Lignocellulosic Bionanomaterials for Biosensor Applications
Abstract
1. Introduction
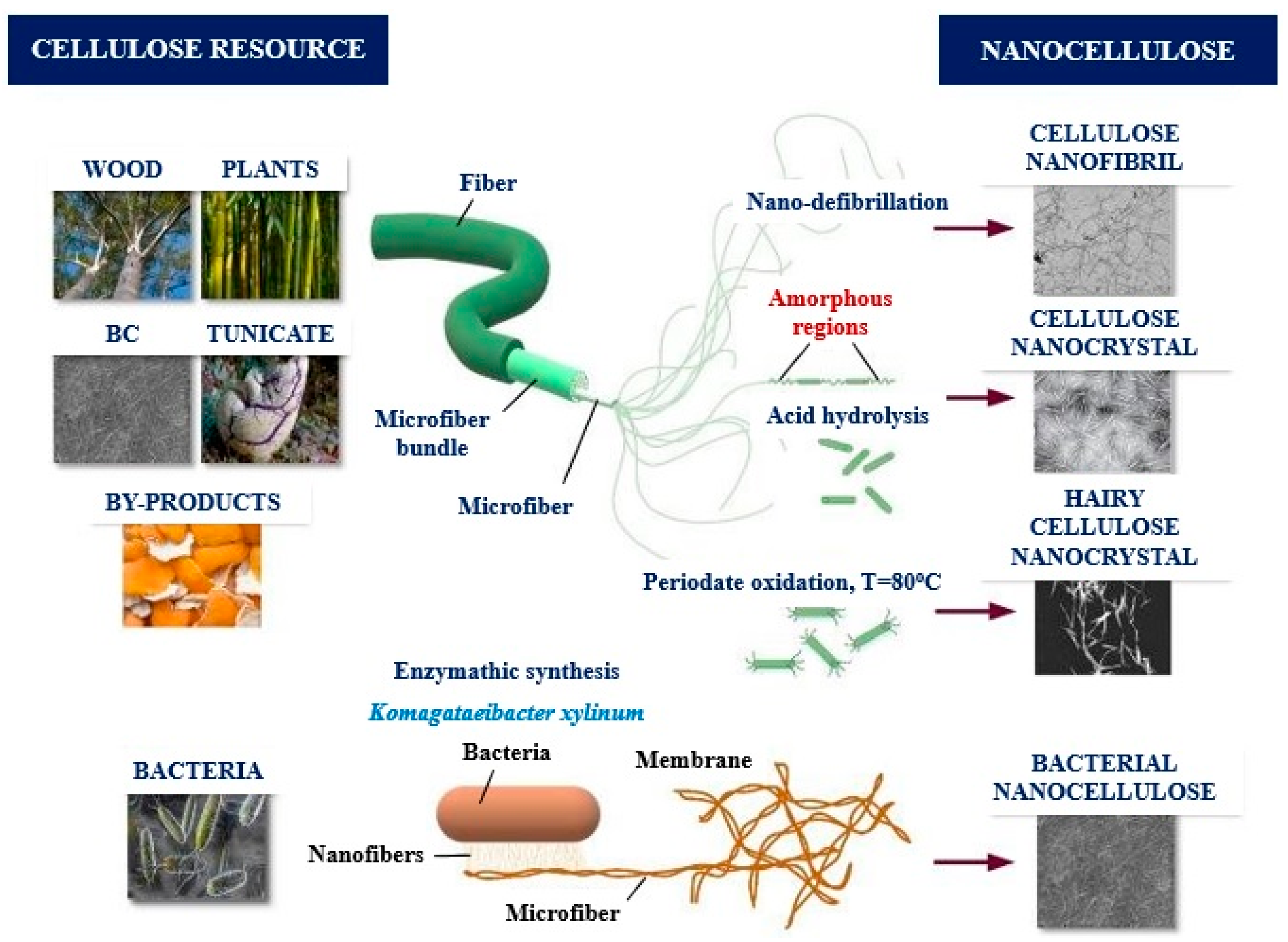
2. Nanocellulose
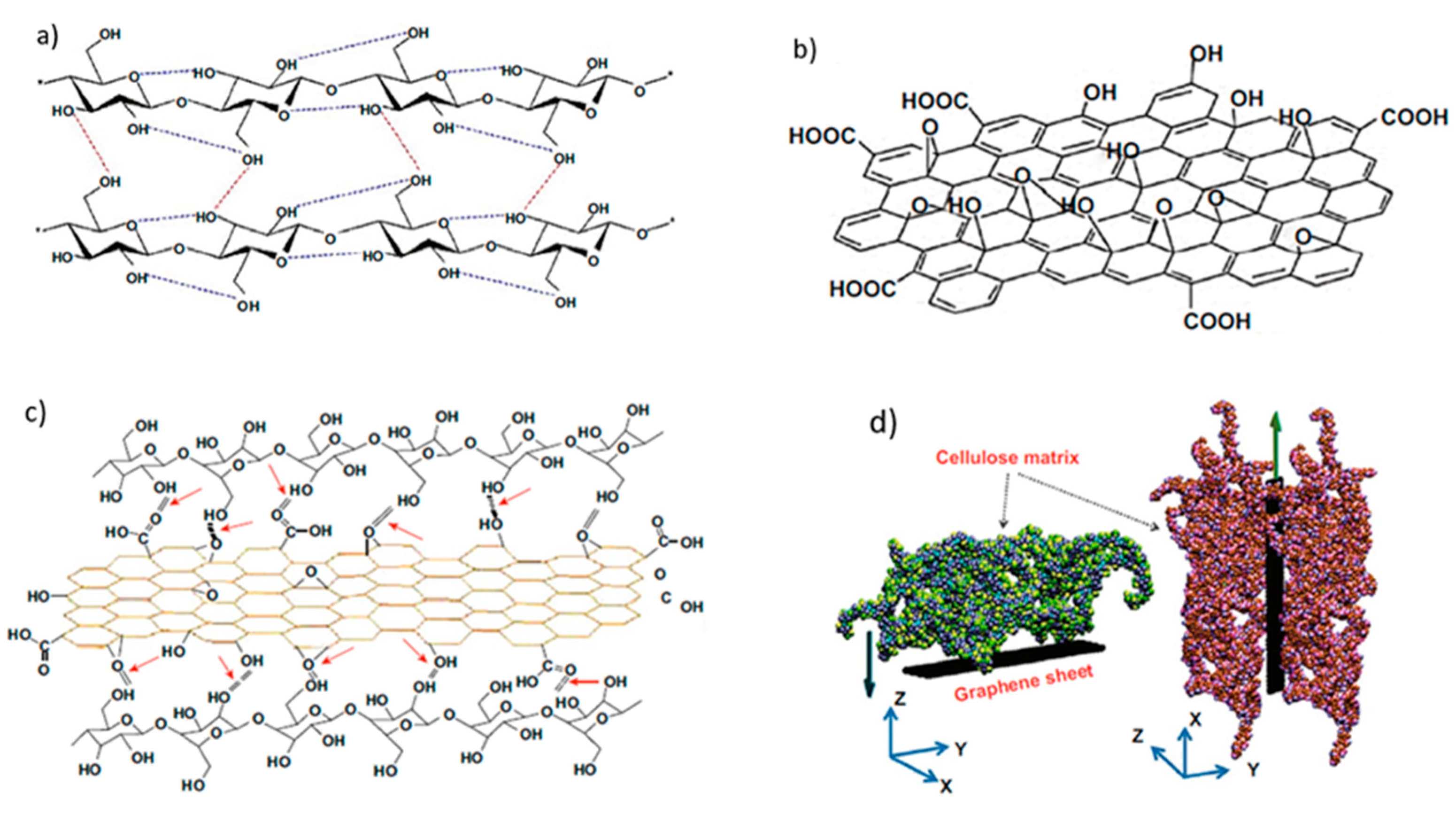
3. Nanolignin
3.1. Synthesis Methods of LNPs
3.1.1. Acid Shifting
3.1.2. Self-Assembly Methods
3.1.3. Mechanical Methods of LNPs Production
3.1.4. Other Preparation Methods
3.2. LNPs Characterization
3.3. LNPs Applications
4. Lignocellulosic Material Based Sensors
4.1. Cellulose Based Sensors
4.1.1. Cellulose-Based Physical and Chemical Sensors
Pressure/Strain Sensors
Proximity Sensors
Temperature Sensors
Humidity Sensors
Gas Sensors
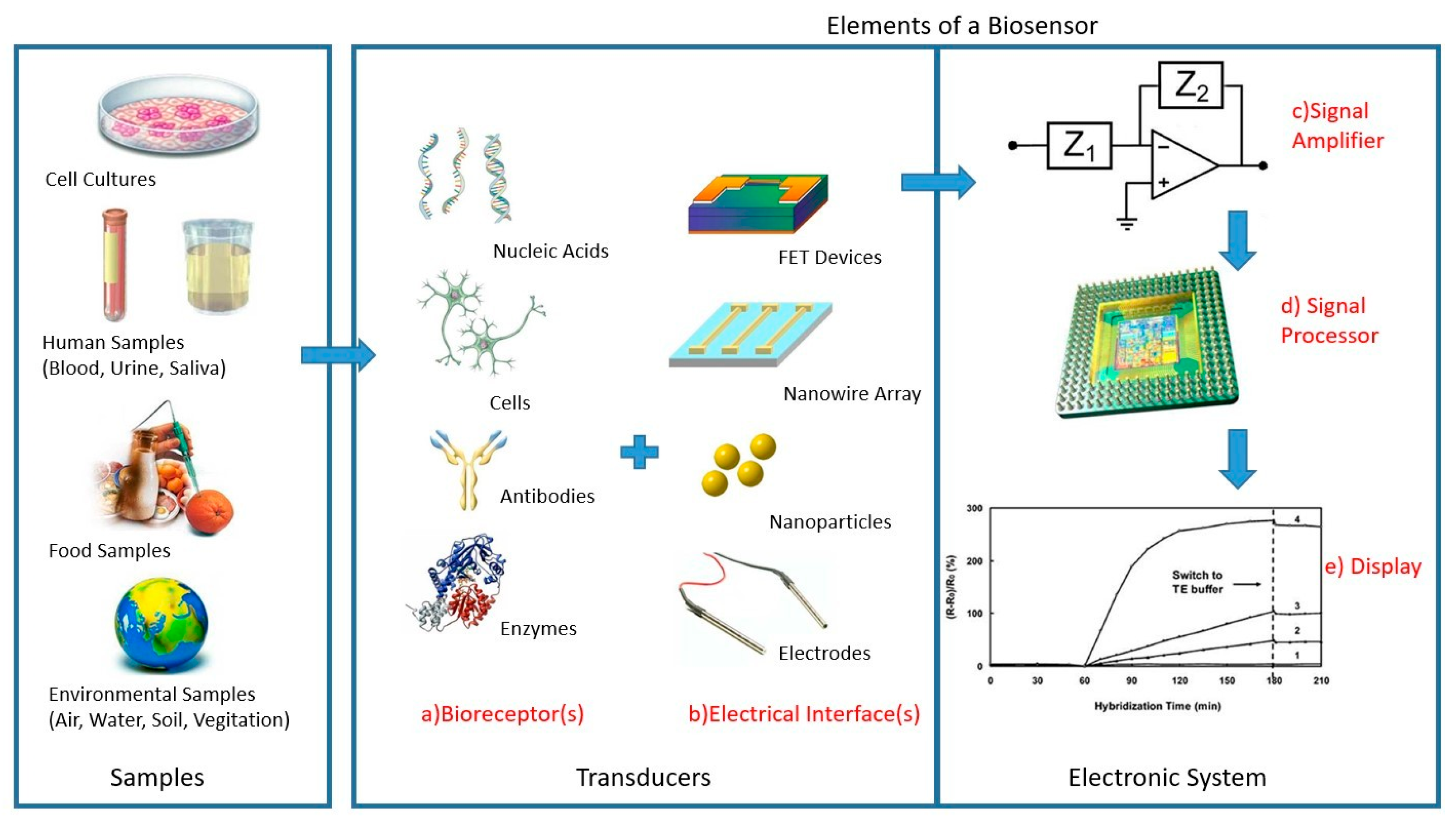
4.1.2. Biosensors
Glucose Sensor
Enzyme Sensors
Cholesterol Sensors
Urea Sensors
Other Biosensors
4.2. Hemicellulose Based Sensors
4.2.1. Hemicellulose Based Physical and Chemical Sensors
4.2.2. Hemicellulose Based Biosensors
4.3. Lignin Based Sensors
4.3.1. Lignin Based Physical and Chemical Sensors
4.3.2. Lignin Based Biosensors
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.Y.; Wang, B.; Ma, M.G.; Wang, B. Review of recent development on preparation, properties, and applications of cellulose-based functional materials. Int. J. Polym. Sci. 2018, 2018, 1–18. [Google Scholar] [CrossRef]
- Barhoum, A.; Jeevanandam, J.; Rastogi, A.; Samyn, P.; Boluk, Y.; Dufresne, A.; Danquah, M.K.; Bechelany, M. Plant celluloses, hemicelluloses, lignins, and volatile oils for the synthesis of nanoparticles and nanostructured materials. Nanoscale 2020, 12, 22845–22890. [Google Scholar] [CrossRef] [PubMed]
- Nechyporchuk, O.; Belgacem, M.N.; Bras, J. Production of cellulose nanofibrils: A review of recent advances. Ind. Crops Prod. 2016, 93, 2–25. [Google Scholar] [CrossRef]
- Abitbol, T.; Rivkin, A.; Cao, Y.; Nevo, Y.; Abraham, E.; Ben-Shalom, T.; Lapidot, S.; Shoseyov, O. Nanocellulose, a tiny fiber with huge applications. Curr. Opin. Biotechnol. 2016, 39, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Abol-Fotouh, D.; Hassan, M.A.; Shokry, H.; Roig, A.; Azab, M.S.; Kashyout, A.E.H.B. Bacterial nanocellulose from agro-industrial wastes: Low-cost and enhanced production by Komagataeibacter saccharivorans MD1. Sci. Rep. 2020, 10, 3491. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y. Key advances in the chemical modification of nanocelluloses. Chem. Soc. Rev. 2014, 43, 1519–1542. [Google Scholar] [CrossRef]
- Heise, K.; Kontturi, E.; Allahverdiyeva, Y.; Tammelin, T.; Linder, M.B.; Nonappa; Ikkala, O. Nanocellulose: Recent Fundamental Advances and Emerging Biological and Biomimicking Applications. Adv. Mater. 2021, 33, 1–30. [Google Scholar] [CrossRef]
- Ioelovich, M. Characterization of Various Kinds of Nanocellulose. In Handbook of Nanocellulose and Cellulose Nanocomposites; Kargarzadeh, H., Ahmad, I., Thomas, S., Dufresne, A., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; Volume 1, pp. 51–100. [Google Scholar] [CrossRef]
- Tayeb, A.H.; Amini, E.; Ghasemi, S.; Tajvidi, M. Cellulose Nanomaterials—Binding Properties and Applications: A Review. Molecules 2018, 10, 2684. [Google Scholar] [CrossRef]
- Lindh, E.L.; Salmén, L. Surface accessibility of cellulose fibrils studied by hydrogen–deuterium exchange with water. Cellulose 2016, 24, 21–33. [Google Scholar] [CrossRef]
- Lindh, E.L.; Bergenstrahle-Wohlert, M.; Terenzi, C.; Salmén, L.; Furó, I. Non-exchanging hydroxyl groups on the surface of cellulose fibrils: The role of interaction with water. Carbohydr. Res. 2016, 434, 136–142. [Google Scholar] [CrossRef]
- Kumar Gupta, P.; Sai Raghunath, S.; Venkatesh Prasanna, D.; Venkat, P.; Shree, V.; Chithananthan, C.; Choudhary, S.; Surender, K.; Geetha, K. An update on overview of cellulose, its structure and applications. In Cellulose; Pascual, A.R., Martín, M.E.E., Eds.; IntechOpen: London, UK, 2019. [Google Scholar]
- Kargarzadeh, H.; Ioelovich, M.; Ahmad, I.; Thomas, S.; Dufresne, A. Methods for extraction of nanocellulose from various sources. In Handbook of Nanocellulose and Cellulose Nanocomposites; Kargarzadeh, H., Ahmad, I., Thomas, S., Dufresne, A., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; Volume 1, pp. 1–49. [Google Scholar] [CrossRef]
- Oprea, M.; Voicu, S.I. Recent advances in composites based on cellulose derivatives for biomedical applications. Carbohydr. Polym. 2020, 247, 116683. [Google Scholar] [CrossRef]
- Al-Harbi, N.; Hussein, M.A.; Al-Hadeethi, Y.; Umar, A. Cellulose Acetate-Hydroxyapatite-Bioglass-Zirconia Nanocomposite Particles as Potential Biomaterial: Synthesis, Characterization, and Biological Properties for Bone Application. Eng. Sci. 2022, 17, 70–82. [Google Scholar] [CrossRef]
- Lievonen, M.; Valle-Delgado, J.J.; Mattinen, M.-L.; Hult, E.-L.; Lintinen, K.; Kostiainen, M.A.; Paananen, A.; Szilvay, G.R.; Setälä, H.; Österberg, M. A Simple Process for Lignin Nanoparticle Preparation. Green Chem. 2016, 18, 1416–1422. [Google Scholar] [CrossRef]
- Zhang, Z.; Terrasson, V.; Guénin, E. Lignin Nanoparticles and Their Nanocomposites. Nanomaterials 2021, 11, 1336. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lahtinen, M.H.; Agustin, M.B.; de Carvalho, D.M.; Hirvonen, S.; Penttilä, P.A.; Mikkonen, K.S. Green Fabrication Approaches of Lignin Nanoparticles from Different Technical Lignins: A Comparison Study. ChemSusChem 2021, 14, 4718–4730. [Google Scholar] [CrossRef]
- Low, L.E.; Teh, K.C.; Siva, S.P.; Chew, I.M.L.; Mwangi, W.W.; Chew, C.L.; Goh, B.-H.; Chan, E.S.; Tey, B.T. Lignin Nanoparticles: The next Green Nanoreinforcer with Wide Opportunity. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100398. [Google Scholar] [CrossRef]
- Ago, M.; Tardy, B.L.; Wang, L.; Guo, J.; Khakalo, A.; Rojas, O.J. Supramolecular Assemblies of Lignin into Nano- and Microparticles. MRS Bull. 2017, 42, 371–378. [Google Scholar] [CrossRef]
- Gao, W.; Fatehi, P. Lignin for Polymer and Nanoparticle Production: Current Status and Challenges. Can. J. Chem. Eng. 2019, 97, 2827–2842. [Google Scholar] [CrossRef]
- Zhou, Y.; Qian, Y.; Wu, S.; Zhong, X.; Huang, J.; Qiu, X. Incorporation of Nano Lignin Reverse Micelles on the Transparency, UV-Blocking and Rheological Properties of High-Density Polyethylene Films. Holzforschung 2020, 74, 513–521. [Google Scholar] [CrossRef]
- Chen, K.; Wang, S.; Qi, Y.; Guo, H.; Guo, Y.; Li, H. State-of-the-Art: Applications and Industrialization of Lignin Micro/Nano Particles. ChemSusChem 2021, 14, 1284–1294. [Google Scholar] [CrossRef]
- Olgun, Ç.; Ateş, S. Characterization and Comparison of Some Kraft Lignins Isolated from Different Sources. Forests 2023, 14, 882. [Google Scholar] [CrossRef]
- Tang, Q.; Qian, Y.; Yang, D.; Qiu, X.; Qin, Y.; Zhou, M. Lignin-Based Nanoparticles: A Review on Their Preparations and Applications. Polymers 2020, 12, 2471. [Google Scholar] [CrossRef] [PubMed]
- Pires, J.R.A.; Souza, V.G.L.; Fernando, A.L. Valorization of energy crops as a source for nanocellulose production–Current knowledge and future prospects. Ind. Crops Prod. 2019, 140, 111642. [Google Scholar] [CrossRef]
- Yasmin, H.; Giwa, S.O.; Noor, S.; Aybar, H.Ş. Reproduction of Nanofluid Synthesis, Thermal Properties and Experiments in Engineering: A Research Paradigm Shift. Energies 2023, 16, 1145. [Google Scholar] [CrossRef]
- Curvello, R.; Raghuwanshi, V.S.; Garnier, G. Engineering nanocellulose hydrogels for biomedical applications. Adv. Colloid Interface Sci. 2019, 267, 47–61. [Google Scholar] [CrossRef]
- Kallel, F.; Bettaieb, F.; Khiari, R.; García, A.; Bras, J.; Chaabouni, S.E. Isolation and structural characterization of cellulose nanocrystals extracted from garlic straw residues. Ind. Crops Prod. 2016, 87, 287–296. [Google Scholar] [CrossRef]
- Silva, F.A.G.S.; Dourado, F.; Gama, M.; Poças, F. Nanocellulose Bio-Based Composites for Food Packaging. Nanomaterials 2020, 10, 2041. [Google Scholar] [CrossRef]
- Phanthong, P.; Reubroycharoen, P.; Hao, X.; Xu, G.; Abudula, A.; Guan, G. Nanocellulose: Extraction and application. Carbon Resour. Convers. 2018, 1, 32–43. [Google Scholar] [CrossRef]
- Norfarhana, A.S.; Ilyas, R.A.; Ngadi, N. A review of nanocellulose adsorptive membrane as multifunctional wastewater treatment. Carbohydr. Polym. 2022, 291, 119563. [Google Scholar] [CrossRef]
- Zielińska, D.; Szentner, K.; Wakiewicz, A.; Borysiak, S. Production of nanocellulose by enzymatic treatment for application in polymer composites. Materials 2021, 14, 2124. [Google Scholar] [CrossRef]
- Ahankari, S.S.; Subhedar, A.R.; Bhadauria, S.S.; Dufresne, A. Nanocellulose in food packaging: A review. Carbohydr. Polym. 2021, 255, 117479. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef]
- Yasmin, H. Design and Research of Biomaterials. Coatings 2022, 12, 1684. [Google Scholar] [CrossRef]
- Dhali, K.; Ghasemlou, M.; Daver, F.; Cass, P.; Adhikari, B. A review of nanocellulose as a new material towards environmental sustainability. Sci. Total Environ. 2021, 775, 145871. [Google Scholar] [CrossRef]
- Du, H.; Liu, W.; Zhang, M.; Si, C.; Zhang, X.; Li, B. Cellulose nanocrystals and cellulose nanofibrils based hydrogels for biomedical applications. Carbohydr. Polym. 2019, 209, 130–144. [Google Scholar] [CrossRef]
- Djafaripetroudy, S.; Shojaeiarani, J.; Chabot, B. Recent advances in isolation, characterization, and potential applications of nanocellulose-based composites: A comprehensive review. J. Nat. Fibers 2023, 20, 2146830. [Google Scholar] [CrossRef]
- Thomas, S.K.; Begum, S.; Midhun Dominic, C.D.; Salim, N.V.; Hameed, N.; Rangappa, S.M.; Siengchin, S.; Parameswaranpillai, J. Isolation and characterization of cellulose nanowhiskers from Acacia caesia plant. J. Appl. Polym. Sci. 2021, 138, 50213. [Google Scholar] [CrossRef]
- Wang, H.; Biswas, S.K.; Zhu, S.; Lu, Y.; Yue, Y.; Han, J.; Xu, X.; Wu, Q.; Xiao, H. Self-healable electro-conductive hydrogels based on core-shell structured nanocellulose/carbon nanotubes hybrids for use as flexible supercapacitors. Nanomaterials 2020, 10, 112. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, J.; Liu, Y.; Hao, X.; Ji, X.; Yang, Q. Biochemical preparation of hydrophobic and lipophilic nanocellulose from hemp stalk. Mater. Today Chem. 2023, 27, 101346. [Google Scholar] [CrossRef]
- Pham, C.D.; Le, T.M.; Nguyen, C.T.X.; Vo, N.; Do, N.H.N.; Le, K.A.; Mai, T.P.; Le, P.T.K. Review of the role of pretreatment step in nanocellulose production from rice straw. Chem. Eng. Trans. 2022, 97, 67–72. [Google Scholar] [CrossRef]
- Mbakop, S.; Nthunya, L.N.; Onyango, M.S. Recent advances in the synthesis of nanocellulose functionalized-hybrid membranes and application in water quality improvement. Processes 2021, 9, 611. [Google Scholar] [CrossRef]
- Zinge, C.; Kandasubramanian, B. Nanocellulose based biodegradable polymers. Eur. Polym. J. 2020, 133, 109758. [Google Scholar] [CrossRef]
- Nasir, M.; Hashim, R.; Sulaiman, O.; Asim, M. Nanocellulose: Preparation methods and applications. In Cellulose-Reinforced Nanofibre Composites: Production Properties and Application; Jawaid, M., Boufi, S., Abdul Khalil, H.P.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 261–276. [Google Scholar] [CrossRef]
- De Amorim, J.D.P.; de Souza, K.C.; Duarte, C.R.; da Silva Duarte, I.; de Assis Sales Ribeiro, F.; Silva, G.S.; de Farias, P.M.A.; Stingl, A.; Costa, A.F.S.; Vinhas, G.M.; et al. Plant and bacterial nanocellulose: Production, properties and applications in medicine, food, cosmetics, electronics and engineering. A review. Environ. Chem. Lett. 2020, 18, 851–869. [Google Scholar] [CrossRef]
- Mohamad Haafiz, M.K.; Hassan, A.; Zakaria, Z.; Inuwa, I.M. Isolation and characterization of cellulose nanowhiskers from oil palm biomass microcrystalline cellulose. Carbohydr. Polym. 2014, 103, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, D.; Jaiswal, A.K.; Jaiswal, S. Nanocellulose Based Green Nanocomposites: Characteristics and Application in Primary Food Packaging. Food Rev. Int. 2022, 1–32. [Google Scholar] [CrossRef]
- Baati, R.; Magnin, A.; Boufi, S. High solid content production of nanofibrillar cellulose via continuous extrusion. ACS Sustain. Chem. Eng. 2017, 5, 2350–2359. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Ishak, M.R.; Zainudin, E.S. Sugar palm nanofibrillated cellulose (Arenga pinnata (Wurmb.) Merr): Effect of cycles on their yield, physic-chemical, morphological and thermal behavior. Int. J. Biol. Macromol. 2019, 123, 379–388. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5566. [Google Scholar] [CrossRef]
- Durmaz, E.; Ates, S. Characterisation of surfaces coated with different nanocellulose-based suspensions. Int. J. Surf. Sci. Eng. 2023, 17, 141–164. [Google Scholar] [CrossRef]
- Lenfant, G.; Heuzey, M.C.; van de Ven, T.G.M.; Carreau, P.J. Gelation of crystalline nanocellulose in the presence of hydroxyethyl cellulose. Can. J. Chem. Eng. 2017, 95, 1891–1900. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Ishak, M.R. Isolation and characterization of nanocrystalline cellulose from sugar palm fibres (Arenga pinnata). Carbohydr. Polym. 2018, 181, 1038–1051. [Google Scholar] [CrossRef]
- Neves, R.M.; Lopes, K.S.; Zimmermann, M.G.V.; Poletto, M.; Zattera, A.J. Cellulose nanowhiskers extracted from TEMPO-oxidized curaua fibers. J. Nat. Fibers 2020, 17, 1355–1365. [Google Scholar] [CrossRef]
- Reshmy, R.; Thomas, D.; Philip, E.; Paul, S.A.; Madhavan, A.; Sindhu, R.; Binod, P.; Pugazhendhi, A.; Sirohi, R.; Tarafdar, A.; et al. Potential of nanocellulose for wastewater treatment. Chemosphere 2021, 281, 1–13. [Google Scholar] [CrossRef]
- Joseph, B.; Sagarika, V.K.; Sabu, C.; Kalarikkal, N.; Thomas, S. Cellulose nanocomposites: Fabrication and biomedical applications. J. Bioresour. Bioprod. 2020, 5, 223–237. [Google Scholar] [CrossRef]
- Jonoobi, M.; Oladi, R.; Davoudpour, Y.; Oksman, K.; Dufresne, A.; Hamzeh, Y.; Davoodi, R. Different preparation methods and properties of nanostructured cellulose from various natural resources and residues: A review. Cellulose 2015, 22, 935–969. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Mariano, M.; Gopakumar, D.; Ahmad, I.; Thomas, S.; Dufresne, A.; Huang, J.; Lin, N. Advances in cellulose nanomaterials. Cellulose 2018, 25, 2151–2189. [Google Scholar] [CrossRef]
- Xie, H.; Du, H.; Yang, X.; Si, C. Recent strategies in preparation of cellulose nanocrystals and cellulose nanofibrils derived from raw cellulose materials. Int. J. Polym. Sci. 2018, 2018, 1–25. [Google Scholar] [CrossRef]
- Perumal, A.B.; Nambiar, R.B.; Moses, J.A.; Anandharamakrishnan, C. Nanocellulose: Recent trends and applications in the food industry. Food Hydrocoll. 2022, 127, 1–23. [Google Scholar] [CrossRef]
- Vanderfleet, O.M.; Cranston, E.D. Production routes to tailor the performance of cellulose nanocrystals. Nat. Rev. Mater. 2021, 6, 124–144. [Google Scholar] [CrossRef]
- Huang, J.; Ma, X.; Yang, G.; Dufresne, A. Introduction to Nanocellulose. In Nanocellulose: From Fundamentals to Advanced Materials; Huang, J., Dufresne, A., Lin, N., Eds.; Boschstr. 12, 69469; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2019; pp. 1–20. [Google Scholar] [CrossRef]
- Durmaz, E.; Ates, S.; Ahsan, L.; He, Z.; Ni, Y. Characterization of Microfibrillarcellulose (MFC) Obtained From Corn Stalk, Sunflower Stalk, Reed And Sesame Husk. Wood Res. 2018, 63, 713–726. [Google Scholar]
- Ludwicka, K.; Kaczmarek, M.; Białkowska, A. Bacterial Nanocellulose—A Biobased Polymer for Active and Intelligent Food Packaging Applications: Recent Advances and Developments. Polymers 2020, 12, 2209. [Google Scholar] [CrossRef] [PubMed]
- Patil, T.V.; Patel, D.K.; Dutta, S.D.; Ganguly, K.; Santra, T.S.; Lim, K.T. Nanocellulose, a versatile platform: From the delivery of active molecules to tissue engineering applications. Bioact. Mater. 2022, 9, 566–589. [Google Scholar] [CrossRef] [PubMed]
- Skočaj, M. Bacterial nanocellulose in papermaking. Cellulose 2019, 26, 6477–6488. [Google Scholar] [CrossRef]
- Lin, S.P.; Calvar, I.L.; Catchmark, J.M.; Liu, J.R.; Demirci, A.; Cheng, K.C. Biosynthesis, production and applications of bacterial cellulose. Cellulose 2013, 20, 2191–2219. [Google Scholar] [CrossRef]
- Gorgieva, S.; Třcek, J. Bacterial cellulose: Production, modification and perspectives in biomedical applications. Nanomaterials 2019, 9, 1352. [Google Scholar] [CrossRef]
- Tang, J.; Li, X.; Bao, L.; Chen, L.; Hong, F.F. Comparison of two types of bioreactors for synthesis of bacterial nanocellulose tubes as potential medical prostheses including artificial blood vessels. J. Chem. Technol. Biotechnol. 2017, 92, 1218–1228. [Google Scholar] [CrossRef]
- Van De Ven, T.G.M.; Sheikhi, A. Hairy cellulose nanocrystalloids: A novel class of nanocellulose. Nanoscale 2016, 8, 15101–15114. [Google Scholar] [CrossRef]
- Koshani, R.; Zhang, J.; Van De Ven, T.G.M.; Lu, X.; Wang, Y. Modified hairy nanocrystalline cellulose as photobactericidal nanofillers for food packaging application. ACS Sustain. Chem. Eng. 2021, 9, 10513–10523. [Google Scholar] [CrossRef]
- Tavakolian, M.; Wiebe, H.; Sadeghi, M.A.; Van De Ven, T.G.M. Dye removal using hairy nanocellulose: Experimental and theoretical investigations. ACS Appl. Mater. Interfaces 2020, 12, 5040–5049. [Google Scholar] [CrossRef]
- Kalia, S.; Boufi, S.; Celli, A.; Kango, S. Nanofibrillated cellulose: Surface modification and potential applications. Colloid Polym. Sci. 2014, 292, 5–31. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Daver, F.; Ivanova, E.P.; Habibi, Y.; Adhikari, B. Surface modifications of nanocellulose: From synthesis to high-performance nanocomposites. Prog. Polym. Sci. 2021, 119, 1–52. [Google Scholar] [CrossRef]
- Negro, C.; MartÃn, B.A.; Sanchez-Salvador, J.L.; Campano, C.; Fuente, E.; Monte, M.C.; Blanco, A. Nanocellulose and Its Potential Use For Sustainable Industrial Applications. Lat. Am. Appl. Res. 2020, 50, 59–64. [Google Scholar] [CrossRef]
- Parray, J.A.; Mohammad, Y.M.; Nowsheen, S. Lignin Nanoparticles: Synthesis and Application. In Nano-Technological Intervention in Agricultural Productivity; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2021; pp. 97–108. [Google Scholar] [CrossRef]
- Lourenço, A.; Jorge, G. Lignin as Feedstock for Nanoparticles Production; Sand, A., Tuteja, J., Eds.; IntechOpen: Rijeka, Croatia, 2023; Chapter 7. [Google Scholar] [CrossRef]
- Perera, U.P.; Mei, L.F.; Khang, W.T.; Irene, M.L. Process Modelling and Economic Evaluation for NanoLignin Production. IOP Conf. Ser. Mater. Sci. Eng. 2019, 652, 012054. [Google Scholar] [CrossRef]
- Behera, S.; Bikash, C.; Hrudayanath, T. Valorization of Lignin Based Nanoparticle from Grass Biomass: An Overview on Synthesis, Characterization and Application. Asian J. Biol. 2023, 17, 34–46. [Google Scholar] [CrossRef]
- Cao, Q.; Ying, L.; Lin, D.; Chuanling, S. Micro/Nanosized Lignin for Biomedical Application. Pap. Biomater. 2021, 6, 77–89. [Google Scholar] [CrossRef]
- Rangan, A.; Manjula, M.V.; Satyanarayana, K.G.; Menon, R. Lignin/Nanolignin and Their Biodegradable Composites. In Biodegradable Green Composites; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 167–198. [Google Scholar] [CrossRef]
- Frangville, C.; Marius, R.; Alexander, P.R.; Orlin, D.V.; Simeon, D.S.; Vesselin, N.P. Fabrication of Environmentally Biodegradable Lignin Nanoparticles. ChemPhysChem 2012, 13, 4235–4243. [Google Scholar] [CrossRef]
- Rahman, O.; Shubin, S.; Jiheng, D.; Donglin, W.; Sharif, A.; Haibin, Y. Lignin Nanoparticles: Synthesis, Characterization and Corrosion Protection Performance. New J. Chem. 2018, 42, 3415–3425. [Google Scholar] [CrossRef]
- Liu, K.; Yuntang, Z.; Jiachuan, C.; Guihua, Y.; Lin, D. Research Progress on the Preparation and High-Value Utilization of Lignin Nanoparticles. Int. J. Mol. Sci. 2022, 23, 7254. [Google Scholar] [CrossRef]
- Gilca, I.A.; Valentin, I.P.; Claudia, C. Obtaining Lignin Nanoparticles by Sonication. Ultrason. Sonochem. 2015, 23, 369–375. [Google Scholar] [CrossRef]
- Matsakas, L.; Anthi, K.; Andrzej, C.; Ulrika, R.; Paul, C. Formation of Lignin Nanoparticles by Combining Organosolv Pretreatment of Birch Biomass and Homogenization Processes. Molecules 2018, 23, 1822. [Google Scholar] [CrossRef]
- Beisl, S.; Angela, M.; Anton, F. Lignin from Micro- to Nanosize: Production Methods. Int. J. Mol. Sci. 2017, 18, 1244. [Google Scholar] [CrossRef] [PubMed]
- Vahabi, H.; Brosse, N.; Abd Latif, N.H.; Fatriasari, W.; Solihat, N.N.; Hashim, R.; Hazwan Hussin, M.; Laoutid, F.; Saeb, M.R. Nanolignin in Materials Science and Technology—Does Flame Retardancy Matter? In Biopolymeric Nanomaterials: Fundamentals and Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 515–559. [Google Scholar] [CrossRef]
- Parvathy, G.; Sethulekshmi, A.S.; Jayan, J.S.; Raman, A.; Saritha, A. Lignin Based Nano-Composites: Synthesis and Applications. Process Saf. Environ. Prot. 2021, 145, 395–410. [Google Scholar] [CrossRef]
- Qian, Y.; Xueqing, Q.; Shiping, Z. Lignin: A Nature-Inspired Sun Blocker for Broad-Spectrum Sunscreens. Green Chem. 2015, 17, 320–324. [Google Scholar] [CrossRef]
- Qian, Y.; Xueqing, Q.; Shiping, Z. Sunscreen Performance of Lignin from Different Technical Resources and Their General Synergistic Effect with Synthetic Sunscreens. ACS Sustain. Chem. Eng. 2016, 4, 4029–4035. [Google Scholar] [CrossRef]
- Morsali, M.; Adrian, M.; Andriana, L.; Ievgen, P.; Mika, H.S. Stabilized Lignin Nanoparticles for Versatile Hybrid and Functional Nanomaterials. Biomacromolecules 2022, 23, 4597–4606. [Google Scholar] [CrossRef]
- Langari, M.M.; Nikzad, M.; Labidi, J. Nanocellulose-Based Sensors in Medical/Clinical Applications: The State-of-the-Art Review. Carbohydr. Polym. 2023, 304, 120509. [Google Scholar] [CrossRef]
- Nadporozhskaya, M.; Kovsh, N.; Paolesse, R.; Lvova, L. Recent Advances in Chemical Sensors for Soil Analysis: A Review. Chemosensors 2022, 10, 35. [Google Scholar] [CrossRef]
- Wang, D.C.; Yu, H.Y.; Jiang, L.; Qi, D.; Zhang, X.; Chen, L.; Lv, W.; Xu, W.; Tam, K.C. Flexible, Anti-Damage, and Non-Contact Sensing Electronic Skin Implanted with MWCNT to Block Public Pathogens Contact Infection. Nano Res. 2022, 15, 2616–2625. [Google Scholar] [CrossRef]
- Batista, E.; Angels Moncusi, M.; López-Aguilar, P.; Martínez-Ballesté, A.; Solanas, A. Sensors for Context-Aware Smart Healthcare: A Security Perspective. Sensors 2021, 21, 6886. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A.; Pratap Singh, R.; Rab, S.; Suman, R. Upgrading the Manufacturing Sector via Applications of Industrial Internet of Things (IIoT). Sens. Int. 2021, 2, 100129. [Google Scholar] [CrossRef]
- Mbunge, E.; Muchemwa, B.; Jiyane, S.; Batani, J. Sensors and Healthcare 5.0: Transformative Shift in Virtual Care through Emerging Digital Health Technologies. Glob. Health J. 2021, 5, 169–177. [Google Scholar] [CrossRef]
- Fang, Z.; Zhu, H.; Preston, C.; Hu, L. Development, Application and Commercialization of Transparent Paper. Transl. Mater. Res. 2014, 1, 015004. [Google Scholar] [CrossRef]
- Chen, A.; Chatterjee, S. Nanomaterials Based Electrochemical Sensors for Biomedical Applications. Chem. Soc. Rev. 2013, 42, 5425. [Google Scholar] [CrossRef]
- Golmohammadi, H.; Morales-Narváez, E.; Naghdi, T.; Merkoçi, A. Nanocellulose in Sensing and Biosensing. Chem. Mater. 2017, 29, 5426–5446. [Google Scholar] [CrossRef]
- Liu, J.; Ji, H.; Lv, X.; Zeng, C.; Li, H.; Li, F.; Qu, B.; Cui, F.; Zhou, Q. Laser-Induced Graphene (LIG)-Driven Medical Sensors for Health Monitoring and Diseases Diagnosis. Mikrochim. Acta 2022, 189, 54. [Google Scholar] [CrossRef]
- Tang, T.Q.; Rooman, M.; Shah, Z.; Khan, S.; Vrinceanu, N.; Alshehri, A.; Racheriu, M. Numerical study of magnetized Powell–Eyring hybrid nanomaterial flow with variable heat transfer in the presence of artificial bacteria: Applications for tumor removal and cancer cell destruction. Front. Mater. 2023, 10, 1144854. [Google Scholar] [CrossRef]
- Ratajczak, K.; Stobiecka, M. High-Performance Modified Cellulose Paper-Based Biosensors for Medical Diagnostics and Early Cancer Screening: A Concise Review. Carbohydr. Polym. 2020, 229, 115463. [Google Scholar] [CrossRef]
- Yuen, J.D.; Shriver-Lake, L.C.; Walper, S.A.; Zabetakis, D.; Breger, J.C.; Stenger, D.A. Microbial Nanocellulose Printed Circuit Boards for Medical Sensing. Sensors 2020, 20, 2047. [Google Scholar] [CrossRef]
- Reshmy, R.; Philip, E.; Paul, S.A.; Madhavan, A.; Sindhu, R.; Binod, P.; Pandey, A.; Sirohi, R. Nanocellulose-Based Products for Sustainable Applications-Recent Trends and Possibilities. Rev. Environ. Sci. Biotechnol. 2020, 19, 779–806. [Google Scholar] [CrossRef]
- Trung, T.Q.; Lee, N.E. Flexible and Stretchable Physical Sensor Integrated Platforms for Wearable Human-Activity Monitoringand Personal Healthcare. Adv. Mater. 2016, 28, 4338–4372. [Google Scholar] [CrossRef]
- Wang, B.; Facchetti, A. Mechanically Flexible Conductors for Stretchable and Wearable E-Skin and E-Textile Devices. Adv. Mater. 2019, 31, 1901408. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xia, K.; Wang, H.; Liang, X.; Yin, Z.; Zhang, Y. Advanced Carbon for Flexible and Wearable Electronics. Adv. Mater. 2019, 31, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhao, M.; Hao, Y.; Wei, Q. Recent Advances in Functional Bacterial Cellulose for Wearable Physical Sensing Applications. Adv. Mater. Technol. 2022, 7, 1–14. [Google Scholar] [CrossRef]
- Dai, L.; Wang, Y.; Zou, X.; Chen, Z.; Liu, H.; Ni, Y. Ultrasensitive Physical, Bio, and Chemical Sensors Derived from 1-, 2-, and 3-D Nanocellulosic Materials. Small 2020, 16, 1–25. [Google Scholar] [CrossRef]
- Norrrahim, M.N.F.; Knight, V.F.; Nurazzi, N.M.; Jenol, M.A.; Misenan, M.S.M.; Janudin, N.; Kasim, N.A.M.; Shukor, M.F.A.; Ilyas, R.A.; Asyraf, M.R.M.; et al. The Frontiers of Functionalized Nanocellulose-Based Composites and Their Application as Chemical Sensors. Polymers 2022, 14, 4461. [Google Scholar] [CrossRef]
- Yan, Q.; Zhou, M.; Fu, H. Study on Mussel-Inspired Tough TA/PANI@CNCs Nanocomposite Hydrogels with Superior Self-Healing and Self-Adhesive Properties for Strain Sensors. Compos. B Eng. 2020, 201, 108356. [Google Scholar] [CrossRef]
- Song, M.; Yu, H.; Zhu, J.; Ouyang, Z.; Abdalkarim, S.Y.H.; Tam, K.C.; Li, Y. Constructing Stimuli-Free Self-Healing, Robust and Ultrasensitive Biocompatible Hydrogel Sensors with Conductive Cellulose Nanocrystals. Chem. Eng. J. 2020, 398, 125547. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Y.; Zhang, Y.; Yang, F.; Liu, Y.; Wang, X.; Yang, J.; Gong, X.; Zheng, J. Highly Stretchable, Self-Adhesive, Biocompatible, Conductive Hydrogels as Fully Polymeric Strain Sensors. J. Mater. Chem. 2020, 8, 20474–20485. [Google Scholar] [CrossRef]
- Su, J.; Zhang, L.; Wan, C.; Deng, Z.; Wei, S.; Yong, K.T.; Wu, Y. Dual-Network Self-Healing Hydrogels Composed of Graphene Oxide@nanocellulose and Poly(AAm-Co-AAc). Carbohydr. Polym. 2022, 296, 1–9. [Google Scholar] [CrossRef]
- Brakat, A.; Zhu, H. Nanocellulose-Graphene Hybrids: Advanced Functional Materials as Multifunctional Sensing Platform. Nanomicro Lett. 2021, 13, 94. [Google Scholar] [CrossRef]
- Sadasivuni, K.K.; Kafy, A.; Zhai, L.; Ko, H.U.; Mun, S.; Kim, J. Transparent and Flexible Cellulose Nanocrystal/Reduced Graphene Oxide Film for Proximity Sensing. Small 2015, 11, 994–1002. [Google Scholar] [CrossRef]
- Kafy, A.; Sadasivuni, K.K.; Mun, S.; Kim, J. A Tactile Sensor Made of Graphene-Cellulose Nanocomposite. In Nanosensors, Biosensors, and Info-Tech Sensors and Systems; Varadan, V.K., Ed.; SPIE: Bellingham, WA, USA, 2015; p. 943403. [Google Scholar]
- Liew, S.Y.; Thielemans, W.; Walsh, D.A. Electrochemical Capacitance of Nanocomposite Polypyrrole/Cellulose Films. J. Phys. Chem. C 2010, 114, 17926–17933. [Google Scholar] [CrossRef]
- McLaurin, E.J.; Bradshaw, L.R.; Gamelin, D.R. Dual-Emitting Nanoscale Temperature Sensors. Chem. Mater. 2013, 25, 1283–1292. [Google Scholar] [CrossRef]
- Zhou, T.; Wei, H.; Tan, H.; Wang, X.; Zeng, H.; Liu, X.; Nagao, S.; Koga, H.; Nogi, M.; Sugahara, T.; et al. Strongly Anisotropic Thermal Conductivity and Adequate Breathability of Bilayered Films for Heat Management of On-Skin Electronics. 2D Mater. 2018, 5, 035013. [Google Scholar] [CrossRef]
- Jung, M.; Kim, K.; Kim, B.; Lee, K.-J.; Kang, J.-W.; Jeon, S. Vertically Stacked Nanocellulose Tactile Sensor. Nanoscale 2017, 9, 17212–17219. [Google Scholar] [CrossRef]
- Pascariu, P.; Airinei, A.; Olaru, N.; Petrila, I.; Nica, V.; Sacarescu, L.; Tudorache, F. Microstructure, Electrical and Humidity Sensor Properties of Electrospun NiO–SnO2 Nanofibers. Sens. Actuators B Chem. 2016, 222, 1024–1031. [Google Scholar] [CrossRef]
- Zhu, P.; Liu, Y.; Fang, Z.; Kuang, Y.; Zhang, Y.; Peng, C.; Chen, G. Flexible and Highly Sensitive Humidity Sensor Based on Cellulose Nanofibers and Carbon Nanotube Composite Film. Langmuir 2019, 35, 4834–4842. [Google Scholar] [CrossRef]
- Ayissi Eyebe, G.; Bideau, B.; Boubekeur, N.; Loranger, É.; Domingue, F. Environmentally-Friendly Cellulose Nanofibre Sheets for Humidity Sensing in Microwave Frequencies. Sens. Actuators B Chem. 2017, 245, 484–492. [Google Scholar] [CrossRef]
- Zhu, P.; Kuang, Y.; Wei, Y.; Li, F.; Ou, H.; Jiang, F.; Chen, G. Electrostatic Self-Assembly Enabled Flexible Paper-Based Humidity Sensor with High Sensitivity and Superior Durability. Chem. Eng. J. 2021, 404, 127105. [Google Scholar] [CrossRef]
- Ginja, G.A.; De Campos Da Costa, J.P.; Gounella, R.H.; Izquierdo, J.E.E.; Carmo, J.P.; Fonseca, F.J.; Cavallari, M.R.; Junior, O.H.A.; Souza, S.S. De A Humidity Sensor Based on Bacterial Nanocellulose Membrane (BNC). IEEE Sens. J. 2023, 23, 3485–3492. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Wang, Y. Strategies for the Performance Enhancement of Graphene-Based Gas Sensors: A Review. Talanta 2021, 235, 122745. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Carmona, E.; Bertuna, A.; Abbatangelo, M.; Sberveglieri, V.; Comini, E.; Sberveglieri, G. BC-MOS: The Novel Bacterial Cellulose Based MOS Gas Sensors. Mater. Lett. 2019, 237, 69–71. [Google Scholar] [CrossRef]
- Liu, H.; He, Y.; Nagashima, K.; Meng, G.; Dai, T.; Tong, B.; Deng, Z.; Wang, S.; Zhu, N.; Yanagida, T.; et al. Discrimination of VOCs Molecules via Extracting Concealed Features from a Temperature-Modulated p-Type NiO Sensor. Sens. Actuators B Chem. 2019, 293, 342–349. [Google Scholar] [CrossRef]
- Koga, H.; Nagashima, K.; Huang, Y.; Zhang, G.; Wang, C.; Takahashi, T.; Inoue, A.; Yan, H.; Kanai, M.; He, Y.; et al. Paper-Based Disposable Molecular Sensor Constructed from Oxide Nanowires, Cellulose Nanofibers, and Pencil-Drawn Electrodes. ACS Appl. Mater. Interfaces 2019, 11, 15044–15050. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Yang, Z.; Chen, Y.; Zhang, J.; Wang, Q.; Huang, F.; Wei, Q. A Room Temperature Ammonia Gas Sensor Based on Cellulose/TiO 2 /PANI Composite Nanofibers. Colloids Surf. A Physicochem. Eng. Asp 2016, 494, 248–255. [Google Scholar] [CrossRef]
- Kumar, S.; Ngasainao, M.R.; Sharma, D.; Sengar, M.; Gahlot, A.P.S.; Shukla, S.; Kumari, P. Contemporary Nanocellulose-Composites: A New Paradigm for Sensing Applications. Carbohydr. Polym. 2022, 298, 120052. [Google Scholar] [CrossRef]
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical Biosensors: Recommended Definitions and Classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef]
- Shukla, S.K.; Govender, P.P.; Tiwari, A. Polymeric Micellar Structures for Biosensor Technology, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 24. [Google Scholar]
- Fatima, T.; Bansal, S.; Husain, S.; Khanuja, M. Biosensors. In Electrochemical Sensors; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–30. ISBN 9780128231487. [Google Scholar]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical Biosensors-Sensor Principles and Architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef]
- Neubauerova, K.; Carneiro, M.C.C.G.; Rodrigues, L.R.; Moreira, F.T.C.; Sales, M.G.F. Nanocellulose- Based Biosensor for Colorimetric Detection of Glucose. Sens. Bio-Sens. Res 2020, 29, 100368. [Google Scholar] [CrossRef]
- Mun, S.; Maniruzzaman, M.; Ko, H.U.; Kafy, A.; Kim, J. Preparation and Characterisation of Cellulose ZnO Hybrid Film by Blending Method and Its Glucose Biosensor Application. Mater. Technol. 2015, 30, B150–B154. [Google Scholar] [CrossRef]
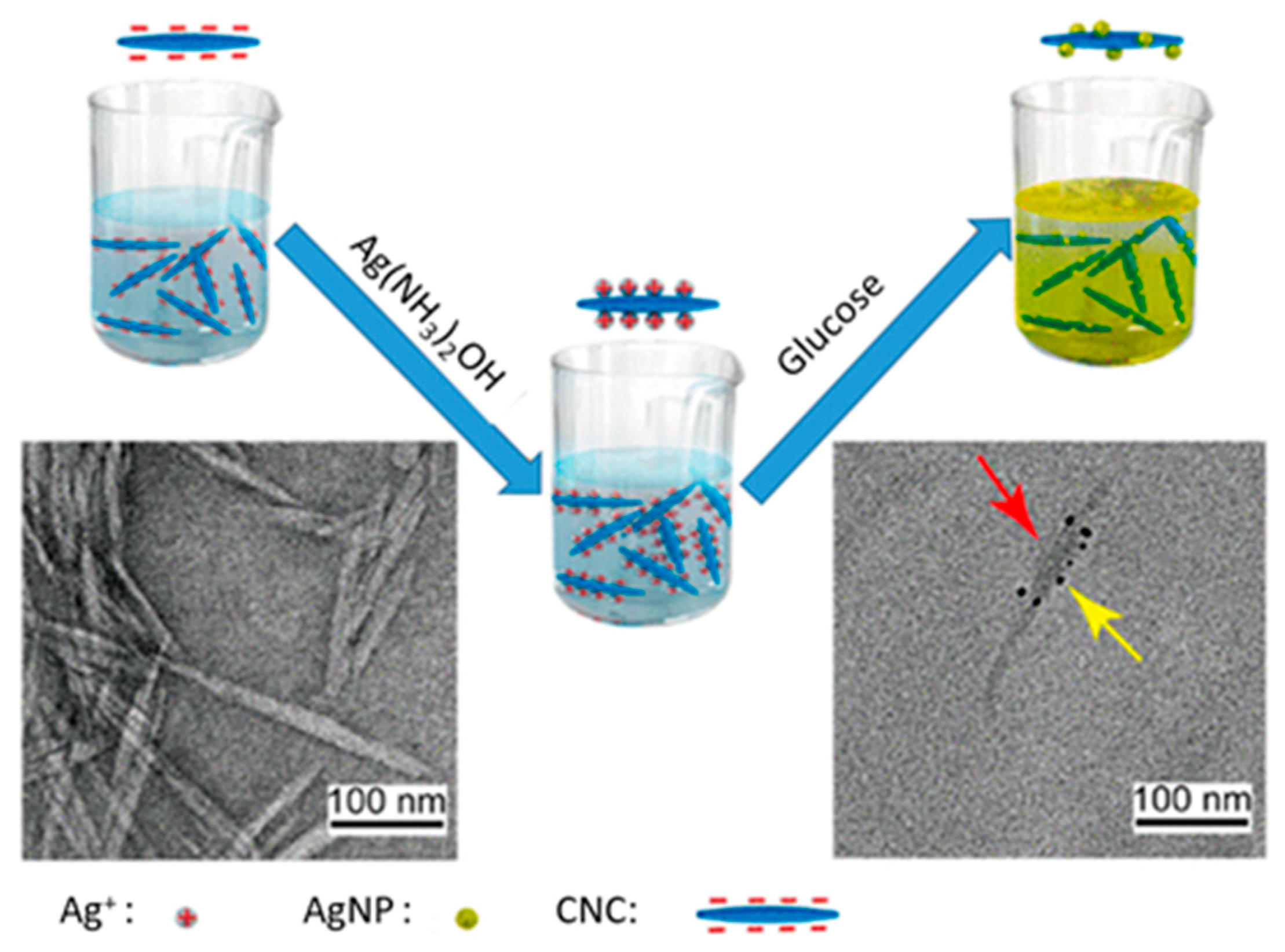
- Wang, S.; Sun, J.; Jia, Y.; Yang, L.; Wang, N.; Xianyu, Y.; Chen, W.; Li, X.; Cha, R.; Jiang, X. Nanocrystalline Cellulose-Assisted Generation of Silver Nanoparticles for Nonenzymatic Glucose Detection and Antibacterial Agent. Biomacromolecules 2016, 17, 2472–2478. [Google Scholar] [CrossRef]
- McLister, A.; McHugh, J.; Cundell, J.; Davis, J. New Developments in Smart Bandage Technologies for Wound Diagnostics. Adv. Mater. 2016, 28, 5732–5737. [Google Scholar] [CrossRef]
- Zhou, J.; Yao, D.; Qian, Z.; Hou, S.; Li, L.; Jenkins, A.T.A.; Fan, Y. Bacteria-Responsive Intelligent Wound Dressing: Simultaneous In Situ Detection and Inhibition of Bacterial Infection for Accelerated Wound Healing. Biomaterials 2018, 161, 11–23. [Google Scholar] [CrossRef]
- Ling, Z.; Xu, F.; Edwards, J.V.; Prevost, N.T.; Nam, S.; Condon, B.D.; French, A.D. Nanocellulose as a Colorimetric Biosensor for Effective and Facile Detection of Human Neutrophil Elastase. Carbohydr. Polym. 2019, 216, 360–368. [Google Scholar] [CrossRef]
- Fontenot, K.R.; Edwards, J.V.; Haldane, D.; Graves, E.; Citron, M.S.; Prevost, N.T.; French, A.D.; Condon, B.D. Human Neutrophil Elastase Detection with Fluorescent Peptide Sensors Conjugated to Cellulosic and Nanocellulosic Materials: Part II, Structure/Function Analysis. Cellulose 2016, 23, 1297–1309. [Google Scholar] [CrossRef]
- Derikvand, F.; Yin, D.T.; Barrett, R.; Brumer, H. Cellulose-based biosensors for esterase detection. Anal. Chem. 2016, 88, 2989–2993. [Google Scholar] [CrossRef]
- Abdi, M.M.; Razalli, R.L.; Tahir, P.M.; Chaibakhsh, N.; Hassani, M.; Mir, M. Optimized Fabrication of Newly Cholesterol Biosensor Based on Nanocellulose. Int. J. Biol. Macromol. 2019, 126, 1213–1222. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Deepa, J.R.; Binussreejayan. Electrochemical Sensing of Cholesterol by Molecularly Imprinted Polymer of Silylated Graphene Oxide and Chemically Modified Nanocellulose Polymer. Mater. Sci. Eng. C 2018, 92, 942–956. [Google Scholar] [CrossRef]
- Khattab, T.A.; Fouda, M.M.G.; Abdelrahman, M.S.; Othman, S.I.; Bin-Jumah, M.; Alqaraawi, M.A.; Al Fassam, H.; Allam, A.A. Co-Encapsulation of Enzyme and Tricyanofuran Hydrazone into Alginate Microcapsules Incorporated onto Cotton Fabric as a Biosensor for Colorimetric Recognition of Urea. React. Funct. Polym. 2019, 142, 199–206. [Google Scholar] [CrossRef]
- Khattab, T.A.; Fouda, M.M.G.; Rehan, M.; Okla, M.K.; Alamri, S.A.; Alaraidh, I.A.; AL-ghamdi, A.A.; Soufan, W.H.; Abdelsalam, E.M.; Allam, A.A. Novel Halochromic Cellulose Nanowhiskers from Rice Straw: Visual Detection of Urea. Carbohydr. Polym. 2020, 231, 115740. [Google Scholar] [CrossRef]
- Abdullahil, K.; Maniruzzaman, M.; Kang, B.W.; Kim, J. Fabrication and Characterization of Titanium Dioxide-Cellulose Composite and Its Urea Biosensing Behavior. Sens. Mater. 2015, 27, 539–548. [Google Scholar] [CrossRef]
- Rehan, M.; Ahmed-Farid, O.A.; Ibrahim, S.R.; Hassan, A.A.; Abdelrazek, A.M.; Khafaga, N.I.M.; Khattab, T.A. Green and Sustainable Encapsulation of Guava Leaf Extracts (Psidium guajava L.) into Alginate/Starch Microcapsules for Multifunctional Finish over Cotton Gauze. ACS Sustain. Chem. Eng. 2019, 7, 18612–18623. [Google Scholar] [CrossRef]
- Dinh Duong, H.; Il Rhee, J. Development of a Ratiometric Fluorescent Urea Biosensor Based on the Urease Immobilized onto the Oxazine 170 Perchlorate-Ethyl Cellulose Membrane. Talanta 2015, 134, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Naghdi, T.; Golmohammadi, H.; Vosough, M.; Atashi, M.; Saeedi, I.; Maghsoudi, M.T. Lab-on-Nanopaper: An Optical Sensing Bioplatform Based on Curcumin Embedded in Bacterial Nanocellulose as an Albumin Assay Kit. Anal. Chim. Acta 2019, 1070, 104–111. [Google Scholar] [CrossRef] [PubMed]
- De Assis, S.C.; Morgado, D.L.; Scheidt, D.T.; de Souza, S.S.; Cavallari, M.R.; Ando Junior, O.H.; Carrilho, E. Review of Bacterial Nanocellulose-Based Electrochemical Biosensors: Functionalization, Challenges, and Future Perspectives. Biosensors 2023, 13, 142. [Google Scholar] [CrossRef]
- Abbasi-Moayed, S.; Golmohammadi, H.; Bigdeli, A.; Hormozi-Nezhad, M.R. A Rainbow Ratiometric Fluorescent Sensor Array on Bacterial Nanocellulose for Visual Discrimination of Biothiols. Analyst 2018, 143, 3415–3424. [Google Scholar] [CrossRef]
- Tian, L.; Jiang, Q.; Liu, K.-K.; Luan, J.; Naik, R.R.; Singamaneni, S. Nanocellulose Films: Bacterial Nanocellulose-Based Flexible Surface Enhanced Raman Scattering Substrate. Adv. Mater. Interfaces 2016, 3, 1600214. [Google Scholar] [CrossRef]
- Ahmad, N.; Tayyeb, D.; Ali, I.; Alruwaili, N.K.; Ahmad, W.; Ur Rehman, A.; Khan, A.H.; Iqbal, M.S. Development and Characterization of Hemicellulose-Based Films for Antibacterial Wound-Dressing Application. Polymers 2020, 12, 548. [Google Scholar] [CrossRef]
- Ling, Z.; Ma, J.; Zhang, S.; Shao, L.; Wang, C.; Ma, J. Stretchable and Fatigue Resistant Hydrogels Constructed by Natural Galactomannan for Flexible Sensing Application. Int. J. Biol. Macromol. 2022, 216, 193–202. [Google Scholar] [CrossRef]
- Zhang, W.; Wen, J.-Y.; Ma, M.-G.; Li, M.-F.; Peng, F.; Bian, J. Anti-Freezing, Water-Retaining, Conductive, and Strain-Sensitive Hemicellulose/Polypyrrole Composite Hydrogels for Flexible Sensors. J. Mater. Res. 2021, 14, 555–566. [Google Scholar] [CrossRef]
- Zhang, W.; Wen, J.; Yang, J.; Li, M.; Peng, F.; Ma, M.; Bian, J. Multifunctional Hybrid Hydrogel with Transparency, Conductivity, and Self-Adhesion for Soft Sensors Using Hemicellulose-Decorated Polypyrrole as a Conductive Matrix. Int. J. Biol. Macromol. 2022, 223, 1–10. [Google Scholar] [CrossRef]
- Gong, X.; Fu, C.; Alam, N.; Ni, Y.; Chen, L.; Huang, L.; Hu, H.-C. Tannic Acid Modified Hemicellulose Nanoparticle Reinforced Ionic Hydrogels with Multi-Functions for Human Motion Strain Sensor Applications. Ind. Crops Prod. 2022, 176, 114412. [Google Scholar] [CrossRef]
- Rao, J.; Gao, H.; Guan, Y.; Li, W.; Liu, Q. Fabrication of Hemicelluloses Films with Enhanced Mechanical Properties by Graphene Oxide for Humidity Sensing. Carbohydr. Polym. 2019, 208, 513–520. [Google Scholar] [CrossRef]
- Guan, Y.; Qi, X.-M.; Chen, G.-G.; Peng, F.; Sun, R.-C. Facile Approach to Prepare Drug-Loading Film from Hemicelluloses and Chitosan. Carbohydr. Polym. 2016, 153, 542–548. [Google Scholar] [CrossRef]
- Kulyk, B.; Matos, M.; Silva, B.F.R.; Carvalho, A.F.; Fernandes, A.J.S.; Evtuguin, D.V.; Fortunato, E.; Costa, F.M. Conversion of Paper and Xylan into Laser-Induced Graphene for Environmentally Friendly Sensors. Diam. Relat. Mater. 2022, 123, 108855. [Google Scholar] [CrossRef]
- Abhilash, M.; Thomas, D. Biopolymers for Biocomposites and Chemical Sensor Applications. In Biopolymer Composites in Electronics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 405–435. [Google Scholar]
- Ren, J.; Dai, Q.; Zhong, H.; Liu, X.; Meng, L.; Wang, X.; Xin, F. Quaternized Xylan/Cellulose Nanocrystal Reinforced Magnetic Hydrogels with High Strength. Cellulose 2018, 25, 4537–4549. [Google Scholar] [CrossRef]
- Seera, S.D.K.; Kundu, D.; Gami, P.; Naik, P.K.; Banerjee, T. Synthesis and Characterization of Xylan-Gelatin Cross-Linked Reusable Hydrogel for the Adsorption of Methylene Blue. Carbohydr. Polym. 2021, 256, 117520. [Google Scholar] [CrossRef]
- Xiang, Z.; Tang, N.; Jin, X.; Gao, W. Fabrications and Applications of Hemicellulose-Based Bio-Adsorbents. Carbohydr. Polym. 2022, 278, 118945. [Google Scholar] [CrossRef]
- Kundu, D.; Mondal, S.K.; Banerjee, T. Development of β-Cyclodextrin-Cellulose/Hemicellulose-Based Hydrogels for the Removal of Cd(II) and Ni(II): Synthesis, Kinetics, and Adsorption Aspects. J. Chem. Eng. Data 2019, 64, 2601–2617. [Google Scholar] [CrossRef]
- Han, G.; Cai, J.; Liu, C.; Ren, J.; Wang, X.; Yang, J.; Wang, X. Highly Sensitive Electrochemical Sensor Based on Xylan-Based Ag@CQDs-RGO Nanocomposite for Dopamine Detection. Appl. Surf. Sci. 2021, 541, 148566. [Google Scholar] [CrossRef]
- Katrlík, J.; Holazová, A.; Medovarská, I.; Seilerová, I.; Gemeiner, P.; Bystrický, S. SPR Biosensor Chip Based on Mannan Isolated from Candida Dubliniensis Yeasts Applied in Immunization Effectiveness Testing. Sens. Actuators B Chem. 2022, 350, 130883. [Google Scholar] [CrossRef]
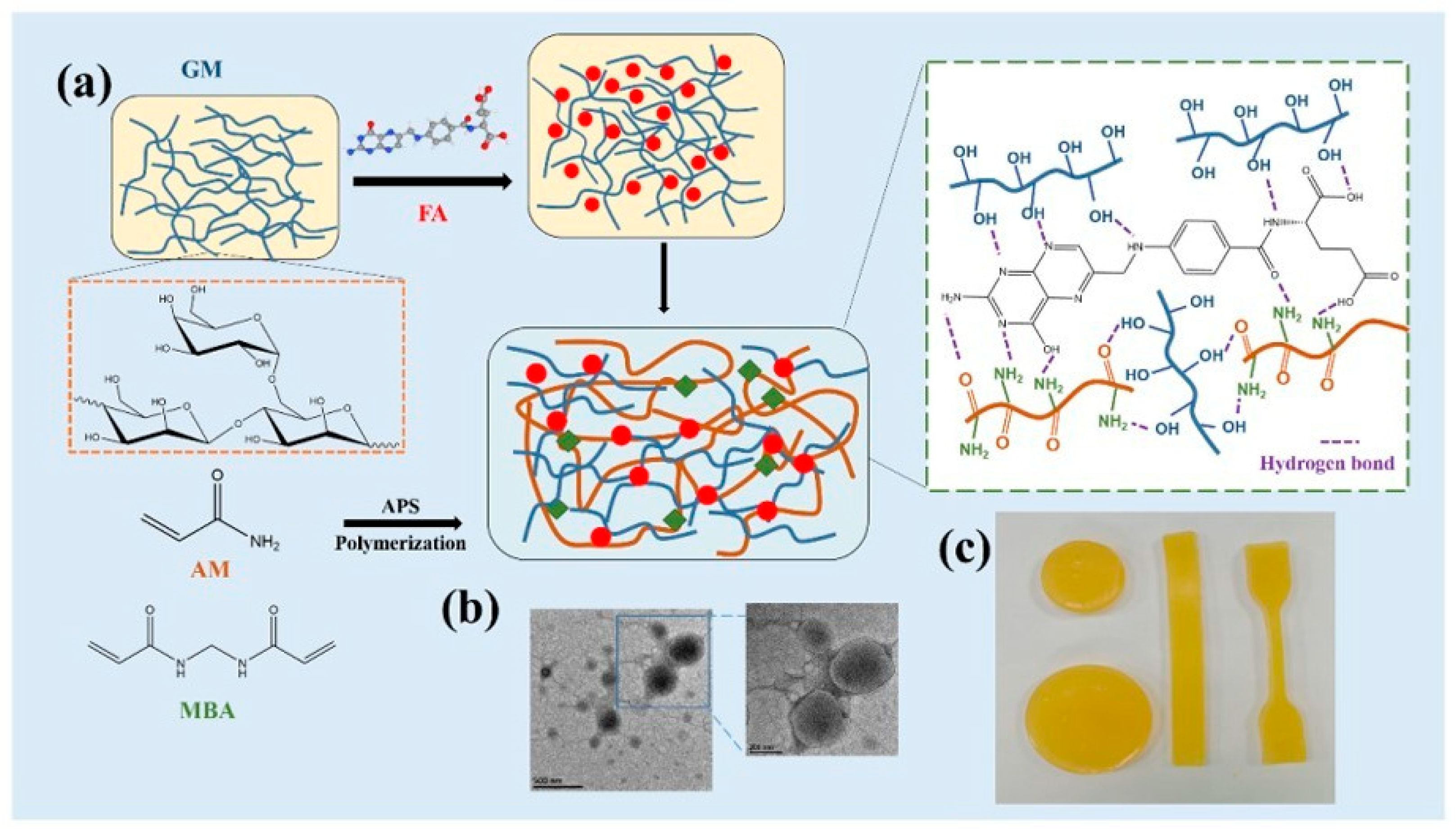
- Han, X.; Lv, Z.; Ran, F.; Dai, L.; Li, C.; Si, C. Green and Stable Piezoresistive Pressure Sensor Based on Lignin-Silver Hybrid Nanoparticles/Polyvinyl Alcohol Hydrogel. Int. J. Biol. Macromol. 2021, 176, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Kim, T.-H.; Liu, K.; Ma, M.-G.; Choi, S.-E.; Si, C. Multifunctional Lignin-Based Composite Materials for Emerging Applications. Front. Bioeng. Biotechnol. 2021, 9, 8976. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Huang, X.-H.; Yao, Y.; Luo, K.-B.; Pan, H.-Z.; Wang, Q. Ringed Electrode Configuration Enhances the Sensitivity of QCM Humidity Sensor Based on Lignin Through Fringing Field Effect. IEEE Sens. J. 2021, 21, 22450–22458. [Google Scholar] [CrossRef]
- Sun, L.; Mo, Z.; Li, Q.; Zheng, D.; Qiu, X.; Pan, X. Facile Synthesis and Performance of PH/Temperature Dual-Response Hydrogel Containing Lignin-Based Carbon Dots. Int. J. Biol. Macromol. 2021, 175, 516–525. [Google Scholar] [CrossRef]
- Joshi, K.M.; Shinde, D.R.; Nikam, L.K.; Panmand, R.; Sethi, Y.A.; Kale, B.B.; Chaskar, M.G. Fragmented Lignin-Assisted Synthesis of a Hierarchical ZnO Nanostructure for Ammonia Gas Sensing. RSC Adv. 2019, 9, 2484–2492. [Google Scholar] [CrossRef]
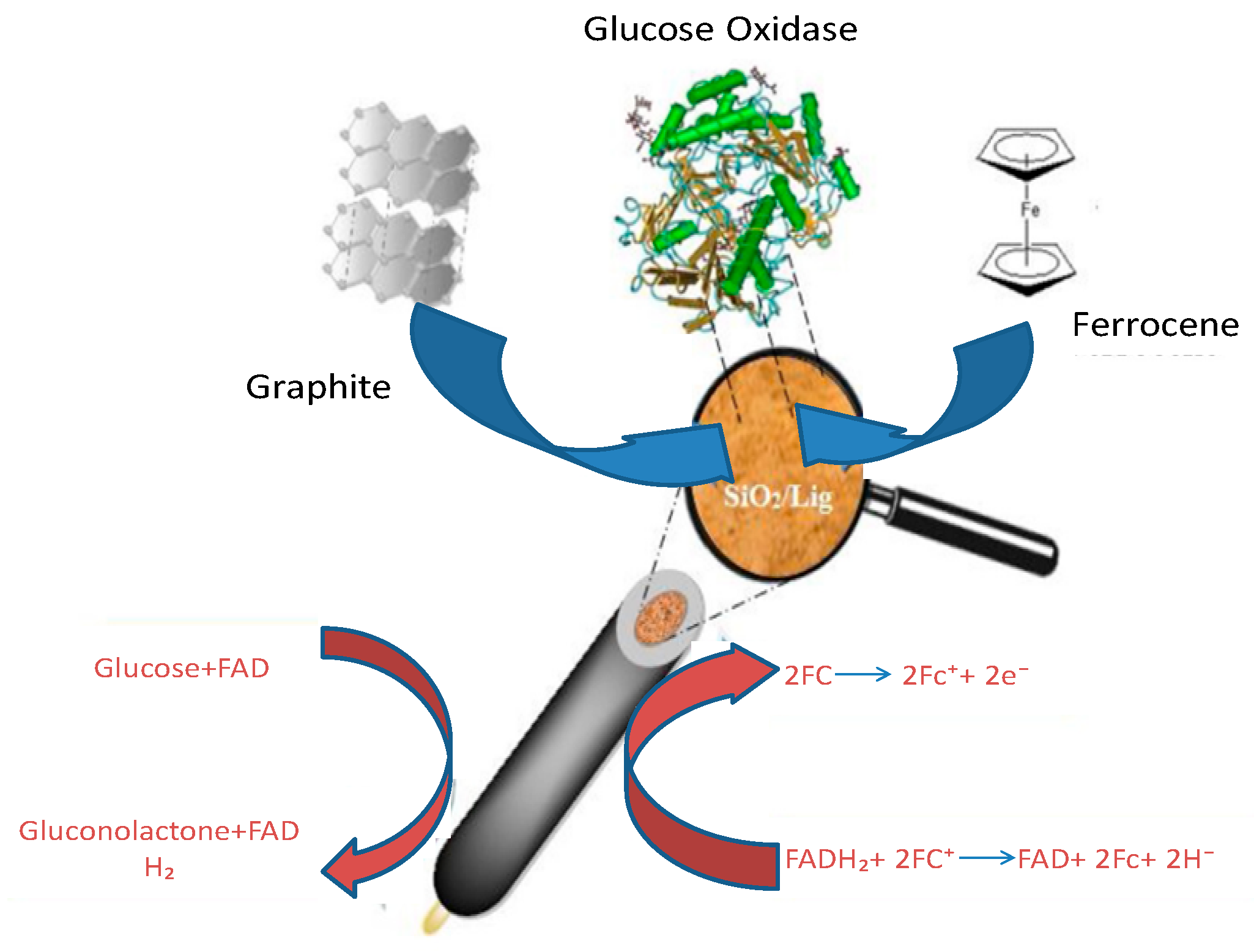
- Jędrzak, A.; Rębiś, T.; Klapiszewski, Ł.; Zdarta, J.; Milczarek, G.; Jesionowski, T. Carbon Paste Electrode Based on Functional GOx/Silica-Lignin System to Prepare an Amperometric Glucose Biosensor. Sens. Actuators B Chem. 2018, 256, 176–185. [Google Scholar] [CrossRef]
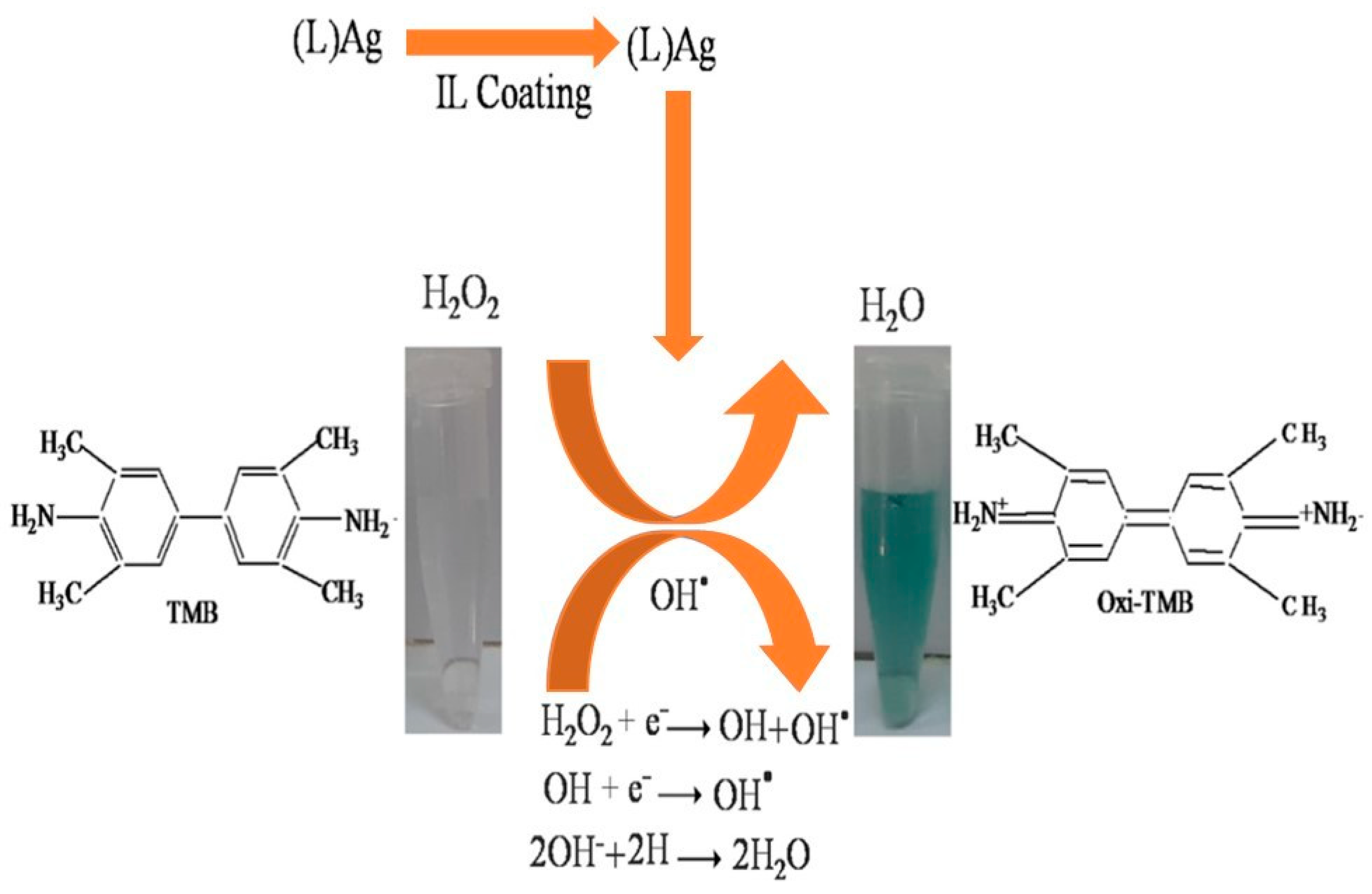
- Nishan, U.; Niaz, A.; Muhammad, N.; Asad, M.; Shah, A.-H.A.; Khan, N.; Khan, M.; Shujah, S.; Rahim, A. Non-Enzymatic Colorimetric Biosensor for Hydrogen Peroxide Using Lignin-Based Silver Nanoparticles Tuned with Ionic Liquid as a Peroxidase Mimic. Arab. J. Chem. 2021, 14, 103164. [Google Scholar] [CrossRef]
- Tai, M.J.Y.; Perumal, V.; Gopinath, S.C.B.; Raja, P.B.; Ibrahim, M.N.M.; Jantan, I.N.; Suhaimi, N.S.H.; Liu, W.-W. Laser-Scribed Graphene Nanofiber Decorated with Oil Palm Lignin Capped Silver Nanoparticles: A Green Biosensor. Sci. Rep. 2021, 11, 5475. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durmaz, E.; Sertkaya, S.; Yilmaz, H.; Olgun, C.; Ozcelik, O.; Tozluoglu, A.; Candan, Z. Lignocellulosic Bionanomaterials for Biosensor Applications. Micromachines 2023, 14, 1450. https://doi.org/10.3390/mi14071450
Durmaz E, Sertkaya S, Yilmaz H, Olgun C, Ozcelik O, Tozluoglu A, Candan Z. Lignocellulosic Bionanomaterials for Biosensor Applications. Micromachines. 2023; 14(7):1450. https://doi.org/10.3390/mi14071450
Chicago/Turabian StyleDurmaz, Ekrem, Selva Sertkaya, Hande Yilmaz, Cagri Olgun, Orhan Ozcelik, Ayhan Tozluoglu, and Zeki Candan. 2023. "Lignocellulosic Bionanomaterials for Biosensor Applications" Micromachines 14, no. 7: 1450. https://doi.org/10.3390/mi14071450
APA StyleDurmaz, E., Sertkaya, S., Yilmaz, H., Olgun, C., Ozcelik, O., Tozluoglu, A., & Candan, Z. (2023). Lignocellulosic Bionanomaterials for Biosensor Applications. Micromachines, 14(7), 1450. https://doi.org/10.3390/mi14071450









