Ultrasound-Mediated Ocular Drug Delivery: From Physics and Instrumentation to Future Directions
Abstract
1. Introduction
2. Ultrasound Physics and Bioeffects
2.1. Properties of Sound Waves
2.2. Wave–Matter Interactions
| Tissue | Cornea | Vitreous | Lens | Retina | Choroid | Sclera |
|---|---|---|---|---|---|---|
| Speed of sound (m·s−1) | 1553 ± 3 | 1506 ± 3 | 1620 ± 3 | 1538 ± 20 | 1527 | 1583 ± 10 |
| Acoustic Impedance (kg·m−2·s−1) 106 | 1.59 ± 0.03 | 1.51 ± 0.01 | 1.71 ± 0.01 | 1.55 ± 0.09 | 1.53 | 1.66 ± 0.02 |
2.2.1. Thermal Effects
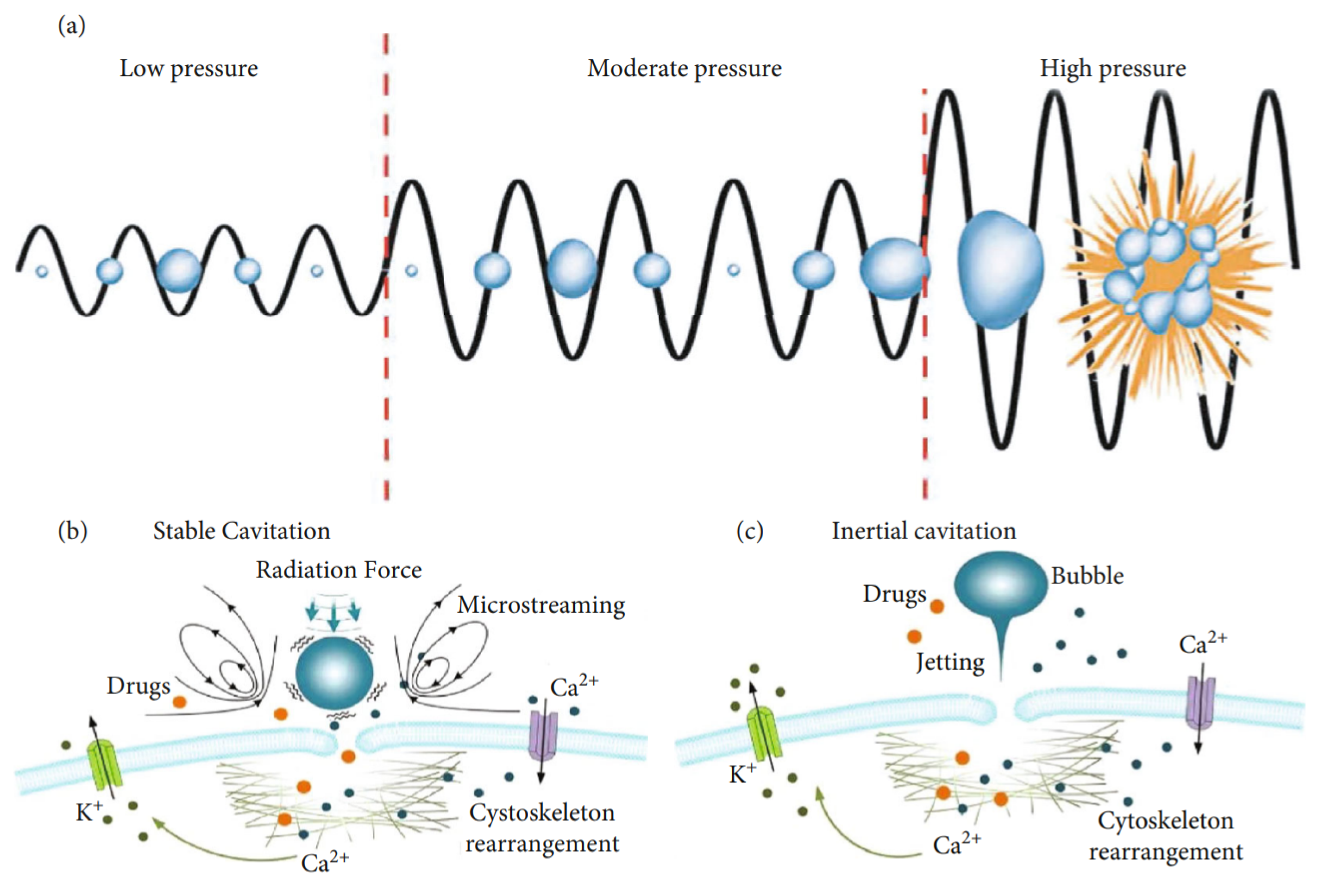
2.2.2. Mechanical Effects
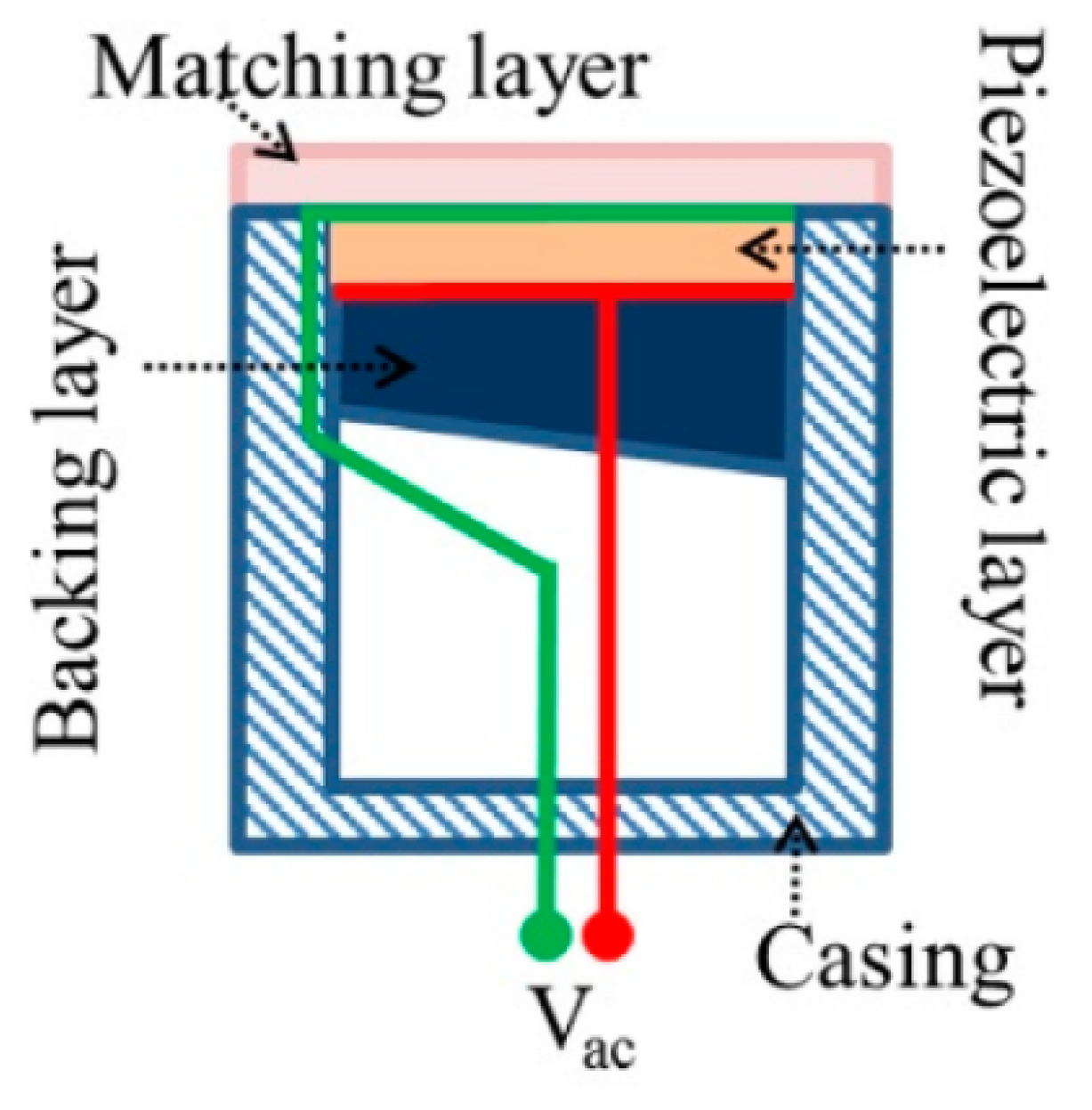
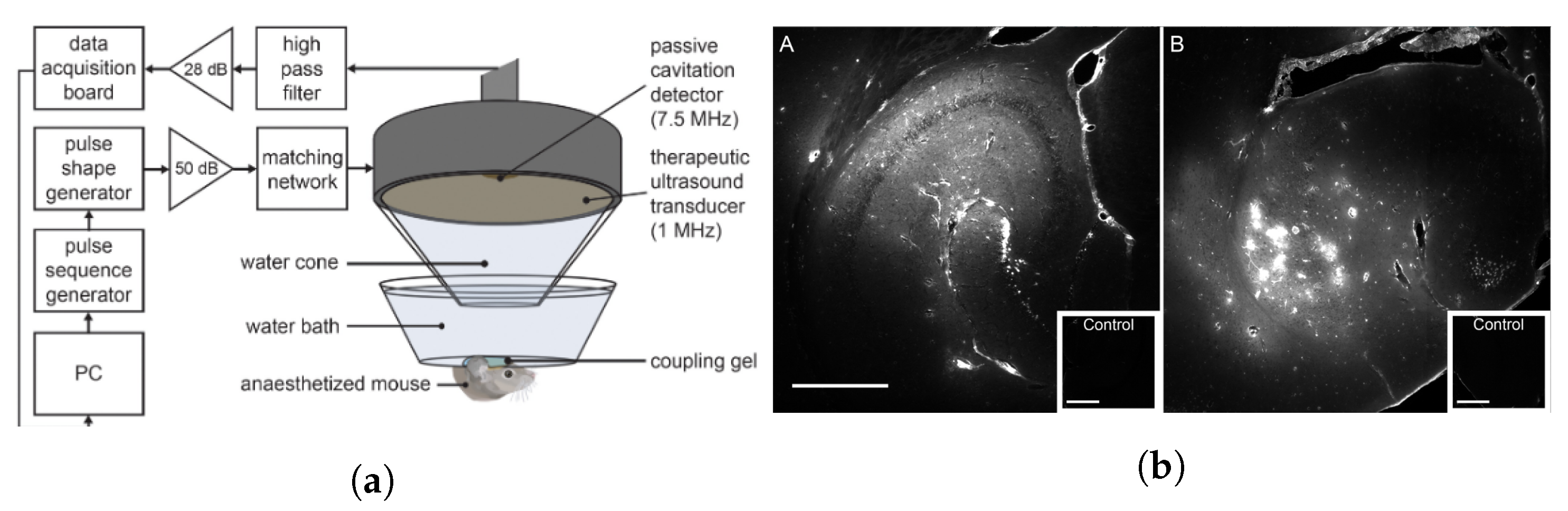
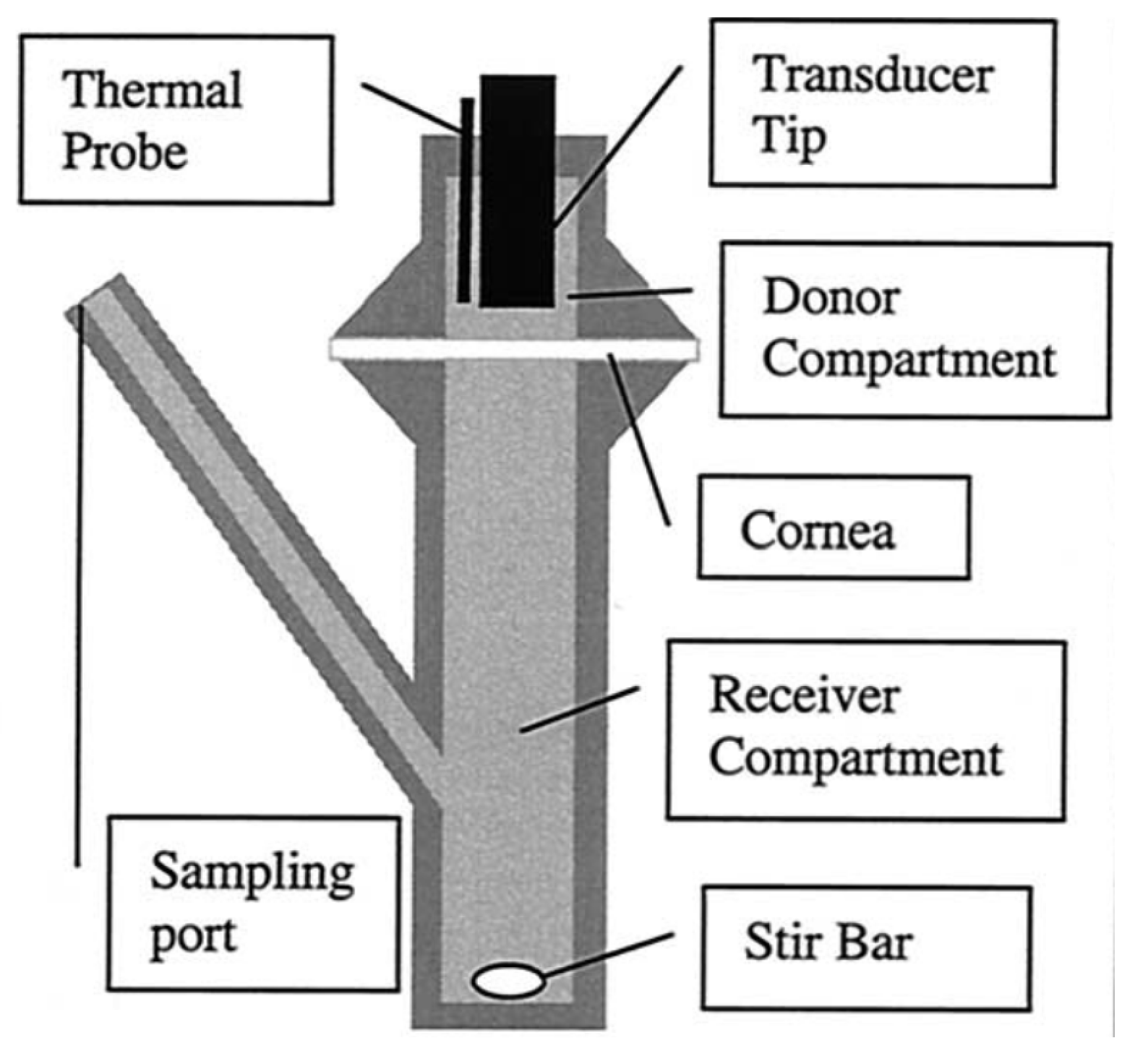
2.3. Instrumentation
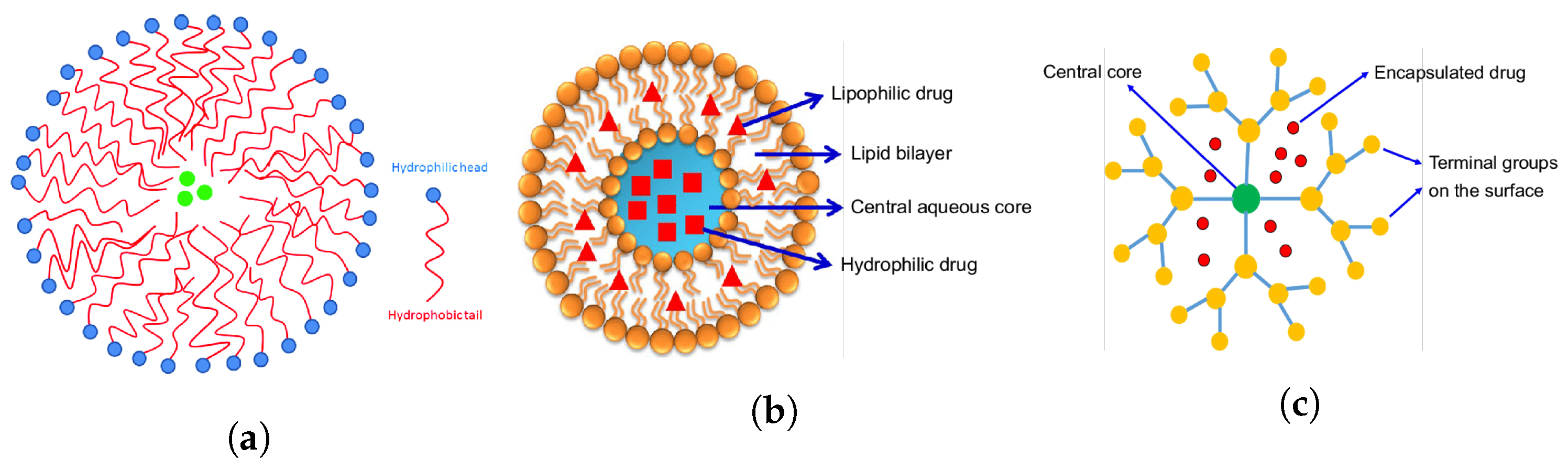
3. Ultrasound-Mediated Drug Delivery
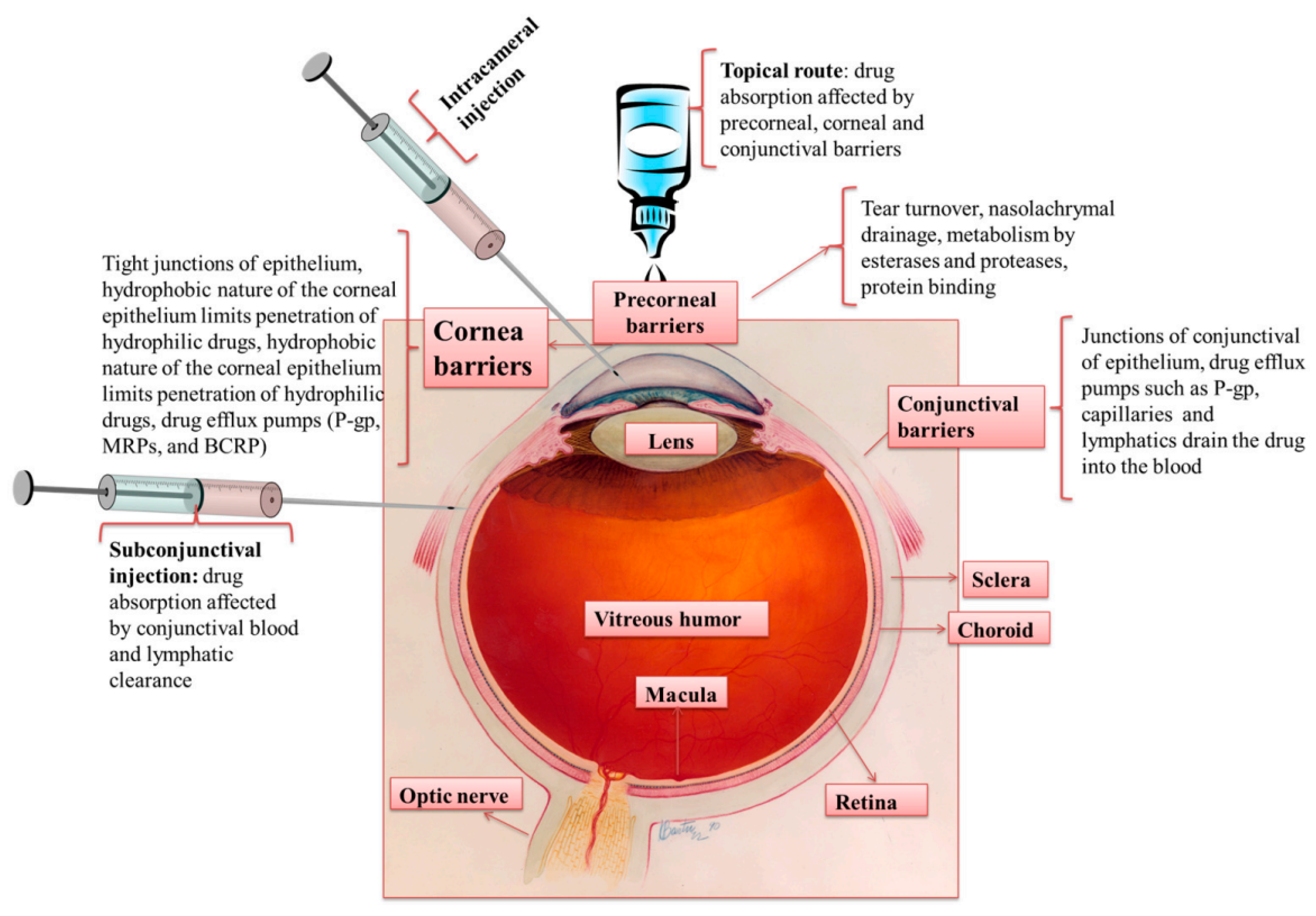
4. Ocular Drug Delivery and Barriers
| Ocular Disease | Affected Tissue | Common Treatment | Limitations |
|---|---|---|---|
| Glaucoma | Optic nerve | Topical -blocker [122] | Systemic side effects [123] |
| Macular degeneration | Retina | Anti-VEGF therapy [124] | Invasive [125] |
| Cataract | Lens | Phacoemulsiphication [126], Intravitreal anti-VEGF injections [127] | Invasive [128] |
| Diabetic retinopathy | Retina | Photocoagulation [129] | Invasive |
| Conjunctivitis | Conjunctiva | Topical antibiotics [130] | Drug clearance, bacterial resistance [131] |
5. Ultrasound-Mediated Ocular Drug Delivery
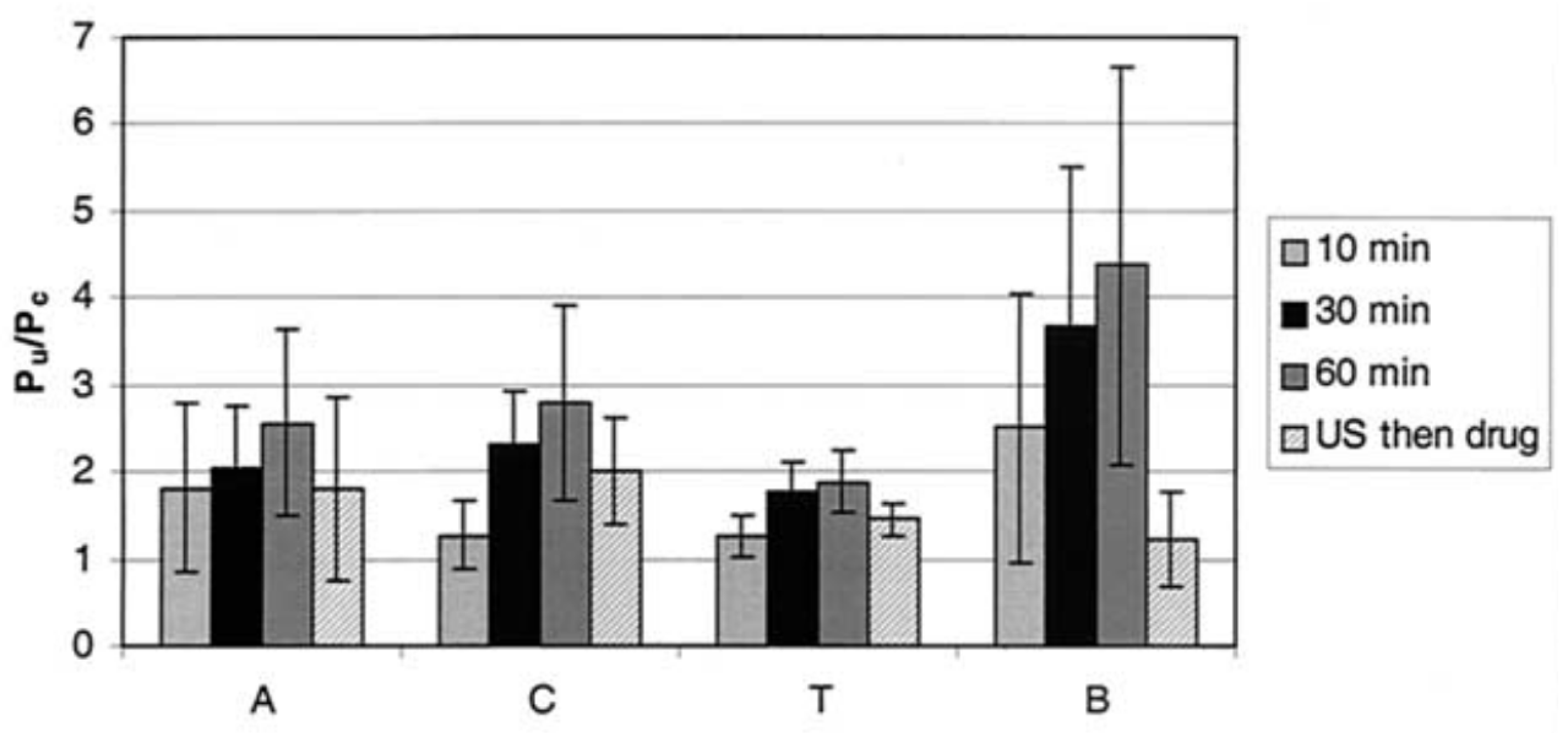
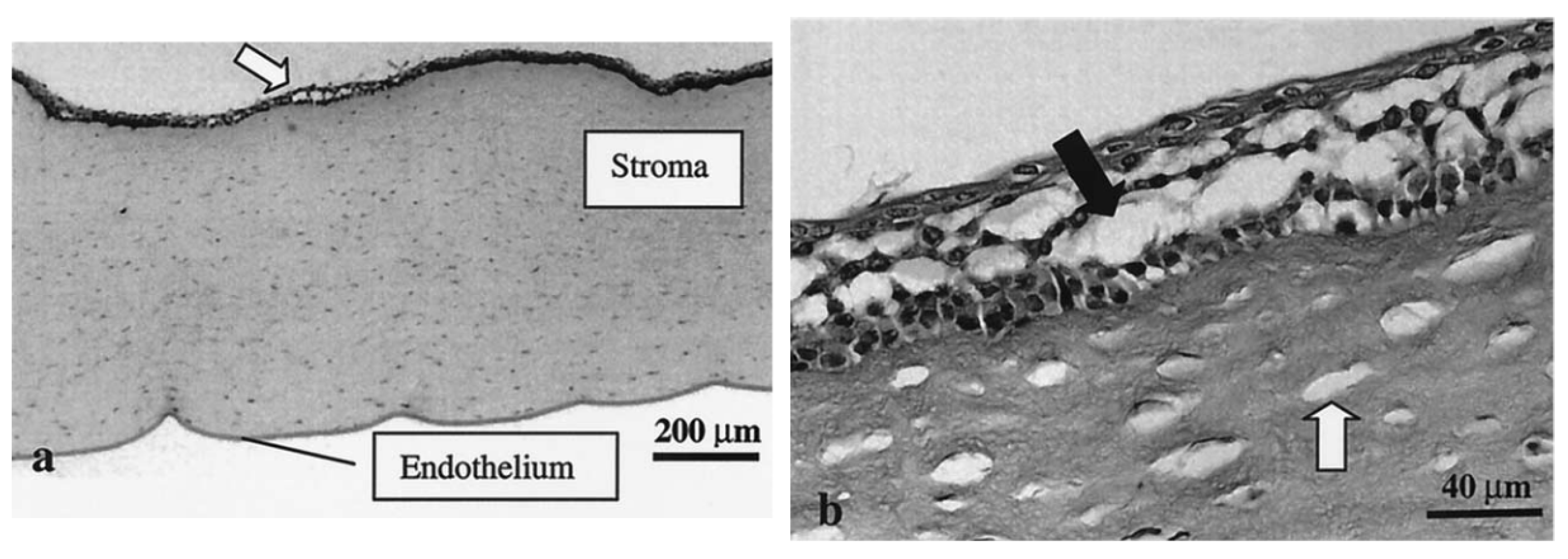
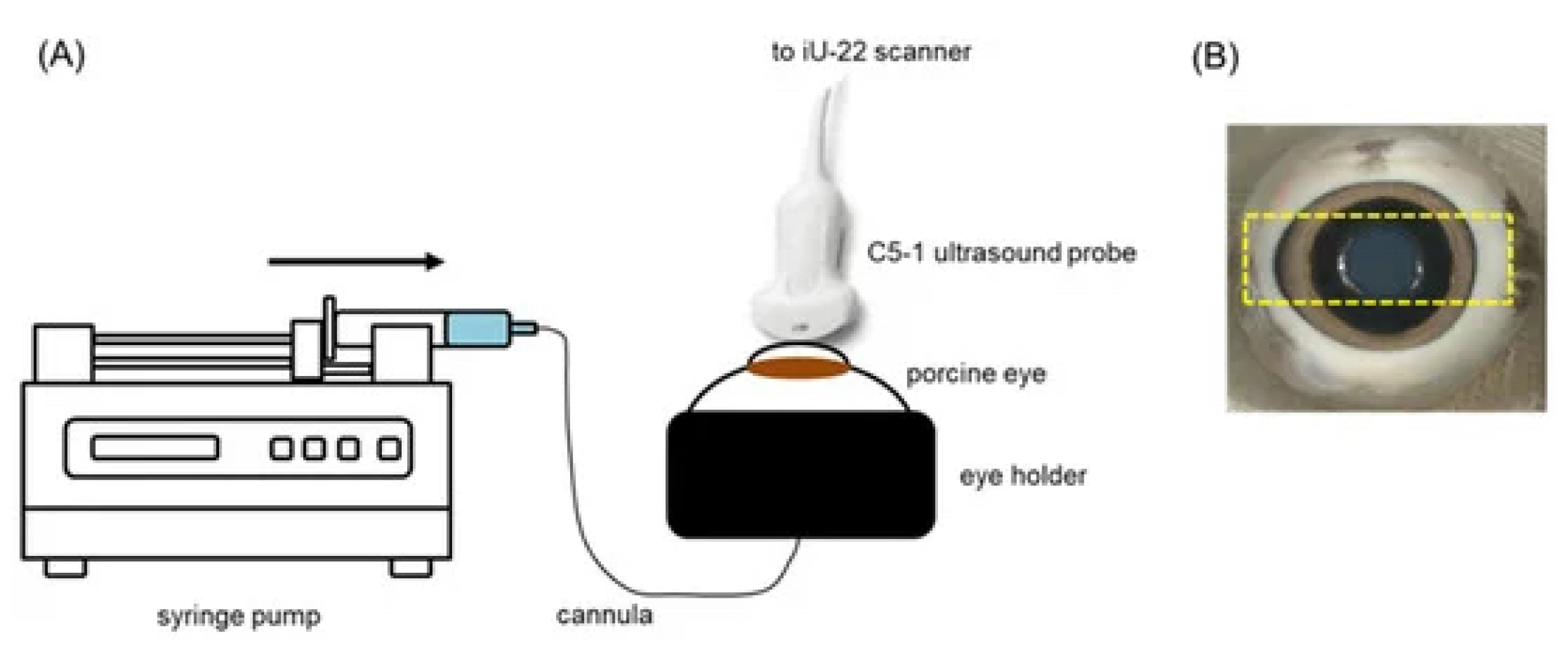
5.1. Ultrasound-Mediated Transcorneal Drug Delivery
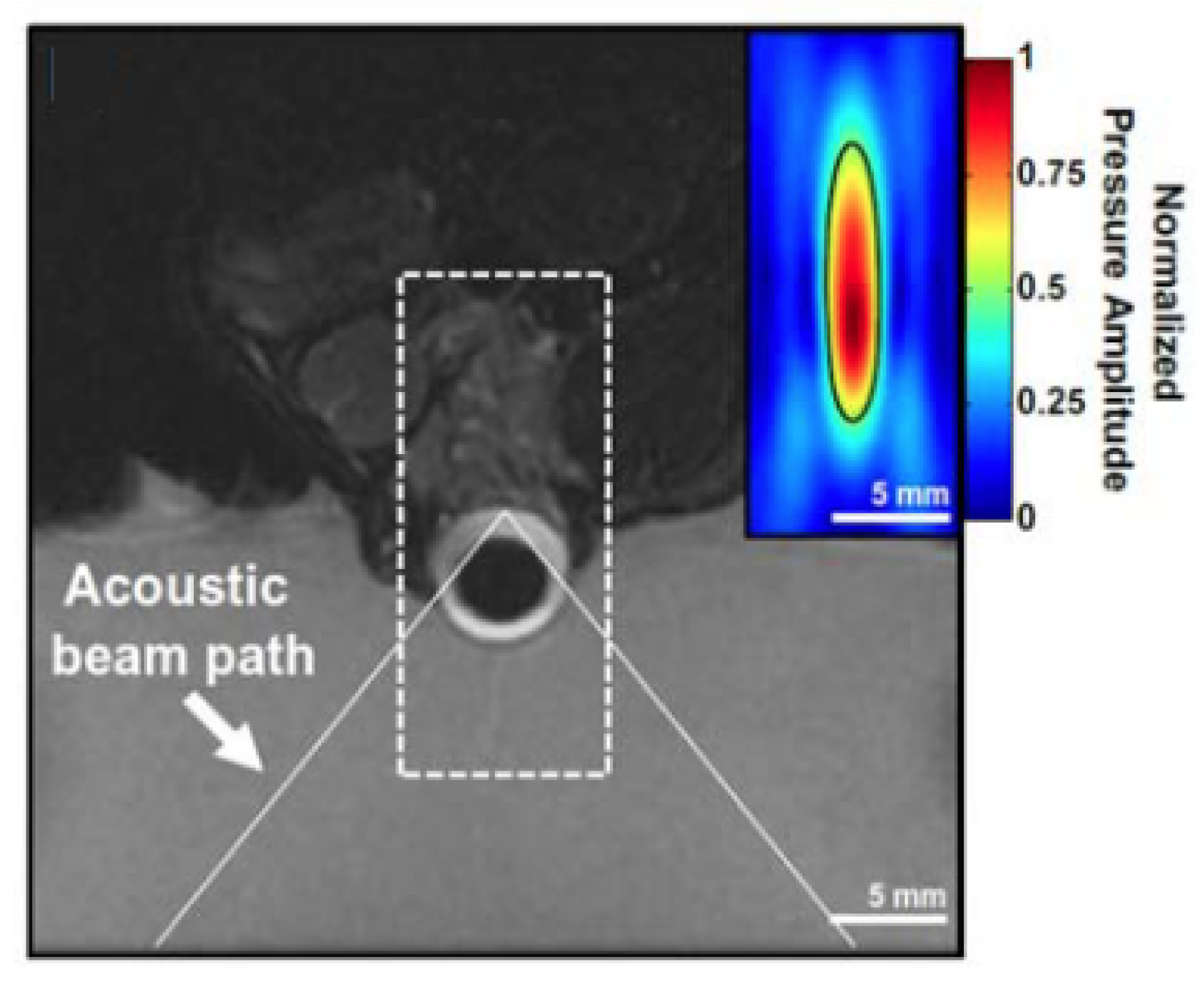
5.2. Ultrasound-Mediated Transscleral Drug Delivery
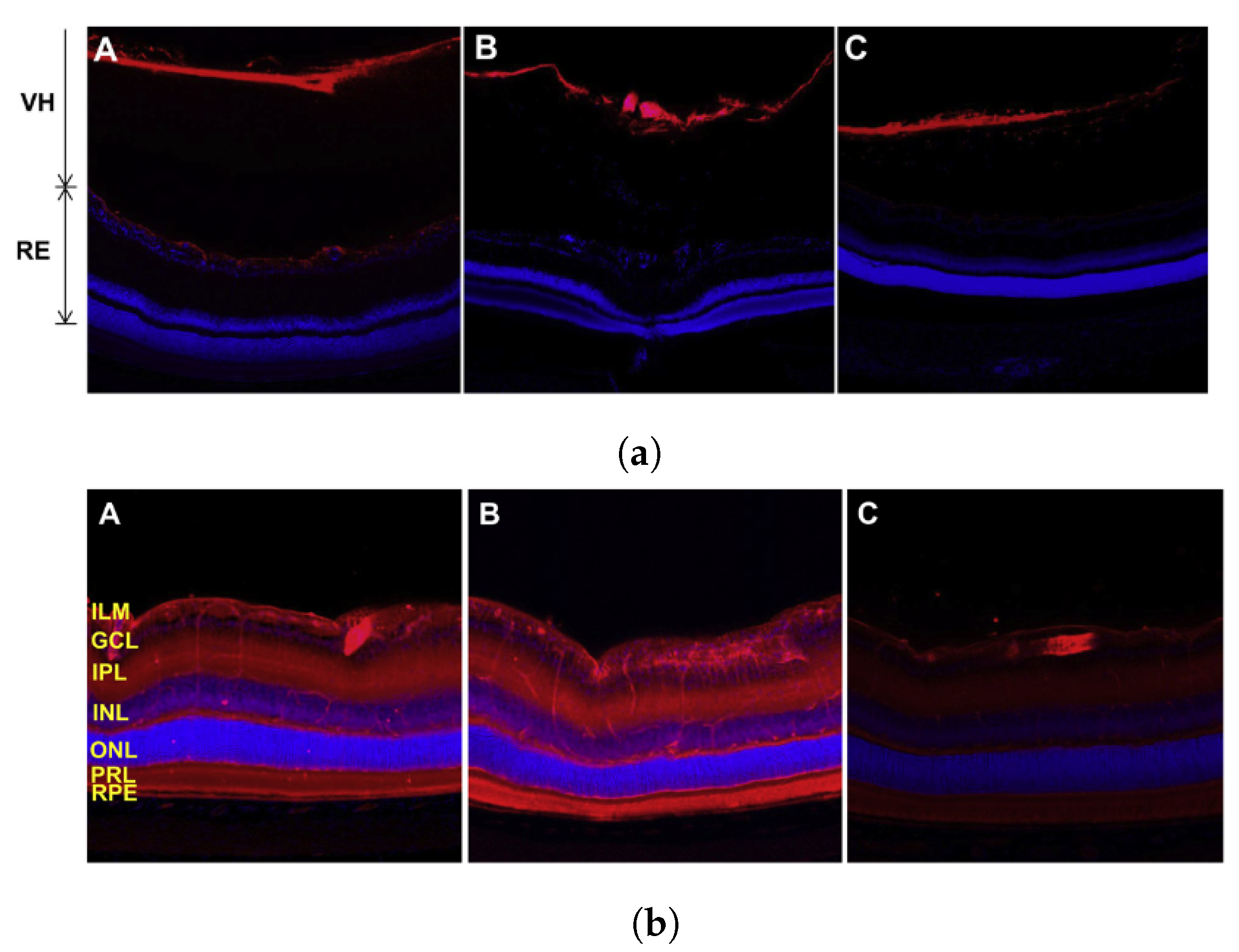
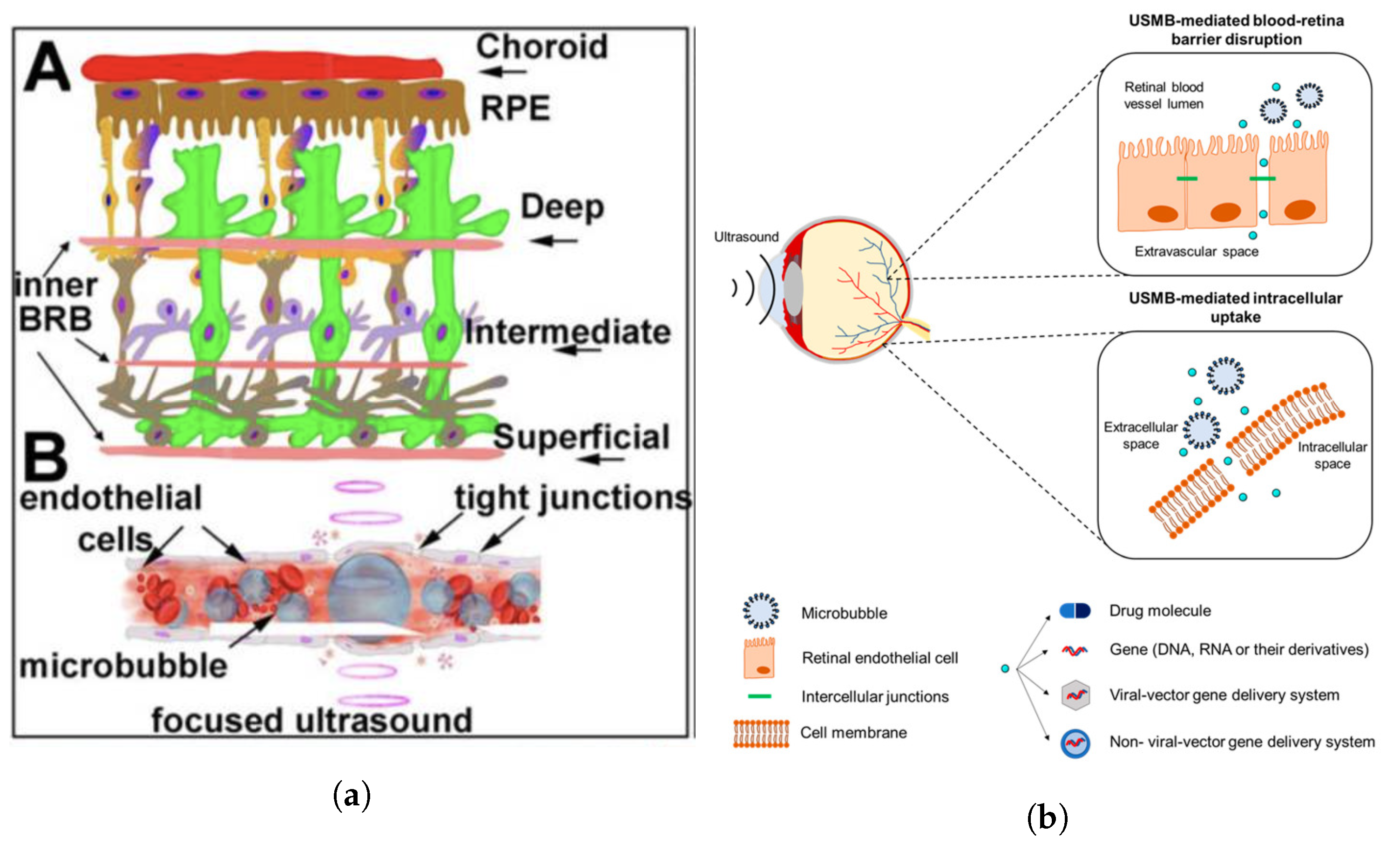
5.3. Ultrasound-Mediated Blood–Retinal Barrier Disruption
| Study | Tissue | Effect | Frequency | Intensity (or Power) | Duty Cycle | MI | Duration | Drug/Model |
|---|---|---|---|---|---|---|---|---|
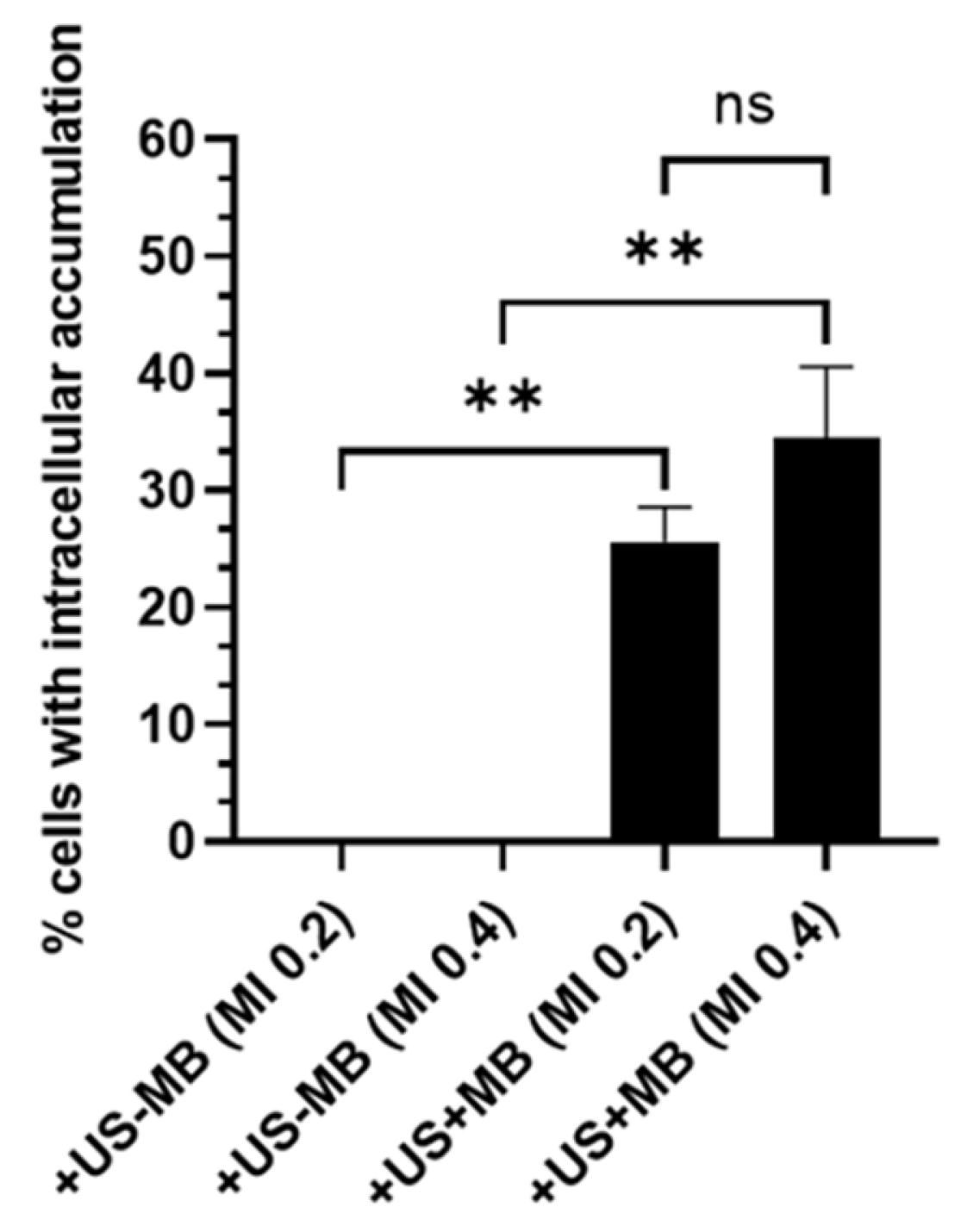
| [172] | Retina | Increased intracellular model drug accumulation. | 2.25 MHz | - | - | 0.2, 0.4 | 2 min | SYTOX green, TRITC dextrans |
| [173] | Sclera | Increased permeability at highest frequency. | 400 kHz, 3 MHz | 1 W·cm−2 | 100% | - | 5 min | Avastin |
| [166] | Sclera | Increased permeability. | 1.1 MHz | 0.5–5.4 W | 2.50% | - | 10 min | Sodium fluorescein |
| [174] | Sclera | Increased drug penetration. | 1 MHz | 0.5 W·cm−2 | 100% | 0.14 | 5 min | FITC-BSA-SFNP |
| [70] | Cornea | Increased permeability. | 20 kHz | 2 W·cm−2 | 14.3% | - | 10, 30, 60 min | Atenolol, carteolol, timolol, betaxolol |
| [168] | Retina | Increased permeability. Retinal damage at 1.1 MPa. | 690 kHz | - | 1% | 0.96, 1.06, 1.32 | 1 min | Magnevist |
| [159] | Cornea | Increased permeability. | 400 kHz–1 MHz | 0.3–1.0 W·cm−2 | 100% | - | 5 min | Tobramycine, dexamethasone, sodium fluorescein |
5.4. Ultrasound-Mediated Intravitreal Diffusion
6. Conclusions and Outlook
Funding
Conflicts of Interest
Abbreviations
| UMODD | Ultrasound-mediated ocular drug delivery |
| BBB | Blood–brain barrier |
| BRB | Blood–retinal barrier |
| BAB | Blood–aqueous barrier |
| TRITC | Tetramethylrhodamine |
| FITC-BSA-SFNP | Fluorescein isothiocynate-labelled bovine serum albumin-loaded silk fibroin nanoparticles |
References
- Newman, P.G.; Rozycki, G.S. The History of Ultrasound. Surg. Clin. N. Am. 1998, 78, 179–195. [Google Scholar] [CrossRef]
- Szabo, T.L. Diagnostic Ultrasound Imaging: Inside Out; Academic Press Series in Biomedical Engineering; Elsevier Academic Press: Amsterdam, The Netherlands; Boston, MA, USA, 2004. [Google Scholar]
- Feng, Z.; Zhou, X.; Xu, S.; Ding, J.; Cao, S.J. Impacts of Humidification Process on Indoor Thermal Comfort and Air Quality Using Portable Ultrasonic Humidifier. Build. Environ. 2018, 133, 62–72. [Google Scholar] [CrossRef]
- Nair, P.; Kannan, N.; Dhinakar, P. Design and Development of Variable Frequency Ultrasonic Pest Repeller. J. Adv. Res. Dyn. Control Syst. 2017, 9, 22–34. [Google Scholar]
- Park, W.J.; Kim, B.S.; Seo, D.E.; Kim, D.S.; Lee, K.H. Parking Space Detection Using Ultrasonic Sensor in Parking Assistance System. In Proceedings of the 2008 IEEE Intelligent Vehicles Symposium, Eindhoven, The Netherlands, 4–6 June 2008; pp. 1039–1044. [Google Scholar] [CrossRef]
- Fuchs, F. Ultrasonic Cleaning and Washing of Surfaces. In Power Ultrasonics; Elsevier: Amsterdam, The Netherlands, 2015; pp. 577–609. [Google Scholar] [CrossRef]
- Perković, T.; Šolić, P.; Zargariasl, H.; Čoko, D.; Rodrigues, J.J.P.C. Smart Parking Sensors: State of the Art and Performance Evaluation. J. Clean. Prod. 2020, 262, 121181. [Google Scholar] [CrossRef]
- Rakkolainen, I.; Freeman, E.; Sand, A.; Raisamo, R.; Brewster, S. A Survey of Mid-Air Ultrasound Haptics and Its Applications. IEEE Trans. Haptics 2021, 14, 2–19. [Google Scholar] [CrossRef]
- Kessel, R.T.; Hollett, R.D. Underwater intruder detection sonar for harbour protection: State of the art review and implications. In Proceedings of the Second IEEE International Conference on Technologies for Homeland Security and Safety, Istanbul, Turkey, 9–13 October 2006. [Google Scholar]
- Huber, A. Non-Destructive Testing of Future Rocket Boosters Using Air-Coupled Ultrasound. In Proceedings of the 19th World Conference on NDT. WCNDT 2016, Munich, Germany, 13–17 June 2016. [Google Scholar]
- Mirchev, Y.; Alexiev, A.; Shekero, A.; Bukharov, S. Long-Range Ultrasonic and Phased Array Technologies. In Non-Destructive Testing and Repair of Pipelines; Springer: Cham, Switzerland, 2018; pp. 3–14. [Google Scholar] [CrossRef]
- Contreras Ortiz, S.H.; Chiu, T.; Fox, M.D. Ultrasound Image Enhancement: A Review. Biomed. Signal Process. Control 2012, 7, 419–428. [Google Scholar] [CrossRef]
- Dieterich, A.V.; Pickard, C.M.; Deshon, L.E.; Strauss, G.R.; Gibson, W.; Davey, P.; McKay, J. M-Mode Ultrasound Used to Detect the Onset of Deep Muscle Activity. J. Electromyogr. Kinesiol. 2015, 25, 224–231. [Google Scholar] [CrossRef]
- Shung, K.K. Diagnostic Ultrasound: Imaging and Blood Flow Measurements; Taylor & Francis: Boca Raton, FL, USA, 2006. [Google Scholar]
- Bailey, M.R.; Khokhlova, V.A.; Sapozhnikov, O.A.; Kargl, S.G.; Crum, L.A. Physical Mechanisms of the Therapeutic Effect of Ultrasound (a Review). Acoust. Phys. 2003, 49, 369–388. [Google Scholar] [CrossRef]
- Fine, I.H.; Packer, M.; Hoffman, R.S. New Phacoemulsification Technologies. J. Cataract. Refract. Surg. 2002, 28, 1054–1060. [Google Scholar] [CrossRef]
- Zhou, Y.F. High Intensity Focused Ultrasound in Clinical Tumor Ablation. World J. Clin. Oncol. 2011, 2, 8–27. [Google Scholar] [CrossRef]
- Warden, S.J.; McMeeken, J.M. Ultrasound Usage and Dosage in Sports Physiotherapy. Ultrasound Med. Biol. 2002, 28, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Deprez, J.; Lajoinie, G.; Engelen, Y.; De Smedt, S.; Lentacker, I. Opening Doors with Ultrasound and Microbubbles: Beating Biological Barriers to Promote Drug Delivery. Adv. Drug Deliv. Rev. 2021, 172, 9–36. [Google Scholar] [CrossRef] [PubMed]
- Shin Low, S.; Nong Lim, C.; Yew, M.; Siong Chai, W.; Low, L.E.; Manickam, S.; Ti Tey, B.; Show, P.L. Recent Ultrasound Advancements for the Manipulation of Nanobiomaterials and Nanoformulations for Drug Delivery. Ultrason. Sonochem. 2021, 80, 105805. [Google Scholar] [CrossRef] [PubMed]
- Sirsi, S.R.; Borden, M.A. State-of-the-Art Materials for Ultrasound-Triggered Drug Delivery. Adv. Drug Deliv. Rev. 2014, 72, 3–14. [Google Scholar] [CrossRef]
- Alexis, F.; Pridgen, E.; Langer, R.; Farokhzad, O. Nanoparticle Technologies for Cancer Therapy. Drug Deliv. 2010, 197, 55–86. [Google Scholar]
- Sagnella, S.M.; McCarroll, J.A.; Kavallaris, M. Drug Delivery: Beyond Active Tumour Targeting. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1131–1137. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Bromberg, L.; Concheiro, A. Light-Sensitive Intelligent Drug Delivery Systems. Photochem. Photobiol. 2009, 85, 848–860. [Google Scholar] [CrossRef]
- Weaver, J.C.; Chizmadzhev, Y.A. Theory of Electroporation: A Review. Bioelectrochem. Bioenerg. 1996, 41, 135–160. [Google Scholar] [CrossRef]
- Price, P.M.; Mahmoud, W.E.; Al-Ghamdi, A.A.; Bronstein, L.M. Magnetic Drug Delivery: Where the Field Is Going. Front. Chem. 2018, 6, 619. [Google Scholar] [CrossRef]
- Jin, Y.; Song, L.; Su, Y.; Zhu, L.; Pang, Y.; Qiu, F.; Tong, G.; Yan, D.; Zhu, B.; Zhu, X. Oxime Linkage: A Robust Tool for the Design of pH-Sensitive Polymeric Drug Carriers. Biomacromolecules 2011, 12, 3460–3468. [Google Scholar] [CrossRef]
- Shahriari, M.; Zahiri, M.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Enzyme Responsive Drug Delivery Systems in Cancer Treatment. J. Control. Release 2019, 308, 172–189. [Google Scholar] [CrossRef] [PubMed]
- Seah, B.C.Q.; Teo, B.M. Recent Advances in Ultrasound-Based Transdermal Drug Delivery. Int. J. Nanomed. 2018, 13, 7749–7763. [Google Scholar] [CrossRef] [PubMed]
- Mo, S.; Coussios, C.C.; Seymour, L.; Carlisle, R. Ultrasound-Enhanced Drug Delivery for Cancer. Expert Opin. Drug Deliv. 2012, 9, 1525–1538. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shriki, J. Ultrasound Physics. Crit. Care Clin. 2014, 30, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Abu-Zidan, F.M.; Hefny, A.F.; Corr, P. Clinical Ultrasound Physics. J. Emergencies Trauma Shock 2011, 4, 501–503. [Google Scholar] [CrossRef]
- Hoskins, P.R.; Martin, K.; Thrush, A. (Eds.) Diagnostic Ultrasound: Physics and Equipment, 3rd ed.; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2019. [Google Scholar]
- Amiri, H.; Makkiabadi, B. A Review of Ultrasound Thermometry Techniques. Front. Biomed. Technol. 2020, 7, 2. [Google Scholar] [CrossRef]
- Sarvazyan, A.; Tatarinov, A.; Sarvazyan, N. Ultrasonic Assessment of Tissue Hydration Status. Ultrasonics 2005, 43, 661–671. [Google Scholar] [CrossRef]
- Landau, L.; Lifshitz, E. Fluid Mechanics. In Course of Theoretical Physics, 2nd ed.; Pergamon Press: Oxford, UK, 1958; Volume 6. [Google Scholar]
- Filippi, P.; Bergassoli, A.; Habault, D.; Lefebvre, J.P. Acoustics: Basic Physics, Theory, and Methods; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Cheeke, J.D.N. Fundamentals and Applications of Ultrasonic Waves, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Kuttruff, H. Ultrasonics: Fundamentals and Applications; Springer: Dordrecht, The Netherlands, 1991. [Google Scholar] [CrossRef]
- Shutilov, V.A. Fundamental Physics of Ultrasound; Gordon and Breach Science Publishers: New York, NY, USA, 1988. [Google Scholar]
- Hunt, J.W. Principles of Ultrasound Used for Hyperthermia. In Physics and Technology of Hyperthermia; Field, S.B., Franconi, C., Eds.; NATO ASI Series; Springer: Dordrecht, The Netherlands, 1987; pp. 354–389. [Google Scholar] [CrossRef]
- Jang, J.; Chang, J. Design and Fabrication of Double-Focused Ultrasound Transducers to Achieve Tight Focusing. Sensors 2016, 16, 1248. [Google Scholar] [CrossRef]
- Elhelf, I.S.; Albahar, H.; Shah, U.; Oto, A.; Cressman, E.; Almekkawy, M. High Intensity Focused Ultrasound: The Fundamentals, Clinical Applications and Research Trends. Diagn. Interv. Imaging 2018, 99, 349–359. [Google Scholar] [CrossRef]
- Jensen, J.A. Medical Ultrasound Imaging. Prog. Biophys. Mol. Biol. 2007, 93, 153–165. [Google Scholar] [CrossRef]
- Hussey, M. Basic Physics and Technology of Medical Diagnostic Ultrasound; Macmillan Education UK: London, UK, 1985. [Google Scholar] [CrossRef]
- Laugier, P.; Haïat, G. Introduction to the Physics of Ultrasound. In Bone Quantitative Ultrasound; Laugier, P., Haïat, G., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 29–45. [Google Scholar] [CrossRef]
- Gutierrez-Vazquez, J.; Perez-Sanchez, L.I.; Satrustegui-Lapetra, M.; Arevalo-Manso, J.J.; Gomez-Herrera, J.J.; Criado-Alvarez, J.J. Measurement of the Anterior Chamber Depth and Ocular Axial Length: Transpalpebral B-mode Ultrasound Using an 18-MHz Linear Probe Compared with the IOL Master 500. Med. Ultrason. 2021, 23, 48. [Google Scholar] [CrossRef]
- Rohrbach, D.; Ito, K.; Lloyd, H.O.; Silverman, R.H.; Yoshida, K.; Yamaguchi, T.; Mamou, J. Material Properties of Human Ocular Tissue at 7-Mm Resolution. Ultrason. Imaging 2017, 39, 313–325. [Google Scholar] [CrossRef]
- Satitpitakul, V.; Taweekitikul, P.; Puangsricharern, V.; Kasetsuwan, N.; Reinprayoon, U.; Kittipibul, T. Alteration of Corneal Biomechanical Properties in Patients with Dry Eye Disease. PLoS ONE 2021, 16, e0254442. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, J.M.; Mol, H.J.M.; Timmer, M.R. Acoustic Parameters of Ocular Tissues. Ultrasound Med. Biol. 1985, 11, 157–161. [Google Scholar] [CrossRef]
- De Bernardo, M.; Vitiello, L.; Cornetta, P.; Rosa, N. Ocular Ultrasound Evaluation of Optic Nerve Sheath Diameter in Military Environments. Mil. Med. Res. 2019, 6, 16. [Google Scholar] [CrossRef]
- Barnett, S.B.; Rott, H.D.; ter Haar, G.R.; Ziskin, M.C.; Maeda, K. The Sensitivity of Biological Tissue to Ultrasound. Ultrasound Med. Biol. 1997, 23, 805–812. [Google Scholar] [CrossRef]
- Zhou, Y. Noninvasive Thermometry in High-Intensity Focused Ultrasound Ablation. Ultrasound Q. 2017, 33, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.H.S.; Sun, J.S.; Chang, S.P.; Lin, J.C. Study of Thermal Effects of Ultrasound Stimulation on Fracture Healing. Bioelectromagnetics 2002, 23, 256–263. [Google Scholar] [CrossRef] [PubMed]
- ter Haar, G. Therapeutic Applications of Ultrasound. Prog. Biophys. Mol. Biol. 2007, 93, 111–129. [Google Scholar] [CrossRef]
- Draper, D.O.; Mahaffey, C.; Kaiser, D.; Eggett, D.; Jarmin, J. Thermal Ultrasound Decreases Tissue Stiffness of Trigger Points in Upper Trapezius Muscles. Physiother. Theory Pract. 2010, 26, 167–172. [Google Scholar] [CrossRef]
- Staruch, R.; Chopra, R.; Hynynen, K. Localised Drug Release Using MRI-controlled Focused Ultrasound Hyperthermia. Int. J. Hyperth. 2011, 27, 156–171. [Google Scholar] [CrossRef] [PubMed]
- Wardlow, R.; Bing, C.; VanOsdol, J.; Maples, D.; Ladouceur-Wodzak, M.; Harbeson, M.; Nofiele, J.; Staruch, R.; Ramachandran, A.; Malayer, J.; et al. Targeted Antibiotic Delivery Using Low Temperature-Sensitive Liposomes and Magnetic Resonance-Guided High-Intensity Focused Ultrasound Hyperthermia. Int. J. Hyperth. 2016, 32, 254–264. [Google Scholar] [CrossRef] [PubMed]
- de Smet, M.; Hijnen, N.M.; Langereis, S.; Elevelt, A.; Heijman, E.; Dubois, L.; Lambin, P.; Grüll, H. Magnetic Resonance Guided High-Intensity Focused Ultrasound Mediated Hyperthermia Improves the Intratumoral Distribution of Temperature-Sensitive Liposomal Doxorubicin. Investig. Radiol. 2013, 48, 395. [Google Scholar] [CrossRef] [PubMed]
- Escoffre, J.M.; Novell, A.; de Smet, M.; Bouakaz, A. Focused Ultrasound Mediated Drug Delivery from Temperature-Sensitive Liposomes: In-Vitro Characterization and Validation. Phys. Med. Biol. 2013, 58, 8135. [Google Scholar] [CrossRef] [PubMed]
- de Smet, M.; Langereis, S.; van den Bosch, S.; Bitter, K.; Hijnen, N.M.; Heijman, E.; Grüll, H. SPECT/CT Imaging of Temperature-Sensitive Liposomes for MR-image Guided Drug Delivery with High Intensity Focused Ultrasound. J. Control. Release 2013, 169, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Gasselhuber, A.; Dreher, M.R.; Partanen, A.; Yarmolenko, P.S.; Woods, D.; Wood, B.J.; Haemmerich, D. Targeted Drug Delivery by High Intensity Focused Ultrasound Mediated Hyperthermia Combined with Temperature-Sensitive Liposomes: Computational Modelling and Preliminary in Vivovalidation. Int. J. Hyperth. 2012, 28, 337–348. [Google Scholar] [CrossRef]
- Ranjan, A.; Jacobs, G.C.; Woods, D.L.; Negussie, A.H.; Partanen, A.; Yarmolenko, P.S.; Gacchina, C.E.; Sharma, K.V.; Frenkel, V.; Wood, B.J.; et al. Image-Guided Drug Delivery with Magnetic Resonance Guided High Intensity Focused Ultrasound and Temperature Sensitive Liposomes in a Rabbit Vx2 Tumor Model. J. Control. Release 2012, 158, 487–494. [Google Scholar] [CrossRef]
- Kheirolomoom, A.; Lai, C.Y.; Tam, S.M.; Mahakian, L.M.; Ingham, E.S.; Watson, K.D.; Ferrara, K.W. Complete Regression of Local Cancer Using Temperature-Sensitive Liposomes Combined with Ultrasound-Mediated Hyperthermia. J. Control. Release 2013, 172, 266–273. [Google Scholar] [CrossRef] [PubMed]
- de Smet, M.; Heijman, E.; Langereis, S.; Hijnen, N.M.; Grüll, H. Magnetic Resonance Imaging of High Intensity Focused Ultrasound Mediated Drug Delivery from Temperature-Sensitive Liposomes: An in Vivo Proof-of-Concept Study. J. Control. Release 2011, 150, 102–110. [Google Scholar] [CrossRef]
- Deng, Z.; Xiao, Y.; Pan, M.; Li, F.; Duan, W.; Meng, L.; Liu, X.; Yan, F.; Zheng, H. Hyperthermia-Triggered Drug Delivery from iRGD-modified Temperature-Sensitive Liposomes Enhances the Anti-Tumor Efficacy Using High Intensity Focused Ultrasound. J. Control. Release 2016, 243, 333–341. [Google Scholar] [CrossRef]
- Sebeke, L.C.; Castillo Gómez, J.D.; Heijman, E.; Rademann, P.; Simon, A.C.; Ekdawi, S.; Vlachakis, S.; Toker, D.; Mink, B.L.; Schubert-Quecke, C.; et al. Hyperthermia-Induced Doxorubicin Delivery from Thermosensitive Liposomes via MR-HIFU in a Pig Model. J. Control. Release 2022, 343, 798–812. [Google Scholar] [CrossRef] [PubMed]
- Starritt, H.C.; Duck, F.A. Safety. In Clinical Ultrasound; Elsevier: Amsterdam, The Netherlands, 2011; pp. 51–60. [Google Scholar] [CrossRef]
- Maruvada, S.; Harris, G.R.; Herman, B.A.; King, R.L. Acoustic Power Calibration of High-Intensity Focused Ultrasound Transducers Using a Radiation Force Technique. J. Acoust. Soc. Am. 2007, 121, 1434–1439. [Google Scholar] [CrossRef] [PubMed]
- Zderic, V.; Vaezy, S.; Martin, R.W.; Clark, J.I. Ocular Drug Delivery Using 20-kHz Ultrasound. Ultrasound Med. Biol. 2002, 28, 823–829. [Google Scholar] [CrossRef]
- Gor’kov, L.P. Hydrodynamics, Technical Report. 1962.
- Torr, G.R. Acoustic Radiation Force; American Association of Physics Teachers: College Park, MD, USA, 1983; pp. 402–408. [Google Scholar]
- Mitome, H. The Mechanism of Generation of Acoustic Streaming. Electron. Commun. Jpn. (Part III: Fundam. Electron. Sci.) 1998, 81, 1–8. [Google Scholar] [CrossRef]
- Matlaga, B.R.; McAteer, J.A.; Connors, B.A.; Handa, R.K.; Evan, A.P.; Williams, J.C.; Lingeman, J.E.; Willis, L.R. Potential for Cavitation-Mediated Tissue Damage in Shockwave Lithotripsy. J. Endourol. 2008, 22, 121–126. [Google Scholar] [CrossRef]
- Roberts, W.W.; Hall, T.L.; Ives, K.; Wolf, J.S.; Fowlkes, J.B.; Cain, C.A. Pulsed Cavitational Ultrasound: A Noninvasive Technology for Controlled Tissue Ablation (Histotripsy) in the Rabbit Kidney. J. Urol. 2006, 175, 734–738. [Google Scholar] [CrossRef]
- Duck, F.A. The Meaning of Thermal Index (TI) and Mechanical Index (MI) Values. BMUS Bull. 1997, 5, 36–40. [Google Scholar] [CrossRef]
- Knapp, R.T. Cavitation and Nuclei. Trans. Am. Soc. Mech. Eng. 2022, 80, 1315–1324. [Google Scholar] [CrossRef]
- Nakamura, K. Ultrasonic Transducers: Materials and Design for Sensors, Actuators and Medical Applications; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Hykes, D.L.; Hendrick, W.R.; Starchman, D.E. Basic Ultrasound Instrumentation. In Ultrasound Physics and Instrumentation; Mosby Year Book: St. Louis, MO, USA, 1992; pp. 3–36. [Google Scholar]
- Manbachi, A.; Cobbold, R.S.C. Development and Application of Piezoelectric Materials for Ultrasound Generation and Detection. Ultrasound 2011, 19, 187–196. [Google Scholar] [CrossRef]
- Lay, R.; Sjoerd Deijs, G.; Malmström, J. The Intrinsic Piezoelectric Properties of Materials – a Review with a Focus on Biological Materials. RSC Adv. 2021, 11, 30657–30673. [Google Scholar] [CrossRef]
- Wild, J.J.; Neal, D. Use of High-Frequency Ultrasonic Waves for Detecting Changes of Texture in Living Tissues. Lancet 1951, 257, 655–657. [Google Scholar] [CrossRef]
- Powers, J.; Kremkau, F. Medical Ultrasound Systems. Interface Focus 2011, 1, 477–489. [Google Scholar] [CrossRef]
- Szabo, T.L.; Lewin, P.A. Ultrasound Transducer Selection in Clinical Imaging Practice. J. Ultrasound Med. 2013, 32, 573–582. [Google Scholar] [CrossRef]
- Stewart, F.; Verbeni, A.; Qiu, Y.; Cox, B.F.; Vorstius, J.; Newton, I.P.; Huang, Z.; Menciassi, A.; Näthke, I.; Cochran, S. A Prototype Therapeutic Capsule Endoscope for Ultrasound-Mediated Targeted Drug Delivery. J. Med. Robot. Res. 2018, 03, 1840001. [Google Scholar] [CrossRef]
- Qiu, Y.; Gigliotti, J.V.; Wallace, M.; Griggio, F.; Demore, C.E.M.; Cochran, S.; Trolier-McKinstry, S. Piezoelectric Micromachined Ultrasound Transducer (PMUT) Arrays for Integrated Sensing, Actuation and Imaging. Sensors 2015, 15, 8020–8041. [Google Scholar] [CrossRef]
- Suen, W.L.L.; Jiang, J.; Wong, H.S.; Qu, J.; Chau, Y. Examination of Effects of Low-Frequency Ultrasound on Scleral Permeability and Collagen Network. Ultrasound Med. Biol. 2016, 42, 2650–2661. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Li, Z.; Chung, J.T.; Lau, L.C.M.; Jhanji, V.; Chau, Y. Low-Intensity Low-Frequency Ultrasound Mediates Riboflavin Delivery during Corneal Crosslinking. Bioeng. Transl. Med. 2023, 8, e10442. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Lin, X.; He, M. Low-Frequency, Low-Intensity Ultrasound Exposure Safety in Animal Eyes. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4110. [Google Scholar]
- Cucevic, V.; Brown, A.S.; Foster, F.S. Thermal Assessment of 40-MHz Pulsed Doppler Ultrasound in Human Eye. Ultrasound Med. Biol. 2005, 31, 565–573. [Google Scholar] [CrossRef]
- Stolnik, S.; Illum, L.; Davis, S.S. Long Circulating Microparticulate Drug Carriers. Adv. Drug Deliv. Rev. 2012, 64, 290–301. [Google Scholar] [CrossRef]
- Dadwal, A.; Baldi, A.; Kumar Narang, R. Nanoparticles as Carriers for Drug Delivery in Cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, Y.; Liu, D.; Wang, Y.; Lu, M.; Zhang, Q.; Huang, J.; Li, Y.; Ma, T.; Yan, F.; et al. In-Vivo Programmable Acoustic Manipulation of Genetically Engineered Bacteria. Nat. Commun. 2023, 14, 3297. [Google Scholar] [CrossRef]
- Galanzha, E.I.; Viegas, M.G.; Malinsky, T.I.; Melerzanov, A.V.; Juratli, M.A.; Sarimollaoglu, M.; Nedosekin, D.A.; Zharov, V.P. In Vivo Acoustic and Photoacoustic Focusing of Circulating Cells. Sci. Rep. 2016, 6, 21531. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Hwang, J. Ultrasound-Targeted Microbubble Destruction for Chemotherapeutic Drug Delivery to Solid Tumors. J. Ther. Ultrasound 2013, 1, 10. [Google Scholar] [CrossRef]
- Graham, S.M.; Carlisle, R.; Choi, J.J.; Stevenson, M.; Shah, A.R.; Myers, R.S.; Fisher, K.; Peregrino, M.B.; Seymour, L.; Coussios, C.C. Inertial Cavitation to Non-Invasively Trigger and Monitor Intratumoral Release of Drug from Intravenously Delivered Liposomes. J. Control. Release 2014, 178, 101–107. [Google Scholar] [CrossRef]
- Krut, Z.; Gazit, D.; Gazit, Z.; Pelled, G. Applications of Ultrasound-Mediated Gene Delivery in Regenerative Medicine. Bioengineering 2022, 9, 190. [Google Scholar] [CrossRef] [PubMed]
- Togtema, M.; Pichardo, S.; Jackson, R.; Lambert, P.F.; Curiel, L.; Zehbe, I. Sonoporation Delivery of Monoclonal Antibodies against Human Papillomavirus 16 E6 Restores P53 Expression in Transformed Cervical Keratinocytes. PLoS ONE 2012, 7, e50730. [Google Scholar] [CrossRef]
- Du, M.; Li, Y.; Chen, Z. Sonoporation-Mediated Gene Transfection: A Novel Direction for Cell Reprogramming In Vivo. Front. Bioeng. Biotechnol. 2022, 9, 803055. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Kumon, R.E.; Deng, C.X. Mechanisms of Microbubble-Facilitated Sonoporation for Drug and Gene Delivery. Ther. Deliv. 2014, 5, 467–486. [Google Scholar] [CrossRef]
- Rich, J.; Tian, Z.; Huang, T.J. Sonoporation: Past, Present, and Future. Adv. Mater. Technol. 2022, 7, 2100885. [Google Scholar] [CrossRef]
- Tu, J.; Yu, A.C.H. Ultrasound-Mediated Drug Delivery: Sonoporation Mechanisms, Biophysics, and Critical Factors. BME Front. 2022, 2022, 9807347. [Google Scholar] [CrossRef]
- Chambers, S.D.; Bartlett, R.H.; Ceccio, S.L. Determination of the in Vivo Cavitation Nuclei Characteristics of Blood. ASAIO J. (Am. Soc. Artif. Intern. Organs 1992) 1999, 45, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Hou, X.; Wong, K.F.; Yang, Y.; Qiu, Z.; Wu, Y.; Hou, S.; Fei, C.; Guo, J.; Sun, L. Gas-Filled Protein Nanostructures as Cavitation Nuclei for Molecule-Specific Sonodynamic Therapy. Acta Biomater. 2021, 136, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.G.; Jonnalagadda, U.S.; Kwan, J.J. Biomedical Applications for Gas-Stabilizing Solid Cavitation Agents. Langmuir 2019, 35, 10106–10115. [Google Scholar] [CrossRef]
- Cardoso, F.L.; Brites, D.; Brito, M.A. Looking at the Blood–Brain Barrier: Molecular Anatomy and Possible Investigation Approaches. Brain Res. Rev. 2010, 64, 328–363. [Google Scholar] [CrossRef]
- Sharif, Y.; Jumah, F.; Coplan, L.; Krosser, A.; Sharif, K.; Tubbs, R.S. Blood Brain Barrier: A Review of Its Anatomy and Physiology in Health and Disease. Clin. Anat. 2018, 31, 812–823. [Google Scholar] [CrossRef]
- Wong, A.; Ye, M.; Levy, A.; Rothstein, J.; Bergles, D.; Searson, P. The Blood-Brain Barrier: An Engineering Perspective. Front. Neuroeng. 2013, 6, 7. [Google Scholar]
- McMahon, D.; Hynynen, K. Acute Inflammatory Response Following Increased Blood-Brain Barrier Permeability Induced by Focused Ultrasound Is Dependent on Microbubble Dose. Theranostics 2017, 7, 3989–4000. [Google Scholar] [CrossRef]
- Morse, S.V.; Pouliopoulos, A.N.; Chan, T.G.; Copping, M.J.; Lin, J.; Long, N.J.; Choi, J.J. Rapid Short-pulse Ultrasound Delivers Drugs Uniformly across the Murine Blood-Brain Barrier with Negligible Disruption. Radiology 2019, 291, 459–466. [Google Scholar] [CrossRef]
- Alli, S.; Figueiredo, C.A.; Golbourn, B.; Sabha, N.; Wu, M.Y.; Bondoc, A.; Luck, A.; Coluccia, D.; Maslink, C.; Smith, C.; et al. Brainstem Blood Brain Barrier Disruption Using Focused Ultrasound: A Demonstration of Feasibility and Enhanced Doxorubicin Delivery. J. Control. Release 2018, 281, 29–41. [Google Scholar] [CrossRef]
- Kobus, T.; Zervantonakis, I.K.; Zhang, Y.; McDannold, N.J. Growth Inhibition in a Brain Metastasis Model by Antibody Delivery Using Focused Ultrasound-Mediated Blood-Brain Barrier Disruption. J. Control. Release 2016, 238, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Kong, C.; Cho, J.S.; Lee, J.; Koh, C.S.; Yoon, M.S.; Na, Y.C.; Chang, W.S.; Chang, J.W. Focused Ultrasound–Mediated Noninvasive Blood-Brain Barrier Modulation: Preclinical Examination of Efficacy and Safety in Various Sonication Parameters. Neurosurg. Focus 2018, 44, E15. [Google Scholar] [CrossRef] [PubMed]
- Bachu, R.D.; Chowdhury, P.; Al-Saedi, Z.H.F.; Karla, P.K.; Boddu, S.H.S. Ocular Drug Delivery Barriers—Role of Nanocarriers in the Treatment of Anterior Segment Ocular Diseases. Pharmaceutics 2018, 10, 28. [Google Scholar] [CrossRef]
- Cunha-Vaz, J.G. The blood-retinal barriers. Doc. Ophthalmol. 1976, 41, 287–327. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, E.; Del Rio-Tsonis, K. Eye Anatomy. In eLS, 1st ed.; John Wiley & Sons, Ltd.: New York, NY, USA, 2012. [Google Scholar] [CrossRef]
- Mishima, S.; Gasset, A.; Klyce, S.D. Determination of Tear Volume and Tear Flow. Investig. Ophthalmol. 1966, 5, 264–276. [Google Scholar]
- Gorantla, S.; Krishna Rapalli, V.; Waghule, T.; Prakash Singh, P.; Kumar Dubey, S.; Saha, R.N.; Singhvi, G. Nanocarriers for Ocular Drug Delivery: Current Status and Translational Opportunity. RSC Adv. 2020, 10, 27835–27855. [Google Scholar] [CrossRef]
- Pflugfelder, S.C.; Stern, M.E. Biological Functions of Tear Film. Exp. Eye Res. 2020, 197, 108115. [Google Scholar] [CrossRef] [PubMed]
- Vaishya, R.D.; Khurana, V.; Patel, S.; Mitra, A.K. Controlled Ocular Drug Delivery with Nanomicelles. WIREs Nanomed. Nanobiotechnol. 2014, 6, 422–437. [Google Scholar] [CrossRef]
- Gan, L.; Wang, J.; Jiang, M.; Bartlett, H.; Ouyang, D.; Eperjesi, F.; Liu, J.; Gan, Y. Recent Advances in Topical Ophthalmic Drug Delivery with Lipid-Based Nanocarriers. Drug Discov. Today 2013, 18, 290–297. [Google Scholar] [CrossRef]
- Lee, D.A.; Higginbotham, E.J. Glaucoma and Its Treatment: A Review. Am. J. Health-Syst. Pharm. 2005, 62, 691–699. [Google Scholar] [CrossRef]
- Stamper, R.L. Primary Drug Treatment for Glaucoma: Beta-Blockers Versus Other Medications. Surv. Ophthalmol. 2002, 47, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.S.; Mitchell, P.; Seddon, J.M.; Holz, F.G.; Wong, T.Y. Age-Related Macular Degeneration. Lancet 2012, 379, 1728–1738. [Google Scholar] [CrossRef]
- Sampat, K.M.; Garg, S.J. Complications of Intravitreal Injections. J. Abbr. 2010, 21, 178–183. [Google Scholar] [CrossRef]
- Lam, D.; Rao, S.K.; Ratra, V.; Liu, Y.; Mitchell, P.; King, J.; Tassignon, M.J.; Jonas, J.; Pang, C.P.; Chang, D.F. Cataract. Nat. Rev. Dis. Prim. 2015, 1, 1–15. [Google Scholar] [CrossRef] [PubMed]
- El Rami, H.; Barham, R.; Sun, J.K.; Silva, P.S. Evidence-Based Treatment of Diabetic Retinopathy. Semin. Ophthalmol. 2017, 32, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Grzybowski, A.; Kanclerz, P.; Huerva, V.; Ascaso, F.J.; Tuuminen, R. Diabetes and Phacoemulsification Cataract Surgery: Difficulties, Risks and Potential Complications. J. Clin. Med. 2019, 8, 716. [Google Scholar] [CrossRef]
- Stitt, A.W.; Curtis, T.M.; Chen, M.; Medina, R.J.; McKay, G.J.; Jenkins, A.; Gardiner, T.A.; Lyons, T.J.; Hammes, H.P.; Simó, R.; et al. The Progress in Understanding and Treatment of Diabetic Retinopathy. Prog. Retin. Eye Res. 2016, 51, 156–186. [Google Scholar] [CrossRef]
- Azari, A.A.; Arabi, A. Conjunctivitis: A Systematic Review. J. Ophthalmic Vis. Res. 2020, 15, 372–395. [Google Scholar] [CrossRef]
- Karpecki, P.; Paterno, M.R.; Comstock, T.L. Limitations of Current Antibiotics for the Treatment of Bacterial Conjunctivitis. Optom. Vis. Sci. 2010, 87, 908–919. [Google Scholar] [CrossRef]
- Durgun, M.E.; Güngör, S.; Özsoy, Y. Micelles: Promising Ocular Drug Carriers for Anterior and Posterior Segment Diseases. J. Ocul. Pharmacol. Ther. 2020, 36, 323–341. [Google Scholar] [CrossRef]
- Washington, N.; Washington, C.; Wilson, C.G. Physiological Pharmaceutics; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Mun, E.A.; Morrison, P.W.J.; Williams, A.C.; Khutoryanskiy, V.V. On the Barrier Properties of the Cornea: A Microscopy Study of the Penetration of Fluorescently Labeled Nanoparticles, Polymers, and Sodium Fluorescein. Mol. Pharm. 2014, 11, 3556–3564. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.L.; Chen, K.H.; Su, W.Y.; Lee, Y.H.; Wu, C.C.; Lin, F.H. Cationic gelatin nanoparticles for drug delivery to the ocular surface: In vitro and in vivo evaluation. J. Nanomater. 2013, 2013, 238351. [Google Scholar] [CrossRef]
- Lallemand, F.; Daull, P.; Benita, S.; Buggage, R.; Garrigue, J.S. Successfully Improving Ocular Drug Delivery Using the Cationic Nanoemulsion, Novasorb. J. Drug Deliv. 2012, 2012, 604204. [Google Scholar] [CrossRef]
- Giannavola, C.; Bucolo, C.; Maltese, A.; Paolino, D.; Vandelli, M.A.; Puglisi, G.; Lee, V.H.L.; Fresta, M. Influence of Preparation Conditions on Acyclovir-Loaded Poly-d,l-Lactic Acid Nanospheres and Effect of PEG Coating on Ocular Drug Bioavailability. Pharm. Res. 2003, 20, 584–590. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, Y.; Yang, Z.; Li, M.; Li, F.; Cui, F.; Liu, T.; Shi, W.; Wu, X. Nanomicelle Formulation for Topical Delivery of Cyclosporine A into the Cornea: In Vitro Mechanism and in Vivo Permeation Evaluation. Sci. Rep. 2015, 5, 12968. [Google Scholar] [CrossRef]
- Lancina, M.G.; Yang, H. Dendrimers for Ocular Drug Delivery. Can. J. Chem. 2017, 95, 897–902. [Google Scholar] [CrossRef]
- Yao, W.J.; Sun, K.X.; Liu, Y.; Liang, N.; Mu, H.J.; Yao, C.; Liang, R.C.; Wang, A.P. Effect of Poly(Amidoamine) Dendrimers on Corneal Penetration of Puerarin. Biol. Pharm. Bull. 2010, 33, 1371–1377. [Google Scholar] [CrossRef]
- Venditti, I. Morphologies and Functionalities of Polymeric Nanocarriers as Chemical Tools for Drug Delivery: A Review. J. King Saud Univ.-Sci. 2019, 31, 398–411. [Google Scholar] [CrossRef]
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective Use of Nanocarriers as Drug Delivery Systems for the Treatment of Selected Tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef]
- Bisht, R.; Mandal, A.; Jaiswal, J.K.; Rupenthal, I.D. Nanocarrier Mediated Retinal Drug Delivery: Overcoming Ocular Barriers to Treat Posterior Eye Diseases. WIREs Nanomed. Nanobiotechnol. 2018, 10, e1473. [Google Scholar] [CrossRef]
- Mains, J.; Wilson, C.G. The Vitreous Humor As a Barrier to Nanoparticle Distribution. J. Ocul. Pharmacol. Ther. 2013, 29, 143–150. [Google Scholar] [CrossRef]
- Hughes, P.; Olejnik, O.; Changlin, J.; Wilson, C. Topical and Systemic Drug Delivery to the Posterior Segments. Adv. Drug Deliv. Rev. 2005, 57, 2010–2032. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.A.; Brereton, H.M.; Farrall, A.; Standfield, S.D.; Taylor, S.D.; Kirk, L.A.; Coster, D.J. Topically Applied Antibody Fragments Penetrate into the Back of the Rabbit Eye. Eye 2005, 19, 910–913. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tan, C.S. Safety and Complications of Intravitreal Injections Performed in an Asian Population in Singapore. Int. Ophthalmol. 2017, 37, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Afarid, M.; Sadegi Sarvestani, A.; Rahat, F.; Azimi, A. Intravitreal Injection of Bevacizumab: Review of Our Previous Experience. Iran. J. Pharm. Res. IJPR 2018, 17, 1093–1098. [Google Scholar]
- Levin, A.M.; Chaya, C.J.; Kahook, M.Y.; Wirostko, B.M. Intraocular Pressure Elevation Following Intravitreal Anti-VEGF Injections: Short- and Long-term Considerations. J. Glaucoma 2021, 30, 1019–1026. [Google Scholar] [CrossRef]
- de Vries, V.A.; Bassil, F.L.; Ramdas, W.D. The Effects of Intravitreal Injections on Intraocular Pressure and Retinal Nerve Fiber Layer: A Systematic Review and Meta-Analysis. Sci. Rep. 2020, 10, 13248. [Google Scholar] [CrossRef]
- Pacella, E.; Loffredo, L.; Malvasi, M.; Trovato Battagliola, E.; Messineo, D.; Pacella, F.; Arrico, L. Effects of Repeated Intravitreal Injections of Dexamethasone Implants on Intraocular Pressure: A 4-Year Study. Clin. Ophthalmol. 2020, 14, 3611–3617. [Google Scholar] [CrossRef]
- Mishra, D.; Gade, S.; Glover, K.; Sheshala, R.; Singh, T.R.R. Vitreous Humor: Composition, Characteristics and Implication on Intravitreal Drug Delivery. Curr. Eye Res. 2023, 48, 208–218. [Google Scholar] [CrossRef]
- Koo, H.; Moon, H.; Han, H.; Na, J.H.; Huh, M.S.; Park, J.H.; Woo, S.J.; Park, K.H.; Chan Kwon, I.; Kim, K.; et al. The Movement of Self-Assembled Amphiphilic Polymeric Nanoparticles in the Vitreous and Retina after Intravitreal Injection. Biomaterials 2012, 33, 3485–3493. [Google Scholar] [CrossRef]
- Pitkänen, L.; Ruponen, M.; Nieminen, J.; Urtti, A. Vitreous Is a Barrier in Nonviral Gene Transfer by Cationic Lipids and Polymers. Pharm. Res. 2003, 20, 576–583. [Google Scholar] [CrossRef]
- Asencio-Duran, M.; Vallejo-Garcia, J.L.; Pastora-Salvador, N.; Fonseca-Sandomingo, A.; Romano, M.R. Vitreous Diagnosis in Neoplastic Diseases. Mediat. Inflamm. 2012, 2012, e930704. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, S.; Saville, B.; Cheng, Y.L. Drug Distribution in the Vitreous Humor of the Human Eye: The Effects of Aphakia and Changes in Retinal Permeability and Vitreous Diffusivity. J. Ocul. Pharmacol. Ther. 1997, 13, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Tabibian, D.; Hoogewoud, F.; Mavrakanas, N.; Schutz, J.S. Misdirected Aqueous Flow in Rhegmatogenous Retinal Detachment: A Pathophysiology Update. Surv. Ophthalmol. 2015, 60, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.W.; Lee, C.J.; Gardiner, B.S. No Flow through the Vitreous Humor: How Strong Is the Evidence? Prog. Retin. Eye Res. 2020, 78, 100845. [Google Scholar] [CrossRef]
- Nabili, M.; Patel, H.; Mahesh, S.P.; Liu, J.; Geist, C.; Zderic, V. Ultrasound-Enhanced Delivery of Antibiotics and Anti-Inflammatory Drugs Into the Eye. Ultrasound Med. Biol. 2013, 39, 638–646. [Google Scholar] [CrossRef]
- Duxfield, L.; Sultana, R.; Wang, R.; Englebretsen, V.; Deo, S.; Rupenthal, I.D.; Al-Kassas, R. Ocular Delivery Systems for Topical Application of Anti-Infective Agents. Drug Dev. Ind. Pharm. 2016, 42, 1–11. [Google Scholar] [CrossRef]
- Seyfoddin, A.; Shaw, J.; Al-Kassas, R. Solid Lipid Nanoparticles for Ocular Drug Delivery. Drug Deliv. 2010, 17, 467–489. [Google Scholar] [CrossRef]
- Zderic, V.; Clark, J.I.; Vaezy, S. Drug Delivery Into the Eye With the Use of Ultrasound. J. Ultrasound Med. 2004, 23, 1349–1359. [Google Scholar] [CrossRef]
- Vazirani, J.; Basu, S. Keratoconus: Current Perspectives. Clin. Ophthalmol. (Auckland, N.Z.) 2013, 7, 2019–2030. [Google Scholar] [CrossRef]
- Kim, S.H.; Lutz, R.J.; Wang, N.S.; Robinson, M.R. Transport Barriers in Transscleral Drug Delivery for Retinal Diseases. Ophthalmic Res. 2007, 39, 244–254. [Google Scholar] [CrossRef]
- Suen, W.L.L.; Wong, H.S.; Yu, Y.; Lau, L.C.M.; Lo, A.C.Y.; Chau, Y. Ultrasound-Mediated Transscleral Delivery of Macromolecules to the Posterior Segment of Rabbit Eye In Vivo. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4358–4365. [Google Scholar] [CrossRef] [PubMed]
- Razavi, A.; Clement, D.; Fowler, R.A.; Birer, A.; Chavrier, F.; Mestas, J.l.; Romano, F.; Chapelon, J.Y.; Béglé, A.; Lafon, C. Contribution of Inertial Cavitation in the Enhancement of In Vitro Transscleral Drug Delivery. Ultrasound Med. Biol. 2014, 40, 1216–1227. [Google Scholar] [CrossRef] [PubMed]
- Lamy, R.; Chan, E.; Lee, O.T.; Phone, A.; Salgaonkar, V.A.; Diederich, C.J.; Stewart, J.M. 880 kHz Ultrasound Treatment for Drug Delivery to the Vitreous Humor. Am. J. Transl. Res. 2018, 10, 3162. [Google Scholar] [PubMed]
- Park, J.; Zhang, Y.; Vykhodtseva, N.; Akula, J.D.; McDannold, N.J. Targeted and Reversible Blood-Retinal Barrier Disruption via Focused Ultrasound and Microbubbles. PLoS ONE 2012, 7, e42754. [Google Scholar] [CrossRef]
- Touahri, Y.; Dixit, R.; Kofoed, R.H.; Mikloska, K.; Park, E.; Raeisossadati, R.; Markham-Coultes, K.; David, L.A.; Rijal, H.; Zhao, J.; et al. Focused Ultrasound as a Novel Strategy for Noninvasive Gene Delivery to Retinal Müller Glia. Theranostics 2020, 10, 2982–2999. [Google Scholar] [CrossRef]
- Rousou, C.; Schuurmans, C.C.L.; Urtti, A.; Mastrobattista, E.; Storm, G.; Moonen, C.; Kaarniranta, K.; Deckers, R. Ultrasound and Microbubbles for the Treatment of Ocular Diseases: From Preclinical Research towards Clinical Application. Pharmaceutics 2021, 13, 1782. [Google Scholar] [CrossRef]
- O’Reilly, M.A.; Hynynen, K. Blood-Brain Barrier: Real-time Feedback-controlled Focused Ultrasound Disruption by Using an Acoustic Emissions–Based Controller. Radiology 2012, 263, 96–106. [Google Scholar] [CrossRef]
- Rousou, C.; van Kronenburg, N.; Sonnen, A.F.P.; van Dijk, M.; Moonen, C.; Storm, G.; Mastrobattista, E.; Deckers, R. Microbubble-Assisted Ultrasound for Drug Delivery to the Retina in an Ex Vivo Eye Model. Pharmaceutics 2023, 15, 1220. [Google Scholar] [CrossRef]
- Almogbil, H.H.; Nasrallah, F.P.; Zderic, V. Feasibility of Therapeutic Ultrasound Application in Topical Scleral Delivery of Avastin. Transl. Vis. Sci. Technol. 2021, 10, 2. [Google Scholar] [CrossRef]
- Huang, D.; Wang, L.; Dong, Y.; Pan, X.; Li, G.; Wu, C. A Novel Technology Using Transscleral Ultrasound to Deliver Protein Loaded Nanoparticles. Eur. J. Pharm. Biopharm. 2014, 88, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Chen, Y.S.; Thakur, S.S.; Rupenthal, I.D. Ultrasound-Mediated Nanoparticle Delivery across Ex Vivo Bovine Retina after Intravitreal Injection. Eur. J. Pharm. Biopharm. 2017, 119, 125–136. [Google Scholar] [CrossRef]
- Thakur, S.S.; Chen, Y.S.; Houston, Z.H.; Fletcher, N.; Barnett, N.L.; Thurecht, K.J.; Rupenthal, I.D.; Parekh, H.S. Ultrasound-Responsive Nanobubbles for Enhanced Intravitreal Drug Migration: An Ex Vivo Evaluation. Eur. J. Pharm. Biopharm. 2019, 136, 102–107. [Google Scholar] [CrossRef]
- Lafond, M.; Aptel, F.; Mestas, J.L.; Lafon, C. Ultrasound-Mediated Ocular Delivery of Therapeutic Agents: A Review. Expert Opin. Drug Deliv. 2017, 14, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadiq, H.; Walters, J.; Alshayeb, A.; Parekh, H.; Veidt, M. Tissue Mimicking Phantom of the Acoustical and Geometrical Properties of the Human Eye. In Proceedings of the Conference of the Acoustical Society of New Zealand, Wellington, New Zealand, 31 October–2 November 2022. [Google Scholar]
- Thakur, S.S.; Shenoy, S.K.; Suk, J.S.; Hanes, J.S.; Rupenthal, I.D. Validation of Hyaluronic Acid-Agar-Based Hydrogels as Vitreous Humor Mimetics for in Vitro Drug and Particle Migration Evaluations. Eur. J. Pharm. Biopharm. 2020, 148, 118–125. [Google Scholar] [CrossRef]
- Murugappan, S.K.; Zhou, Y. Transsclera Drug Delivery by Pulsed High-Intensity Focused Ultrasound (HIFU): An Ex Vivo Study. Curr. Eye Res. 2015, 40, 1172–1180. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duncan, B.; Al-Kassas, R.; Zhang, G.; Hughes, D.; Qiu, Y. Ultrasound-Mediated Ocular Drug Delivery: From Physics and Instrumentation to Future Directions. Micromachines 2023, 14, 1575. https://doi.org/10.3390/mi14081575
Duncan B, Al-Kassas R, Zhang G, Hughes D, Qiu Y. Ultrasound-Mediated Ocular Drug Delivery: From Physics and Instrumentation to Future Directions. Micromachines. 2023; 14(8):1575. https://doi.org/10.3390/mi14081575
Chicago/Turabian StyleDuncan, Blair, Raida Al-Kassas, Guangming Zhang, Dave Hughes, and Yongqiang Qiu. 2023. "Ultrasound-Mediated Ocular Drug Delivery: From Physics and Instrumentation to Future Directions" Micromachines 14, no. 8: 1575. https://doi.org/10.3390/mi14081575
APA StyleDuncan, B., Al-Kassas, R., Zhang, G., Hughes, D., & Qiu, Y. (2023). Ultrasound-Mediated Ocular Drug Delivery: From Physics and Instrumentation to Future Directions. Micromachines, 14(8), 1575. https://doi.org/10.3390/mi14081575






