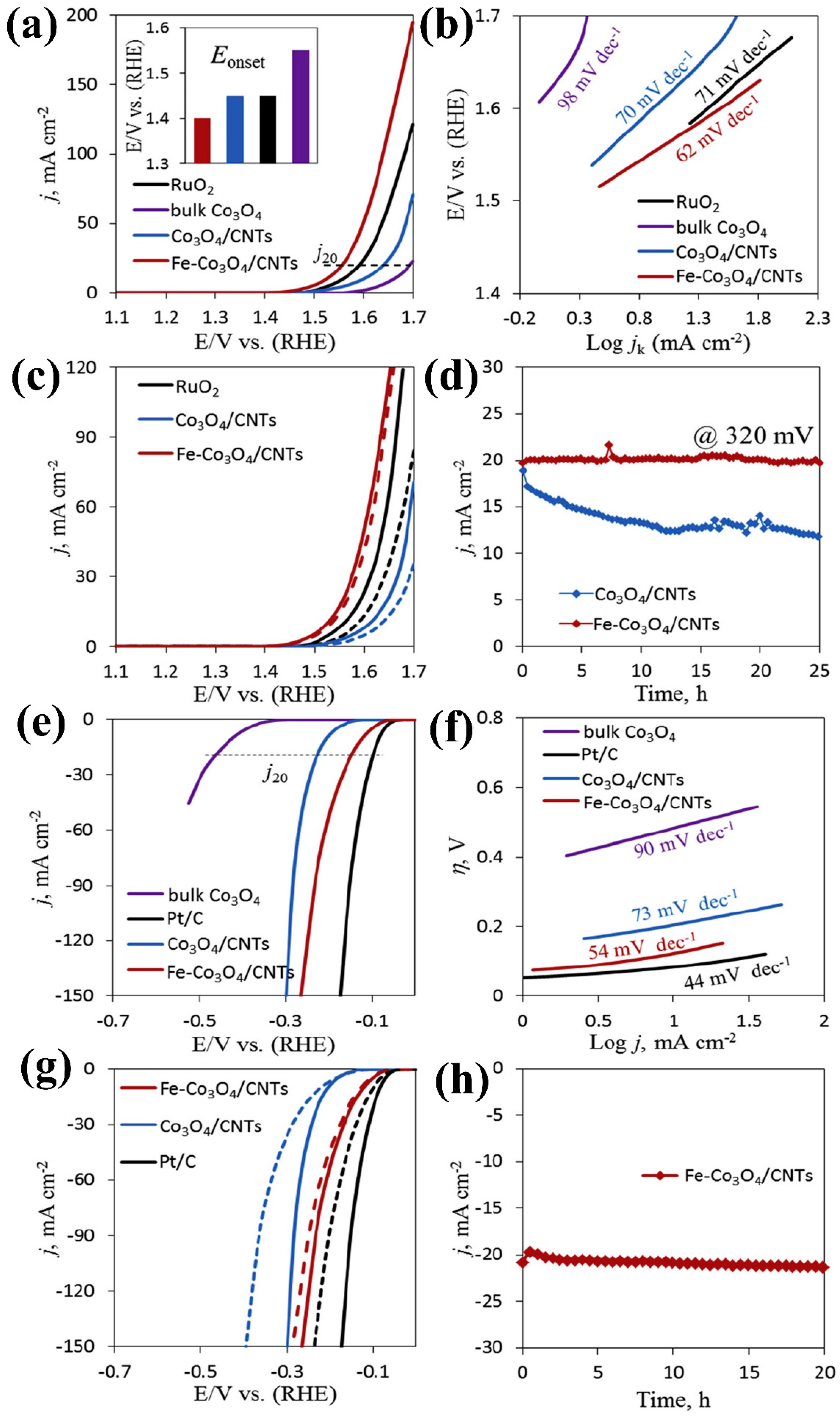
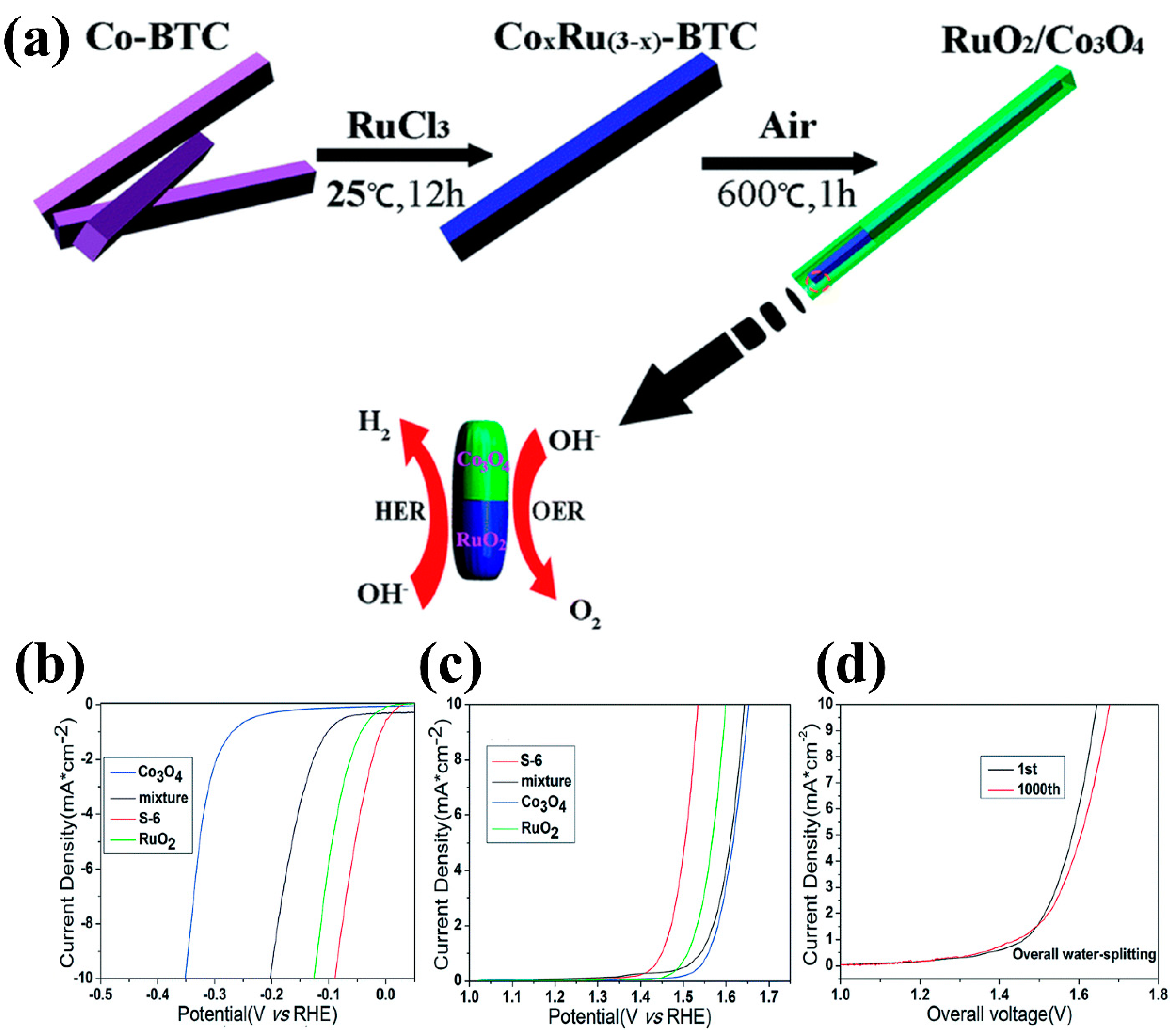
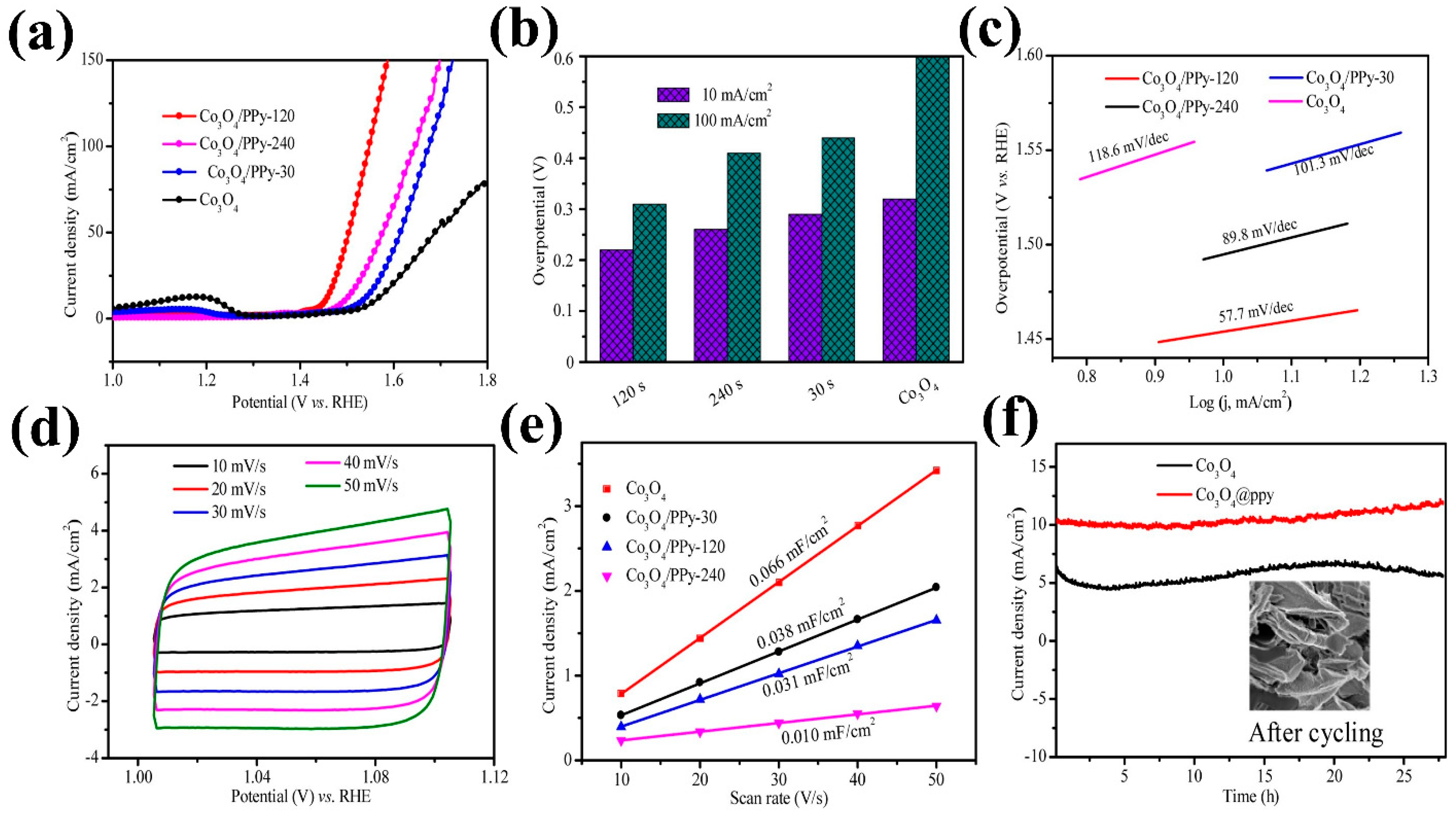

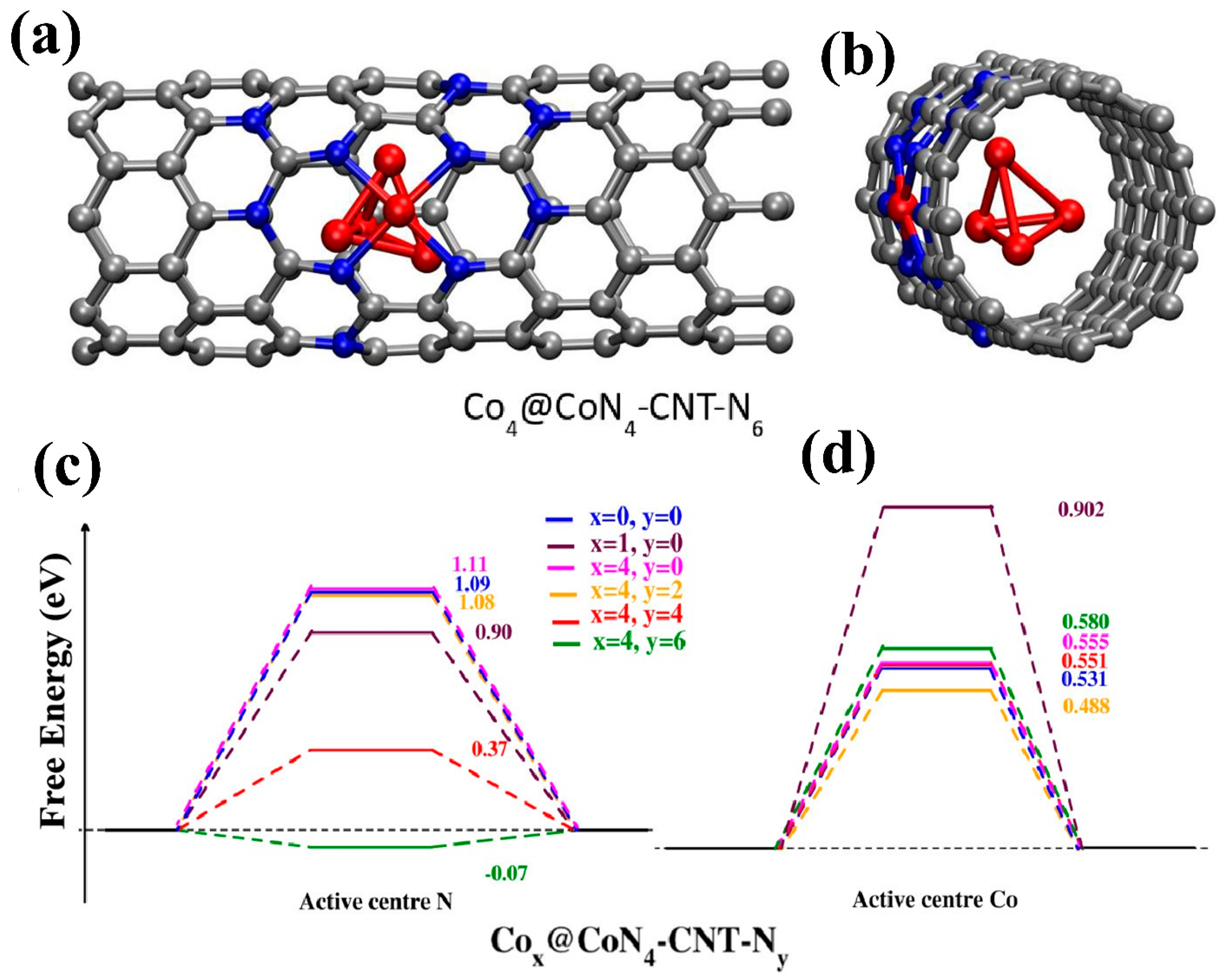
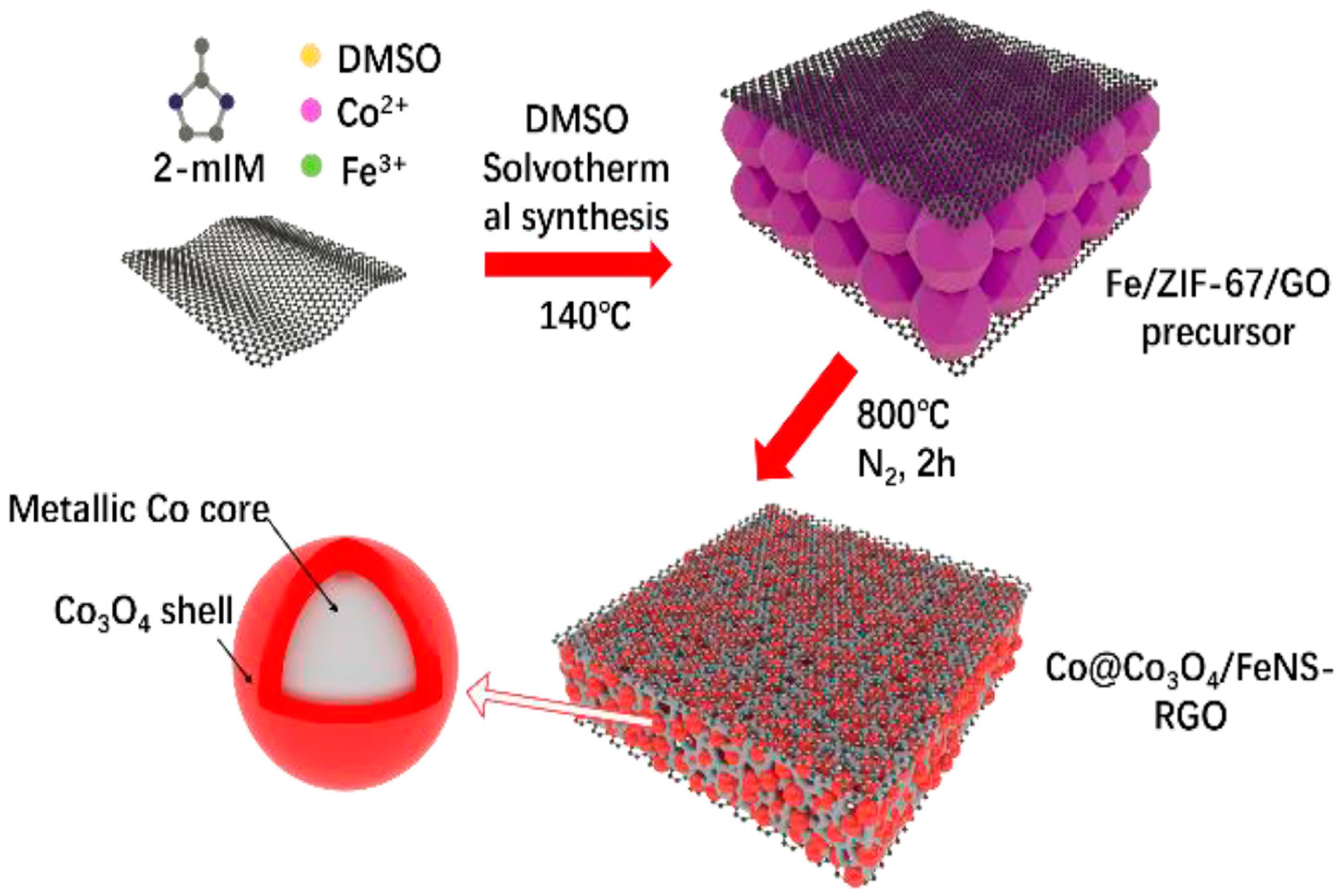
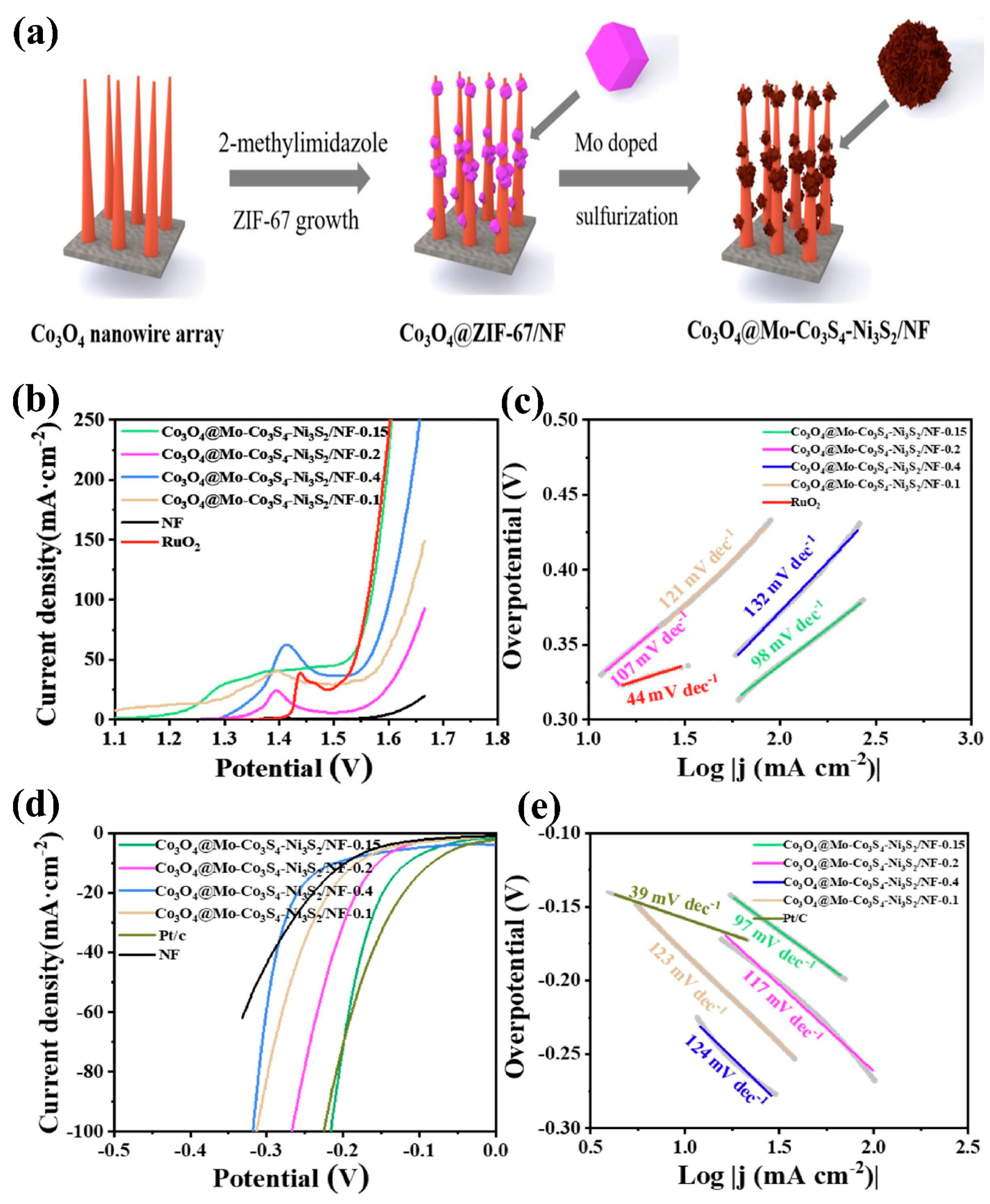

| Composite Material | Electrolyte | η (mV)/Current Density (mA cm−2) | Tafel Slope (mV dec−1) | Reference | ||
|---|---|---|---|---|---|---|
| OER | HER | OER | HER | |||
| Co3O4/CC | 0.1 M KOH (OER) 0.1 M H2SO4 (HER) | 357/10 | 291/10 | 283 | 202 | [56] |
| Fe-Co3O4/CNT | 1 M KOH | 300/10 | 120/10 | 62 | 54 | [61] |
| Co3O4/g-C3N4 | 1 M KOH | 170/20 | 151/20 | 188 | 176 | [62] |
| C–Co3O4 | 1 M KOH | 250/10 | 163/10 | 54 | 89 | [63] |
| Co3O4/Ppy/MWCNT | 0.1 M KOH | 340/10 | 490/10 | 87 | 110 | [64] |
| Co3O4/WO3/C | 1 M KOH (OER) 0.5 M H2SO4 (OER & HER) | 229/10 295/10 | 123/10 | 63 77 | 36 | [65] |
| Co3O4/MoO3/g-C3N4 | 1 M KOH (OER) 0.5 M H2SO4 (HER) | 206/10 | 125/10 | 60 | 94 | [66] |
| Co3O4/CF | 1 M KOH | 155/10 | 380/10 | 101 | 116 | [67] |
| Co3O4/graphene | 1 M KOH | 283/10 | 108/10 | 25 | 90 | [70] |
| Co3O4–carbon microtubes | 1 M KOH | 158/10 | 210/10 | - | - | [71] |
| N–Co3O4@C@NF | 1 M KOH | 96/10 | 42/10 | 89 | 56 | [72] |
| Composite Material | Electrolyte | η (mV)/Current Density (mA cm−2) | Tafel Slope (mV dec−1) | Reference | ||
|---|---|---|---|---|---|---|
| OER | HER | OER | HER | |||
| Au@C/Co3O4 | 1 M KOH | 452/10 | 482/10 | 74.7 | 76.1 | [22] |
| RuO2–Co3O4 heterojunction | 1 M KOH | 305/10 | 89/10 | - | - | [30] |
| Co3O4–N doped carbon | 1 M KOH | 318/10 | 106/10 | 97 | 109 | [73] |
| Ni/Co/Co3O4@C | 1 M KOH | 246/30 | 143/30 | 50.2 | 114 | [74] |
| Co3O4/PPy | 1 M KOH | 220/10 | 140/10 | 57.7 | 83 | [75] |
| Co3O4@Co/NCNT | 0.5 H2SO4 | 161/10 | 171/10 | - | 121 | [76] |
| Co@Co3O4/FeNS–rGO | 1 M KOH | 287/10 | 130/10 | 69 | 111 | [77] |
| Co3O4/MoS2 | 1 M KOH | 230/20 | 205/10 | 45 | 89 | [78] |
| Co3O4@Mo-Co3S4–Ni3S2/NF | 1 M KOH | 295/10 | 116/10 | 98 | 97 | [79] |
| Co/Co3O4@C | 1 M KOH | 192/10 | 357/10 | - | - | [80] |
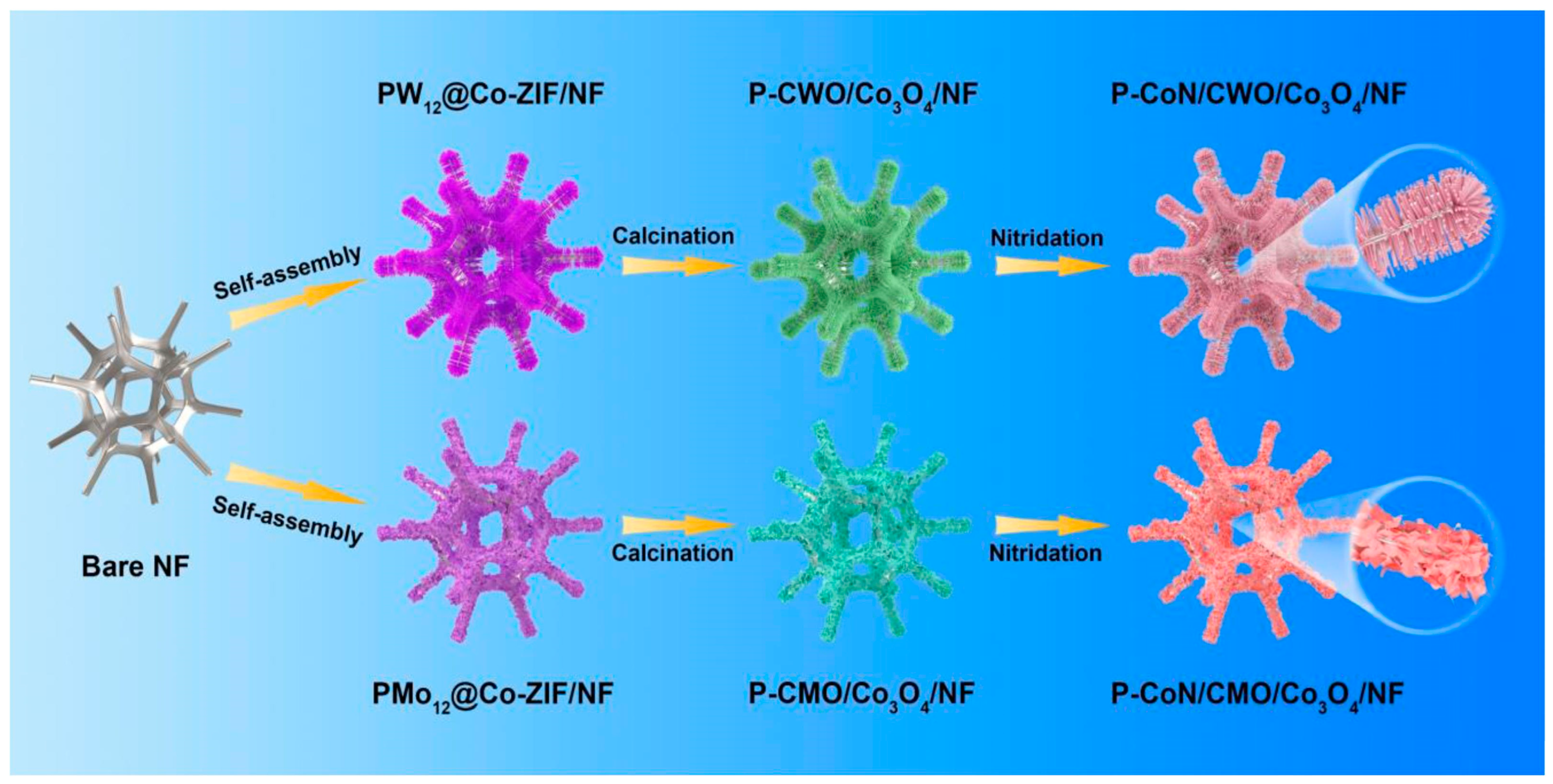
| P–CoN/CWO/Co3O4 | 1 M KOH | 175/10 | - | 98.9 | - | [83] |
| P–CoN/CMO/Co3O4 | 1 M KOH | - | 109/10 | - | 89.1 | [83] |
| Composite Material | Electrolyte | η (mV)/Current Density (mA cm−2) | Tafel Slope (mV dec−1) | Reference | ||
|---|---|---|---|---|---|---|
| OER | HER | OER | HER | |||
| PtRu−Co3O4 | 0.5 M H2SO4 | 143/10 | 99/10 | 67 | 59 | [14] |
| NiCoP@Co3O4/NF | 1 M KOH | 120/10 | 72/10 | 62.85 | 56.26 | [17] |
| P-Co3O4/NF | 1 M KOH | 260/20 | 97/10 | 60 | 86 | [32] |
| N/S-VO-Co3O4 | 1 M KOH | 294/10 | 181/10 | 71 | 79 | [33] |
| RuO2@Co3O4 | 1 M KOH 0.5 M H2SO4 | 152/10 219/10 | 90/10 33/10 | 68 73 | 103 95 | [34] |
| CoP/Co3O4 | 1 M KOH | 257/20 | 98/10 | 61.2 | 53.6 | [35] |
| DV (dual vacancies)-Co3O4/Co3S4 | 1 M KOH | 233/100 | 26/10 | 75 | 58 | [40] |
| Ni–Co3O4 film | 1 M KOH | 353/10 | 190/10 | 51 | 148 | [60] |
| Electric-field-treated Ni–Co3O4 film | 1 M KOH | 311/10 | 93/10 | 43 | 69 | [60] |
| P–Co3O4 | 1 M KOH | 280/10 | 120/10 | 51.6 | 52 | [85] |
| NiCoO2@Co3O4 | 1 M KOH | 79.9/10 | 88.2/10 | 46.2 | 72.2 | [86] |
| Ni-Co3O4 NFs | 1 M KOH | 330/10 | 74/10 | 97 | 52 | [87] |
| WCoSe/WCo3O4 | 1 M KOH | 175/10 | 98/10 | 62 | 72 | [88] |
| W-Co3S4@Co3O4 | 1 M KOH | 260/10 | 140/10 | 39.8 | 94 | [89] |
| Co3O4–MoSe2@C | 1 M KOH | 360/10 | 144/10 | 75.7 | 95.5 | [90] |
| Co3O4@NiO | 1 M KOH | 156/10 | 65/10 | 101 | 61 | [91] |
| Mo–Co3O4/NC | 1 M KOH | 276/10 | 91/10 | 66.7 | 139.7 | [92] |
| FeOOH@Co3O4 | 1 M KOH | 370/10 | 174/10 | 71 | 57 | [93] |
| P–Co3O4 | 1 M KOH | 310/20 | 111/20 | 122.5 | 136.5 | [94] |
| Ru-Co3O4 | 1 M KOH | 270/100 | 48.6/100 | 103.8 | 27.7 | [95] |
| Co3O4–Ag | 1 M KOH | 206/10 | 51/10 | 55 | 49 | [96] |
| Ru/Co3O4 heterojunction | 1 M KOH | 253/10 | 11/10 | 77 | 51 | [97] |
| RuO2–Co3O4 | 1 M KOH | 260/10 | 75/10 | 73 | 54.4 | [98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagh, K.S.; Mane, S.M.; Teli, A.M.; Shin, J.C.; Lee, J. Recent Advancements in Co3O4-Based Composites for Enhanced Electrocatalytic Water Splitting. Micromachines 2024, 15, 1450. https://doi.org/10.3390/mi15121450
Wagh KS, Mane SM, Teli AM, Shin JC, Lee J. Recent Advancements in Co3O4-Based Composites for Enhanced Electrocatalytic Water Splitting. Micromachines. 2024; 15(12):1450. https://doi.org/10.3390/mi15121450
Chicago/Turabian StyleWagh, Komal S., Sagar M. Mane, Aviraj M. Teli, Jae Cheol Shin, and Jaewoong Lee. 2024. "Recent Advancements in Co3O4-Based Composites for Enhanced Electrocatalytic Water Splitting" Micromachines 15, no. 12: 1450. https://doi.org/10.3390/mi15121450
APA StyleWagh, K. S., Mane, S. M., Teli, A. M., Shin, J. C., & Lee, J. (2024). Recent Advancements in Co3O4-Based Composites for Enhanced Electrocatalytic Water Splitting. Micromachines, 15(12), 1450. https://doi.org/10.3390/mi15121450







