A Highly Flexible Piezoelectric Ultrasonic Sensor for Wearable Bone Density Testing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Piezoelectric Materials
2.2. Fabrication of Piezoelectric Sensor
2.3. Bone Density Testing Method
3. Results and Discussion
3.1. Piezoelectric Properties of La/Sm-PMN-PZT
3.2. HSFU Design
3.3. Ultrasonic Energy Transfer
3.4. Hardware Design and Wireless Transmission
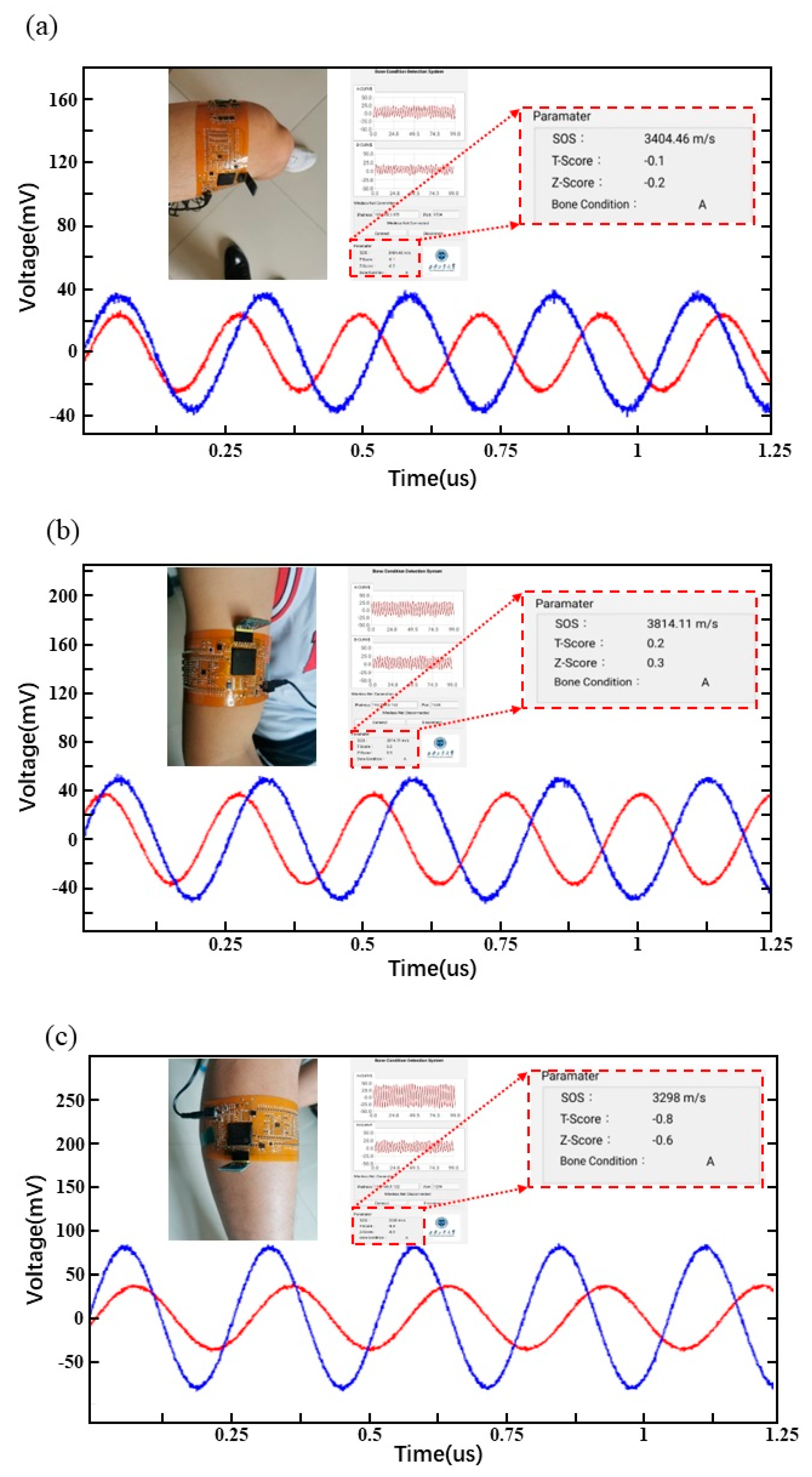
3.5. Real-Time Display of Bone Testing
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Perkel, J.M. The Internet of Things comes to the lab. Nature 2017, 542, 125–126. [Google Scholar] [CrossRef]
- Seth, I.; Panda, S.N.; Guleria, K. IoT based Smart Applications and Recent Research Trends. In Proceedings of the 2021 6th International Conference on Signal Processing, Computing and Control (ISPCC), Solan, India, 7–9 October 2021; pp. 407–412. [Google Scholar]
- Shafique, K.; Khawaja, B.A.; Sabir, F.; Qazi, S.; Mustaqim, M. Internet of Things (IoT) for Next-Generation Smart Systems: A Review of Current Challenges, Future Trends and Prospects for Emerging 5G-IoT Scenarios. IEEE Access 2020, 8, 23022–23040. [Google Scholar] [CrossRef]
- Khanna, A.; Kaur, S. Internet of Things (IoT), Applications and Challenges: A Comprehensive Review. Wirel. Pers. Commun. 2020, 114, 1687–1762. [Google Scholar] [CrossRef]
- Yuan, X.; Ouaskioud, O.; Yin, X.; Li, C.; Ma, P.; Yang, Y.; Yang, P.-F.; Xie, L.; Ren, L. Epidermal Wearable Biosensors for the Continuous Monitoring of Biomarkers of Chronic Disease in Interstitial Fluid. Micromachines 2023, 14, 1452. [Google Scholar] [CrossRef] [PubMed]
- Dao, N.-N. Internet of wearable things: Advancements and benefits from 6G technologies. Future Gener. Comput. Syst. 2023, 138, 172–184. [Google Scholar] [CrossRef]
- Krzysiak, R.; Nguyen, S.; Chen, Y. XAIoT—The Future of Wearable Internet of Things. In Proceedings of the 2022 18th IEEE/ASME International Conference on Mechatronic and Embedded Systems and Applications (MESA), Taipei, Taiwan, 30 November 2022; pp. 1–6. [Google Scholar]
- Rahmani, A.M.; Szu-Han, W.; Yu-Hsuan, K.; Haghparast, M. The Internet of Things for Applications in Wearable Technology. IEEE Access 2022, 10, 123579–123594. [Google Scholar] [CrossRef]
- Surantha, N.; Atmaja, P.; David; Wicaksono, M. A Review of Wearable Internet-of-Things Device for Healthcare. Procedia Comput. Sci. 2021, 179, 936–943. [Google Scholar] [CrossRef]
- Nan, X.; Wang, X.; Kang, T.; Zhang, J.; Dong, L.; Dong, J.; Xia, P.; Wei, D. Review of Flexible Wearable Sensor Devices for Biomedical Application. Micromachines 2022, 13, 1395. [Google Scholar] [CrossRef]
- Phan, D.T.; Nguyen, C.H.; Nguyen, T.D.P.; Tran, L.H.; Park, S.; Choi, J.; Lee, B.-I.; Oh, J. A Flexible, Wearable, and Wireless Biosensor Patch with Internet of Medical Things Applications. Biosensors 2022, 12, 139. [Google Scholar] [CrossRef]
- Rani, S.; Bandyopadhyay-Ghosh, S.; Ghosh, S.B.; Liu, G. Advances in Sensing Technologies for Monitoring of Bone Health. Biosensors 2020, 10, 42. [Google Scholar] [CrossRef]
- Fasihi, L.; Tartibian, B.; Eslami, R.; Fasihi, H. Artificial intelligence used to diagnose osteoporosis from risk factors in clinical data and proposing sports protocols. Sci. Rep. 2022, 12, 18330. [Google Scholar] [CrossRef] [PubMed]
- Jang, R.; Choi, J.H.; Kim, N.; Chang, J.S.; Yoon, P.W.; Kim, C.-H. Prediction of osteoporosis from simple hip radiography using deep learning algorithm. Sci. Rep. 2021, 11, 19997. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, T.; Alharbi, B.; Quan, L.; Esquela-Kerscher, A.; Slaughter, G. Plasmonic-Based Biosensor for the Early Diagnosis of Prostate Cancer. ACS Omega 2022, 7, 2411–2418. [Google Scholar] [CrossRef] [PubMed]
- Huddleston, A.L. Dual-Photon Absorptiometry. In Quantitative Methods in Bone Densitometry; Springer: Boston, MA, USA, 1988; pp. 57–83. [Google Scholar]
- Velchik, M.G. Photon absorptiometry. Am. J. Physiol. Imaging 1987, 2, 118–126. [Google Scholar] [PubMed]
- Lorente Ramos, R.M.; Azpeitia Armán, J.; Arévalo Galeano, N.; Muñoz Hernández, A.; García Gómez, J.M.; Gredilla Molinero, J. Dual energy X-ray absorptimetry: Fundamentals, methodology, and clinical applications. Radiologia 2012, 54, 410–423. [Google Scholar] [CrossRef]
- Ritt, P.; Kuwert, T. Quantitative SPECT/CT—Technique and Clinical Applications. In Molecular Imaging in Oncology; Schober, O., Kiessling, F., Debus, J., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 565–590. [Google Scholar]
- Yamaguchi, T. Basic concept and clinical applications of quantitative ultrasound (QUS) technologies. J. Med. Ultrason. 2021, 48, 391–402. [Google Scholar] [CrossRef]
- Hong, Y.; Wang, B.; Lin, W.; Jin, L.; Liu, S.; Luo, X.; Pan, J.; Wang, W.; Yang, Z. Highly anisotropic and flexible piezoceramic kirigami for preventing joint disorders. Sci. Adv. 2021, 7, eabf0795. [Google Scholar] [CrossRef]
- Ates, H.C.; Nguyen, P.Q.; Gonzalez-Macia, L.; Morales-Narváez, E.; Güder, F.; Collins, J.J.; Dincer, C. End-to-end design of wearable sensors. Nat. Rev. Mater. 2022, 7, 887–907. [Google Scholar] [CrossRef]
- Gao, F.; Liu, C.; Zhang, L.; Liu, T.; Wang, Z.; Song, Z.; Cai, H.; Fang, Z.; Chen, J.; Wang, J.; et al. Wearable and flexible electrochemical sensors for sweat analysis: A review. Microsyst. Nanoeng. 2023, 9, 1. [Google Scholar] [CrossRef]
- Du, Y.; Du, W.; Lin, D.; Ai, M.; Li, S.; Zhang, L. Recent Progress on Hydrogel-Based Piezoelectric Devices for Biomedical Applications. Micromachines 2023, 14, 167. [Google Scholar] [CrossRef]
- Liu, T.; Dangi, A.; Kim, J.N.; Kothapalli, S.-R.; Choi, K.; Trolier-McKinstry, S.; Jackson, T. Flexible Thin-Film PZT Ultrasonic Transducers on Polyimide Substrates. Sensors 2021, 21, 1014. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.-F.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, A.; Ronca, V.; Vozzi, A.; Sciaraffa, N.; di Florio, A.; Tamborra, L.; Simonetti, I.; Aricò, P.; Di Flumeri, G.; Rossi, D.; et al. Wearable Technologies for Mental Workload, Stress, and Emotional State Assessment during Working-Like Tasks: A Comparison with Laboratory Technologies. Sensors 2021, 21, 2332. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, S.; Bailey, S.G.; Parvizi, H.; Ghannam, R. Wearable Sensors for Learning Enhancement in Higher Education. Sensors 2022, 22, 7633. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Fu, J.; Wang, F.; Zhang, Y.; Wang, P.; Liu, X.; Jiao, Y.; Li, H.; Chen, Y.; Ma, Y.; et al. A flexible, stretchable system for simultaneous acoustic energy transfer and communication. Sci. Adv. 2021, 7, eabg2507. [Google Scholar] [CrossRef]
- Mishra, A.K.; Janani Kavi Priya, V.S.; Pradeep, K.; Sai Vaishnav, J.; Kabhilesh, G. Smart materials for ultrasonic piezoelectric composite transducer: A short review. Mater. Today Proc. 2022, 62, 2064–2069. [Google Scholar] [CrossRef]
- Li, J.; Ma, Y.; Zhang, T.; Shung, K.K.; Zhu, B. Recent Advancements in Ultrasound Transducer: From Material Strategies to Biomedical Applications. BME Front. 2022, 2022, 9764501. [Google Scholar] [CrossRef]
- Muleki-Seya, P.; Han, A.; Andre, M.P.; Erdman, J.W., Jr.; O’Brien, W.D., Jr. Analysis of Two Quantitative Ultrasound Approaches. Ultrason. Imaging 2018, 40, 84–96. [Google Scholar] [CrossRef]
- Lin, D.; Li, Z.; Li, F.; Xu, Z.; Yao, X. Characterization and piezoelectric thermal stability of PIN–PMN–PT ternary ceramics near the morphotropic phase boundary. J. Alloys Compd. 2010, 489, 115–118. [Google Scholar] [CrossRef]
- Lin, D.; Chen, H.; Li, Z.; Xu, Z. Phase diagram and dielectric properties of Pb(In1/2Nb1/2)O3-Pb(Mg1/3Nb2/3)O3-PbTiO3 ceramics. J. Adv. Dielectr. 2015, 5, 1550014. [Google Scholar] [CrossRef]
- Yu, Y.; Niu, Q.; Li, X.; Xue, J.; Liu, W.; Lin, D. A Review of Fingerprint Sensors: Mechanism, Characteristics, and Applications. Micromachines 2023, 14, 1253. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, K.; Pazhamalai, P.; Mariappan, V.K.; Nardekar, S.S.; Sahoo, S.; Kim, S.-J. Probing the energy conversion process in piezoelectric-driven electrochemical self-charging supercapacitor power cell using piezoelectrochemical spectroscopy. Nat. Commun. 2020, 11, 2351. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Zhang, J.; Wu, Y.; Wang, C.; Koval, V.; Shi, B.; Ye, H.; McKinnon, R.; Viola, G.; Yan, H. Unfolding grain size effects in barium titanate ferroelectric ceramics. Sci. Rep. 2015, 5, 9953. [Google Scholar] [CrossRef]
- Sözen, T.; Özışık, L.; Başaran, N. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Gourlay, M.L.; Fine, J.P.; Preisser, J.S.; May, R.C.; Li, C.; Lui, L.-Y.; Ransohoff, D.F.; Cauley, J.A.; Ensrud, K.E. Bone-Density Testing Interval and Transition to Osteoporosis in Older Women. N. Engl. J. Med. 2012, 366, 225–233. [Google Scholar] [CrossRef]
- Carovac, A.; Smajlovic, F.; Junuzovic, D. Application of ultrasound in medicine. Acta Inform. Medica AIM J. Soc. Med. Inform. Bosnia Herzeg. Cas. Drus. Za Med. Inform. BiH 2011, 19, 168–171. [Google Scholar] [CrossRef]
- Hadzik, R.; Bombiński, P.; Brzewski, M. Double aorta artifact in sonography—A diagnostic challenge. J. Ultrason. 2017, 17, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Patey, S.J.; Corcoran, J.P. Physics of ultrasound. Anaesth. Intensive Care Med. 2021, 22, 58–63. [Google Scholar] [CrossRef]





| La/Sm-PMN-PZ-PT | Tc (°C) | d33 (pC/N) | εr | kp | kt | k33 |
|---|---|---|---|---|---|---|
| 0.0125/0.34/0.32/0.34 | 187 | 380 | 2262 | 0.54 | 0.48 | 0.67 |
| 0.0125/0.31/0.35/0.34 | 196 | 460 | 2347 | 0.58 | 0.51 | 0.71 |
| 0.0125/0.285/0.37/0.345 | 212 | 445 | 2353 | 0.59 | 0.52 | 0.72 |
| 0.0125/0.265/0.39/0.345 | 218 | 510 | 2442 | 0.59 | 0.53 | 0.73 |
| 0.01/0.24/0.41/0.35 | 221 | 400 | 2147 | 0.61 | 0.53 | 0.74 |
| 0.01/0.20/0.45/0.35 | 234 | 415 | 2053 | 0.61 | 0.54 | 0.74 |
| 0.01/0.17/0.47/0.36 | 255 | 525 | 2676 | 0.63 | 0.58 | 0.77 |
| 0.01/0.14/0.50/0.36 | 264 | 445 | 1947 | 0.59 | 0.57 | 0.74 |
| Transmit Deepness (mm) | 1 | 2 | 4 | 5 |
|---|---|---|---|---|
| Response Peak-Peak Voltage (mV) | 1420 | 1180 | 1980 | 2280 |
| Response Voltage Root Mean Square (mV) | 626 | 531 | 893 | 979 |
| Excitation Peak-Peak Voltage (mV) | 2000 | 4000 | 6000 | 8000 |
| Media Layer | Water | Water | Water | Water |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Z.; Wang, B.; Zhang, Z.; Yu, Y.; Lin, D. A Highly Flexible Piezoelectric Ultrasonic Sensor for Wearable Bone Density Testing. Micromachines 2023, 14, 1798. https://doi.org/10.3390/mi14091798
Song Z, Wang B, Zhang Z, Yu Y, Lin D. A Highly Flexible Piezoelectric Ultrasonic Sensor for Wearable Bone Density Testing. Micromachines. 2023; 14(9):1798. https://doi.org/10.3390/mi14091798
Chicago/Turabian StyleSong, Zhiqiang, Bozhi Wang, Zhuo Zhang, Yirong Yu, and Dabin Lin. 2023. "A Highly Flexible Piezoelectric Ultrasonic Sensor for Wearable Bone Density Testing" Micromachines 14, no. 9: 1798. https://doi.org/10.3390/mi14091798
APA StyleSong, Z., Wang, B., Zhang, Z., Yu, Y., & Lin, D. (2023). A Highly Flexible Piezoelectric Ultrasonic Sensor for Wearable Bone Density Testing. Micromachines, 14(9), 1798. https://doi.org/10.3390/mi14091798










