Exploring the Connection Between Nanomaterials and Neurodegenerative Disorders
Abstract
1. Introduction
1.1. Parkinson’s Disease
1.2. Alzheimer’s Disease
1.3. Tauopathy and Autophagy
1.4. Huntington’s (HTT) Disease
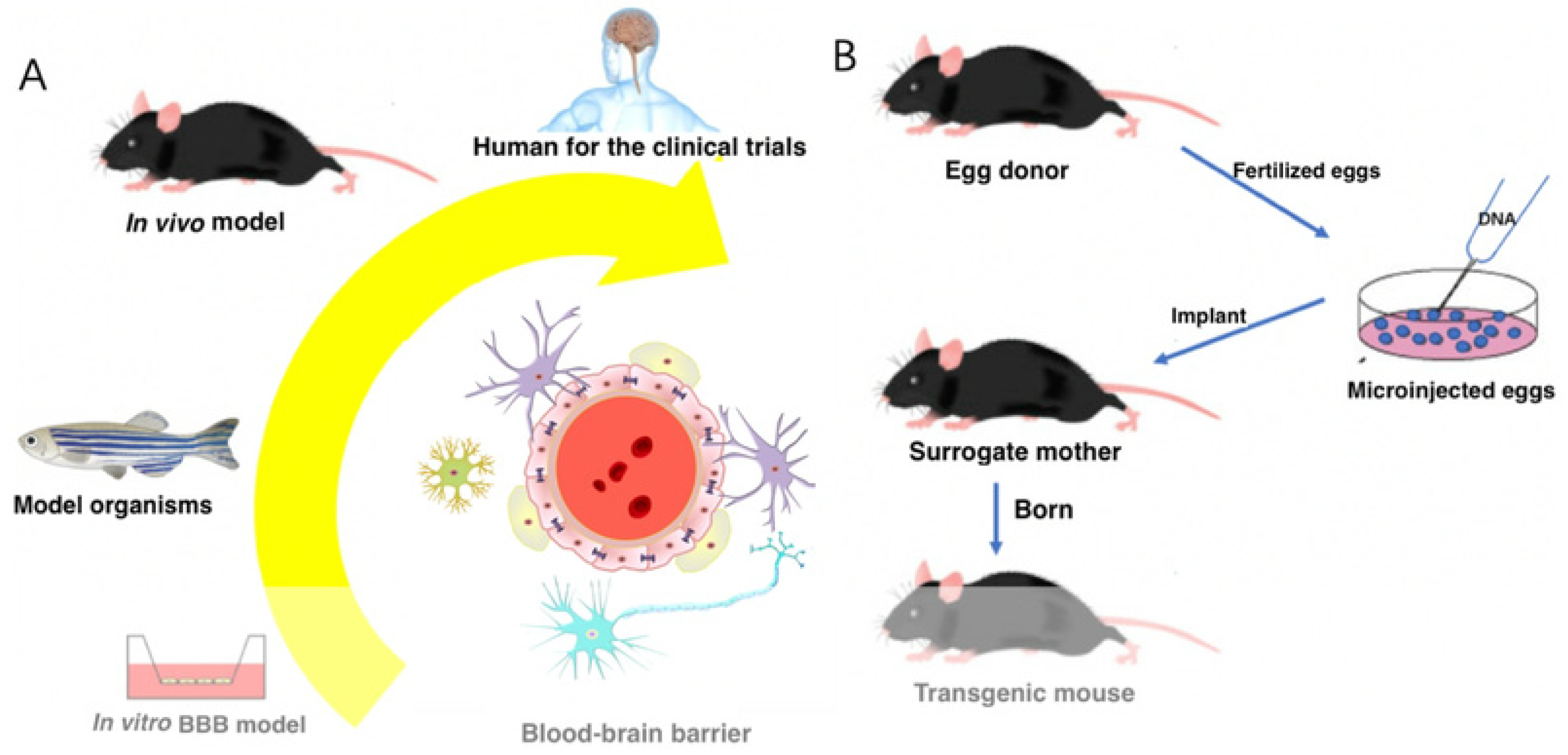
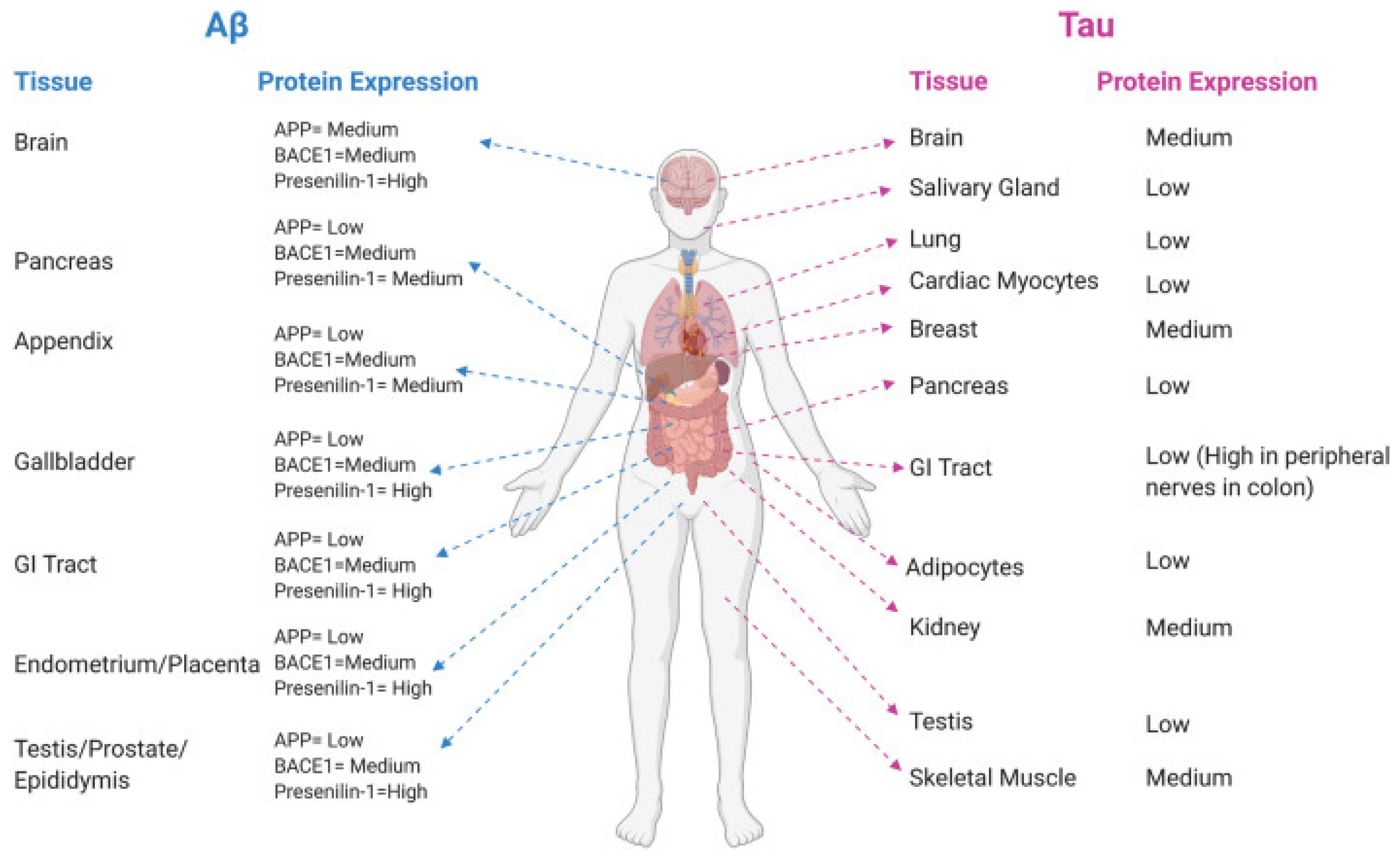
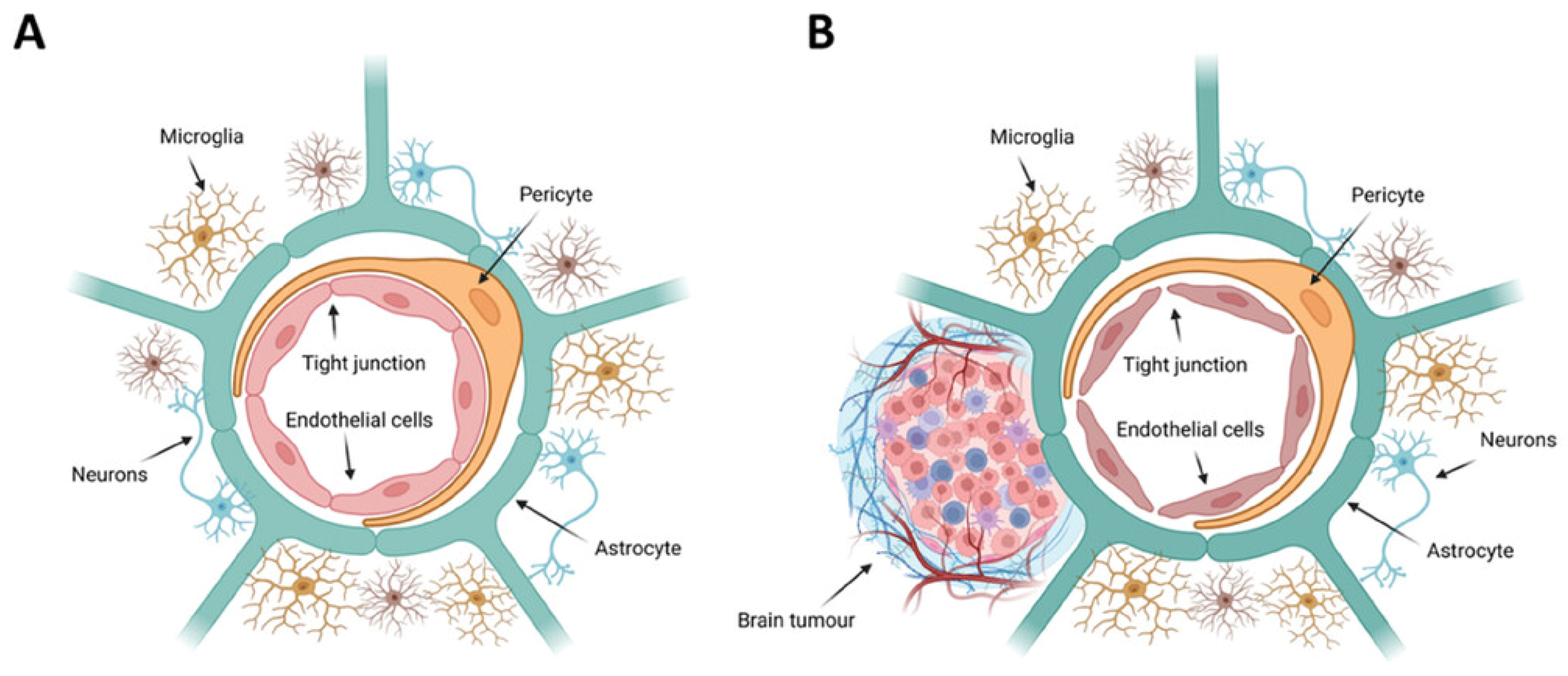
2. Implication of Blood–Brain Barrier
3. Nanomaterials for Targeted Therapy of Neurodegenerative Diseases (NDDs)
3.1. The Potential of siRNA in Treating Neurodegenerative Disease
3.2. A Nanomaterials Strategy for Overcoming Barriers
3.3. Barrier Between Blood and Brain Tumors
4. Conclusions
Funding
Conflicts of Interest
References
- Ransohoff, R.M. How neuroinflammation contributes to neurodegeneration. Science 2016, 353, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Singla, R.; Abidi, S.M.; Dar, A.I.; Acharya, A. Inhibition of glycation-induced aggregation of human serum albumin by organic–inorganic hybrid nanocomposites of iron oxide-functionalized nanocellulose. ACS Omega 2019, 4, 14805–14819. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, N.; Shah, J.; Mishra, V.; Amin, M.C.I.M.; Iyer, A.K.; Tekade, R.K.; Kesharwani, P. Dendrimer-mediated approaches for the treatment of brain tumor. J. Biomater. Sci. Polym. Ed. 2016, 27, 557–580. [Google Scholar] [CrossRef] [PubMed]
- Neuwelt, E.; Abbott, N.J.; Abrey, L.; Banks, W.A.; Blakley, B.; Davis, T.; Engelhardt, B.; Grammas, P.; Nedergaard, M.; Nutt, J.; et al. Strategies to advance translational research into brain barriers. Lancet Neurol. 2008, 7, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Workman, M.J.; Svendsen, C.N. Recent advances in human iPSC- derived models of the blood−brain barrier. Fluids Barriers CNS 2020, 17, 30. [Google Scholar] [CrossRef]
- Bellat, V.; Alcaina, Y.; Tung, C.H.; Ting, R.; Michel, A.O.; Souweidane, M.; Law, B. A combined approach of convection- enhanced delivery of peptide nanofiber reservoir to prolong local DM1 retention for diffuse intrinsic pontine glioma treatment. Neuro-Oncology 2020, 22, 1495–1504. [Google Scholar] [CrossRef]
- Di Marco, A.; Vignone, D.; Paz, O.G.; Fini, I.; Battista, M.R.; Cellucci, A.; Bracacel, E.; Auciello, G.; Veneziano, M.; Khetarpal, V.; et al. Establishment of an in vitro human blood-brain barrier model derived from induced pluripotent stem cells and comparison to a porcine cell-based system. Cells 2020, 9, 994. [Google Scholar] [CrossRef]
- Ochekpe, N.A.; Olorunfemi, P.O.; Ngwuluka, N.C. Nanotechnology and drug delivery part 1: Background and applications. Trop. J. Pharm. Res. 2009, 8, 265–274. [Google Scholar] [CrossRef]
- Xie, J.B.; Shen, Z.Y.; Anraku, Y.; Kataoka, K.; Chen, X.Y. Nanomaterial-based blood-brain-barrier (BBB) crossing strategies. Biomaterials 2019, 224, 119491. [Google Scholar] [CrossRef]
- Qiao, S.P.; Liu, Y.; Han, F.T.; Guo, M.; Hou, X.L.; Ye, K.R.; Deng, S.; Shen, Y.J.; Zhao, Y.F.; Wei, H.Y.; et al. An intelligent neural stem cell delivery system for neurodegenerative diseases treatment. Adv. Healthc. Mater. 2018, 7, 1800080. [Google Scholar] [CrossRef]
- Prades, R.; Guerrero, S.; Araya, E.; Molina, C.; Salas, E.; Zurita, E.; Selva, J.; Egea, G.; López-Iglesias, C.; Teixidó, M.; et al. Delivery of gold nanoparticles to the brain by conjugation with a peptide that recognizes the transferrin receptor. Biomaterials 2012, 33, 7194–7205. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Lower Respiratory Infections Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1211–1228. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, T. A master regulator of α-synuclein aggregation. ACS Chem. Neurosci. 2020, 11, 1376–1378. [Google Scholar] [CrossRef] [PubMed]
- Bloch, D.N.; Miller, Y. Study of molecular mechanisms of α-synuclein assembly: Insight into a cross-β structure in the N-termini of new α-synuclein fibrils. ACS Omega 2017, 2, 3363–3370. [Google Scholar] [CrossRef] [PubMed]
- Pupyshev, A.B.; Korolenko, T.A.; Akopyan, A.A.; Amstislavskaya, T.G.; Tikhonova, M.A. Suppression of autophagy in the brain of transgenic mice with overexpression of A53T-mutant α-synuclein as an early event at synucleinopathy progression. Neurosci. Lett. 2018, 672, 140–144. [Google Scholar] [CrossRef]
- Wanli, W.S.; Haibing, J.; Zhong, P.; Yuji, T.; Hokuto, M.; Akira, S.; Valina, L.D.; Ted, M.D.; Christopher, A.R. Endoplasmic reticulum stress and mitochondrial cell death pathways mediate A53T mutant alpha-synuclein-induced toxicity. Hum. Mol. Genet. 2005, 24, 3801–3811. [Google Scholar]
- Zondler, L.; Kostka, M.; Garidel, P.; Heinzelmann, U.; Hengerer, B.; Mayer, B.; Weishaupt, J.H.; Gillardon, F.; Danzer, K.M. Proteasome impairment by α-synuclein. PLoS ONE 2017, 12, e0184040. [Google Scholar] [CrossRef]
- Matsumoto, J.; Stewart, T.; Sheng, L.; Li, N.; Bullock, K.; Song, N.; Shi, M.; Banks, W.A.; Zhang, J. Transmission of α-synuclein-containing erythrocyte-derived extracellular vesicles across the blood-brain barrier via adsorptive mediated transcytosis: Another mechanism for initiation and progression of Parkinson’s disease? Acta Neuropathol. Commun. 2017, 5, 71. [Google Scholar] [CrossRef]
- Maddahi, A.; Polyvyana, A.; Nassiri, A.M.; Maddahi, Y.; Choukou, M.A. Caring4Dementia: A Mobile Application to Train People Caring for Patients with Dementia. In Proceedings of the 11th Augmented Human International Conference, Winnipeg, MB, Canada, 27–29 May 2020; pp. 1–7. [Google Scholar]
- Kent, S.A.; Spires-Jones, T.L.; Durrant, C.S. The physiological roles of tau and Aβ: Implications for Alzheimer’s disease pathology and therapeutics. Acta Neuropathol. 2020, 140, 417–447. [Google Scholar] [CrossRef]
- KoSIK, K.S.; Joachim, C.L.; Selkoe, D.J. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc. Natl. Acad. Sci. USA 1986, 83, 4044–4048. [Google Scholar] [CrossRef]
- Masters, C.L.; Simms, G.; Weinman, N.A.; Multhaup, G.; McDonald, B.L.; Beyreuther, K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc. Natl. Acad. Sci. USA 1985, 82, 4245–4249. [Google Scholar] [CrossRef]
- Spires-Jones, T.L.; Attems, J.; Thal, D.R. Interactions of pathological proteins in neurodegenerative diseases. Acta Neuropathol. 2017, 134, 187–205. [Google Scholar] [CrossRef] [PubMed]
- Tanzi, R.E.; Gusella, J.F.; Watkins, P.C.; Bruns, G.A.; St George-Hyslop, P.; Van Keuren, M.L.; Patterson, D.; Pagan, S.; Kurnit, D.M.; Neve, R.L. Amyloid β protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science 1987, 235, 880–884. [Google Scholar] [CrossRef] [PubMed]
- Vassar, R.; Bennett, B.D.; Babu-Khan, S.; Kahn, S.; Mendiaz, E.A.; Denis, P.; Teplow, D.B.; Ross, S.; Amarante, P.; Loeloff, R.; et al. β-Secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 1999, 286, 735–741. [Google Scholar] [CrossRef] [PubMed]
- DeKosky, S.T.; Scheff, S.W. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: Correlation with cognitive severity. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 1990, 27, 457–464. [Google Scholar] [CrossRef]
- Liu, L.; Ding, L.; Rovere, M.; Wolfe, M.S.; Selkoe, D.J. A cellular complex of BACE1 and γ-secretase sequentially generates Aβ from its full-length precursor. J. Cell Biol. 2019, 218, 644–663. [Google Scholar] [CrossRef]
- Guo, T.; Zhang, D.; Zeng, Y.; Huang, T.Y.; Xu, H.; Zhao, Y. Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease. Mol. Neurodegener. 2020, 15, 40. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.; Yuan, P.; Yang, J.; Xu, Y.; Grutzendler, J.; Shao, Y.; Moore, A.; Ran, C. Oxalate-curcumin–based probe for micro-and macroimaging of reactive oxygen species in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2017, 114, 12384–12389. [Google Scholar] [CrossRef]
- Costa, R.O.; Lacor, P.N.; Ferreira, I.L.; Resende, R.; Auberson, Y.P.; Klein, W.L.; Oliveira, C.R.; Rego, A.C.; Pereira, C.M. Endoplasmic reticulum stress occurs downstream of GluN2B subunit of N-methyl-D-aspartate receptor in mature hippocampal cultures treated with amyloid-β oligomers. Aging Cell 2012, 11, 823–833. [Google Scholar] [CrossRef]
- Lee, S.J.C.; Nam, E.; Lee, H.J.; Savelieff, M.G.; Lim, M.H. Towards an understanding of amyloid-β oligomers: Characterization, toxicity mechanisms, and inhibitors. Chem. Soc. Rev. 2017, 46, 310–323. [Google Scholar] [CrossRef]
- Meng, X.; Song, Q.; Liu, Z.; Liu, X.; Wang, Y.; Liu, J. Neurotoxic β-amyloid oligomers cause mitochondrial dysfunction—The trigger for PANoptosis in neurons. Front. Aging Neurosci. 2024, 16, 1400544. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, A.; Dev, K.; Tanwar, R.S.; Selwal, K.K.; Tyagi, P.K. Copper mediated neurological disorder: Visions into amyotrophic lateral sclerosis, Alzheimer and Menkes disease. J. Trace Elem. Med. Biol. 2015, 29, 11–23. [Google Scholar] [CrossRef]
- Sharoar, M.G.; Palko, S.; Ge, Y.; Saido, T.C.; Yan, R. Accumulation of saposin in dystrophic neurites is linked to impaired lysosomal functions in Alzheimer’s disease brains. Mol. Neurodegener. 2021, 16, 45. [Google Scholar] [CrossRef] [PubMed]
- Congdon, E.E.; Sigurdsson, E.M. Tau-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2018, 14, 399–415. [Google Scholar] [CrossRef]
- Wang, L.; Yin, Y.L.; Liu, X.Z.; Shen, P.; Zheng, Y.G.; Lan, X.R.; Lu, C.-B.; Wang, J.Z. Current understanding of metal ions in the pathogenesis of Alzheimer’s disease. Transl. Neurodegener. 2020, 9, 10. [Google Scholar] [CrossRef]
- Zhang, F.; Gannon, M.; Chen, Y.; Yan, S.; Zhang, S.; Feng, W.; Tao, J.; Sha, B.; Liu, Z.; Saito, T.; et al. β-amyloid redirects norepinephrine signaling to activate the pathogenic GSK3β/tau cascade. Sci. Transl. Med. 2020, 12, eaay6931. [Google Scholar] [CrossRef] [PubMed]
- Arnsten, A.F.; Datta, D.; Del Tredici, K.; Braak, H. Hypothesis: Tau pathology is an initiating factor in sporadic Alzheimer’s disease. Alzheimer’s Dement. 2021, 17, 115–124. [Google Scholar] [CrossRef]
- Jin, M.; Shiwaku, H.; Tanaka, H.; Obita, T.; Ohuchi, S.; Yoshioka, Y.; Jin, X.; Kondo, K.; Fujita, K.; Homma, H.; et al. Tau activates microglia via the PQBP1-cGAS-STING pathway to promote brain inflammation. Nat. Commun. 2021, 12, 6565. [Google Scholar] [CrossRef]
- Elliott, J.H.; Wightman, F.; Solomon, A.; Ghneim, K.; Ahlers, J.; Cameron, M.J.; Smith, M.Z.; Tim Spelman, T.; McMahon, J.; Velayudham, P.; et al. Activation of HIV transcription with short-course vorinostat in HIV-infected patients on suppressive antiretroviral therapy. PLoS Pathog. 2014, 10, e1004473. [Google Scholar] [CrossRef]
- Uytterhoeven, V.; Lauwers, E.; Maes, I.; Miskiewicz, K.; Melo, M.N.; Swerts, J.; Kuenen, S.; Wittocx, R.; Corthout, N.; Marrink, S.-J. Hsc70-4 deforms membranes to promote synaptic protein turnover by endosomal microautophagy. Neuron 2015, 88, 735–748. [Google Scholar] [CrossRef]
- Zhang, L.D.; Han, W.; Zhu, C.F.; Song, X.G.; Cheng, H.; Yang, K.; Qin, X.F.; Zhang, J.Y.; Gui, L. Moxibustion at acpoints of governor vessel on regulating PI3K/Akt/mTOR signaling pathway and enhancing autophagy process in APP/PS1 double-transgenic Alzheimer’s disease mice. Zhongguo Zhen Jiu Chin. Acupunct. Moxibustion 2019, 39, 1313–1318. [Google Scholar]
- Dayalu, P.; Albin, R.L. Huntington Disease: Pathogenesis and Treatment. Neurol. Clin. 2015, 33, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Harjes, P.; Wanker, E.E. The hunt for huntingtin function: Interaction partners tell many different stories. Trends Biochem. Sci. 2003, 28, 425–433. [Google Scholar] [CrossRef]
- Cattaneo, E.; Zuccato, C.; Tartari, M. Normal huntingtin function: An alternative approach to Huntington’s disease. Nat. Rev. Neurosci. 2005, 6, 919–930. [Google Scholar] [CrossRef]
- Rui, Y.N.; Xu, Z.; Patel, B.; Chen, Z.; Chen, D.; Tito, A.; David, G.; Sun, Y.; Stimming, E.F.; Bellen, H.J.; et al. Huntingtin functions as a scaffold for selective macroautophagy. Nat. Cell Biol. 2015, 17, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Clabough, E.B.; Sarkar, S.; Futter, M.; Rubinsztein, D.C.; Zeitlin, S.O. Deletion of the huntingtin polyglutamine stretch enhances neuronal autophagy and longevity in mice. PLoS Genet. 2010, 6, e1000838. [Google Scholar] [CrossRef]
- Zrinzo, L. Pitfalls in precision stereotactic surgery. Surg. Neurol. Int. 2012, 3 (Suppl. 1), S53. [Google Scholar] [CrossRef]
- Lozano, A.M.; Lipsman, N.; Bergman, H.; Brown, P.; Chabardes, S.; Chang, J.W.; Matthews, K.; Mcintyre, C.C.; Schlaepfer, T.E.; Schulder, M.; et al. Deep brain stimulation: Current challenges and future directions. Nat. Rev. Neurol. 2019, 15, 148–160. [Google Scholar] [CrossRef]
- Dichiara, M.; Amata, B.; Turnaturi, R.; Marrazzo, A.; Amata, E. Tuning properties for blood–brain barrier permeation: A statistics-based analysis. ACS Chem. Neurosci. 2019, 11, 34–44. [Google Scholar] [CrossRef]
- Banks, W.A. Characteristics of compounds that cross the blood-brain barrier. BMC Neurol. 2009, 9 (Suppl. 1), S3. [Google Scholar] [CrossRef]
- Pulgar, V.M. Transcytosis to cross the blood brain barrier, new advancements and challenges. Front. Neurosci. 2019, 12, 1019. [Google Scholar] [CrossRef] [PubMed]
- Kou, L.; Bhutia, Y.D.; Yao, Q.; He, Z.; Sun, J.; Ganapathy, V. Transporter-guided delivery of nanoparticles to improve drug permeation across cellular barriers and drug exposure to selective cell types. Front. Pharmacol. 2018, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Soto, C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat. Rev. Neurosci. 2003, 4, 49–60. [Google Scholar] [CrossRef]
- Singer, O.; Marr, R.A.; Rockenstein, E.; Crews, L.; Coufal, N.G.; Gage, F.H.; Verma, I.M.; Masliah, E. Targeting BACE1 with siRNAs ameliorates Alzheimer disease neuropathology in a transgenic model. Nat. Neurosci. 2005, 8, 1343–1349. [Google Scholar] [CrossRef]
- Shyam, R.; Ren, Y.; Lee, J.; Braunstein, K.E.; Mao, H.Q.; Wong, P.C. Intraventricular delivery of siRNA nanoparticles to the central nervous system. Mol. Ther. -Nucleic Acids 2015, 4, e242. [Google Scholar] [CrossRef]
- Wang, P.; Zheng, X.; Guo, Q.; Yang, P.; Pang, X.; Qian, K.; Lu, W.; Zhang, Q.; Jiang, X. Systemic delivery of BACE1 siRNA through neuron-targeted nanocomplexes for treatment of Alzheimer’s disease. J. Control. Release 2018, 279, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Xiong, N.; Zhao, Y.; Dong, X.; Zheng, J.; Sun, Y. Design of a molecular hybrid of dual peptide inhibitors coupled on AuNPs for enhanced inhibition of amyloid β-protein aggregation and cytotoxicity. Small 2017, 13, 1601666. [Google Scholar] [CrossRef]
- Gomez-Gualdrón, D.A.; Burgos, J.C.; Yu, J.; Balbuena, P.B. Carbon nanotubes: Engineering biomedical applications. Prog. Mol. Biol. Transl. Sci. 2011, 104, 175–245. [Google Scholar] [PubMed]
- Chen, D.; Wang, J.; Wang, Y.; Zhang, F.; Dong, X.; Jiang, L.; Yin, T.; Huojun, Z.; Li, W. Promoting inter-/intra-cellular process of nanomedicine through its physicochemical properties optimization. Curr. Drug Metab. 2018, 19, 75–82. [Google Scholar] [CrossRef]
- Sabourian, P.; Yazdani, G.; Ashraf, S.S.; Frounchi, M.; Mashayekhan, S.; Kiani, S.; Kakkar, A. Effect of physico-chemical properties of nanoparticles on their intracellular uptake. Int. J. Mol. Sci. 2020, 21, 8019. [Google Scholar] [CrossRef]
- Price, J.P.; Kruger, D.F.; Saravolatz, L.D.; Whitehouse, F.W. Evaluation of the insulin jet injector as a potential source of infection. Am. J. Infect. Control 1989, 17, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Volceanov, A.; Teleanu, R.I. Blood-brain delivery methods using nanotechnology. Pharmaceutics 2018, 10, 269. [Google Scholar] [CrossRef] [PubMed]
- Gaba, B.; Khan, T.; Haider, M.F.; Alam, T.; Baboota, S.; Parvez, S.; Ali, J. Vitamin E Loaded Naringenin Nanoemulsion via Intranasal Delivery for the Management of Oxidative Stress in a 6-OHDA Parkinson’s Disease Model. BioMed Res. Int. 2019, 2019, 2382563. [Google Scholar] [CrossRef] [PubMed]
- Marcuzzo, S.; Isaia, D.; Bonanno, S.; Malacarne, C.; Cavalcante, P.; Zacheo, A.; Laquintana, V.; Denora, N.; Sanavio, B.; Salvati, E.; et al. FM19G11-loaded gold nanoparticles enhance the proliferation and self-renewal of ependymal stem progenitor cells derived from ALS mice. Cells 2019, 8, 279. [Google Scholar] [CrossRef]
- Inglut, C.T.; Sorrin, A.J.; Kuruppu, T.; Vig, S.; Cicalo, J.; Ahmad, H.; Huang, H.C. Immunological and toxicological considerations for the design of liposomes. Nanomaterials 2020, 10, 190. [Google Scholar] [CrossRef]
- DeMattos, R.B.; Bales, K.R.; Cummins, D.J.; Dodart, J.C.; Paul, S.M.; Holtzman, D.M. Peripheral anti-Aβ antibody alters CNS and plasma Aβ clearance and decreases brain Aβ burden in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2001, 98, 8850–8855. [Google Scholar] [CrossRef]
- Helmschrodt, C.; Höbel, S.; Schöniger, S.; Bauer, A.; Bonicelli, J.; Gringmuth, M.; Fietz, S.A.; Aigner, A.; Richter, A.; Richter, F. Polyethylenimine nanoparticle-mediated siRNA delivery to reduce α-Synuclein expression in a model of Parkinson’s disease. Mol. Ther. Nucleic Acids 2017, 9, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Drummond, C.J.; Zhai, J.; Tran, N. Lipid Nanoparticles: Versatile Drug Delivery Vehicles for Traversing the Blood Brain Barrier to Treat Brain Cancer. Adv. Funct. Mater. 2024, 34, 2404234. [Google Scholar] [CrossRef]
- Xuan, L.; Ju, Z.; Skonieczna, M.; Zhou, P.; Huang, R. Nanoparticles-induced potential toxicity on human health: Applications, toxicity mechanisms, and evaluation models. MedComm 2023, 4, e327. [Google Scholar] [CrossRef]
- Senapati, S.; Secchi, V.; Cova, F.; Richman, M.; Villa, I.; Yehuda, R.; Shenberger, Y.; Campione, M.; Rahimipour, S.; Monguzzi, A. Noninvasive Treatment of Alzheimer’s Disease with Scintillating Nanotubes. Adv. Healthc. Mater. 2023, 12, 2301527. [Google Scholar] [CrossRef]
- Fabel, K.; Dietrich, J.; Hau, P.; Wismeth, C.; Winner, B.; Przywara, S.; Steinbrecher, A.; Ullrich, W.; Bogdahn, U. Long-term stabilization in patients with malignant glioma after treatment with liposomal doxorubicin. Cancer 2001, 92, 1936. [Google Scholar] [CrossRef] [PubMed]
- Hau, P.; Fabel, K.; Baumgart, U.; Rümmele, P.; Grauer, O.; Bock, A.; Dietmaier, C.; Dietmaier, W.; Dietrich, J.; Dudel, C. Pegylated liposomal doxorubicin-efficacy in patients with recurrent high-grade glioma. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2004, 100, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Brandsma, D.; Kerklaan, B.M.; Diéras, V.; Altintas, S.; Anders, C.K.; Ballester, M.A.; Gelderblom, H.; Soetekouw, P.M.M.B.; Gladdines, W.; Lonnqvist, F. Phase 1/2a study of glutathione pegylated liposomal doxorubicin (2b3-101) in patients with brain metastases (BM) from solid tumors or recurrent high grade gliomas (HGG). Ann. Oncol. 2014, 25, iv157. [Google Scholar] [CrossRef]
- Clarke, J.L.; Molinaro, A.M.; Cabrera, J.R.; DeSilva, A.A.; Rabbitt, J.E.; Chang, S.M.; Butowski, N.A.; Taylor, J.W.; Prados, M.D. A phase 1 trial of intravenous liposomal irinotecan in patients with recurrent high-grade glioma. Cancer Chemother. Pharmacol. 2017, 79, 603–610. [Google Scholar] [CrossRef]
- Kasenda, B.; König, D.; Manni, M.; Ritschard, R.; Duthaler, U.; Bartoszek, E.; Bärenwaldt, A.; Deuster, S.; Hutter, G.; Cordier, D.; et al. Targeting immunoliposomes to EGFR-positive glioblastoma. ESMO Open 2022, 7, 100365. [Google Scholar] [CrossRef]
- Senapati, S.; Tripathi, K.; Awad, K.; Rahimipour, S. Multifunctional Liposomes Targeting Amyloid-β Oligomers for Early Diagnosis and Therapy of Alzheimer’s Disease. Small 2024, 20, 2311670. [Google Scholar] [CrossRef]

| Product Name | Nanoparticles | Cargo | Brain Tumor | Status of Clinical Trial | Refs. |
|---|---|---|---|---|---|
| Caelyx (Schering Plough, Weimar, Germany) | Liposomes | Doxorubicin | Recurrent malignant glioma | Commercialized | [72] |
| Caelyx (Essex Pharma, Munich, Germany) | Commercialized | [73] | |||
| 2B3-101 | Phase II | [74] | |||
| CPT-11 | Irinotecan | Phase I | [75] | ||
| Anti-EGFR ILs-dox | Doxorubicin | Phase I | [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nanda, S.S.; Yi, D.K. Exploring the Connection Between Nanomaterials and Neurodegenerative Disorders. Micromachines 2024, 15, 1382. https://doi.org/10.3390/mi15111382
Nanda SS, Yi DK. Exploring the Connection Between Nanomaterials and Neurodegenerative Disorders. Micromachines. 2024; 15(11):1382. https://doi.org/10.3390/mi15111382
Chicago/Turabian StyleNanda, Sitansu Sekhar, and Dong Kee Yi. 2024. "Exploring the Connection Between Nanomaterials and Neurodegenerative Disorders" Micromachines 15, no. 11: 1382. https://doi.org/10.3390/mi15111382
APA StyleNanda, S. S., & Yi, D. K. (2024). Exploring the Connection Between Nanomaterials and Neurodegenerative Disorders. Micromachines, 15(11), 1382. https://doi.org/10.3390/mi15111382







