Classification-Based Evaluation of Multi-Ingredient Dish Using Graphene-Modified Interdigital Electrodes
Abstract
:1. Introduction
- (i)
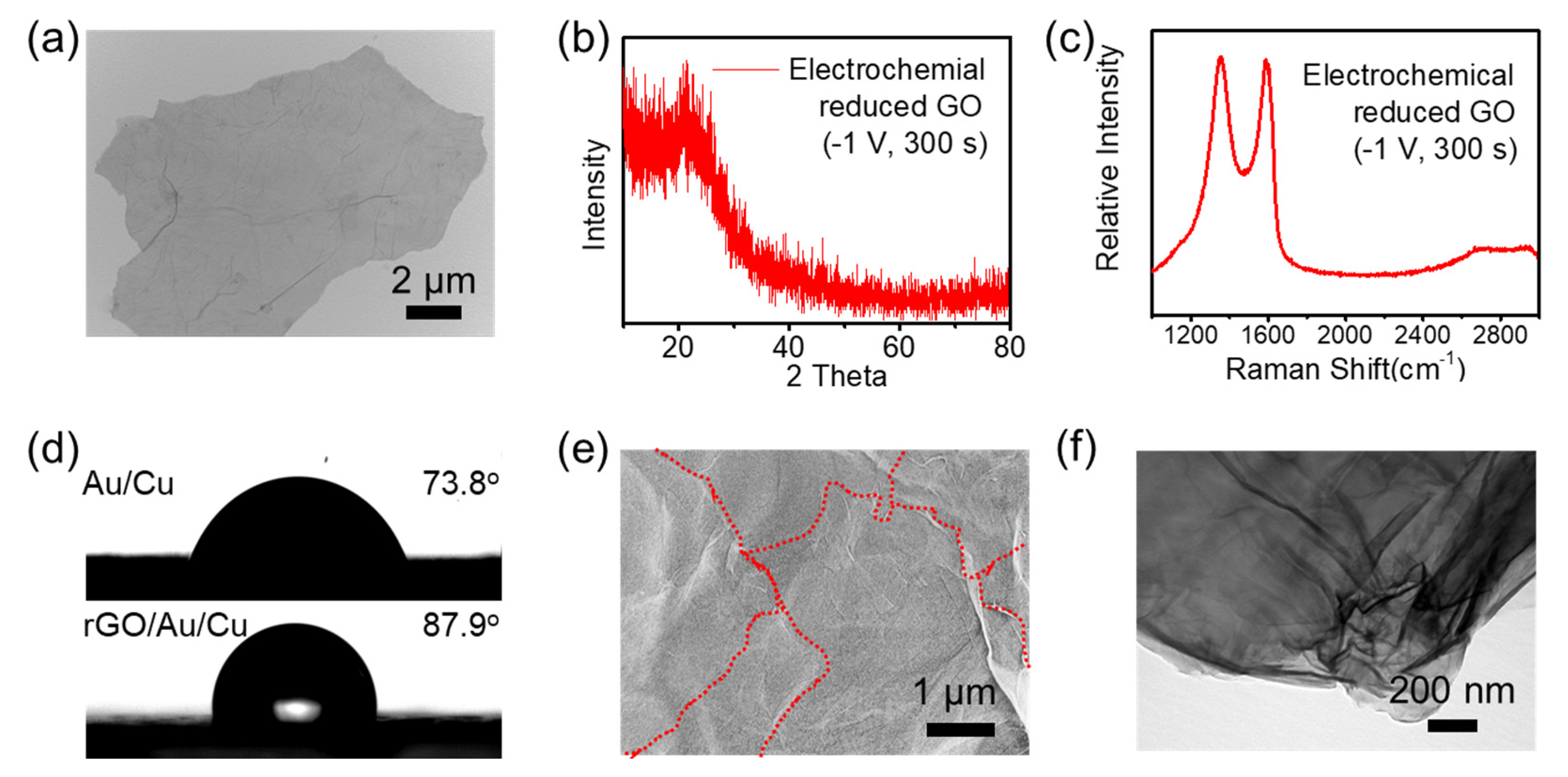
- The ERGO showed high graphitization and good conductivity, which facilitates the electron transfer between the interface of ions in solution and the electrode and the interface of graphene and gold.
- (ii)
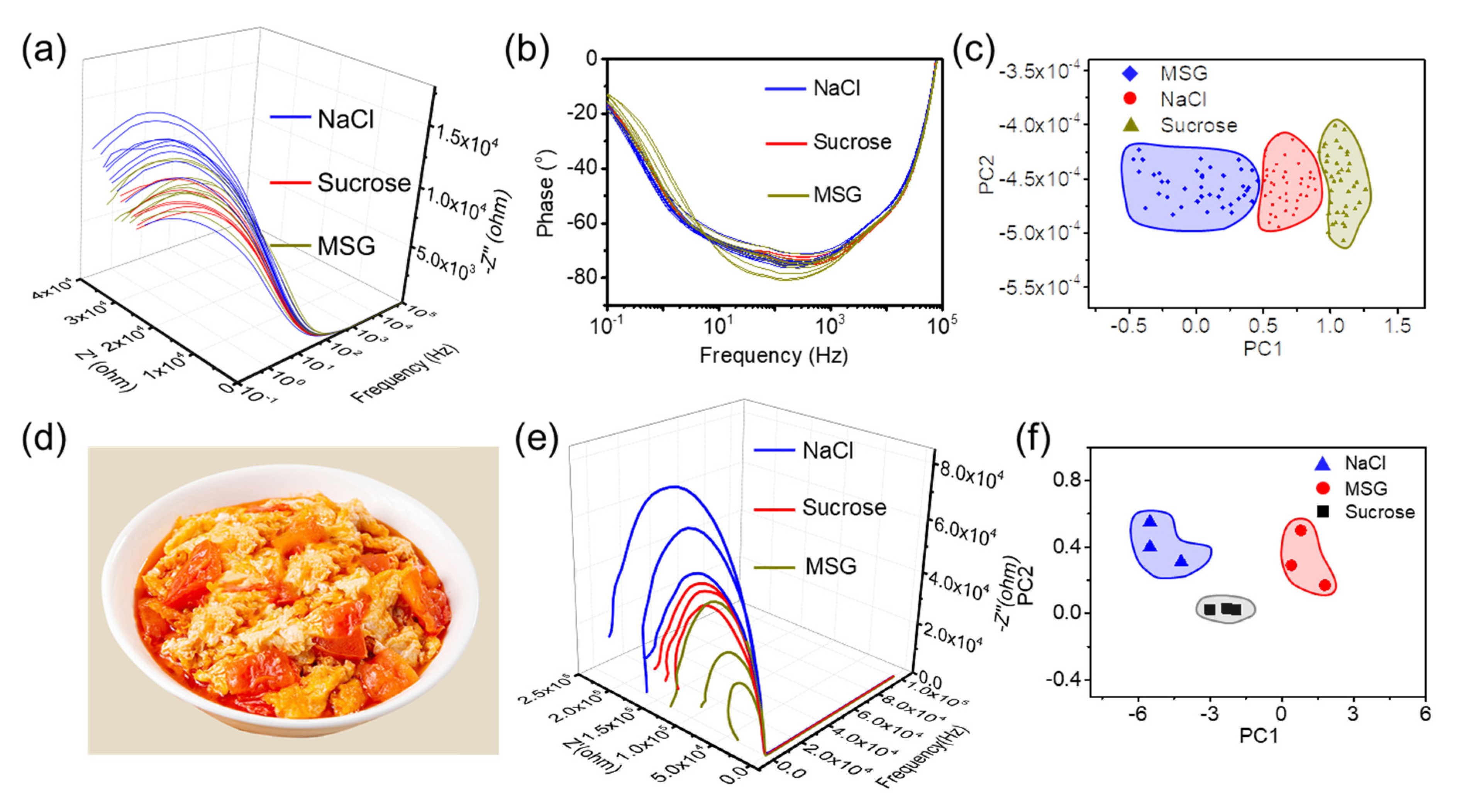
- The sensing system could successfully evaluate the deviations from a standard of a sample without quantitative analysis. Our electronic tongue is facile and offers a more universal approach to simultaneously identifying multiple ingredients.
2. Materials and Methods
2.1. Materials and Characterization
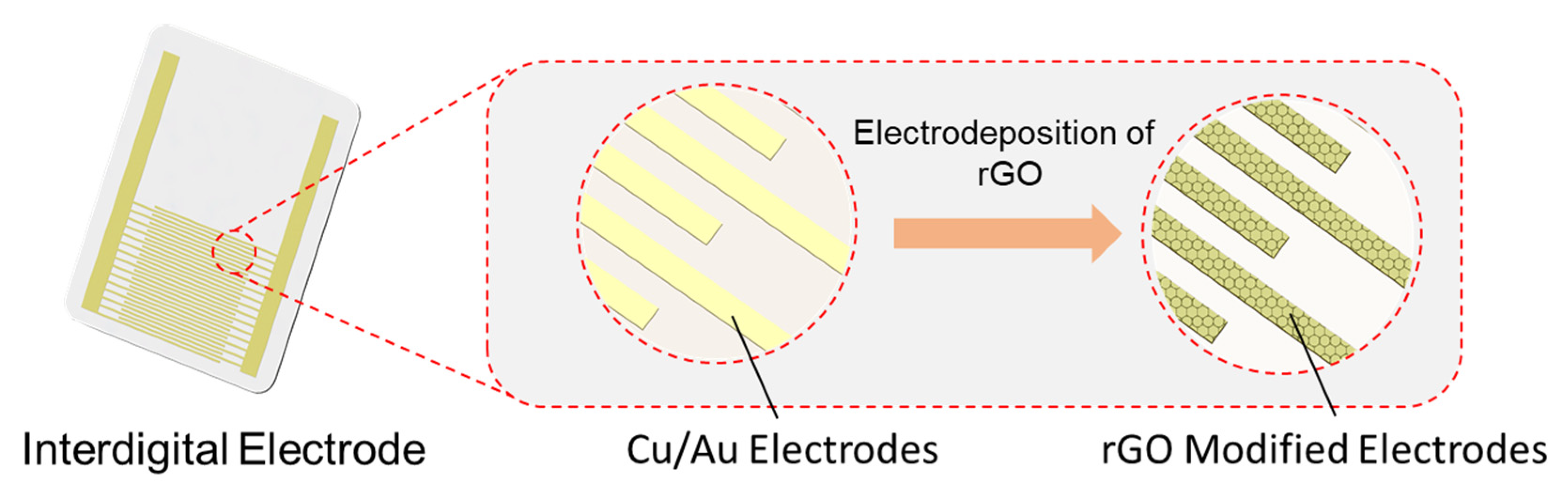
2.2. Preparation of ERGO Modified IE
2.3. Method for Database Establishment for Dish Evaluation
3. Results and Discussions
- (i)
- Modification of IE electrodes with ERGO
- (ii)
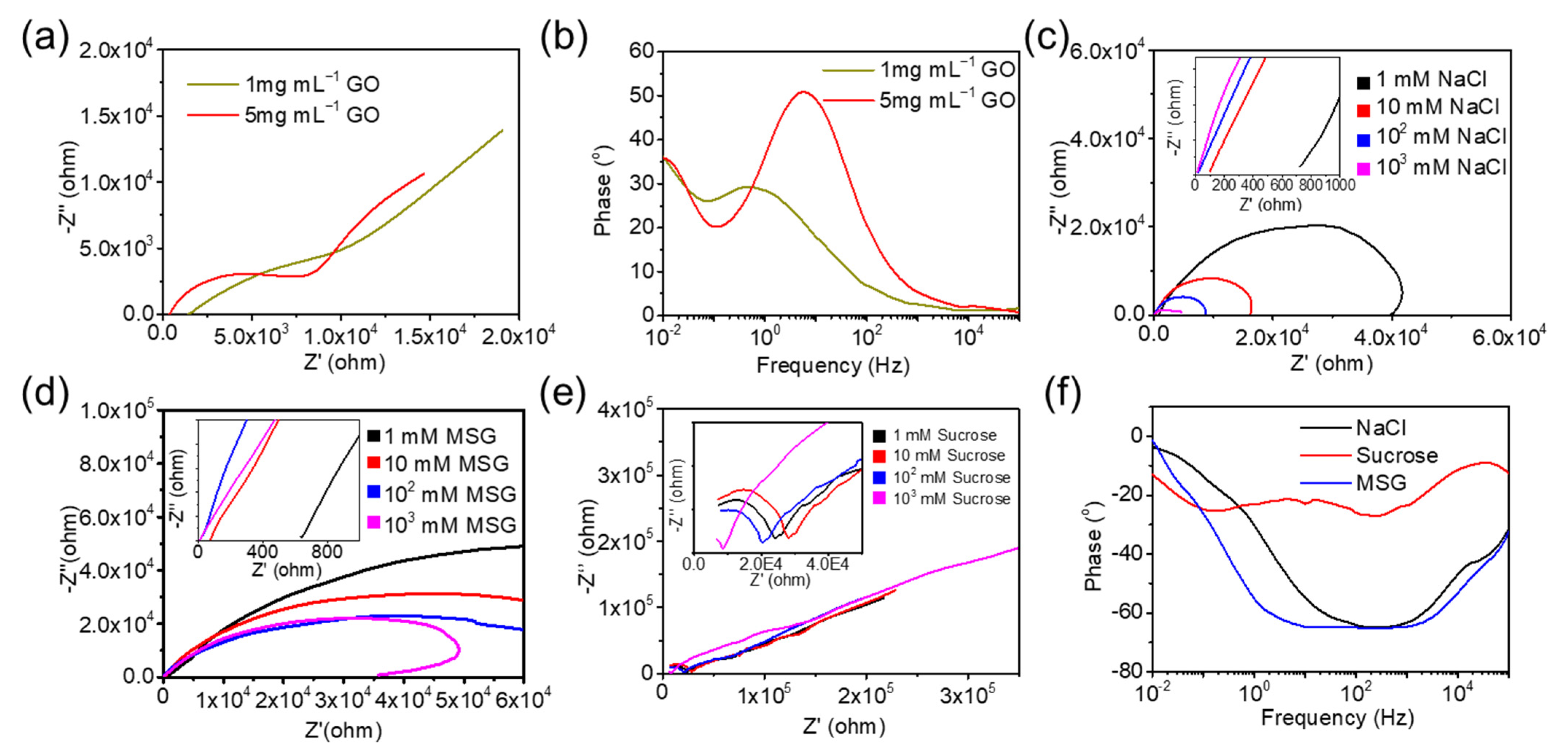
- Electrochemical characterization of individual ingredient
- (iii)
- Classification-based evaluation in dimensions of cooking seasoning
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Silver, D.; Huang, A.; Maddison, C.J.; Guez, A.; Sifre, L.; van den Driessche, G.; Schrittwieser, J.; Antonoglou, I.; Panneershelvam, V.; Lanctot, M.; et al. Mastering the game of Go with deep neural networks and tree search. Nature 2016, 529, 484. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, M.; O’Hare, G. How Smart Is Your City? Science 2012, 335, 1581–1582. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.-Z. Robot learning—Beyond imitation. Sci. Robot. 2019, 4, eaaw3520. [Google Scholar] [CrossRef]
- Jung, Y.H.; Park, B.; Kim, J.U.; Kim, T.-i. Bioinspired Electronics for Artificial Sensory Systems. Adv. Mater. 2018, 31, 1803637. [Google Scholar] [CrossRef]
- Savage, N. Technology: The taste of things to come. Nature 2012, 486, S18. [Google Scholar] [CrossRef]
- Toko, K. Taste sensor with global selectivity. Mat. Sci. Eng. C-Mater. 1996, 4, 69–82. [Google Scholar] [CrossRef]
- Chandrashekar, J.; Hoon, M.A.; Ryba, N.J.P.; Zuker, C.S. The receptors and cells for mammalian taste. Nature 2006, 444, 288–294. [Google Scholar] [CrossRef]
- Da, H.; Sun, Q.; Su, K.; Wan, H.; Li, H.; Xu, N.; Sun, F.; Zhuang, L.; Hu, N.; Wang, P. Recent achievements in electronic tongue and bioelectronic tongue as taste sensors. Sens. Actuat. B-Chem. 2015, 207, 1136–1146. [Google Scholar]
- Ciosek, P.; Augustyniak, E.; Wroblewski, W. Polymeric membrane ion-selective and cross-sensitive electrode-based electronic tongue for qualitative analysis of beverages. Analyst 2004, 129, 639–644. [Google Scholar] [CrossRef]
- Szpakowska, M.; Szwacki, J.; Lisowska-Oleksiak, A. Investigation of some taste substances using a set of electrodes with lipid-modified membranes. Desalination 2004, 163, 55–59. [Google Scholar] [CrossRef]
- Nery, E.W.; Guimarães, J.A.; Kubota, L.T. Paper—Based Electronic Tongue. Electroanalysis 2015, 27, 2357–2362. [Google Scholar] [CrossRef]
- Zheng, L.; Suslick, K.S. A Hand-Held Optoelectronic Nose for the Identification of Liquors. ACS Sens. 2017, 3, 121–127. [Google Scholar]
- Kobayashi, Y.; Habara, M.; Ikezazki, H.; Chen, R.; Naito, Y.; Toko, K. Advanced taste sensors based on artificial lipids with global selectivity to basic taste qualities and high correlation to sensory scores. Sensors 2010, 10, 3411–3443. [Google Scholar] [CrossRef]
- Tahara, Y.; Nakashi, K.; Ji, K.; Ikeda, A.; Toko, K. Development of a portable taste sensor with a lipid/polymer membrane. Sensors 2013, 13, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Podrazka, M.; Baczynska, E.; Kundys, M.; Jelen, P.S.; Witkowska Nery, E. Electronic Tongue—A Tool for All Tastes? Biosensors 2017, 8, 3. [Google Scholar] [CrossRef]
- Krantz-Rülcker, C.; Stenberg, M.; Winquist, F.; Lundström, I. Electronic tongues for environmental monitoring based on sensor arrays and pattern recognition: A review. Anal. Chim. Acta 2001, 426, 217–226. [Google Scholar] [CrossRef]
- Tahara, Y.; Toko, K. Electronic Tongues—A Review. IEEE Sens. J. 2013, 13, 3001–3011. [Google Scholar] [CrossRef]
- Stoikova, E.E.; Dolgova, N.N.; Savel’Ev, A.A. Beer classification based on the array of solid-contact potentiometric sensors with thiacalixarene receptors. Russ. Chem. Bull. 2014, 63, 223–231. [Google Scholar] [CrossRef]
- Yu, Y.; Zhao, H.; Yang, R.; Dong, G.; Li, L.; Yang, J.; Jin, T.; Zhang, W.; Liu, Y. Pure Milk Brands Classification by Means of a Voltammetric Electronic Tongue and Multivariate Analysis. Int. J. Electrochem. Sci. 2015, 10, 4381–4392. [Google Scholar] [CrossRef]
- Wu, J.; Liu, J.; Fu, M.; Li, G.; Lou, Z. Classification of Chinese Yellow Wines by Chemometric Analysis of Cyclic Voltammogram of Copper Electrodes. Sensors 2005, 5, 529–536. [Google Scholar] [CrossRef]
- Ivarsson, P.; Holmin, S.; Höjer, N.E.; Krantz-Rülcker, C.; Winquist, F. Discrimination of tea by means of a voltammetric electronic tongue and different applied waveforms. Sens. Actuat. B-Chem. 2001, 76, 449–454. [Google Scholar] [CrossRef]
- Cole, M.; Spulber, I.; Gardner, J.W. Surface acoustic wave electronic tongue for robust analysis of sensory components. Sens. Actuat. B-Chem. 2015, 207, 1147–1153. [Google Scholar] [CrossRef]
- Litvinenko, S.V.; Bielobrov, D.O.; Lysenko, V.; Skryshevsky, V.A. Optical Addressing Electronic Tongue based on Low Selective Photovoltaic Transducer with Nanoporous Silicon Layer. Nanoscale Res. Lett. 2016, 11, 374. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, C.; Petersson, H.; Sundgren, H.; Björefors, F.; Krantz-Rülcker, C. Simultaneous estimation of soot and diesel contamination in engine oil using electrochemical impedance spectroscopy. Sens. Actuat. B-Chem. 2007, 127, 613–618. [Google Scholar] [CrossRef]
- Nallon, E.C.; Schnee, V.P.; Bright, C.; Polcha, M.P.; Li, Q. Chemical Discrimination with an Unmodified Graphene Chemical Sensor. ACS Sens. 2016, 1, 26–31. [Google Scholar] [CrossRef]
- Silva, M.F.P.D.; Braga, G.D.S. A Hybrid Electronic Nose and Tongue for the Detection of Ketones: Improved Sensor Orthogonality Using Graphene Oxide-Based Detectors. IEEE Sens. J. 2017, 17, 1971–1980. [Google Scholar]
- Facure, M.H.M.; Mercante, L.A.; Mattoso, L.H.C.; Correa, D.S. Detection of trace levels of organophosphate pesticides using an electronic tongue based on graphene hybrid nanocomposites. Talanta 2017, 167, 59–66. [Google Scholar] [CrossRef]
- Nag, A.; Mukhopadhyay, S.C. Fabrication and implementation of printed sensors for taste sensing applications. Sens. Actuators A Phys. 2018, 269, 53–61. [Google Scholar] [CrossRef]
- Cui, G.; Bi, Z.; Zhang, R.; Liu, J.; Yu, X.; Li, Z. A comprehensive review on graphene-based anti-corrosive coatings. Chem. Eng. J. 2019, 373, 104–121. [Google Scholar] [CrossRef]
- Zhao, Z.; Bai, P.; Du, W.; Liu, B.; Pan, D.; Das, R.; Liu, C.; Guo, Z. An overview of graphene and its derivatives reinforced metal matrix composites: Preparation, properties and applications. Carbon 2020, 170, 302–326. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhang, X.; He, Y.; Peng, H.; Wang, G.; Xin, G. Simultaneously Armored and Active Graphene for Transparent and Flexible Supercapacitors. Adv. Funct. Mater. 2018, 28, 1801998–1802008. [Google Scholar] [CrossRef]
- Liu, C.; Wang, K.; Luo, S.; Tang, Y.; Chen, L. Direct Electrodeposition of Graphene Enabling the One-Step Synthesis of Graphene–Metal Nanocomposite Films. Small 2011, 7, 1203–1206. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, F.; Sairin, M.A.; Nizar, N.N.A.; Aziz, S.A.; Hashim, D.M.; Misbahulmunir, S.; Rokhani, F.Z. Investigation on interdigitated electrode design for impedance spectroscopy technique targeting lard detection application. In Proceedings of the 2015 9th International Conference on Sensing Technology (ICST), Auckland, New Zealand, 8–10 December 2015; pp. 739–744. [Google Scholar]
- Pershina, E.D.; Burda, V.E.; Kazdobin, K.A. Identification of the sugars content in the production of champagne by the electrochemical impedance spectroscopy method. Surf. Eng. Appl. Electrochem. 2013, 49, 348–354. [Google Scholar] [CrossRef]
- Valle, M.D. Bioelectronic Tongues Employing Electrochemical Biosensors; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Jacques, J.; Preda, C. Functional data clustering: A survey. Adv. Data Anal. Classif. 2014, 8, 231–255. [Google Scholar] [CrossRef]
- Park, S.; Ruoff, R.S. Chemical methods for the production of graphenes. Nat. Nanotechnol. 2009, 4, 217. [Google Scholar] [CrossRef]
- ISO/TR 10271:1993; Dentistry—Determination of Tarnish and Corrosion of Metals and Alloys. ISO: Geneva, Switzerland, 1993.
- Sochacki, G.; Abdulali, A.; Iida, F. Mastication-Enhanced Taste-Based Classification of Multi-Ingredient Dishes for Robotic Cooking. Front. Robot. AI 2022, 9, 886074. [Google Scholar] [CrossRef]
| Categories | Concentrations of Subsets | |||
|---|---|---|---|---|
| 1 mM (Ω) | 10 mM (Ω) | 100 mM (Ω) | 1000 mM (Ω) | |
| NaCl | 688.5 | 91 | 25 | 7.5 |
| MSG | 631.1 | 71.7 | 7.4 | 1.8 |
| Sucrose | 23,278 | 19,875 | 16,434 | 779 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; Xu, Y.; Liu, S.; He, X.; Ding, N. Classification-Based Evaluation of Multi-Ingredient Dish Using Graphene-Modified Interdigital Electrodes. Micromachines 2023, 14, 1624. https://doi.org/10.3390/mi14081624
Zhu H, Xu Y, Liu S, He X, Ding N. Classification-Based Evaluation of Multi-Ingredient Dish Using Graphene-Modified Interdigital Electrodes. Micromachines. 2023; 14(8):1624. https://doi.org/10.3390/mi14081624
Chicago/Turabian StyleZhu, Hongwu, Yongyuan Xu, Shengkai Liu, Xuchun He, and Ning Ding. 2023. "Classification-Based Evaluation of Multi-Ingredient Dish Using Graphene-Modified Interdigital Electrodes" Micromachines 14, no. 8: 1624. https://doi.org/10.3390/mi14081624
APA StyleZhu, H., Xu, Y., Liu, S., He, X., & Ding, N. (2023). Classification-Based Evaluation of Multi-Ingredient Dish Using Graphene-Modified Interdigital Electrodes. Micromachines, 14(8), 1624. https://doi.org/10.3390/mi14081624





