Ag2S-Ag2O-Ag/poly-2-aminobenzene-1-thiol Nanocomposite as a Promising Two-Electrode Symmetric Supercapacitor: Tested in Acidic and Basic Mediums
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of P2ABT and Ag2S-Ag2O-Ag/P2ABT
2.3. Supercapacitor Fabrication
2.4. Characterization
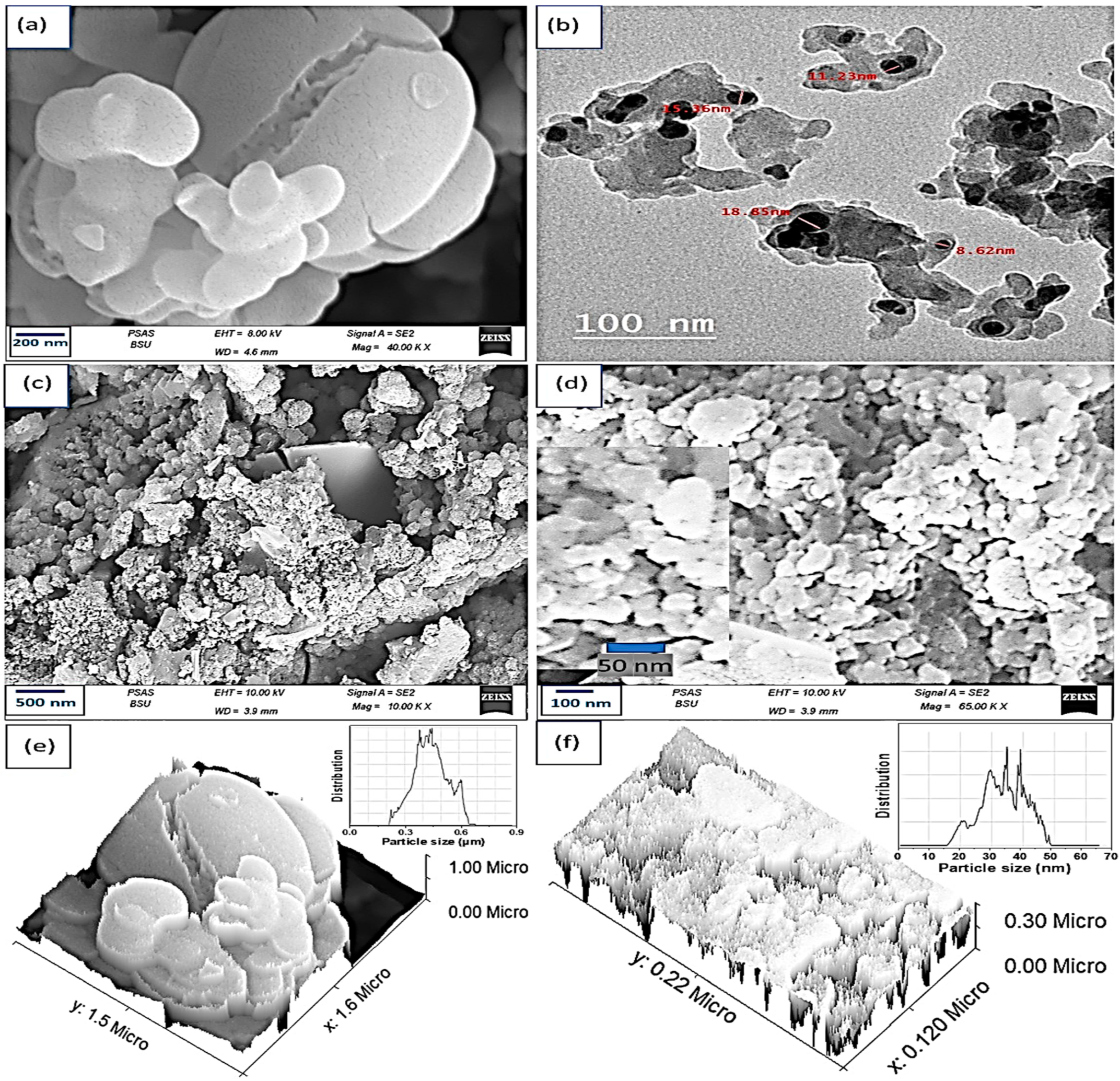
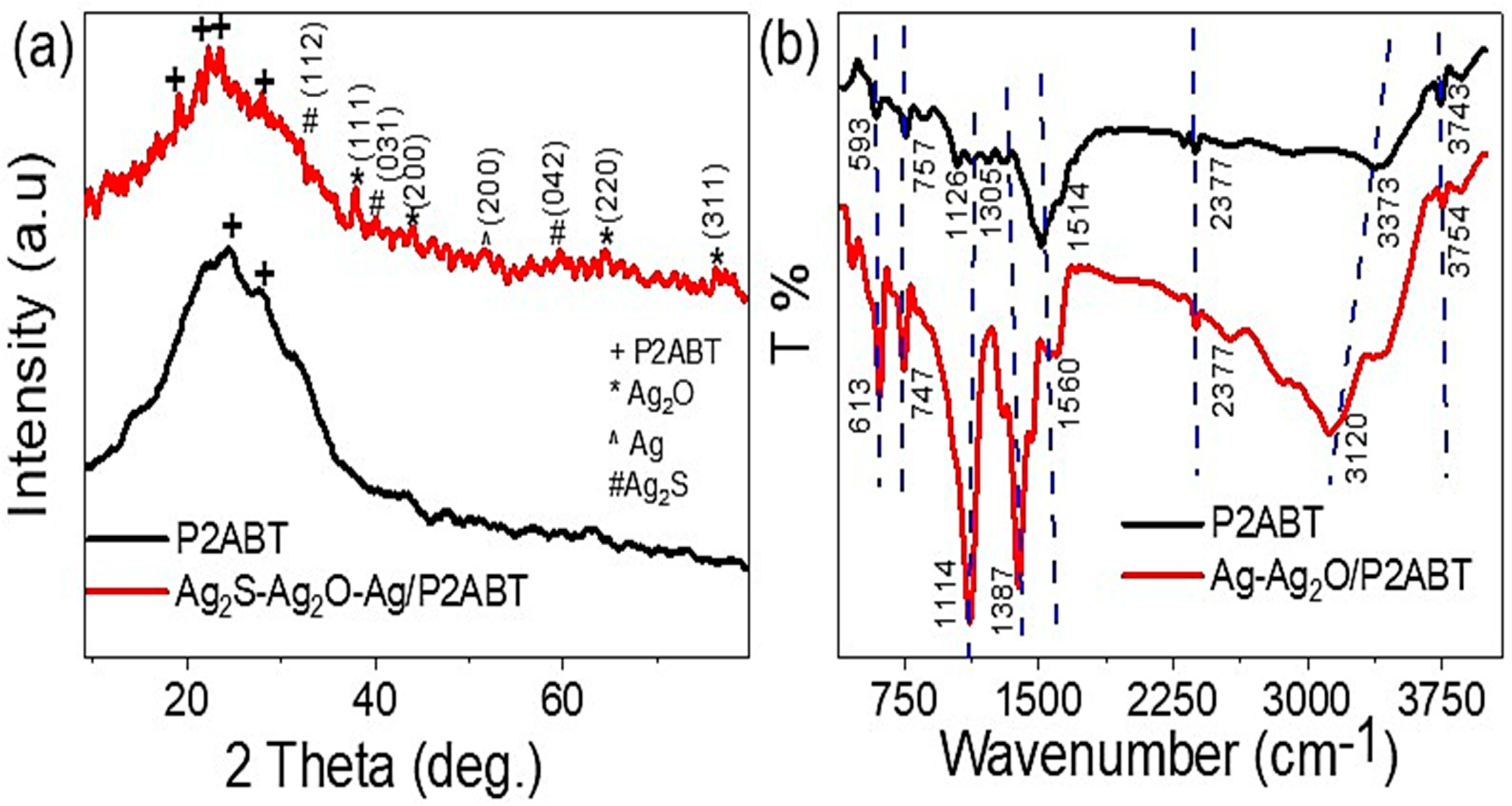
3. Results and Discussion
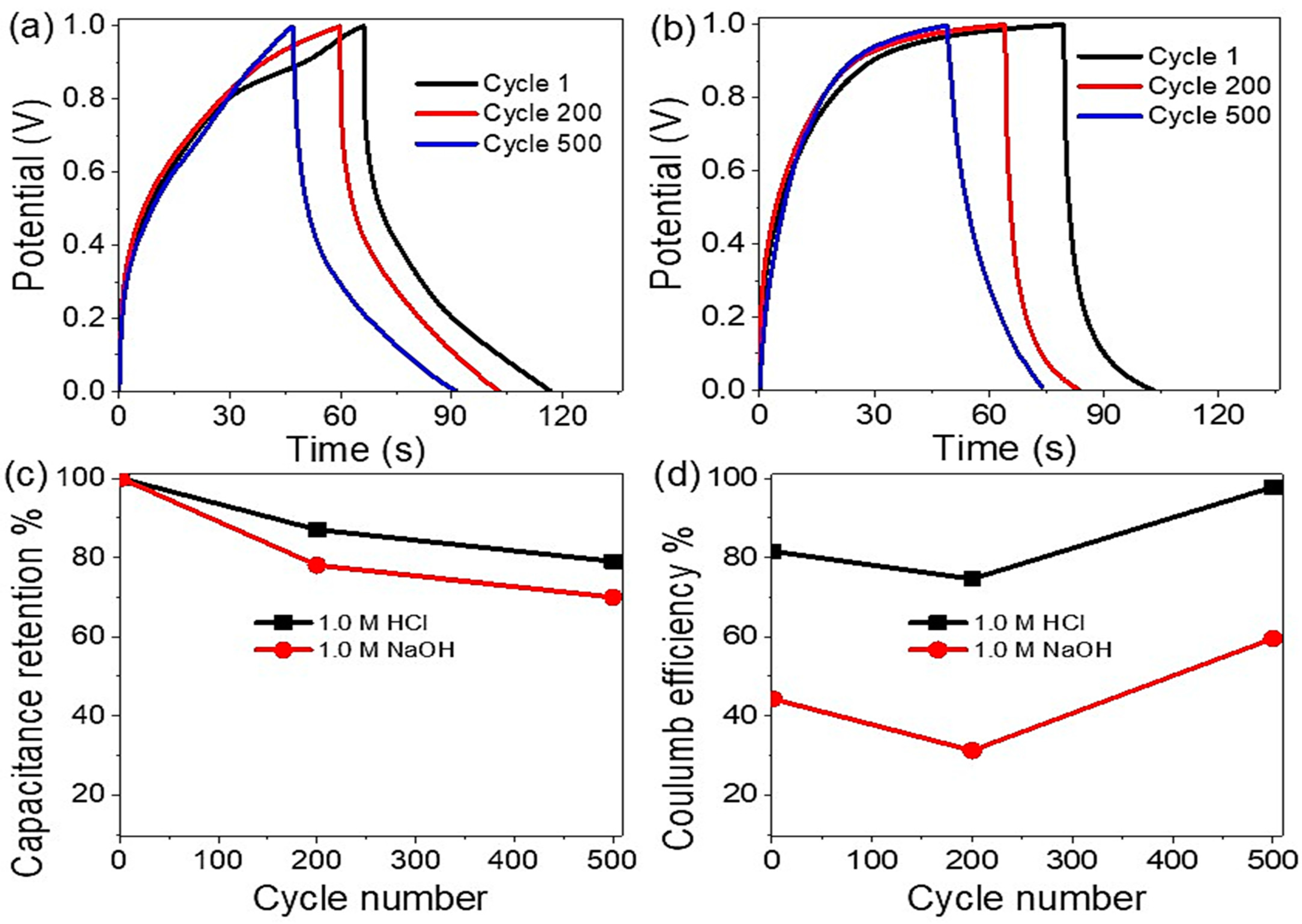
The Electrochemical Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hadia, N.M.A.; Hajjiah, A.; Elsayed, A.M.; Mohamed, S.H.; Alruqi, M.; Shaban, M.; Alzahrani, F.M.; Abdelazeez, A.A.A.; Rabia, M. Bunch of Grape-Like Shape PANI/Ag2O/Ag Nanocomposite Photocatalyst for Hydrogen Generation from Wastewater. Adsorpt. Sci. Technol. 2022, 2022, 4282485. [Google Scholar] [CrossRef]
- Hadia, N.M.A.; Eid, S.; Shaban, M.; Mohamed, S.H.; Elsayed, A.M.; Ahmed, A.M.; Alzaid, M.; Abdelazeez, A.A.A.; El Malti, W.; Rabia, M. Poly-3-Methyl Aniline-Assisted Spherical PbS Quantum Dots through the Ionic Adsorption Deposition Method as a Novel and Highly Efficient Photodetector in UV, Vis, and NIR Regions. Adsorpt. Sci. Technol. 2022, 2022, 7693472. [Google Scholar] [CrossRef]
- Katsuyama, Y.; Takehi, T.; Sokabe, S.; Tanaka, M.; Ishizawa, M.; Abe, H.; Watanabe, M.; Honma, I.; Nakayasu, Y. Series Module of Quinone-Based Organic Supercapacitor (>6 V) with Practical Cell Structure. Sci. Rep. 2022, 12, 3915. [Google Scholar] [CrossRef] [PubMed]
- El Nady, J.; Shokry, A.; Khalil, M.; Ebrahim, S.; Elshaer, A.M.; Anas, M. One-Step Electrodeposition of a Polypyrrole/NiO Nanocomposite as a Supercapacitor Electrode. Sci. Rep. 2022, 12, 3611. [Google Scholar] [CrossRef]
- Hameed, S.A.; Ewais, H.A.; Rabia, M. Dumbbell-like Shape Fe2O3/Poly-2-Aminothiophenol Nanocomposite for Two-Symmetric Electrode Supercapacitor Application. J. Mater. Sci. Mater. Electron. 2023, 34, 1–8. [Google Scholar] [CrossRef]
- Qin, H.; Liu, P.; Chen, C.; Cong, H.P.; Yu, S.H. A Multi-Responsive Healable Supercapacitor. Nat. Commun. 2021, 12, 4297. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Pazhamalai, P.; Mariappan, V.K.; Nardekar, S.S.; Sahoo, S.; Kim, S.J. Probing the Energy Conversion Process in Piezoelectric-Driven Electrochemical Self-Charging Supercapacitor Power Cell Using Piezoelectrochemical Spectroscopy. Nat. Commun. 2020, 11, 2351. [Google Scholar] [CrossRef]
- Holes Help Supercapacitor. Nature 2013, 497, 291. [CrossRef]
- Kakani, V.; Ramesh, S.; Yadav, H.M.; Bathula, C.; Basivi, P.K.; Palem, R.R.; Kim, H.S.; Pasupuletti, V.R.; Lee, H.; Kim, H. Hydrothermal Synthesis of CuO@MnO2 on Nitrogen-Doped Multiwalled Carbon Nanotube Composite Electrodes for Supercapacitor Applications. Sci. Rep. 2022, 12, 12951. [Google Scholar] [CrossRef]
- Doroodmand, M.M.; Owji, S. Alternate Layer by Layered Self Assembly of Conjugated and Unconjugated Salen Based Nanowires as Capacitive Pseudo Supercapacitor. Sci. Rep. 2021, 11, 18768. [Google Scholar] [CrossRef]
- Rabia, M.; Essam, D.; Alkallas, F.H.; Shaban, M.; Elaissi, S.; Ben Gouider Trabelsi, A. Flower-Shaped CoS-Co2O3/G-C3N4 Nanocomposite for Two-Symmetric-Electrodes Supercapacitor of High Capacitance Efficiency Examined in Basic and Acidic Mediums. Micromachines 2022, 13, 2234. [Google Scholar] [CrossRef]
- Gamal, A.; Shaban, M.; BinSabt, M.; Moussa, M.; Ahmed, A.M.; Rabia, M.; Hamdy, H. Facile Fabrication of Polyaniline/Pbs Nanocomposite for High-Performance Supercapacitor Application. Nanomaterials 2022, 12, 817. [Google Scholar] [CrossRef]
- Trabelsi, A.B.G.; Essam, D.; Alkallas, F.H.; Ahmed, A.M.; Rabia, M. Petal-like NiS-NiO/G-C3N4 Nanocomposite for High-Performance Symmetric Supercapacitor. Micromachines 2022, 13, 2134. [Google Scholar] [CrossRef]
- Ben Gouider Trabelsi, A.; Elsayed, A.M.; Alkallas, F.H.; Al-Noaimi, M.; Kusmartsev, F.V.; Rabia, M. A Fractal, Flower Petal-like CuS-CuO/G-C3N4 Nanocomposite for High Efficiency Supercapacitors. Coatings 2022, 12, 1834. [Google Scholar] [CrossRef]
- Mohd Abdah, M.A.A.; Azman, N.H.N.; Kulandaivalu, S.; Sulaiman, Y. Asymmetric Supercapacitor of Functionalised Electrospun Carbon Fibers/Poly(3,4-Ethylenedioxythiophene)/Manganese Oxide//Activated Carbon with Superior Electrochemical Performance. Sci. Rep. 2019, 9, 16782. [Google Scholar] [CrossRef] [PubMed]
- Basivi, P.K.; Ramesh, S.; Kakani, V.; Yadav, H.M.; Bathula, C.; Afsar, N.; Sivasamy, A.; Kim, H.S.; Pasupuleti, V.R.; Lee, H. Ultrasonication-Mediated Nitrogen-Doped Multiwalled Carbon Nanotubes Involving Carboxy Methylcellulose Composite for Solid-State Supercapacitor Applications. Sci. Rep. 2021, 11, 9918. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Zhang, Y.; Li, H.; Pan, L.; Li, Y.; Sun, Z. Electrochemical Behaviors of Graphene–ZnO and Graphene–SnO2 Composite Films for Supercapacitors. Electrochim. Acta 2010, 55, 4170–4173. [Google Scholar] [CrossRef]
- Lu, T.; Pan, L.; Li, H.; Zhu, G.; Lv, T.; Liu, X.; Sun, Z.; Chen, T.; Chua, D.H.C. Microwave-Assisted Synthesis of Graphene–ZnO Nanocomposite for Electrochemical Supercapacitors. J. Alloys Compd. 2011, 509, 5488–5492. [Google Scholar] [CrossRef]
- Praveena, P.; Mathew, S.A.; Narayanan, V.; Stephen, A. Pseudocapacitive Polycarbazole/Ag2O Nanocomposite for Supercapacitor Applications. AIP Conf. Proc. 2019, 2115, 030611. [Google Scholar] [CrossRef]
- Karaca, E.; Gökcen, D.; Pekmez, N.Ö.; Pekmez, K. Galvanostatic Synthesis of Nanostructured Ag-Ag2O Dispersed PPy Composite on Graphite Electrode for Supercapacitor Applications. Int. J. Energy Res. 2020, 44, 158–170. [Google Scholar] [CrossRef]
- Shahi, M.; Paterson, A.F. Small Molecule versus Polymer Semiconductors. Encycl. Mater. Electron. 2023, 1, 95–107. [Google Scholar] [CrossRef]
- Atta, A.; Negm, H.; Abdeltwab, E.; Rabia, M.; Abdelhamied, M.M. Facile Fabrication of Polypyrrole/NiOx Core-Shell Nanocomposites for Hydrogen Production from Wastewater. Polym. Adv. Technol. 2023, 34, 1633–1641. [Google Scholar] [CrossRef]
- Abdelazeez, A.A.A.; El-Fatah, G.A.; Shaban, M.; Ahmed, A.M.; Rabia, M. ITO/Poly-3-Methylaniline/Au Electrode for Electrochemical Water Splitting and Dye Removal. ECS J. Solid State Sci. Technol. 2021, 10, 123009. [Google Scholar] [CrossRef]
- Sayyah, S.M.; Shaban, M.; Rabia, M. Electropolymerization of m-Toluidin on Platinum Electrode from Aqueous Acidic Solution and Character of the Obtained Polymer. Adv. Polym. Technol. 2018, 37, 126–136. [Google Scholar] [CrossRef]
- Shaban, M.; Abukhadra, M.R.; Rabia, M.; Elkader, Y.A.; Abd El-Halim, M.R. Investigation the Adsorption Properties of Graphene Oxide and Polyaniline Nano/Micro Structures for Efficient Removal of Toxic Cr(VI) Contaminants from Aqueous Solutions; Kinetic and Equilibrium Studies. Rend. Lincei 2018, 29, 141–154. [Google Scholar] [CrossRef]
- Kamaraj, R.; Vasudevan, S. Sulfur-Doped Carbon Chain Network as High-Performance Electrocatalyst for Electro-Fenton System. ChemistrySelect 2019, 4, 2428–2435. [Google Scholar] [CrossRef]
- Fang, L.; Han, D.; Kang, S.; Heo, U.-S.; Nam, K.-W.; Kang, Y.-M. Non-Monotonic First-Cycle Irreversible Capacity Governed by Delithiation Depth in Li-Rich Layered Cathodes. Energy Environ. Sci. 2023. [Google Scholar] [CrossRef]
- Xiong, C.; Yang, Q.; Dang, W.; Zhou, Q.; Jiang, X.; Sun, X.; Wang, Z.; An, M.; Ni, Y. A Multifunctional Paper-Based Supercapacitor with Excellent Temperature Adaptability, Plasticity, Tensile Strength, Self-Healing, and High Thermoelectric Effects. J. Mater. Chem. A 2023, 11, 4769–4779. [Google Scholar] [CrossRef]
- Xiong, C.; Zhang, Y.; Xu, J.; Dang, W.; Sun, X.; An, M.; Ni, Y.; Mao, J. Kinetics Process for Structure-Engineered Integrated Gradient Porous Paper-Based Supercapacitors with Boosted Electrochemical Performance. Nano Res. 2023, 1–9. [Google Scholar] [CrossRef]
- Almutairi, M.M.; Ebraheim, E.E.; Mahmoud, M.S.; Atrees, M.S.; Ali, M.E.M.; Khawassek, Y.M. Nanocomposite of TiO2 @ Ni- or Co-Doped Graphene Oxide for Efficient Photocatalytic Water Splitting. Egypt. J. Chem. 2019, 62, 1649–1658. [Google Scholar] [CrossRef]
- Madani, A.; Böttner, S.; Jorgensen, M.R.; Schmidt, O.G. Rolled-up TiO2 Optical Microcavities for Telecom and Visible Photonics. Opt. Lett. 2014, 39, 189–192. [Google Scholar] [CrossRef]
- He, H.L.; Liu, J.; Liu, H.; Pan, Q.; Zhang, G. The Development of High-Performance Room Temperature NOX One-Dimensional Na0.23TiO2/TiO2 Compound Gas Sensor. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129444. [Google Scholar] [CrossRef]
- Mohamed, H.S.H.; Rabia, M.; Zhou, X.-G.; Qin, X.-S.; Khabiri, G.; Shaban, M.; Younus, H.A.; Taha, S.; Hu, Z.-Y.; Liu, J.; et al. Phase-Junction Ag/TiO2 Nanocomposite as Photocathode for H2 Generation. J. Mater. Sci. Technol. 2021, 83, 179–187. [Google Scholar] [CrossRef]
- Maake, P.J.; Mokoena, T.P.; Bolokang, A.S.; Hintsho-Mbita, N.; Tshilongo, J.; Cummings, F.R.; Swart, H.C.; Iwuoha, E.I.; Motaung, D.E. Fabrication of AgCu/TiO2 Nanoparticle-Based Sensors for Selective Detection of Xylene Vapor. Mater. Adv. 2022, 3, 7302–7318. [Google Scholar] [CrossRef]
- Rabia, M.; Mohamed, S.H.; Zhao, H.; Shaban, M.; Lei, Y.; Ahmed, A.M. TiO2/TiOxNY Hollow Mushrooms-like Nanocomposite Photoanode for Hydrogen Electrogeneration. J. Porous Mater. 2019, 27, 133–139. [Google Scholar] [CrossRef]
- Abdelhamied, M.M.; Ghobashy, M.M.; Hadia, N.M.A.; Mohamed, W.S.; Sharshir, A.I.; Nady, N.; Mohamed, S.H.; Shaban, M.; Rabia, M. Chemical Deposition of Ag and Ag2O on Grafting Film of PET-COOH by Photografting Polymerization for Optoelectronic Application. J. Mater. Sci. Mater. Electron. 2023, 34, 41. [Google Scholar] [CrossRef]
- Shaikh, N.S.; Ubale, S.B.; Mane, V.J.; Shaikh, J.S.; Lokhande, V.C.; Praserthdam, S.; Lokhande, C.D.; Kanjanaboos, P. Novel Electrodes for Supercapacitor: Conducting Polymers, Metal Oxides, Chalcogenides, Carbides, Nitrides, MXenes, and Their Composites with Graphene. J. Alloys Compd. 2022, 893, 161998. [Google Scholar] [CrossRef]
- Kim, K.S.; Park, S.J. Bridge Effect of Silver Nanoparticles on Electrochemical Performance of Graphite Nanofiber/Polyaniline for Supercapacitor. Synth. Met. 2012, 162, 2107–2111. [Google Scholar] [CrossRef]
- Atta, A.; Abdelhamied, M.M.; Essam, D.; Shaban, M.; Alshammari, A.H.; Rabia, M. Structural and Physical Properties of Polyaniline/Silver Oxide/Silver Nanocomposite Electrode for Supercapacitor Applications. Int. J. Energy Res. 2022, 46, 6702–6710. [Google Scholar] [CrossRef]
- Elsayed, A.M.; Rabia, M.; Shaban, M.; Aly, A.H.; Ahmed, A.M. Preparation of Hexagonal Nanoporous Al2O3/TiO2/TiN as a Novel Photodetector with High Efficiency. Sci. Rep. 2021, 11, 17572. [Google Scholar] [CrossRef]
- Elsayed, A.M.; Alkallas, F.H.; Trabelsi, A.B.G.; AlFaify, S.; Shkir, M.; Alrebdi, T.A.; Almugren, K.S.; Kusmatsev, F.V.; Rabia, M. Photodetection Enhancement via Graphene Oxide Deposition on Poly 3-Methyl Aniline. Micromachines 2023, 14, 606. [Google Scholar] [CrossRef]
- Elsayed, A.M.; Shaban, M.; Aly, A.H.; Ahmed, A.M.; Rabia, M. Preparation and Characterization of a High-Efficiency Photoelectric Detector Composed of Hexagonal Al2O3/TiO2/TiN/Au Nanoporous Array. Mater. Sci. Semicond. Process. 2022, 139, 106348. [Google Scholar] [CrossRef]
- Altowyan, A.S.; Shaban, M.; Gamel, A.; Gamal, A.; Ali, M.; Rabia, M. High-Performance PH Sensor Electrodes Based on a Hexagonal Pt Nanoparticle Array-Coated Nanoporous Alumina Membrane. Materials 2022, 15, 6515. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, H.; Ahmed, A.M.; Shaban, M.; Abdel-Khaliek, A.A.; Hasan, F.; Mohammed Alzahrani, F.; Rabia, M. Design and Characterization of Nanostructured Ag2O-Ag/Au Based on Al2O3 Template Membrane for Photoelectrochemical Water Splitting and Hydrogen Generation. Photonics 2022, 9, 968. [Google Scholar] [CrossRef]
- Vinay, S.P.; Udayabhanu; Sumedha, H.N.; Nagaraju, G.; Harishkumar, S.; Chandrasekhar, N. Facile Combustion Synthesis of Ag2O Nanoparticles Using Cantaloupe Seeds and Their Multidisciplinary Applications. Appl. Organomet. Chem. 2020, 34, e5830. [Google Scholar] [CrossRef]
- Fan, W.; Jewell, S.; She, Y.; Leung, M.K.H. In Situ Deposition of Ag–Ag2S Hybrid Nanoparticles onto TiO2 Nanotube Arrays towards Fabrication of Photoelectrodes with High Visible Light Photoelectrochemical Properties. Phys. Chem. Chem. Phys. 2013, 16, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Govarthanan, M.; Selvankumar, T.; Manoharan, K.; Rathika, R.; Shanthi, K.; Lee, K.J.; Cho, M.; Kamala-Kannan, S.; Oh, B.T. Biosynthesis and Characterization of Silver Nanoparticles Using Panchakavya, an Indian Traditional Farming Formulating Agent. Int. J. Nanomed. 2014, 9, 1593–1599. [Google Scholar] [CrossRef] [PubMed]
- Azzam, E.M.S.; Abd El-Salam, H.M.; Aboad, R.S. Kinetic Preparation and Antibacterial Activity of Nanocrystalline Poly(2-Aminothiophenol). Polym. Bull. 2019, 76, 1929–1947. [Google Scholar] [CrossRef]
- Shaban, M.; Rabia, M.; Fathallah, W.; El-Mawgoud, N.A.; Mahmoud, A.; Hussien, H.; Said, O. Preparation and Characterization of Polyaniline and Ag/ Polyaniline Composite Nanoporous Particles and Their Antimicrobial Activities. J. Polym. Environ. 2018, 26, 434–442. [Google Scholar] [CrossRef]
- Different Types of Supercapacitors, 978-620-2-67505-5, 6202675055, 9786202675055 by Mohamed Mosaad Hamid, Eman Ali, Mohamed Rabia. Available online: https://www.morebooks.de/store/gb/book/different-types-of-supercapacitors/isbn/978-620-2-67505-5 (accessed on 23 January 2021).
- Hao, J.; Huang, Y.; He, C.; Xu, W.; Yuan, L.; Shu, D.; Song, X.; Meng, T. Bio-Templated Fabrication of Three-Dimensional Network Activated Carbons Derived from Mycelium Pellets for Supercapacitor Applications. Sci. Rep. 2018, 8, 562. [Google Scholar] [CrossRef]
- Ramadan, M.; Abdellah, A.M.; Mohamed, S.G.; Allam, N.K. 3D Interconnected Binder-Free Electrospun MnO@C Nanofibers for Supercapacitor Devices. Sci. Rep. 2018, 8, 7988. [Google Scholar] [CrossRef]
- Rabbani, M.A.; Oladipo, A.A.; Kusaf, M. N and P Co-Doped Green Waste Derived Hierarchical Porous Carbon as a Supercapacitor Electrode for Energy Storage: Electrolyte Effects. ChemistrySelect 2023, 8, e202204288. [Google Scholar] [CrossRef]
- Oladipo, A.A. N,S Co–Doped Biocarbon for Supercapacitor Application: Effect of Electrolytes Concentration and Modelling with Artificial Neural Network. Mater. Chem. Phys. 2021, 260, 124129. [Google Scholar] [CrossRef]
- Fallah, A.; Oladipo, A.A.; Gazi, M. Boron-Doped Sucrose Carbons for Supercapacitor Electrode: Artificial Neural Network-Based Modelling Approach. J. Mater. Sci. Mater. Electron. 2020, 31, 14563–14576. [Google Scholar] [CrossRef]
- Sayyah, S.M.; Shaban, M.; Rabia, M. A Sensor of M-Toluidine/m-Cresol Polymer Film for Detection of Lead Ions by Potentiometric Methods. Sens. Lett. 2016, 14, 522–529. [Google Scholar] [CrossRef]
- Yu, J.; Fu, N.; Zhao, J.; Liu, R.; Li, F.; Du, Y.; Yang, Z. High Specific Capacitance Electrode Material for Supercapacitors Based on Resin-Derived Nitrogen-Doped Porous Carbons. ACS Omega 2019, 4, 15904–15911. [Google Scholar] [CrossRef]
- Naveed ur Rehman, M.; Munawar, T.; Nadeem, M.S.; Mukhtar, F.; Maqbool, A.; Riaz, M.; Manzoor, S.; Ashiq, M.N.; Iqbal, F. Facile Synthesis and Characterization of Conducting Polymer-Metal Oxide Based Core-Shell PANI-Pr2O–NiO–Co3O4 Nanocomposite: As Electrode Material for Supercapacitor. Ceram. Int. 2021, 47, 18497–18509. [Google Scholar] [CrossRef]
- Mai, L.Q.; Yang, F.; Zhao, Y.L.; Xu, X.; Xu, L.; Luo, Y.Z. Hierarchical MnMoO4/CoMoO4 Heterostructured Nanowires with Enhanced Supercapacitor Performance. Nat. Commun. 2011, 2, 381. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Huang, J.; Xiao, Y.; Zhang, G.; Dai, C.; Li, Z.; Zhao, Y.; Jiang, L.; Qu, L. A Seamlessly Integrated Device of Micro-Supercapacitor and Wireless Charging with Ultrahigh Energy Density and Capacitance. Nat. Commun. 2021, 12, 2647. [Google Scholar] [CrossRef]
- El-Kady, M.F.; Ihns, M.; Li, M.; Hwang, J.Y.; Mousavi, M.F.; Chaney, L.; Lech, A.T.; Kaner, R.B. Engineering Three-Dimensional Hybrid Supercapacitors and Microsupercapacitors for High-Performance Integrated Energy Storage. Proc. Natl. Acad. Sci. USA 2015, 112, 4233–4238. [Google Scholar] [CrossRef]
- Rajkumar, M.; Hsu, C.T.; Wu, T.H.; Chen, M.G.; Hu, C.C. Advanced Materials for Aqueous Supercapacitors in the Asymmetric Design. Prog. Nat. Sci. Mater. Int. 2015, 25, 527–544. [Google Scholar] [CrossRef]
- Lei, Y.; Huang, Z.H.; Yang, Y.; Shen, W.; Zheng, Y.; Sun, H.; Kang, F. Porous Mesocarbon Microbeads with Graphitic Shells: Constructing a High-Rate, High-Capacity Cathode for Hybrid Supercapacitor. Sci. Rep. 2013, 3, 2477. [Google Scholar] [CrossRef]
- Scarabelot, L.T.; Muller, D.; de Souza, L.V.; Hotza, D.; Rambo, C.R. Ni(OH)2 Aerogels Incorporated with Polypyrrole as Electrodes for Supercapacitors. J. Electron. Mater. 2017, 46, 5232–5239. [Google Scholar] [CrossRef]
- Santos, R.S.; Suresh Babu, R.; Devendiran, M.; Haddad, D.B.; de Barros, A.L.F. Facile Synthesis of Transition Metal (M = Cu, Co) Oxide Grafted Graphitic Carbon Nitride Nanosheets for High Performance Asymmetric Supercapacitors. Mater. Lett. 2022, 308, 131156. [Google Scholar] [CrossRef]
- Mozaffari, S.A.; Mahmoudi Najafi, S.H.; Norouzi, Z. Hierarchical NiO@Ni(OH)2 Nanoarrays as High-Performance Supercapacitor Electrode Material. Electrochim. Acta 2021, 368, 137633. [Google Scholar] [CrossRef]
- Roshni, C.P.; Jithesh, K.; Manuraj, M.; Govind Raj, K.; Rakhi, R.B. β-Ni(OH)2 Supported over g-C3N4: A Novel Catalyst for Para-Nitrophenol Reduction and Supercapacitor Electrode. Results Chem. 2022, 4, 100498. [Google Scholar] [CrossRef]
- Perez-Gonzalez, R.; Araujo, E.; Ge, W.; Cherepanov, S.; Zakhidov, A.; Rodriguez-Gonzalez, V.; Encinas, A.; Oliva, J. Carbon Nanotube Anodes Decorated with Ag NWs/Ni(OH)2 NWs for Efficient Semitransparent Flexible Solid State Supercapacitors. Electrochim. Acta 2020, 354, 136684. [Google Scholar] [CrossRef]




| Supercapacitor Material | Used Electrolyte | Current Density A/g | Capacitance (First Cycle) (F/g) | Capacitance (500th Cycle) (F/g) |
|---|---|---|---|---|
| Ppy/metal composite [64] | poly(vinyl alcohol)/H3PO4 | 0.005 | 70 | 50 |
| CaO/G-C3N4 [65] | 6 M NaOH | 0.5 | 84 | -- |
| NiO/nanowalls [66] | 1 M KOH | - | - | -- |
| G-C3N4 [67] | 1 M NaOH | 1 | 20.5 | 19 |
| β-Ni(OH)2 [67] | 1 M NaOH | 1 | 14.2 | 13.1 |
| Carbon nanotube/Ag [68] | Gel electrolyte (polymethyl methacrylate, acetone, H2O, H3PO4) | 0.001 | 88 | -- |
| Ag2S-Ag2O-Ag/P2ABT (current work) | 1 M HCl | 0.3 | 92.5 | 74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabia, M.; Elsayed, A.M.; Abdallah Alnuwaiser, M.; Abdelazeez, A.A.A. Ag2S-Ag2O-Ag/poly-2-aminobenzene-1-thiol Nanocomposite as a Promising Two-Electrode Symmetric Supercapacitor: Tested in Acidic and Basic Mediums. Micromachines 2023, 14, 1423. https://doi.org/10.3390/mi14071423
Rabia M, Elsayed AM, Abdallah Alnuwaiser M, Abdelazeez AAA. Ag2S-Ag2O-Ag/poly-2-aminobenzene-1-thiol Nanocomposite as a Promising Two-Electrode Symmetric Supercapacitor: Tested in Acidic and Basic Mediums. Micromachines. 2023; 14(7):1423. https://doi.org/10.3390/mi14071423
Chicago/Turabian StyleRabia, Mohamed, Asmaa M. Elsayed, Maha Abdallah Alnuwaiser, and Ahmed Adel A. Abdelazeez. 2023. "Ag2S-Ag2O-Ag/poly-2-aminobenzene-1-thiol Nanocomposite as a Promising Two-Electrode Symmetric Supercapacitor: Tested in Acidic and Basic Mediums" Micromachines 14, no. 7: 1423. https://doi.org/10.3390/mi14071423
APA StyleRabia, M., Elsayed, A. M., Abdallah Alnuwaiser, M., & Abdelazeez, A. A. A. (2023). Ag2S-Ag2O-Ag/poly-2-aminobenzene-1-thiol Nanocomposite as a Promising Two-Electrode Symmetric Supercapacitor: Tested in Acidic and Basic Mediums. Micromachines, 14(7), 1423. https://doi.org/10.3390/mi14071423






