Effect of Particle Migration on the Stress Field in Microfluidic Flows of Blood Analog Fluids at High Reynolds Numbers
Abstract
1. Introduction

- The main focus is the general study of particle migration and CFL formation of pBAFs in microfluidic systems at high Reynolds numbers (). This Reynolds number range has not been studied in the literature before, but is highly interesting since similar can occur in narrow gaps of VADs.
- By performing optical measurements using astigmatism particle tracking velocimetry (APTV), we measure the CFL height and determine the particle distribution in the microchannel flow. With this distribution, an inhomogeneous viscosity distribution in the pBAF flow can be determined.
- Direct measurements of wall shear stress (WSS) in the pBAF flow allow the assessment of shear stress changes due to CFL formation. For this purpose, the pBAF flow is compared to a flow of an equivalent homogeneous blood analog fluid (hBAF) with the same density and corresponding bulk viscosity . With these measurements, we are able to combine the optically determined results (CFL heights and viscosity distributions) with their effects on the flow (stress reduction).
- In the final step, pressure loss measurements across the microchannel are used to determine the extent to which CFL formation and shear stress reduction lead to a reduction in pressure losses in the channel. Based on these results, an apparent viscosity is determined for the pBAF to quantify the Fåhræus–Lindqvist effect for the investigated parameter range. To our knowledge, this is the first time that the complete chain leading to this effect has been described for a pBAF flow as well as for high Reynolds number flows.
2. Materials and Methods
2.1. Microchannel Geometries for the Experimental Analysis of the Particle-Laden Flow
2.2. Fluids
2.3. Fluid Properties and Rheology
2.4. Optical Determination of the Particle Migration and CFL Formation by Means of APTV
2.5. Determination of the Impact of the CFL on the Flow Using Pressure Loss and Wall Shear Stress Measurements
2.6. Flow Simulations of the hBAF Flow
2.7. Determination of the Global, Apparent Viscosity and Local Viscosity
3. Results and Discussion
3.1. Characterization of the Particle Distributions in the pBAF Flows
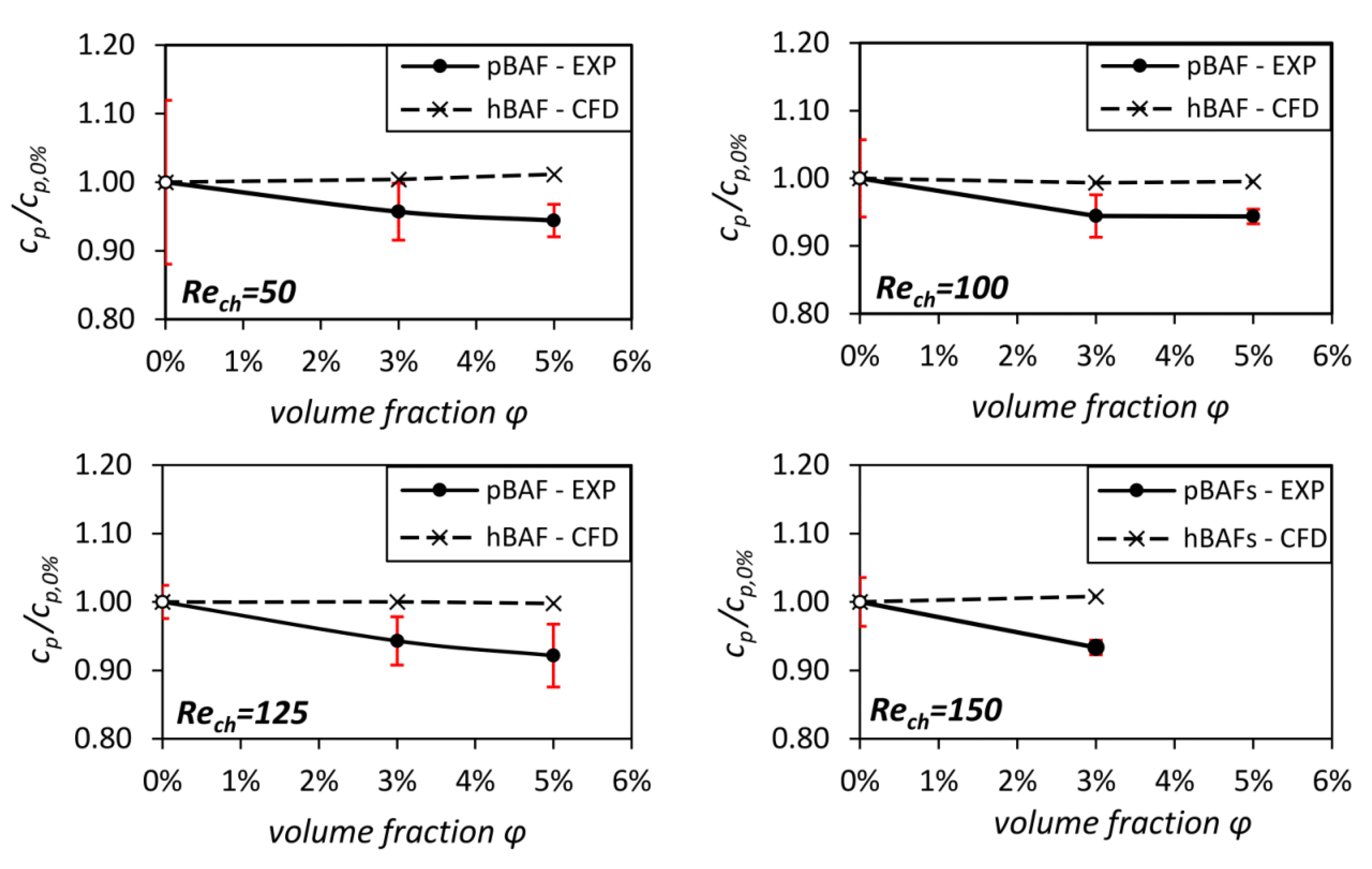
3.2. Global Influence of Particle Migration on the Flow Losses in the Channel
4. Summary of the CFL Characteristics of the pBAFs at High Reynolds Numbers
5. Significance of the Results for the Understanding of the Shear Stress Distribution in VAD Gaps
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature and Abbreviations
| Bulk Velocity | (m/s) | |
| Skin Friction Coefficient | (-) | |
| Pressure Coefficient | (-) | |
| Particle Diameter | (m) | |
| Hydraulic Diameter | (m) | |
| h | Channel Height | (m) |
| L | Channel Length | (m) |
| Channel Reynolds Number | (-) | |
| Particle Reynolds Number | (-) | |
| Velocities | (m/s) | |
| w | Channel Width | (m) |
| Spatial Directions | (m) | |
| Apparent Viscosity | (mPas) | |
| Local Viscosity | (mPas) | |
| Bulk Viscosity (from the Rheometer) | (mPas) | |
| Density | (kg/m) | |
| Shear Stress | (Pa) | |
| Wall Shear Stress | (Pa) | |
| Particle Volume Fraction | (%) | |
| Local Particle Distribution | (-) |
| APTV | Astigmatism Particle Tracking Velocimetry |
| BAF | Blood Analog Fluid |
| hBAF | Homogeneous Blood Analog Fluid |
| pBAF | Particulate Blood Analog Fluid |
| CFD | Computational Fluid Dynamics |
| CFL | Cell-Free Layer |
| DNS | Direct Numerical Simulation |
| EXP | Experiment |
| PMMA | Polymethylmethacrylat |
| PS | Polystyrol |
| PSSA | Pseudo Serge Silberberg Annulus |
| RIM | Refractive Index Matched |
| RBC | Red Blood Cell |
| VAD | Ventricular Assist Device |
| WSS | Wall Shear Stress |
References
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2023, 118, 3272–3287. [Google Scholar] [CrossRef] [PubMed]
- Newsletter Transplant: International Figures on Donation and Transplantation. 2021. Available online: https://www.transplant-observatory.org/download/newsletter-transplant-2022-2/ (accessed on 15 July 2023).
- Perschall, M. Numerische Untersuchung des Wellenpumpenkonzeptes und der Mechanischen Herzunterstützung. Ph.D. Thesis, Karlsruher Institut für Technologie, Karlsruhe, Germany, 2010. [Google Scholar] [CrossRef]
- Vidakovic, S.; Ayre, P.; Woodard, J.; Lingard, N.; Tansley, G.; Reizes, J. Paradoxical effects of viscosity on the VentrAssist rotary blood pump. Artif. Organs 2000, 24, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, Z.; Griffith, B.P.; Wu, Z.J. Computational characterization of flow and blood damage potential of the new maglev CH-VAD pump versus the HVAD and HeartMate II pumps. Int. J. Artif. Organs 2020, 43, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Antaki, J.F.; Diao, C.G.; Shu, F.J.; Wu, J.C.; Zhao, R.; Kameneva, M.V. Microhaemodynamics within the blade tip clearance of a centrifugal turbodynamic blood pump. Proc. Inst. Mech. Eng. Part J. Eng. Med. 2008, 222, 573–581. [Google Scholar] [CrossRef]
- Thamsen, B.; Plamondon, M.; Granegger, M.; Schmid Daners, M.; Kaufmann, R.; Neels, A.; Meboldt, M. Investigation of the Axial Gap Clearance in a Hydrodynamic-Passive Magnetically Levitated Rotary Blood Pump Using X-Ray Radiography. Artif. Organs 2018, 42, 510–515. [Google Scholar] [CrossRef]
- Thamsen, B.; Granneger, M.; Kretzscher, U. Blood damage in ventricular assist devices. Int. J. Artif. Organs 2016, 39, 147–149. [Google Scholar] [CrossRef]
- Stergiou, Y.G.; Keramydas, A.T.; Anastasiou, A.D.; Mouza, A.A.; Paras, S.V. Experimental and Numerical Study of Blood Flow in m-vessels: Influence of the Fahraeus–Lindqvist Effect. Fluids 2019, 4, 143. [Google Scholar] [CrossRef]
- Gliah, O.R. In Vitro Investigation of Cell-Free Layer Formation in Microchannels: Dependency on the Red Blood Cell Aggregation and Field of Shear. Ph.D Thesis, University of Ottawa, Ottawa, ON, Canada, 2018. [Google Scholar]
- Fink, K. Microfluidic Analysis of Vertebrate Red Blood Cell Characteristics. Ph.D. Thesis, University of California, Berkeley, CA, USA, 2016. [Google Scholar]
- Gracka, M.; Lima, R.; Miranda, J.M.; Student, S.; Melka, B.; Ostrowski, Z. Red blood cells tracking and cell-free layer formation in a microchannel with hyperbolic contraction: A CFD model validation. Comput. Methods Programs Biomed. 2022, 226, 107117. [Google Scholar] [CrossRef]
- Köry, J.; Maini, P.K.; Pitt-Francis, J.M.; Byrne, H.M. Dependence of cell-free-layer width on rheological parameters: Combining empirical data on flow separation at microvascular bifurcations with geometrical considerations. Phys. Rev. 2022, 105, 14414. [Google Scholar] [CrossRef]
- Recktenwald, S.M.; Graessel, K.; Rashidi, Y.; Steuer, J.N.; John, T.; Gekle, S.; Wagner, C. Cell-free layer of red blood cells in a constricted microfluidic channel under steady and time-dependent flow conditions. Fluid Dyn. 2023; in press. [Google Scholar] [CrossRef]
- Fåhræus, R.; Lindqvist, T. The viscosity of the blood in narrow capillary tubes. Am. J. Physiol.-Leg. Content 1931, 96, 562–568. [Google Scholar] [CrossRef]
- Maeda, N. Erythrocyte Rheology in Microcirculation. Jpn. J. Physiol. 1996, 46, 1–14. [Google Scholar] [CrossRef]
- Rubenstein, D.; Yin, W.; Frame, M.D. Biofluid Mechanics: An Introduction to Fluid Mechanics, Macrocirculation, and Microcirculation. In Biomedical Engineering, 3rd ed.; Academic Press: London, UK, 2022. [Google Scholar]
- Tanishita, K.; Yamamoto, K. (Eds.) Vascular Engineering: New Prospects of Vascular Medicine and Biology with a Multidiscipline Approach, 1st ed.; Springer: Tokyo, Japan, 2015. [Google Scholar] [CrossRef]
- Roselli, R.J. Biotransport: Principles and Applications; SpringerLink Bücher, Springer Science+Business Media LLC: New York, NY, USA, 2011. [Google Scholar] [CrossRef]
- Pinho, D.; Campo-Deaño, L.; Lima, R.; Pinho, F.T. In vitro particulate analogue fluids for experimental studies of rheological and hemorheological behavior of glucose-rich RBC suspensions. Biomicrofluidics 2017, 11, 54105. [Google Scholar] [CrossRef]
- Froese, V.; Gabel, G.; Parnell, J.; Prause, A.; Lommel, M.; Kertzscher, U. Flow study on a transparent two-phase blood model fluid based on alginate microspheres. Exp. Fluids 2022, 63, 188. [Google Scholar] [CrossRef]
- Sadek, S.H.; Rubio, M.; Lima, R.; Vega, E.J. Blood Particulate Analogue Fluids: A Review. Materials 2021, 14, 2451. [Google Scholar] [CrossRef]
- Torner, B.; Konnigk, L.; Abroug, N.; Wurm, H. Turbulence and turbulent flow structures in a ventricular assist device-A numerical study using the large-eddy simulation. Int. J. Numer. Methods Biomed. Eng. 2021, 37, e3431. [Google Scholar] [CrossRef]
- Dean, R.B. Reynolds number dependence of skin friction and other bulk flow variables in two-dimensional rectangular duct flow. J. Fluids Eng. 1978, 100, 215–223. [Google Scholar] [CrossRef]
- Çengel, Y.A.; Cimbala, J.M. Fluid Mechanics: Fundamentals and Applications, 3rd ed.; McGraw-Hill: New York, NY, USA, 2014. [Google Scholar]
- Schonberg, J.A.; Hinch, E. Inertial migration of a sphere in Poiseuille flow. J. Fluid Mech. 1989, 203, 517–524. [Google Scholar] [CrossRef]
- Bhagat, A.A.S.; Kuntaegowdanahalli, S.S.; Papautsky, I. Inertial microfluidics for continuous particle filtration and extraction. Microfluid. Nanofluidics 2009, 7, 217–226. [Google Scholar] [CrossRef]
- Di Carlo, D. Inertial microfluidics. Lab Chip 2009, 9, 3038–3046. [Google Scholar] [CrossRef]
- Oertel, H.; Ruck, S. Bioströmungsmechanik: Grundlagen, Methoden und Phänomene; Vieweg+Teubner: Wiesbaden, German, 2012. [Google Scholar] [CrossRef]
- Brockmann, P.; Symanczyk, C.; Ennayar, H.; Hussong, J. Utilizing APTV to investigate the dynamics of polydisperse suspension flows beyond the dilute regime: Applying APTV to polydisperse suspensions flows. Exp. Fluids 2022, 63, 129. [Google Scholar] [CrossRef]
- Bailey, B.; Yoda, M. An aqueous low-viscosity density-and refractive index-matched suspension system. Exp. Fluids 2003, 35, 1–3. [Google Scholar] [CrossRef]
- Primary Fluid Systems Inc. Chemical Resistance Guide; Primary Fluid Systems Inc.: Burlington, ON, Canada, 2016. [Google Scholar]
- Kenner, T. The measurement of blood density and its meaning. Basic Res. Cardiol. 1989, 84, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Stanley, N.; Ciero, A.; Timms, W.; Hewlin, R.L.J. A 3-D Printed Optically Clear Rigid Diseased Carotid Bifurcation Arterial Mock Vessel Model for Particle Image Velocimetry Analysis in Pulsatile Flow. ASME Open Eng. 2023, 2, 21010. [Google Scholar] [CrossRef]
- Errill, E.W. Rheology of blood. Physiol. Rev. 1969, 49, 863–888. [Google Scholar] [CrossRef]
- Cardoso, A.V.; Camargos, A.O. Geometrical Aspects During Formation of Compact Aggregates of Red Blood Cells. Mater. Res. 2002, 5, 263–268. [Google Scholar] [CrossRef]
- Falbe, J.; Regitz, M.; Römpp, H. (Eds.) Römpp Chemie Lexikon: 3: H-L, 9th ed.; Römpp-Chemie-Lexikon, Thieme: Stuttgart, German, 1990. [Google Scholar]
- Cierpka, C.; Kahler, C.J. Particle imaging techniques for volumetric three-component (3D3C) velocity measurements in microfluidics. J. Vis. 2012, 15, 1–31. [Google Scholar] [CrossRef]
- Brockmann, P.; Hussong, J. On the calibration of Astigmatism particle tracking velocimetry for suspensions of different volume fractions. Exp. Fluids 2021, 62, 23. [Google Scholar] [CrossRef]
- Rossi, M.; Kähler, C.J. Optimization of astigmatic particle tracking velocimeters. Exp. Fluids 2014, 55, 1–13. [Google Scholar] [CrossRef]
- Roscoe, R. The viscosity of suspensions of rigid spheres. Br. J. Appl. Phys. 1952, 3, 267–269. [Google Scholar] [CrossRef]
- Gülich, J.F. Centrifugal Pumps, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Torner, B.; Konnigk, L.; Wurm, F.H. Influence of turbulent shear stresses on the numerical blood damage prediction in a ventricular assist device. Int. J. Artif. Organs 2019, 42, 391398819861395. [Google Scholar] [CrossRef]
- Zhang, J.; Li, W.; Alici, G. Advanced Mechatronics Mems Devices; Springer: Cham, Switzerland, 2017; pp. 563–593. [Google Scholar] [CrossRef]
- Amini, H.; Lee, W.; Di Carlo, D. Inertial microfluidic physics. Lab Chip 2014, 14, 2739–2761. [Google Scholar] [CrossRef]
- Vasseur, P.; Cox, R. The lateral migration of spherical particles sedimenting in a stagnant bounded fluid. J. Fluid Mech. 1977, 80, 561–591. [Google Scholar] [CrossRef]
- Matas, J.; Morris, J.; Guazzelli, E. Lateral forces on a sphere. Oil Gas Sci. Technol. 2004, 59, 59–70. [Google Scholar] [CrossRef]
- Feng, J.; Hu, H.H.; Joseph, D.D. Direct simulation of initial value problems for the motion of solid bodies in a Newtonian fluid. Part 2. Couette and Poiseuille flows. J. Fluid Mech. 1994, 277, 271–301. [Google Scholar] [CrossRef]
- Segre, G.; Silberberg, A. Behaviour of macroscopic rigid spheres in Poiseuille flow Part 2. Experimental results and interpretation. J. Fluid Mech. 1962, 14, 136–157. [Google Scholar] [CrossRef]
- Nott, P.R.; Brady, J.F. Pressure-driven flow of suspensions: Simulation and theory. J. Fluid Mech. 1994, 275, 157–199. [Google Scholar] [CrossRef]
- Asmolov, E.S. The inertial lift on a spherical particle in a plane Poiseuille flow at large channel Reynolds number. J. Fluid Mech. 1999, 381, 63–87. [Google Scholar] [CrossRef]
- Di Carlo, D.; Edd, J.F.; Humphry, K.J.; Stone, H.A.; Toner, M. Particle segregation and dynamics in confined flows. Phys. Rev. Lett. 2009, 102, 094503. [Google Scholar] [CrossRef] [PubMed]
- Matas, J.P.; Morris, J.F.; Guazzelli, É. Inertial migration of rigid spherical particles in Poiseuille flow. J. Fluid Mech. 2004, 515, 171–195. [Google Scholar] [CrossRef]


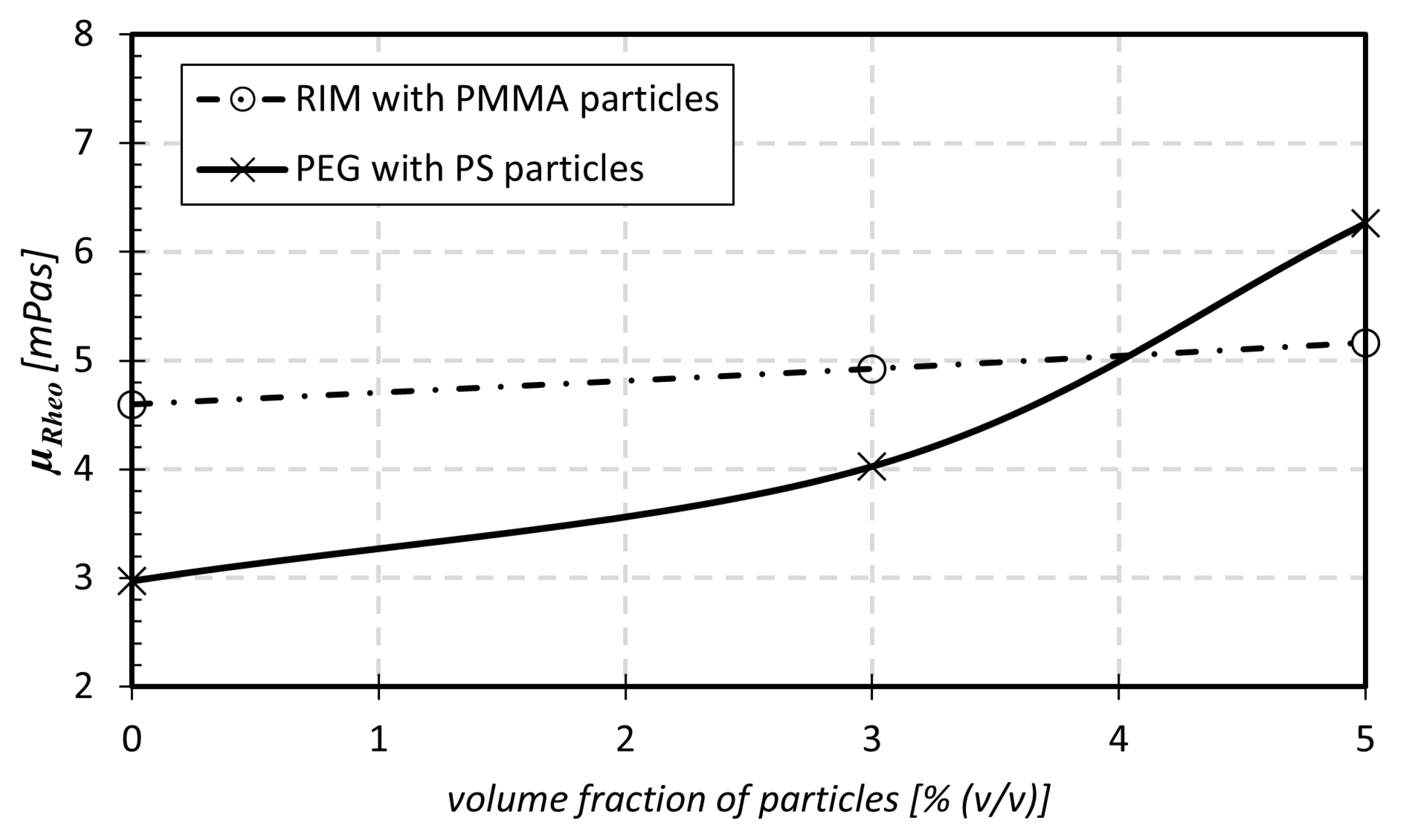

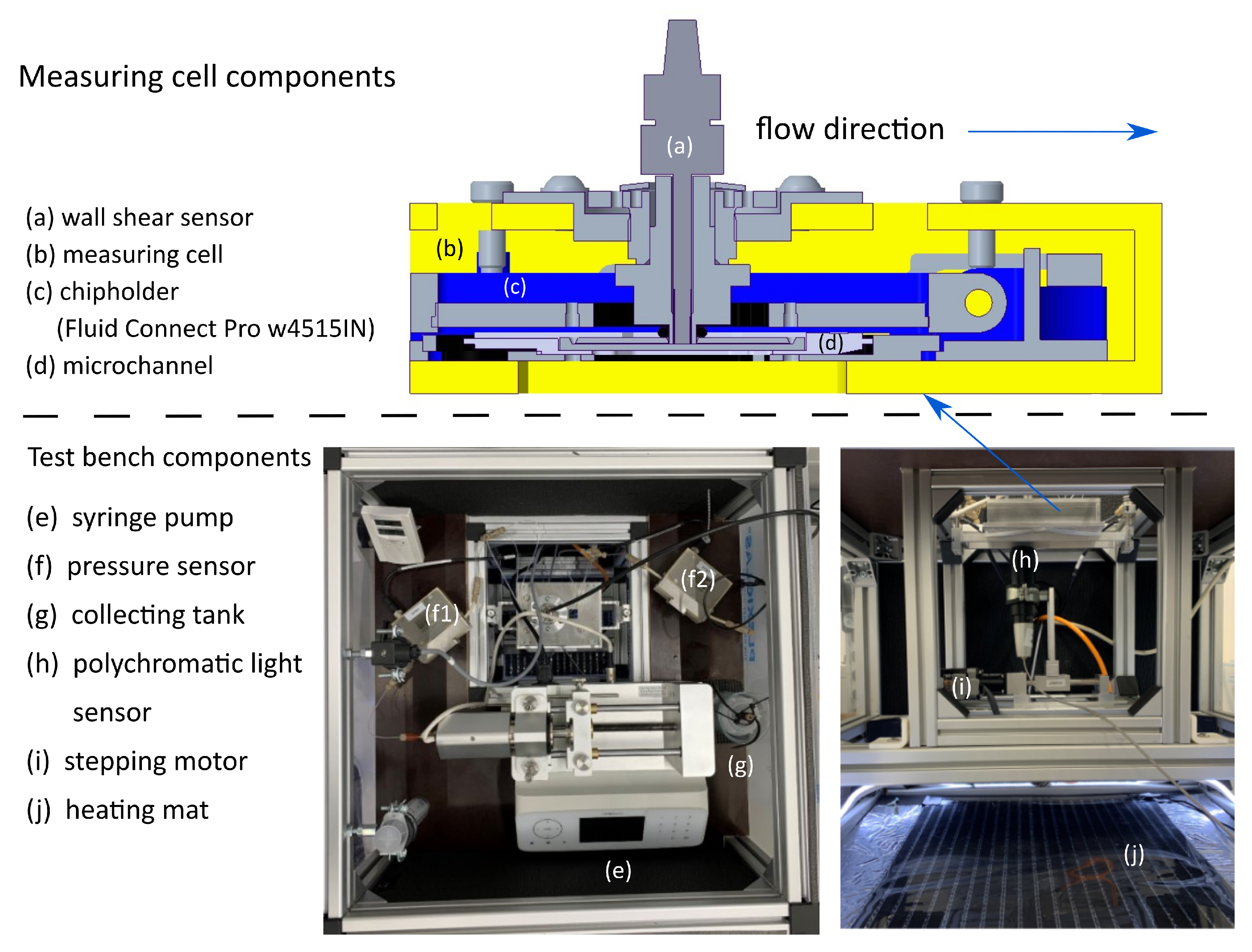
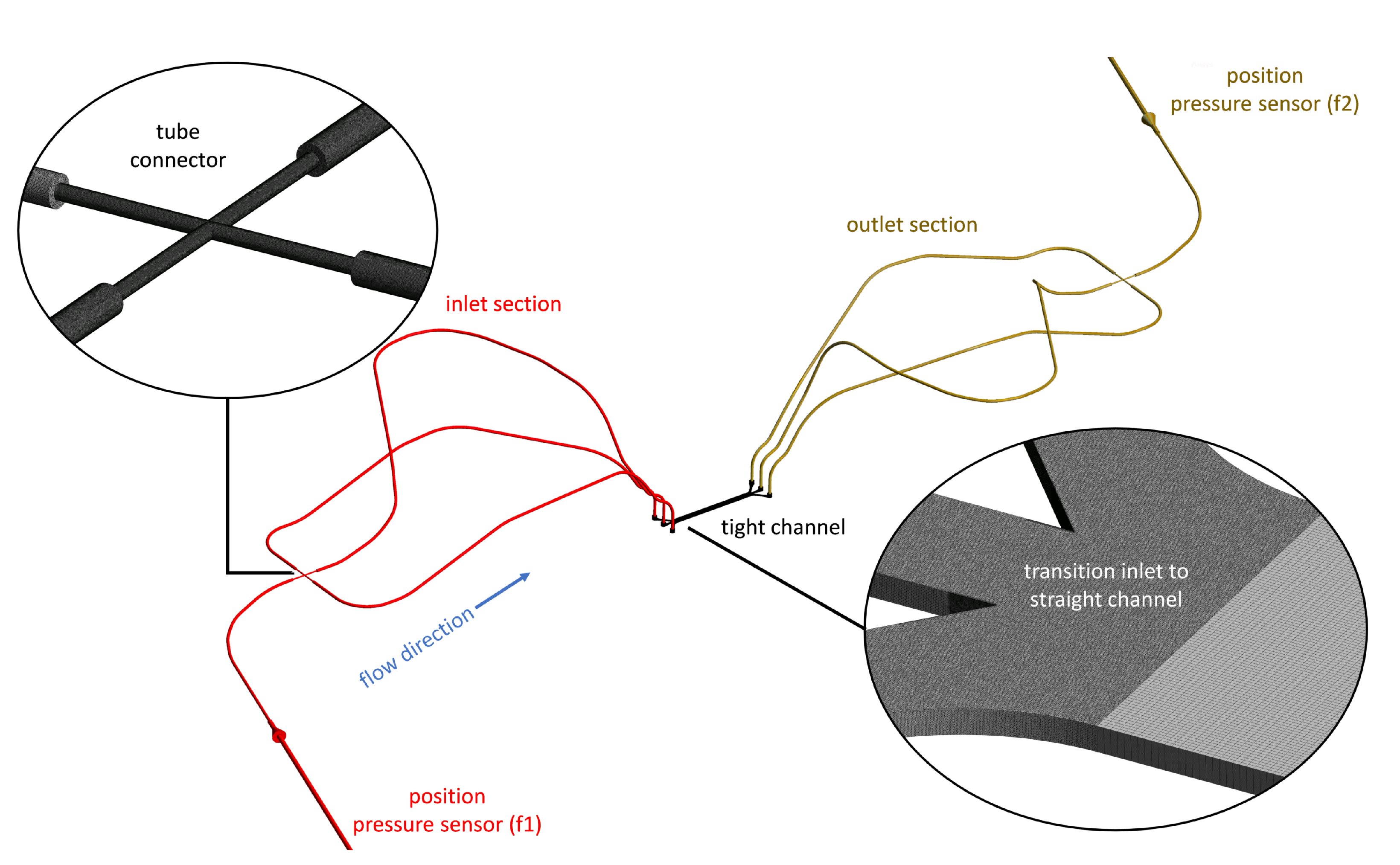

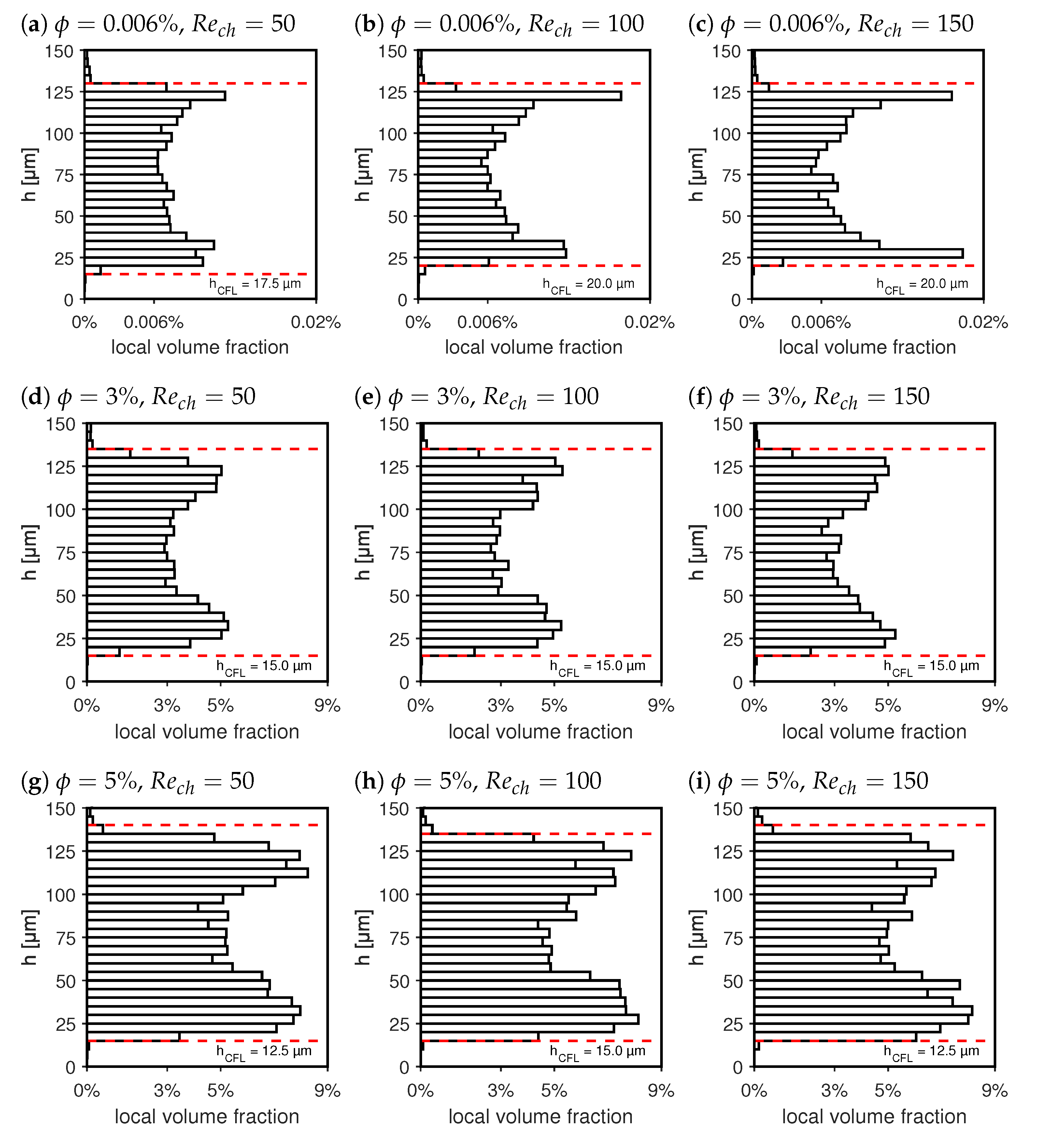

| Acronym | Carrier Fluid | Particle Volume Fraction | Density |
|---|---|---|---|
| RIM-0 (hBAF) | water–glycerol–ammonium thiocyanate | 0.006% () PMMA | 1178 kg/m |
| RIM-3 (pBAF) | 3% () PMMA | 1178 kg/m | |
| RIM-5 (pBAF) | 5% () PMMA | 1179 kg/m | |
| PEG-0 (hBAF) | water–polyethylene glycol 200 | 0% () PS | 1047 kg/m |
| PEG-3 (pBAF) | 3% () PS | 1054 kg/m | |
| PEG-5 (pBAF) | 5% () PS | 1065 kg/m |
| Normalized Friction Coefficient | ||||
|---|---|---|---|---|
| Reynolds Number | Fluid (Method) | |||
| hBAF (CFD) | 1 | 1 | 1 | |
| pBAF (EXP) | 1 | 0.90 (−10%) | 0.87 (−13%) | |
| hBAF (CFD) | 1 | 1 | - | |
| pBAF (EXP) | 1 | 0.88 (−12%) | - | |
| hBAF (CFD) | 1 | 1 | - | |
| pBAF (EXP) | 1 | 0.93 (−7%) | - | |
| hBAF (CFD) | 1 | 1 | - | |
| pBAF (EXP) | 1 | 0.90 (−10%) | - | |
| Absolute WSS Value | |||
|---|---|---|---|
| Reynolds Number | Fluid (Method) | ||
| hBAF (CFD) | 105 Pa | 189 Pa | |
| pBAF (EXP) | 105 Pa | 167 Pa (−12%) | |
| hBAF (CFD) | 131 Pa | 237 Pa | |
| pBAF (EXP) | 131 Pa | 219 Pa (−7%) | |
| hBAF (CFD) | 157 Pa | 283 Pa | |
| pBAF (EXP) | 159 Pa | 257 Pa (−10%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knüppel, F.; Sun, A.; Wurm, F.-H.; Hussong, J.; Torner, B. Effect of Particle Migration on the Stress Field in Microfluidic Flows of Blood Analog Fluids at High Reynolds Numbers. Micromachines 2023, 14, 1494. https://doi.org/10.3390/mi14081494
Knüppel F, Sun A, Wurm F-H, Hussong J, Torner B. Effect of Particle Migration on the Stress Field in Microfluidic Flows of Blood Analog Fluids at High Reynolds Numbers. Micromachines. 2023; 14(8):1494. https://doi.org/10.3390/mi14081494
Chicago/Turabian StyleKnüppel, Finn, Ang Sun, Frank-Hendrik Wurm, Jeanette Hussong, and Benjamin Torner. 2023. "Effect of Particle Migration on the Stress Field in Microfluidic Flows of Blood Analog Fluids at High Reynolds Numbers" Micromachines 14, no. 8: 1494. https://doi.org/10.3390/mi14081494
APA StyleKnüppel, F., Sun, A., Wurm, F.-H., Hussong, J., & Torner, B. (2023). Effect of Particle Migration on the Stress Field in Microfluidic Flows of Blood Analog Fluids at High Reynolds Numbers. Micromachines, 14(8), 1494. https://doi.org/10.3390/mi14081494





