ZnO Hollow Quasi-Spheres Modified Screen-Printed Graphite Electrode for Determination of Carmoisine
Abstract
1. Introduction
2. Experimental
2.1. Chemicals and Solutions
2.2. Equipments
2.3. Synthesis of ZnO HQSs
2.4. Preparation of ZnO HQSs/SPGE
2.5. Preparation of Real Samples
3. Results and Discussions
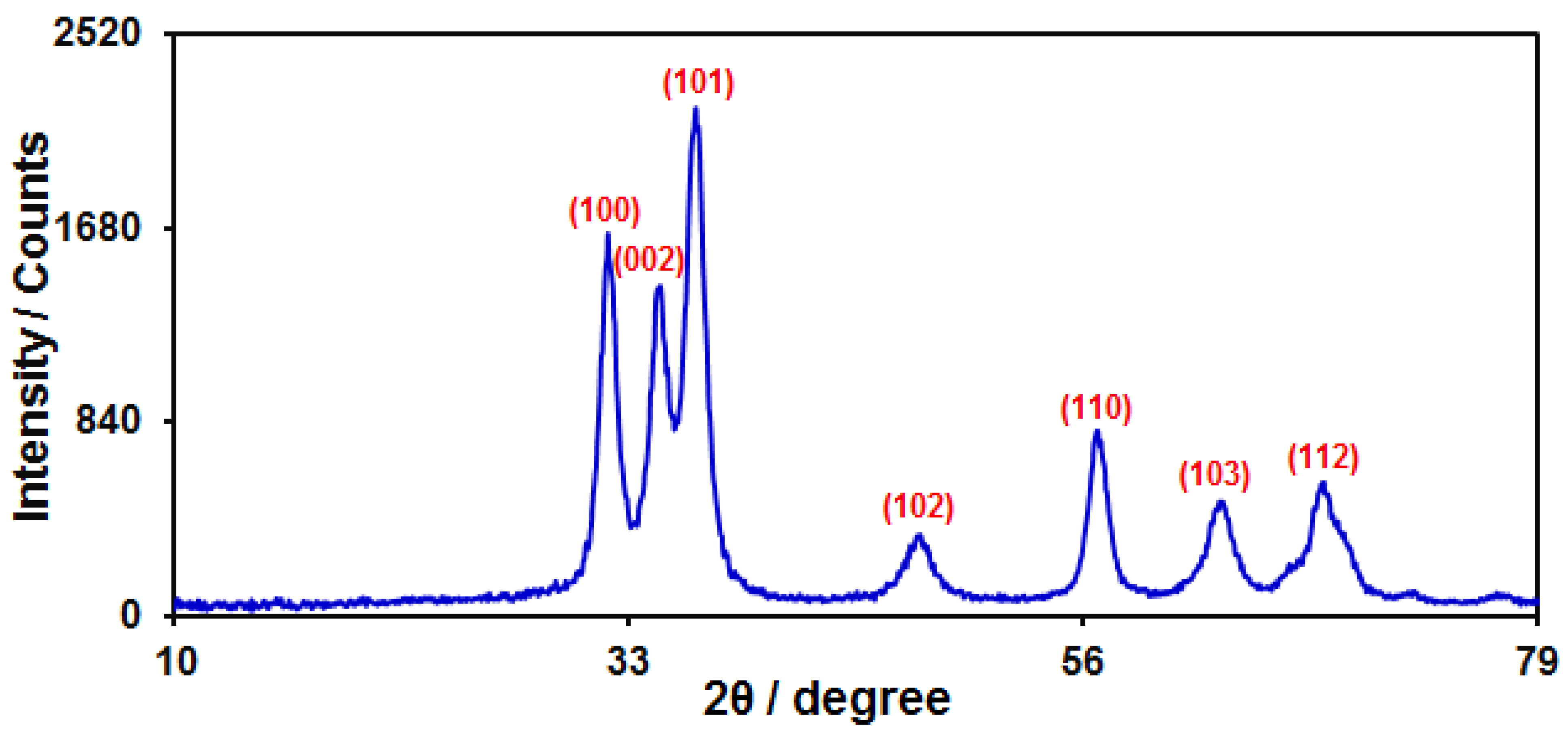
3.1. Characterization of ZnO HQSs
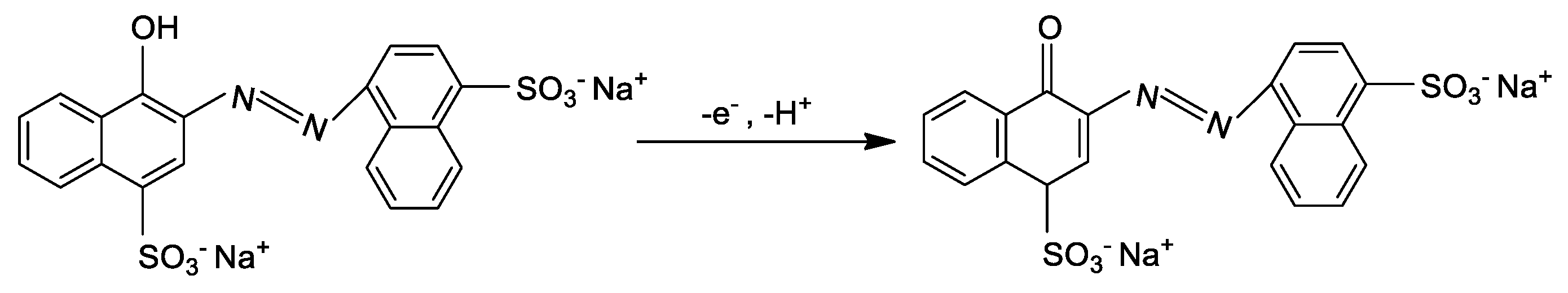
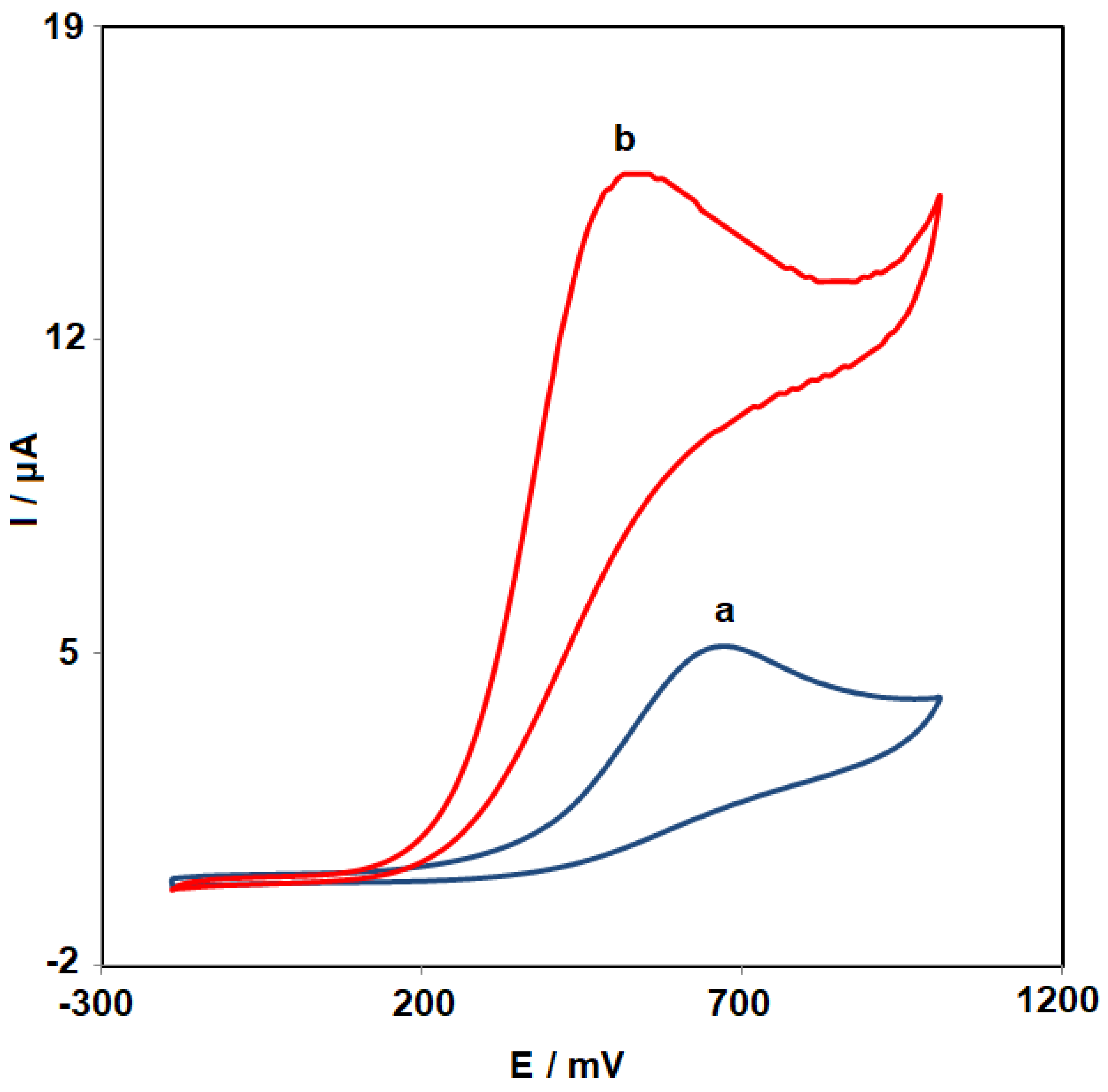
3.2. Performance of the ZnO HQSs/SPGE Sensor for Carmoisine Determination
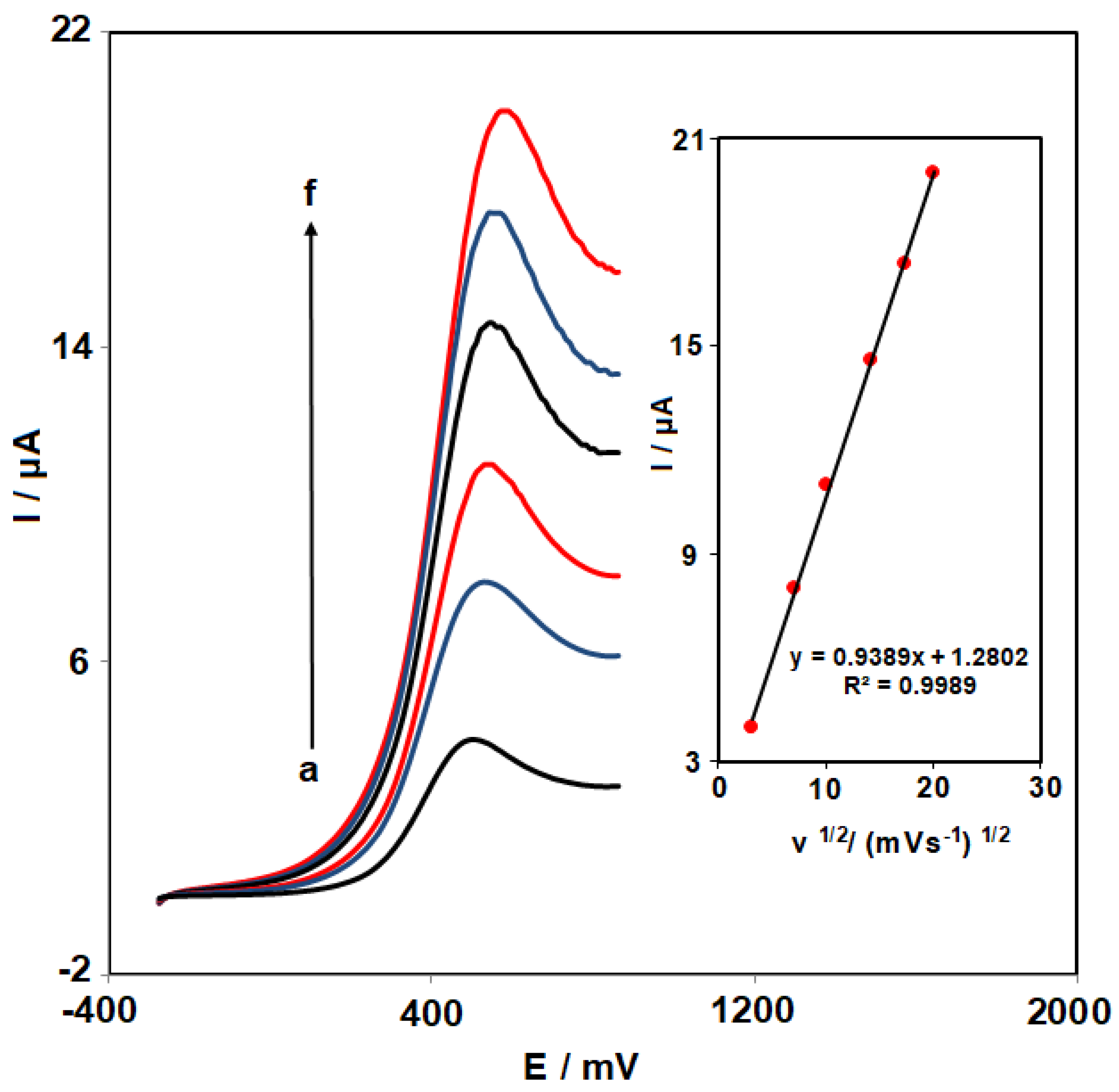
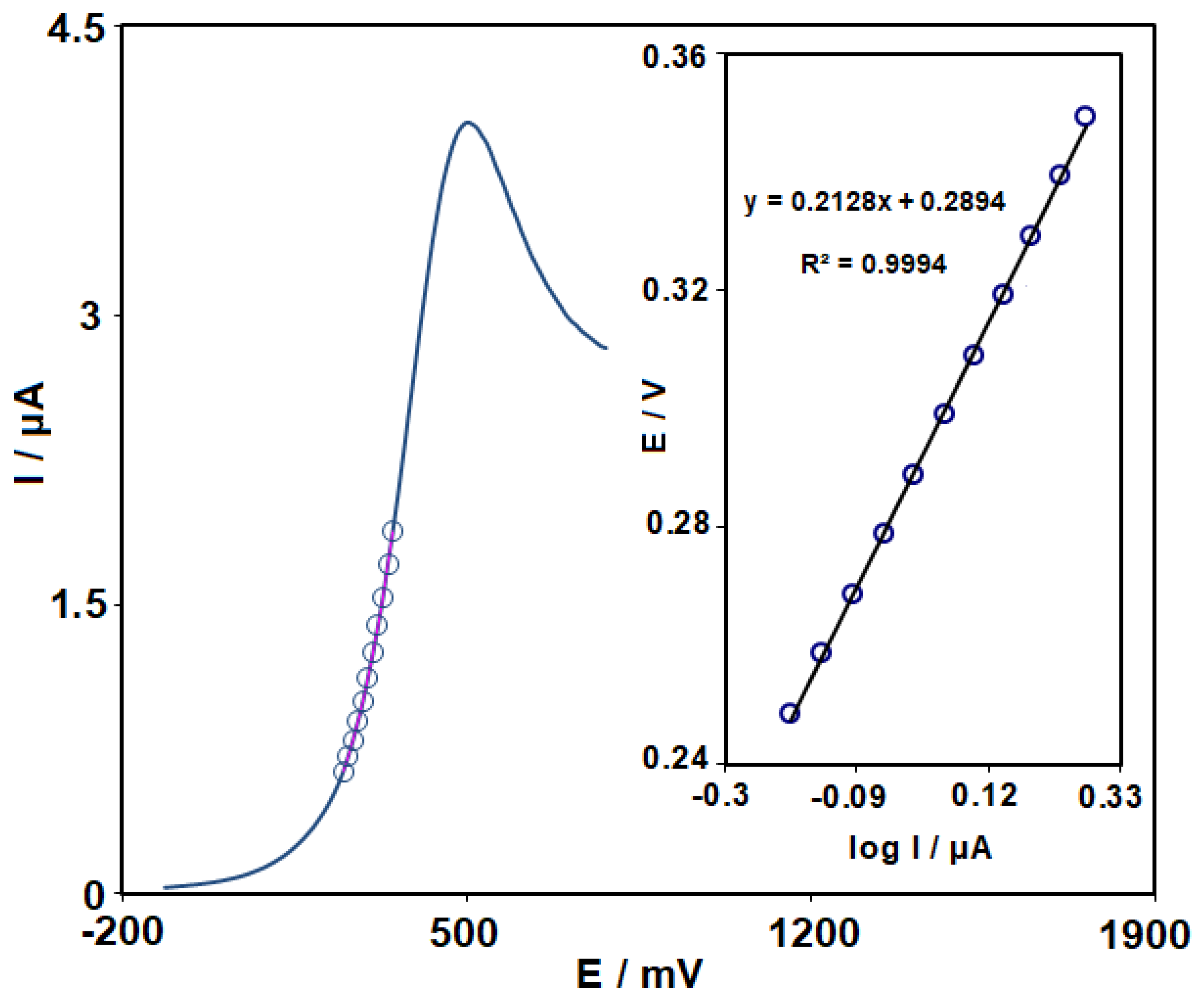
3.3. Effects of Scan Rate
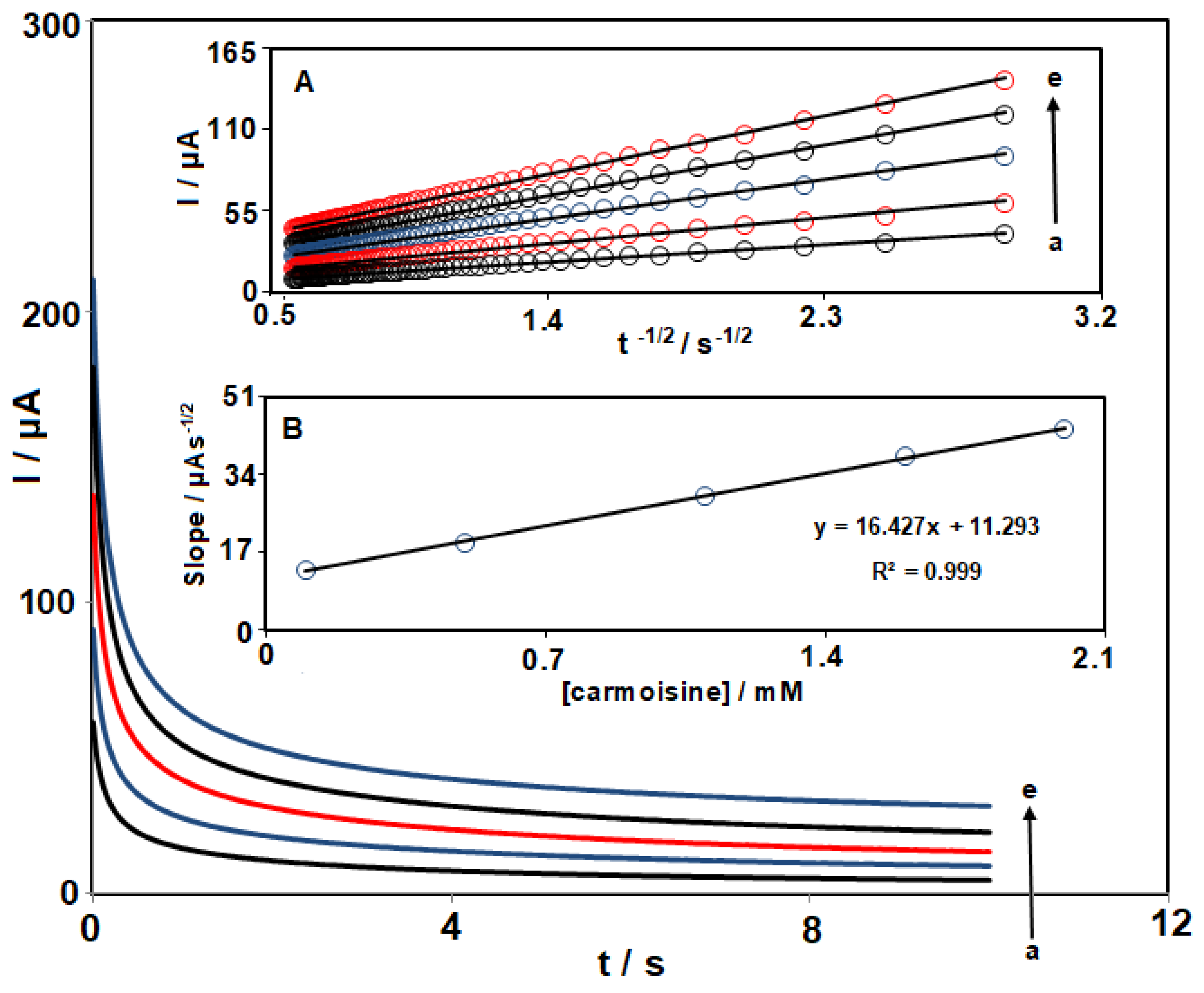
3.4. Chronoamperometric Measurements
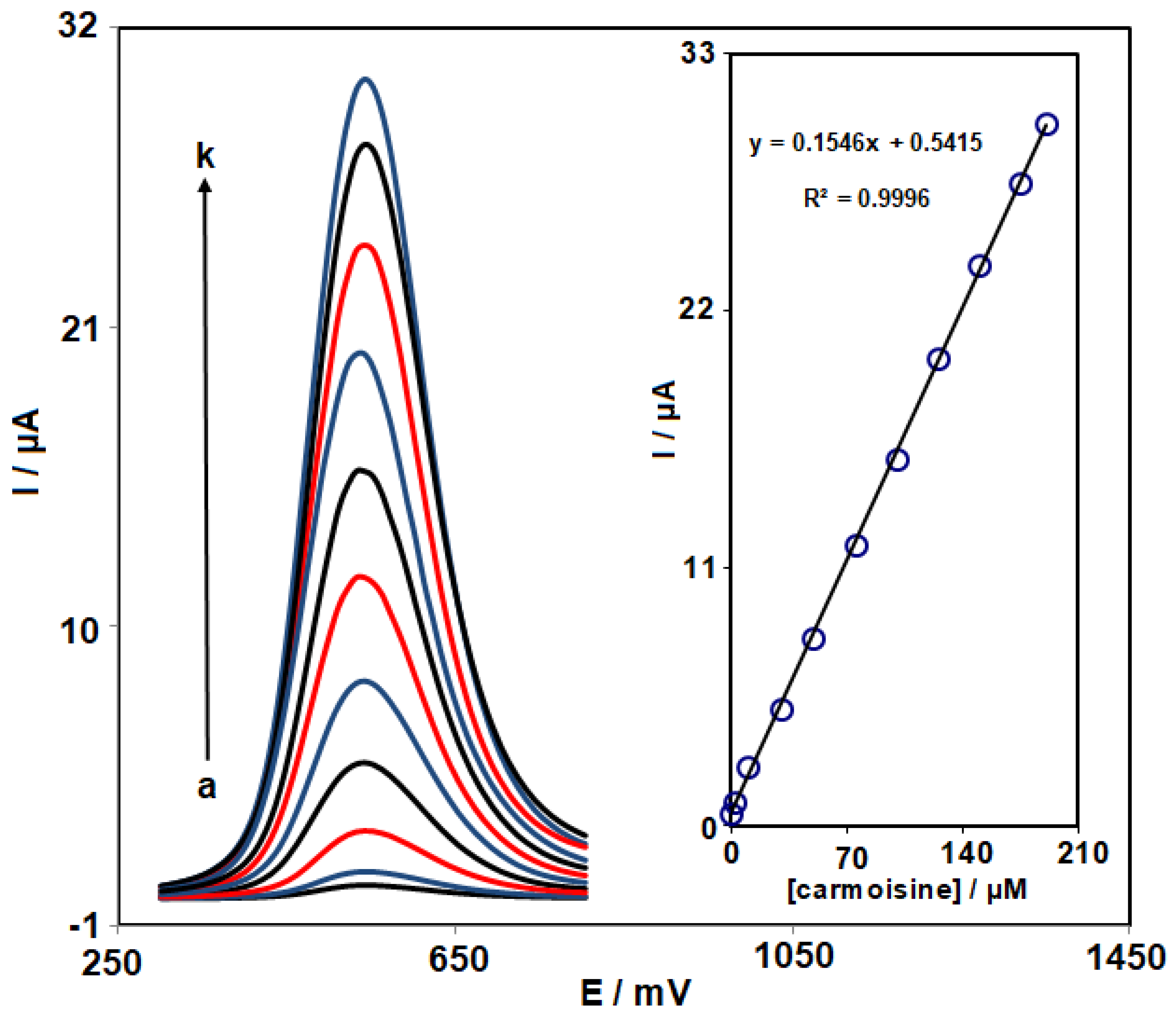
3.5. Determination of Carmoisine by DPV
3.6. Repeatability, Reproducibility, and Stability
3.7. Interference Studies
3.8. Analysis of the Real Samples
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mohammadi, S.Z.; Baghelani, Y.M.; Mousazadeh, F.; Rahimi, S.; Mohammad-Hassani, M. Electrochemical determination of amaranth in food samples by using modified electrode. J. Electrochem. Sci. Eng. 2022, 12, 1165–1177. [Google Scholar]
- Rubio, L.; Sanllorente, S.; Sarabia, L.A.; Ortiz, M.C. Fluorescence determination of cochineal in strawberry jam in the presence of carmoisine as a quencher by means of four-way PARAFAC decomposition. Food Chem. 2019, 290, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Karimi, F.; Demir, E.; Aydogdu, N.; Shojaei, M.; Taher, M.A.; Asrami, P.N.; Cheraghi, S. Advancement in electrochemical strategies for quantification of Brown HT and carmoisine (acid red 14) drom azo dyestuff class. Food Chem. Toxicol. 2022, 165, 113075. [Google Scholar] [CrossRef] [PubMed]
- Chebotarev, A.N.; Pliuta, K.V.; Snigur, D.V. Determination of carmoisine onto carbon-paste electrode modified by silica impregnated with cetylpyridinium chloride. ChemistrySelect 2020, 5, 3688–3693. [Google Scholar] [CrossRef]
- Alsantali, R.I.; Raja, Q.A.; Alzahrani, A.Y.; Sadiq, A.; Naeem, N.; Mughal, E.U.; AlRooqi, M.M.; El Guesmi, N.; Moussa, Z.; Ahmed, S.A. Miscellaneous azo dyes: A comprehensive review on recent advancements in biological and industrial Applications. Dyes Pigm. 2021, 199, 110050. [Google Scholar] [CrossRef]
- Griess, P. XLII.—On a new series of bodies in which nitrogen is substituted for hydrogen. J. Chem. Soc. 1865, 18, 268–272. [Google Scholar] [CrossRef]
- El Harfi, S.; El Harfi, A. Classifications, properties and applications of textile dyes: A review. Appl. J. Environ. Eng. Sci. 2017, 3, 311–320. [Google Scholar]
- Shi, Y.; Yang, Z.; Xing, L.; Zhang, X.; Li, X.; Zhang, D. Recent advances in the biodegradation of azo dyes. World J. Microbiol. Biotechnol. 2021, 37, 137. [Google Scholar] [CrossRef]
- Vineis, P.; Pirastu, R. Aromatic amines and cancer. Cancer Causes Control 1997, 8, 346–355. [Google Scholar] [CrossRef]
- Micheletti, L.; Coldibeli, B.; Salamanca-Neto, C.A.R.; Almeida, L.C.; Sartori, E.R. Assessment of the use of boron-doped diamond electrode for highly sensitive voltammetric determination of the azo-dye carmoisine E−122 in food and environmental matrices. Talanta 2020, 220, 121417. [Google Scholar] [CrossRef]
- Gupta, V.K.; Mittal, A.; Malviya, A.; Mittal, J. Adsorption of carmoisine A from wastewater using waste materials—Bottom ash and deoiled soya. J. Colloid Interface Sci. 2009, 335, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.V.; Rao, N.C.; Karthikeyan, J. Adsorptive removal of direct azo dye from aqueous phase onto coal based sorbents: A kinetic and mechanistic study. J. Hazard. Mater. 2002, 90, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Biswas, M.M.; Taylor, K.E.; Bewtra, J.K.; Biswas, N. Enzymatic treatment of sulfonated aromatic amines generated from reductive degradation of reactive azo dyes. Water Environ. Res. 2007, 79, 351–356. [Google Scholar] [CrossRef]
- Gaunt, I.F.; Farmer, M.; Grasso, P.; Gangolli, S.D. Acute (mouse and rat) and short-term (rat) toxicity studies on carmoisine. Food Cosmet. Toxicol. 1967, 5, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Jangju, A.; Farhadi, K.; Hatami, M.; Amani, S.; Esma-ali, F.; Moshkabadi, A.; Hajilari, F. Application of zein-modified magnetite nanoparticles in dispersive magnetic micro-solid-phase extraction of synthetic food dyes in foodstuffs. J. Sep. Sci. 2017, 40, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wan, S.; Xu, O.; Song, H.; Yang, J.; Zhu, X. Pyridine ionic liquid functionalized bimetallic MOF solid-phase extraction coupled with high performance liquid chromatography for separation/analysis sunset yellow. RSC Adv. 2022, 12, 30928–30935. [Google Scholar] [CrossRef] [PubMed]
- Soponar, F.; Mot¸, A.C.; Sarbu, C. Quantitative determination of some food dyes using digital processing of images obtained by thin-layer chromatography. J. Chromatogr. A 2008, 1188, 295–300. [Google Scholar] [CrossRef]
- Asadpour-Zeynali, K.; Manafi-Khoshmanesh, S. Simultaneous standard addition method for novel determination of components in a single step: Application in analysis of Sunset yellow and Carmoisine by a spectrophotometric technique. Anal. Methods 2014, 6, 6110–6115. [Google Scholar] [CrossRef]
- Del Giovine, L.; Bocca, A.P. Determination of synthetic dyes in ice-cream by capillary electrophoresis. Food Control 2003, 14, 131–135. [Google Scholar] [CrossRef]
- Bişgin, A.T. Simultaneous extraction and determination of allura red (E129) and brilliant blue FCF (E133) in foodstuffs by column solid-phase spectrophotometry. J. AOAC Int. 2019, 102, 181–188. [Google Scholar] [CrossRef]
- Xu, H.; Yang, X.; Li, G.; Zhao, C.; Liao, X. Green synthesis of fluorescent carbon dots for selective detection of tartrazine in food samples. J. Agric. Food Chem. 2015, 63, 6707–6714. [Google Scholar] [CrossRef]
- Rovina, K.; Siddiquee, S.; Shaarani, S.M. Highly sensitive electrochemical determination of sunset yellow in commercial food products based on CHIT/GO/ MWCNTs/AuNPs/GCE. Food Control 2017, 82, 66–73. [Google Scholar] [CrossRef]
- Ahmadi, S.; Hasanzadeh, M.; Ghasempour, Z. Sub-micro electrochemical recognition of carmoisine, sunset yellow, and tartrazine in fruit juices using P (β-CD/Arg)/CysA-AuNPs/AuE. Food Chem. 2023, 402, 134501. [Google Scholar] [CrossRef]
- Okeke, E.S.; Ezeorba, T.P.C.; Okoye, C.O.; Chen, Y.; Mao, G.; Feng, W.; Wu, X. Analytical detection methods for azo dyes: A focus on comparative limitations and prospects of bio-sensing and electrochemical nano-detection. J. Food Compos. Anal. 2022, 114, 104778. [Google Scholar] [CrossRef]
- Kazemipour, M.; Ansari, M.; Mohammadi, A.; Beitollahi, H.; Ahmadi, R. Use of adsorptive square-wave anodic stripping voltammetry at carbon paste electrode for the determination of amlodipine besylate in pharmaceutical preparations. J. Anal. Chem. 2009, 64, 65–70. [Google Scholar] [CrossRef]
- Hasanpour, F.; Taei, M.; Fouladgar, M.; Salehi, M. Au nano dendrites/composition optimized nd-dopped cobalt oxide as an efficient electrocatalyst for ethanol oxidation. J. Appl. Organomet. Chem. 2022, 2, 188–196. [Google Scholar]
- Ariavand, S.; Ebrahimi, M.; Foladi, E. Design and construction of a novel and an efficient potentiometric sensor for determination of sodium ion in urban water samples. Chem. Methodol. 2022, 6, 886–904. [Google Scholar]
- Karimi-Maleh, H.; Liu, Y.; Li, Z.; Darabi, R.; Orooji, Y.; Karaman, C.; Karimi, F.; Baghayeri, M.; Rouhi, J.; Fu, L. Calf thymus ds-DNA intercalation with pendimethalin herbicide at the surface of ZIF-8/Co/rGO/C3N4/ds-DNA/SPCE; A bio-sensing approach for pendimethalin quantification confirmed by molecular docking study. Chemosphere 2023, 332, 138815. [Google Scholar] [CrossRef]
- Peyman, H.; Roshanfekr, H.; Babakhanian, A.; Jafari, H. PVC membrane electrode modified by lawson as synthetic derivative ionophore for determination of cadmium in alloy and wastewater. Chem. Methodol. 2021, 5, 446–453. [Google Scholar]
- Mazloum-Ardakani, M.; Beitollahi, H.; Amini, M.K.; Mirkhalaf, F.; Mirjalili, B.F.; Akbari, A. Application of 2-(3, 4-dihydroxyphenyl)-1,3-dithialone self-assembled monolayer on gold electrode as a nanosensor for electrocatalytic determination of dopamine and uric acid. Analyst 2011, 136, 1965–1970. [Google Scholar] [CrossRef] [PubMed]
- Peyman, H. Design and fabrication of modified DNA-Gp nano-biocomposite electrode for industrial dye measurement and optical confirmation. Prog. Chem. Biochem. Res. 2022, 5, 391–405. [Google Scholar]
- Sun, R.; Lv, R.; Li, Y.; Du, T.; Chen, L.; Zhang, Y.; Qi, Y. Simple and sensitive electrochemical detection of sunset yellow and Sudan I in food based on AuNPs/Zr-MOF-Graphene. Food Control 2023, 145, 109491. [Google Scholar] [CrossRef]
- Arivazhagan, M.; Maduraiveeran, G. Hierarchical gold dispersed nickel oxide nanodendrites microarrays as a potential platform for the sensitive electrochemical detection of glucose and lactate in human serum and urine. Mater. Chem. Phys. 2023, 295, 127084. [Google Scholar] [CrossRef]
- Beitollahi, H.; Mahmoudi Moghaddam, H.; Tajik, S. Voltammetric determination of bisphenol A in water and juice using a lanthanum (III)-doped cobalt (II, III) nanocube modified carbon screen-printed electrode. Anal. Lett. 2019, 52, 1432–1444. [Google Scholar] [CrossRef]
- Mohanraj, J.; Durgalakshmi, D.; Rakkesh, R.A.; Balakumar, S.; Rajendran, S.; Karimi-Maleh, H. Facile synthesis of paper based graphene electrodes for point of care devices: A double stranded DNA (dsDNA) biosensor. J. Colloid Interface Sci. 2020, 566, 463–472. [Google Scholar] [CrossRef]
- Mehdizadeh, Z.; Shahidi, S.; Ghorbani-HasanSaraei, A.; Limooei, M.; Bijad, M. Monitoring of amaranth in drinking samples using voltammetric amplified electroanalytical sensor. Chem. Methodol. 2022, 6, 246–252. [Google Scholar]
- Zhang, Z.; Karimi-Maleh, H. Label-free electrochemical aptasensor based on gold nanoparticles/titanium carbide MXene for lead detection with its reduction peak as index signal. Adv. Compos. Hybrid Mater. 2023, 6, 68. [Google Scholar] [CrossRef]
- Magar, H.S.; Hassan, R.Y.; Abbas, M.N. Non-enzymatic disposable electrochemical sensors based on CuO/Co3O4@MWCNTs nanocomposite modified screen-printed electrode for the direct determination of urea. Sci. Rep. 2023, 13, 2034. [Google Scholar] [CrossRef]
- Moru, S.; Sunil Kumar, V.; Kummari, S.; Yugender Goud, K. A disposable screen printed electrodes with hexagonal Ni (OH)2 nanoplates embedded chitosan layer for the detection of depression biomarker. Micromachines 2023, 14, 146. [Google Scholar]
- Beitollahi, H.; Garkani-Nejad, F.; Dourandish, Z.; Tajik, S. A novel voltammetric amaranth sensor based on screen printed electrode modified with polypyrrole nanotubes. Environ. Res. 2022, 214, 113725. [Google Scholar] [CrossRef]
- Maksuk, C.; Tinala, C.; Somboot, W.; Jakmunee, J.; Marken, F.; Kanyanee, T. Rapid determination of hydrogen peroxide in milk with non-enzymatic amperometric sensor based on porous gold modified screen-printed electrode in online dialysis system. Electroanalysis 2023, 35, e202100691. [Google Scholar] [CrossRef]
- Hosseini Fakhrabad, A.; Sanavi Khoshnood, R.; Abedi, M.R.; Ebrahimi, M. Fabrication a composite carbon paste electrodes (CPEs) modified with Multi-Wall Carbon Nano-Tubes (MWCNTs/N, N-Bis (salicyliden)-1,3-propandiamine) for determination of lanthanum (III). Eurasian Chem. Commun. 2021, 3, 627–634. [Google Scholar]
- Buledi, J.A.; Mahar, N.; Mallah, A.; Solangi, A.R.; Palabiyik, I.M.; Qambrani, N.; Karimi, F.; Vasseghian, Y.; Karimi-Maleh, H. Electrochemical quantification of mancozeb through tungsten oxide/reduced graphene oxide nanocomposite: A potential method for environmental remediation. Food Chem. Toxicol. 2022, 161, 112843. [Google Scholar] [CrossRef] [PubMed]
- Tajik, S.; Orooji, Y.; Karimi, F.; Ghazanfari, Z.; Beitollahi, H.; Shokouhimehr, M.; Varma, R.S.; Jang, H.W. High performance of screen-printed graphite electrode modified with Ni–Mo-MOF for voltammetric determination of amaranth. J. Food Meas. Charact. 2021, 15, 4617–4622. [Google Scholar] [CrossRef]
- Cheraghi, S.; Taher, M.A.; Karimi-Maleh, H.; Karimi, F.; Shabani-Nooshabadi, M.; Alizadeh, M.; Al-Othman, A.; Erk, N.; Raman, P.K.Y.; Karaman, C. Novel enzymatic graphene oxide based biosensor for the detection of glutathione in biological body fluids. Chemosphere 2022, 287, 132187. [Google Scholar]
- Zhong, R.; Xu, M.; Fu, N.; Liu, R.; Wang, X.; Yang, Z. A flexible high-performance symmetric quasi-solid supercapacitor based on Ni-doped MnO2 nano-array@carbon cloth. Electrochim. Acta 2020, 348, 136209. [Google Scholar] [CrossRef]
- Alwan, L.; Al Samarrai, E.; Mahmood, M.; Ali, Q.; Al Samarrai, O. Estimation and development of some biophysical characteristics of the drug Favipiravir used in the treatment of corona-virus using green chemistry technology. Eurasian Chem. Commun. 2022, 4, 835–851. [Google Scholar]
- Janitabar Darzi, S.; Bastami, H. Au decorated mesoporous TiO2 as a high performance photocatalyst towards crystal violet dye. Adv. J. Chem. A 2022, 5, 22–30. [Google Scholar]
- Wang, F.; Hu, Z.; Mao, L.; Mao, J. Nano-silicon@soft carbon embedded in graphene scaffold: High-performance 3D free-standing anode for lithium-ion batteries. J. Power Sources 2020, 450, 227692. [Google Scholar] [CrossRef]
- Sheikhshoaie, I.; Rezazadeh, A.; Ramezanpour, S. Removal of Pb (II) from aqueous solution by gel combustion of a new nano sized Co3O4/ZnO composite. Asian J. Nanosci. Mater. 2022, 5, 336–345. [Google Scholar]
- Tallapaneni, V.; Mude, L.; Pamu, D.; Karri, V.V.S.R. Formulation, characterization and in vitro evaluation of dual-drug loaded biomimetic chitosan-collagen hybrid nanocomposite scaffolds. J. Med. Chem. Sci. 2022, 5, 1059–1074. [Google Scholar]
- Shete, R.; Fernandes, P.; Borhade, B.; Pawar, A.; Sonawane, M.; Warude, N. Review of cobalt oxide nanoparticles: Green synthesis, biomedical applications, and toxicity studies. J. Chem. Rev. 2022, 4, 331. [Google Scholar]
- Shayegan, H.; Safarifard, V.; Taherkhani, H.; Rezvani, M.A. Efficient removal of cobalt(II) ion from aqueous solution using amide-functionalized metal-organic framework. J. Appl. Organomet. Chem. 2022, 2, 109–118. [Google Scholar]
- Nareetsile, F.; Matshwele, J.T.; Odisitse, S. Metallo-drugs as promising antibacterial agents and their modes of action. J. Med. Chem. Sci. 2022, 5, 1109–1131. [Google Scholar]
- Sharma, M.; Yadav, S.; Srivastava, M.; Ganesh, N.; Srivastava, S. Promising anti-inflammatory bio-efficacy of saponin loaded silver nanoparticles prepared from the plant Madhuca longifolia. Asian J. Nanosci. Mater. 2022, 5, 313–326. [Google Scholar]
- Yan, R.; Lu, N.; Han, S.; Lu, Z.; Xiao, Y.; Zhao, Z.; Zhang, M. Simultaneous detection of dual biomarkers using hierarchical MoS2 nanostructuring and nano-signal amplification-based electrochemical aptasensor toward accurate diagnosis of prostate cancer. Biosens. Bioelectron. 2022, 197, 113797. [Google Scholar] [CrossRef] [PubMed]
- Foroughi, M.M.; Beitollahi, H.; Tajik, S.; Akbari, A.; Hosseinzadeh, R. Electrochemical determination of N-acetylcysteine and folic acid in pharmaceutical and biological samples using a modified carbon nanotube paste electrode. Int. J. Electrochem. Sci. 2014, 9, 8407. [Google Scholar] [CrossRef]
- Roshanfekr, H. A simple specific dopamine aptasensor based on partially reduced graphene oxide–Au NPs composite. Prog. Chem. Biochem. Res. 2023, 6, 79–88. [Google Scholar]
- Rajaji, U.; Ganesh, P.S.; Kim, S.Y.; Govindasamy, M.; Alshgari, R.A.; Liu, T.Y. MoS2 sphere/2D S-Ti3C2 MXene nanocatalysts on laser-induced graphene electrodes for hazardous aristolochic acid and roxarsone electrochemical detection. ACS Appl. Nano Mater. 2022, 5, 3252–3264. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Fakude, C.T.; Mabuba, N.; Peleyeju, G.M.; Arotiba, O.A. The determination of 2-phenylphenol in the presence of 4-chlorophenol using nano-Fe3O4/ionic liquid paste electrode as an electrochemical sensor. J. Colloid Interface Sci. 2019, 554, 603–610. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Nouralishahi, A.; Shalbaf, M.; Shabani Shayeh, J.; Nouralishahi, A. An electrochemical aptasensor for detection of prostate-specific antigen-based on carbon quantum dots-gold nanoparticles. Biotechnol. Appl. Biochem. 2023, 70, 175–183. [Google Scholar] [CrossRef]
- Zhang, Z.; Karimi-Maleh, H. In situ synthesis of label-free electrochemical aptasensor-based sandwich-like AuNPs/PPy/Ti3C2Tx for ultrasensitive detection of lead ions as hazardous pollutants in environmental fluids. Chemosphere 2023, 324, 138302. [Google Scholar] [CrossRef]
- Báez, R.M.; Morales, M.A.; Flores, A.L.; Serrano, R.A. Multiscale modeling of ZnO nanoparticle synthesis: Chemical kinetics and turing instability. Mater. Today Commun. 2021, 29, 102748. [Google Scholar] [CrossRef]
- Kar, J.P.; Das, S.N.; Choi, J.H.; Myoung, J.M. Growth and characterization of vertically aligned ZnO microtubes on silicon substrate. Mater. Lett. 2009, 63, 2327–2330. [Google Scholar] [CrossRef]
- Yue, S.; Zhang, L.; Lu, J.; Zhang, J. Polymer-controlled crystallization of dumbbell-like ZnO hollow architectures. Mater. Lett. 2009, 63, 1217–1220. [Google Scholar] [CrossRef]
- Ameen, S.; Akhtar, M.S.; Seo, H.K.; Shin, H.S. An electrochemical sensing platform based on hollow mesoporous ZnO nanoglobules modified glassy carbon electrode: Selective detection of piperidine chemical. Chem. Eng. J. 2015, 270, 564–571. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, H.; Zhang, L.; Leng, D.; Zhang, Y.; Wang, W.; Wang, C. Metal–organic framework-derived ZnO hollow nanocages functionalized with nanoscale Ag catalysts for enhanced ethanol sensing properties. Sens. Actuators B Chem. 2019, 291, 458–469. [Google Scholar] [CrossRef]
- Rao, J.; Yu, A.; Shao, C.; Zhou, X. Construction of hollow and mesoporous ZnO microsphere: A facile synthesis and sensing property. ACS Appl. Mater. Interfaces 2012, 4, 5346–5352. [Google Scholar] [CrossRef]
- Chen, X.; Jing, X.; Wang, J.; Liu, J.; Song, D.; Liu, L. Self-assembly of ZnO nanoparticles into hollow microspheres via a facile solvothermal route and their application as gas sensor. CrystEngComm 2013, 15, 7243–7249. [Google Scholar] [CrossRef]
- Cho, G.R.; Kim, D.H.; Lee, D.H. A facile approach to fabrication of hollow ZnO nanoparticles. Compos. Res. 2018, 31, 94–98. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Movaghar Nezhad, H.; Shahidi, S.A.; Bijad, M. Fabrication of a nanostructure voltammetric sensor for carmoisine analysis as a food dye additive. Anal. Bioanal. Electrochem. 2018, 10, 220–229. [Google Scholar]
- Bijad, M.; Karimi-Maleh, H.; Farsi, M.; Shahidi, S.A. An electrochemical-amplified-platform based on the nanostructure voltammetric sensor for the determination of carmoisine in the presence of tartrazine in dried fruit and soft drink samples. J. Food Meas. Charact. 2018, 12, 634–640. [Google Scholar] [CrossRef]
- Asadpour-Zeynali, K.; Mollarasouli, F. Bismuth and Bismuth-Chitosan modified electrodes for determination of two synthetic food colorants by net analyte signal standard addition method. Cent. Eur. J. Chem. 2014, 12, 711–718. [Google Scholar] [CrossRef]
- Sierra-Rosales, P.; Toledo-Neira, C.; Squella, J.A. Electrochemical determination of food colorants in soft drinks using MWCNT-modified GCEs. Sens. Actuators B Chem. 2017, 240, 1257–1264. [Google Scholar] [CrossRef]

| Electrochemical Sensor | Linear Range | LOD | Ref. |
|---|---|---|---|
| Silica/cetylpyridinium chloride (CPCl)/carbon paste electrode (CPE) | 0.08 μM to 1.0 μM | 0.01 μM | [4] |
| Cathodically pretreated boron-doped diamond electrode | 0.0591 μM to 1.31 µM | 0.007 µM | [10] |
| CdO/carbon nanotubes (CNTs)/ionic liquid (IL)/CPE | 0.1 μM to 700.0 μM | 40.0 nM | [72] |
| NiO-CNTs/ILCPE | 70.0 μM to 650.0 μM | 20 nM | [73] |
| Bismuth–chitosan/glassy carbon electrode (GCE) | 1 µM to 41 µM | 10 µM | [74] |
| Multi-walled carbon nanotubes (MWCNTs)/GCE | 0.54 µM to 5.0 µM | 0.11 µM | [75] |
| ZnO HQSs/SPGE | 0.08 µM to 190.0 µM | 0.02 µM | Present work |
| Sample | Spiked Concentration (μM) | Found Concentration (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Powdered juice | 0 | 3.4 | - | 3.2 |
| 1.0 | 4.3 | 97.7 | 1.8 | |
| 2.0 | 5.5 | 101.8 | 2.3 | |
| 3.0 | 6.7 | 104.7 | 2.9 | |
| 4.0 | 8.3 | 98.8 | 2.1 | |
| Lemon juice | 0 | 4.0 | - | 2.8 |
| 1.0 | 5.1 | 102.0 | 3.5 | |
| 2.0 | 5.9 | 98.3 | 2.7 | |
| 3.0 | 7.3 | 104.3 | 2.0 | |
| 4.0 | 7.9 | 98.7 | 1.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammadi, S.Z.; Tajik, S.; Mousazadeh, F.; Baghadam-Narouei, E.; Garkani Nejad, F. ZnO Hollow Quasi-Spheres Modified Screen-Printed Graphite Electrode for Determination of Carmoisine. Micromachines 2023, 14, 1433. https://doi.org/10.3390/mi14071433
Mohammadi SZ, Tajik S, Mousazadeh F, Baghadam-Narouei E, Garkani Nejad F. ZnO Hollow Quasi-Spheres Modified Screen-Printed Graphite Electrode for Determination of Carmoisine. Micromachines. 2023; 14(7):1433. https://doi.org/10.3390/mi14071433
Chicago/Turabian StyleMohammadi, Sayed Zia, Somayeh Tajik, Farideh Mousazadeh, Elaheh Baghadam-Narouei, and Fariba Garkani Nejad. 2023. "ZnO Hollow Quasi-Spheres Modified Screen-Printed Graphite Electrode for Determination of Carmoisine" Micromachines 14, no. 7: 1433. https://doi.org/10.3390/mi14071433
APA StyleMohammadi, S. Z., Tajik, S., Mousazadeh, F., Baghadam-Narouei, E., & Garkani Nejad, F. (2023). ZnO Hollow Quasi-Spheres Modified Screen-Printed Graphite Electrode for Determination of Carmoisine. Micromachines, 14(7), 1433. https://doi.org/10.3390/mi14071433






