Processes for the 3D Printing of Hydrodynamic Flow-Focusing Devices
Abstract
1. Introduction
2. Materials and Methods
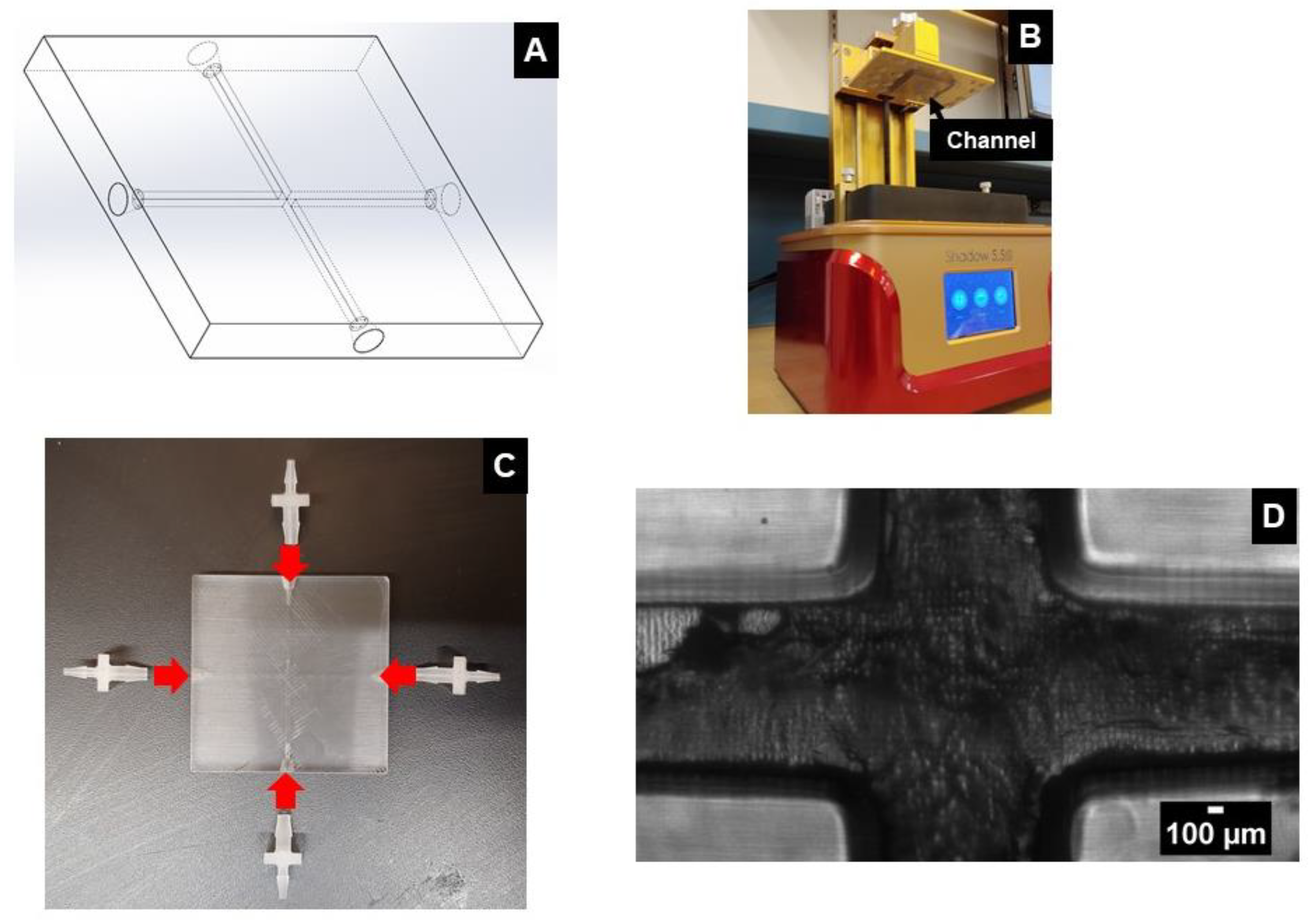
2.1. Monolithic SLA Method
2.2. Hybrid Molding Method
2.3. Sample Preparation
2.4. Experimental Setup
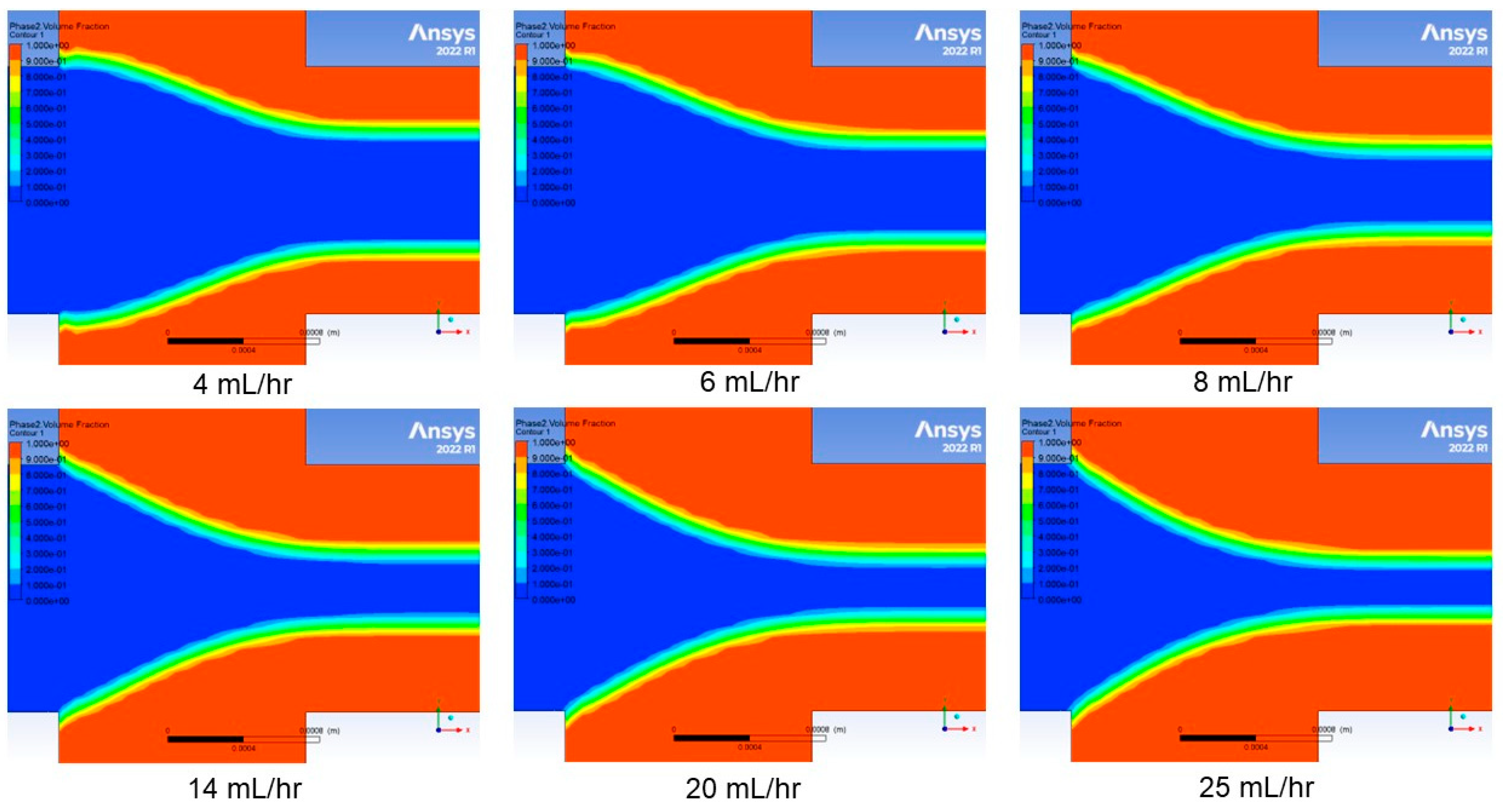
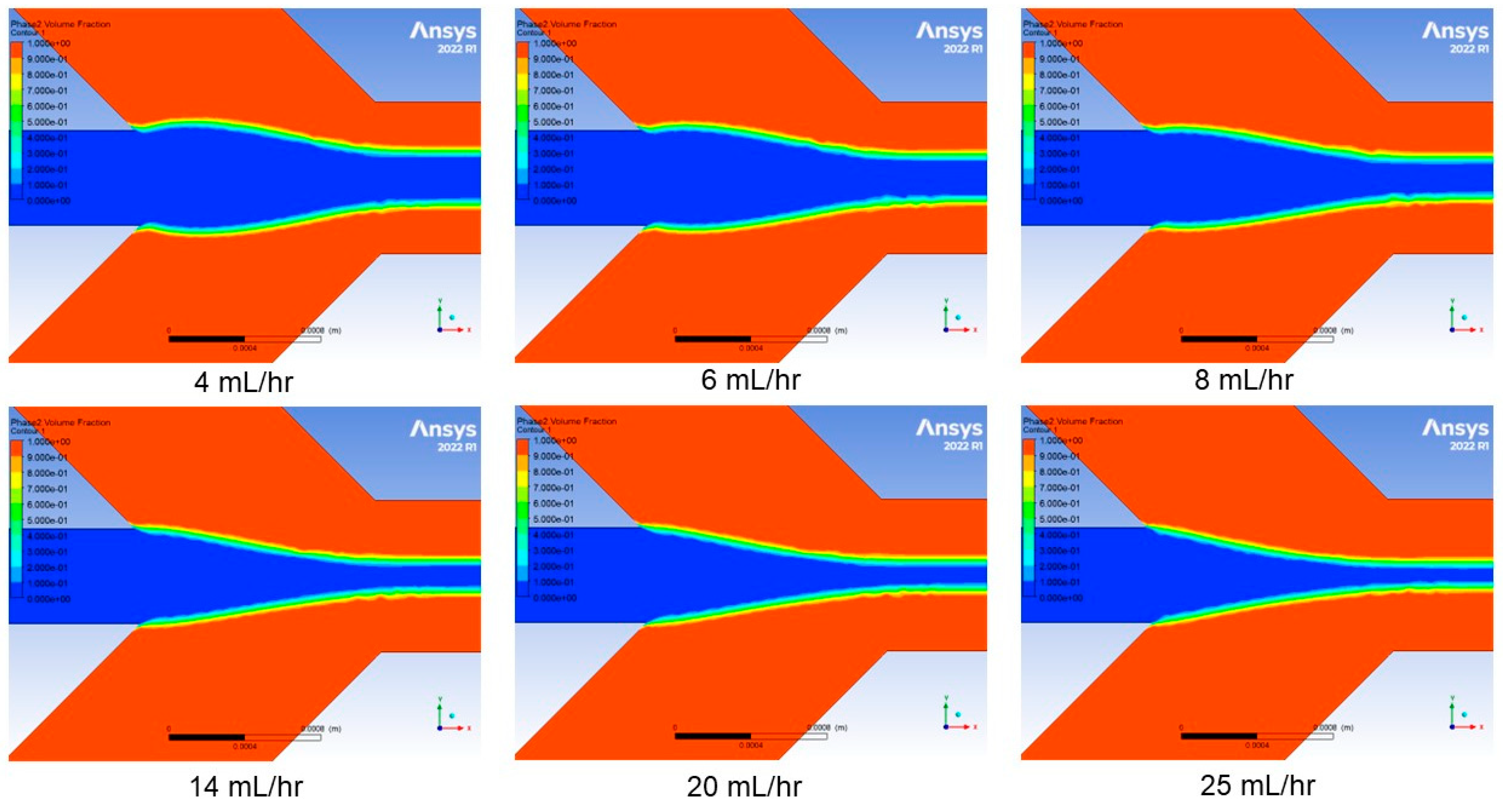
3. Modeling and Simulation
4. Results and Discussion
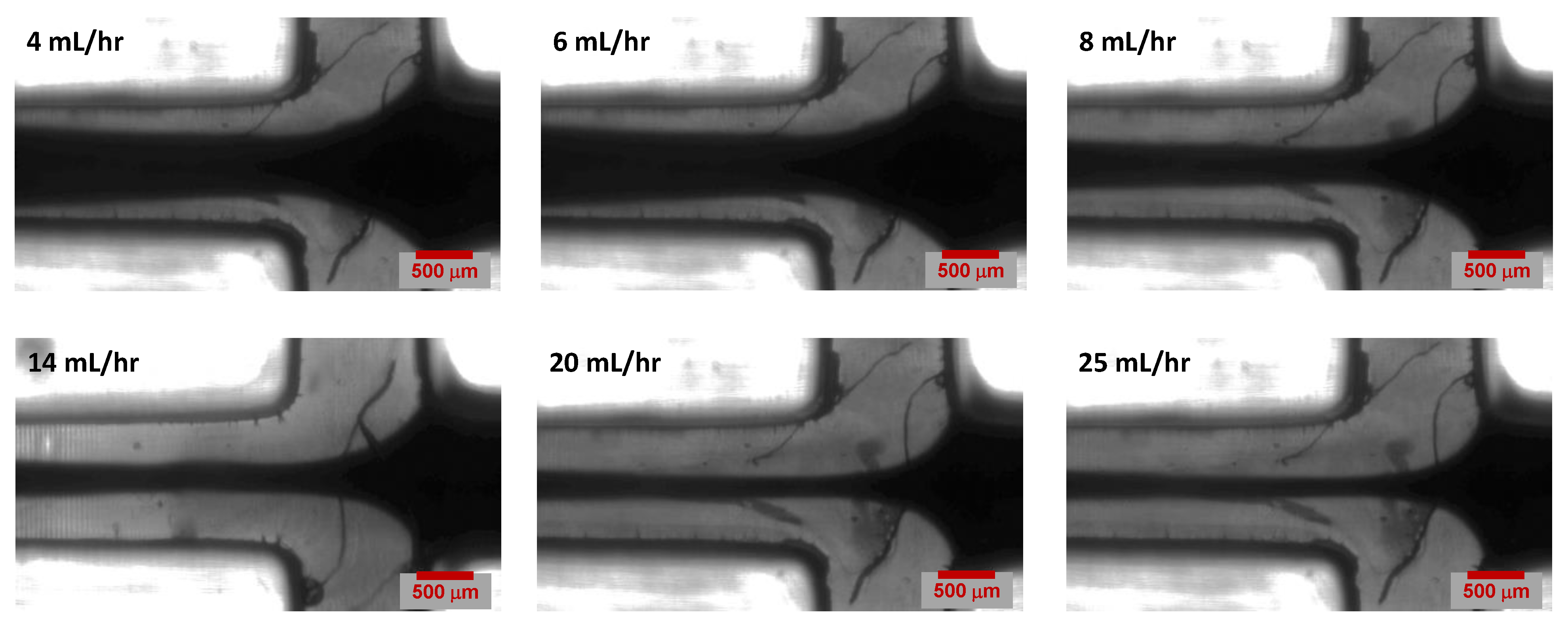
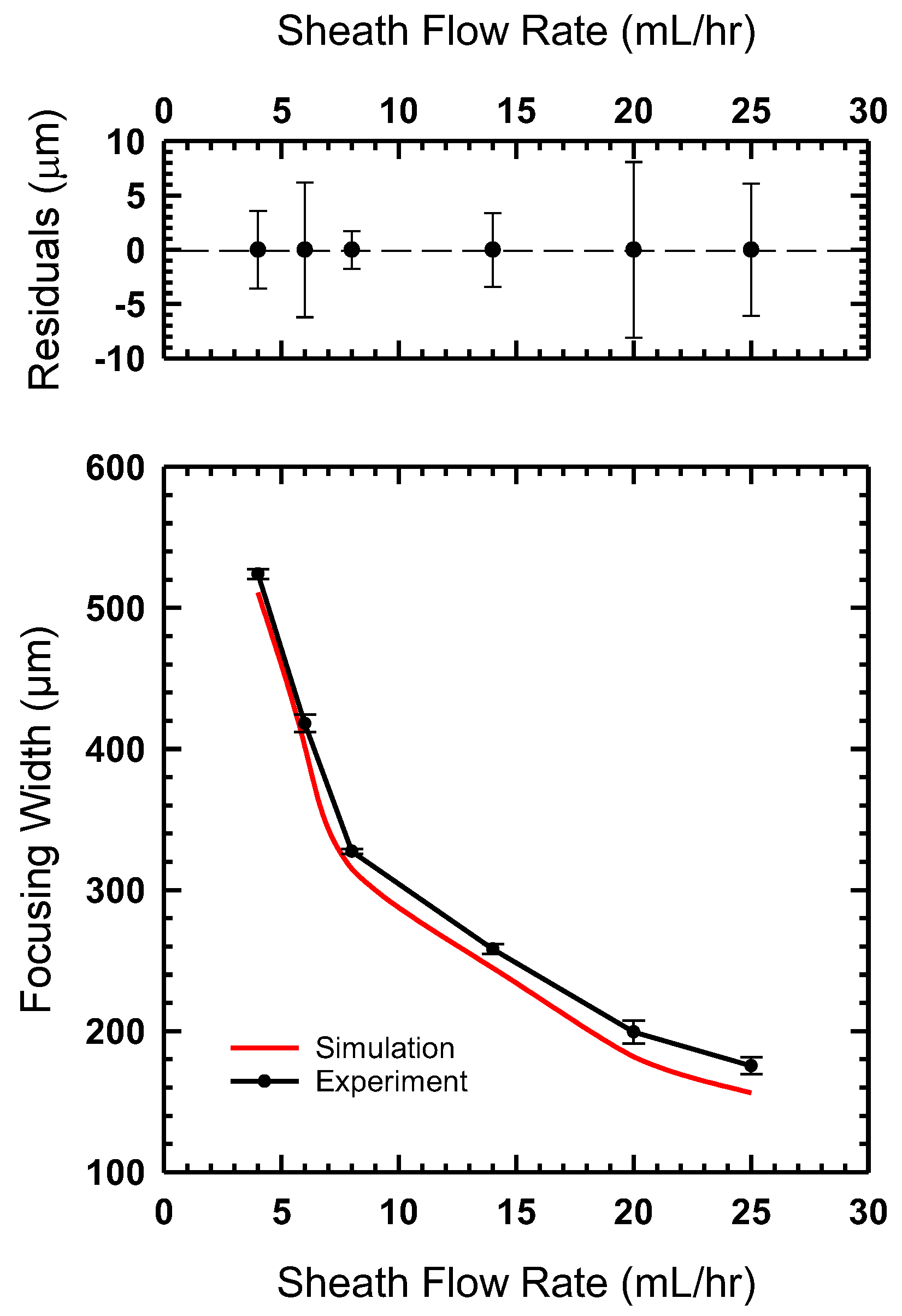
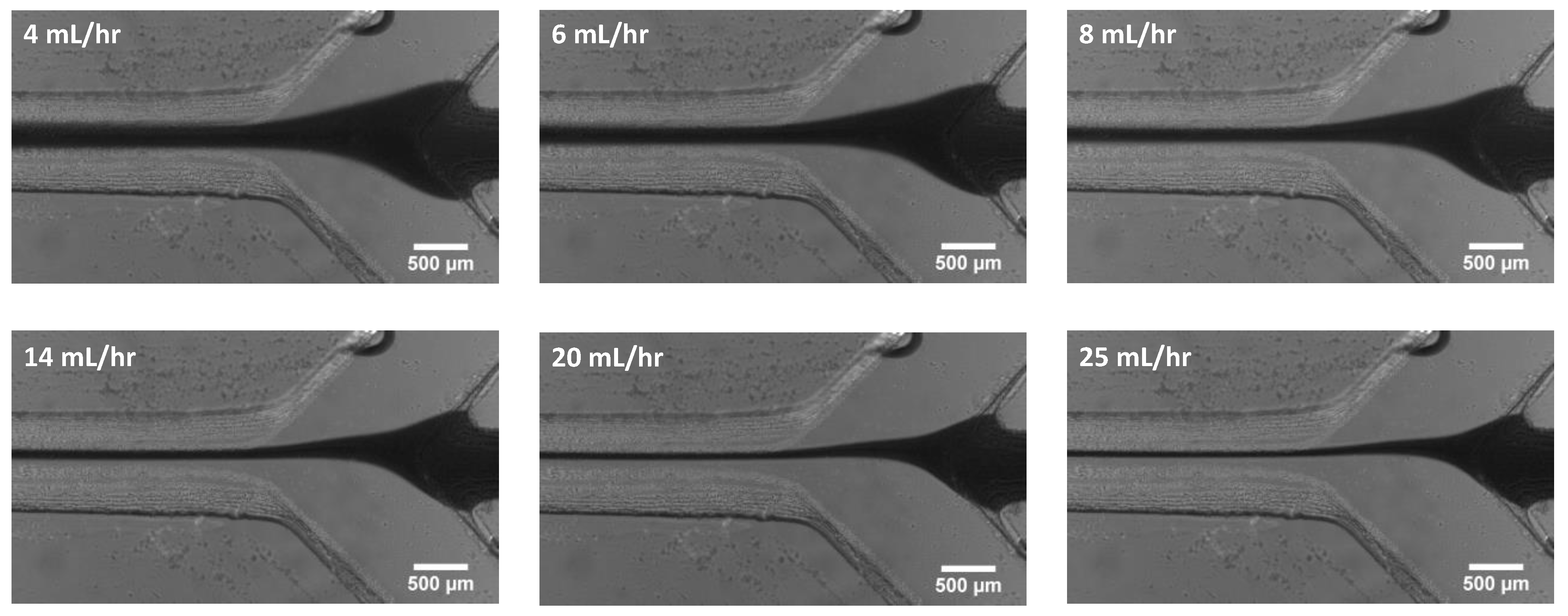
4.1. Effect of Sheath Flow Rate
4.2. Experimental Results
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Daniele, M.A.; Boyd, D.A.; Mott, D.R.; Ligler, F.S. 3D Hydrodynamic Focusing Microfluidics for Emerging Sensing Technologies. Biosens. Bioelectron. 2015, 67, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Crosland-Taylor, P. A Device for Counting Small Particles Suspended in a Fluid through a Tube. Nature 1953, 171, 37–38. [Google Scholar] [CrossRef]
- Schrum, D.P.; Culbertson, C.T.; Jacobson, S.C.; Ramsey, J.M. Microchip Flow Cytometry Using Electrokinetic Focusing. Anal. Chem. 1999, 71, 4173–4177. [Google Scholar] [CrossRef]
- Huh, D.; Tung, Y.; Wei, H. Use of Air-Liquid Two-Phase Flow in Hydrophobic Microfluidic Channels for Disposable Flow Cytometers. Biomed. Microdevices 2002, 4, 141–149. [Google Scholar] [CrossRef]
- Lin, C.-H.; Lee, G.-B. Micromachined Flow Cytometers with Embedded Etched Optic Fibers for Optical Detection. J. Micromech. Microeng. 2003, 13, 447–453. [Google Scholar] [CrossRef]
- Fu, L.-M.; Yang, R.-J.; Lin, C.-H.; Pan, Y.-J.; Lee, G.-B. Electrokinetically Driven Micro Flow Cytometers with Integrated Fiber Optics for On-Line Cell/Particle Detection. Anal. Chim. Acta 2004, 507, 163–169. [Google Scholar] [CrossRef]
- Lin, C.-H.; Lee, G.-B.; Fu, L.-M.; Hwey, B.-H. Vertical Focusing Device Utilizing Dielectrophoretic Force and Its Application on Microflow Cytometer. J. Microelectromech. Syst. 2004, 13, 923–932. [Google Scholar] [CrossRef]
- Lee, G.-B.; Lin, C.-H.; Chang, S.-C. Micromachine-Based Multi-Channel Flow Cytometers for Cell/Particle Counting and Sorting. J. Micromech. Microeng. 2005, 15, 447–454. [Google Scholar] [CrossRef]
- Huh, D.; Gu, W.; Kamotani, Y.; Grotberg, J.B.; Takayama, S. Microfluidics for Flow Cytometric Analysis of Cells and Particles. Physiol. Meas. 2005, 26, R73–R98. [Google Scholar] [CrossRef]
- Piyasena, M.E.; Graves, S.W. The Intersection of Flow Cytometry with Microfluidics and Microfabrication. Lab Chip 2014, 14, 1044–1059. [Google Scholar] [CrossRef]
- Knight, J.B.; Vishwanath, A.; Brody, J.P.; Austin, R.H. Hydrodynamic Focusing on a Silicon Chip: Mixing Nanoliters in Microseconds. Phys. Rev. Lett. 1998, 80, 3863. [Google Scholar] [CrossRef]
- Pollack, L.; Tate, M.W.; Darnton, N.C.; Knight, J.B.; Gruner, S.M.; Eaton, W.A.; Austin, R.H. Compactness of the Denatured State of a Fast-Folding Protein Measured by Submillisecond Small-Angle X-ray Scattering. Proc. Natl. Acad. Sci. USA 1999, 96, 10115–10117. [Google Scholar] [CrossRef] [PubMed]
- Hertzog, D.E.; Michalet, X.; Jäger, M.; Kong, X.; Santiago, J.G.; Weiss, S.; Bakajin, O. Femtomole Mixer for Microsecond Kinetic Studies of Protein Folding. Anal. Chem. 2004, 76, 7169–7178. [Google Scholar] [CrossRef] [PubMed]
- Takayama, S.; Mcdonald, J.C.; Ostuni, E.; Liang, M.N.; Kenis, P.J.A.; Ismagilov, R.F.; Whitesides, G.M. Patterning Cells and Their Environments Using Multiple Laminar Fluid Flows in Capillary Networks. Proc. Natl. Acad. Sci. USA 1999, 4, 5545–5548. [Google Scholar] [CrossRef] [PubMed]
- Regenberg, B.; Krühne, U.; Beyer, M.; Pedersen, L.H.; Simón, M.; Thomas, O.R.T.; Nielsen, J.; Ahl, T. Use of Laminar Flow Patterning for Miniaturised Biochemical Assays. Lab Chip 2004, 4, 654–657. [Google Scholar] [CrossRef]
- Wong, P.; Lee, Y.-K.; Ho, C.-M. Deformation of DNA Molecules by Hydrodynamic Focusing. J. Fluid Mech. 2003, 497, 55–65. [Google Scholar] [CrossRef]
- Blankenstein, G.; Darling Larsen, U. Modular Concept of a Laboratory on a Chip for Chemical and Biochemical Analysis. Biosens. Bioelectron. 1998, 13, 427–438. [Google Scholar] [CrossRef]
- Lee, G.-B.; Hung, C.-I.; Ke, B.-J.; Huang, G.-R.; Hwei, B.-H. Micromachined Pre-Focused 1× N Flow Switches for Continuous Sample Injection. J. Micromech. Microeng. 2001, 11, 567–573. [Google Scholar] [CrossRef]
- Lee, G.-B.; Hwei, B.-H.; Huang, G.-R. Micromachined Pre-Focused M × N Flow Switches for Continuous Multi-Sample Injection. J. Micromech. Microeng. 2001, 11, 654–661. [Google Scholar] [CrossRef]
- Vestad, T.; Marr, D.W.M.; Oakey, J. Flow Control for Capillary-Pumped Microfluidic Systems. J. Micromech. Microeng. 2004, 14, 1503–1506. [Google Scholar] [CrossRef]
- Garstecki, P.; Gitlin, I.; DiLuzio, W.; Whitesides, G.M.; Kumacheva, E.; Stone, H.A. Formation of Monodisperse Bubbles in a Microfluidic Flow-Focusing Device. Appl. Phys. Lett. 2004, 85, 2649–2651. [Google Scholar] [CrossRef]
- Takeuchi, S.; Garstecki, P.; Weibel, D.B.; Whitesides, G.M. An Axisymmetric Flow-Focusing Microfluidic Device. Adv. Mater. 2005, 17, 1067–1072. [Google Scholar] [CrossRef]
- Raj, M.K.; Chakraborty, S. PDMS Microfluidics: A Mini Review. J. Appl. Polym. Sci. 2020, 137, 48958. [Google Scholar] [CrossRef]
- Xia, Y.; Whitesides, G.M. Soft Lithography. Annu. Rev. Mater. Sci. 1998, 33, 153–184. [Google Scholar] [CrossRef]
- Szydzik, C.; Niego, B.; Dalzell, G.; Knoerzer, M.; Ball, F.; Nesbitt, W.S.; Medcalf, R.L.; Khoshmanesh, K.; Mitchell, A. Fabrication of Complex PDMS Microfluidic Structures and Embedded Functional Substrates by One-Step Injection Moulding. RSC Adv. 2016, 6, 87988–87994. [Google Scholar] [CrossRef]
- Weerakoon-Ratnayake, K.M.; O’Neil, C.E.; Uba, F.I.; Soper, S.A. Thermoplastic Nanofluidic Devices for Biomedical Applications. Lab Chip 2017, 17, 362–381. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Cho, Y.H.; Park, M.S. Microchannel Fabrication on Glass Materials for Microfluidic Devices. Int. J. Precis. Eng. Manuf. 2019, 20, 479–495. [Google Scholar] [CrossRef]
- Hansen, C.J.; Saksena, R.; Kolesky, D.B.; Vericella, J.J.; Kranz, S.J.; Muldowney, G.P.; Christensen, K.T.; Lewis, J.A. High-Throughput Printing via Microvascular Multinozzle Arrays. Adv. Mater. 2013, 25, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Teh, K.S. Additive Direct-Write Microfabrication for MEMS: A Review. Front. Mech. Eng. 2017, 12, 490–509. [Google Scholar] [CrossRef]
- Zhang, Q.; Austin, R.H. Applications of Microfluidics in Stem Cell Biology. Bionanoscience 2012, 2, 277–286. [Google Scholar] [CrossRef]
- Montanero, J.M.; Gañán-Calvo, A.M.; Acero, A.J.; Vega, E.J. Micrometer Glass Nozzles for Flow Focusing. J. Micromech. Microeng. 2010, 20, 075035. [Google Scholar] [CrossRef]
- Austin Suthanthiraraj, P.P.; Piyasena, M.E.; Woods, T.A.; Naivar, M.A.; Lopez, G.P.; Graves, S.W. One-Dimensional Acoustic Standing Waves in Rectangular Channels for Flow Cytometry. Methods 2012, 57, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Piyasena, M.E.; Austin Suthanthiraraj, P.P.; Applegate, R.W.; Goumas, A.M.; Woods, T.A.; López, G.P.; Graves, S.W. Multinode Acoustic Focusing for Parallel Flow Cytometry. Anal. Chem. 2012, 84, 1831–1839. [Google Scholar] [CrossRef]
- Kalb, D.M.; Fencl, F.A.; Woods, T.A.; Swanson, A.; Maestas, G.C.; Juárez, J.J.; Edwards, B.S.; Shreve, A.P.; Graves, S.W. Line-Focused Optical Excitation of Parallel Acoustic Focused Sample Streams for High Volumetric and Analytical Rate Flow Cytometry. Anal. Chem. 2017, 89, 9967–9975. [Google Scholar] [CrossRef]
- Bruus, H. Acoustofluidics 10: Scaling Laws in Acoustophoresis. Lab Chip 2012, 12, 1578–1586. [Google Scholar] [CrossRef]
- Au, A.K.; Huynh, W.; Horowitz, L.F.; Folch, A. 3D-Printed Microfluidics. Angew. Chem. Int. Ed. 2016, 55, 3862–3881. [Google Scholar] [CrossRef] [PubMed]
- Gyimah, N.; Scheler, O.; Rang, T.; Pardy, T. Can 3D Printing Bring Droplet Microfluidics to Every Lab?—A Systematic Review. Micromachines 2021, 12, 339. [Google Scholar] [CrossRef]
- Amin, R.; Knowlton, S.; Hart, A.; Yenilmez, B.; Ghaderinezhad, F.; Katebifar, S.; Messina, M.; Khademhosseini, A.; Tasoglu, S. 3D-Printed Microfluidic Devices. Biofabrication 2016, 8, 022001. [Google Scholar] [CrossRef]
- Manzanares Palenzuela, C.L.; Pumera, M. (Bio)Analytical Chemistry Enabled by 3D Printing: Sensors and Biosensors. TrAC Trends Anal. Chem. 2018, 103, 110–118. [Google Scholar] [CrossRef]
- Gaal, G.; Mendes, M.; de Almeida, T.P.; Piazzetta, M.H.O.; Gobbi, Â.L.; Riul, A.; Rodrigues, V. Simplified Fabrication of Integrated Microfluidic Devices Using Fused Deposition Modeling 3D Printing. Sens. Actuators B Chem. 2017, 242, 35–40. [Google Scholar] [CrossRef]
- Duong, L.H.; Chen, P.-C. Simple and Low-Cost Production of Hybrid 3D-Printed Microfluidic Devices. Biomicrofluidics 2019, 13, 024108. [Google Scholar] [CrossRef] [PubMed]
- Gross, B.C.; Erkal, J.L.; Lockwood, S.Y.; Chen, C.; Spence, D.M. Evaluation of 3D Printing and Its Potential Impact on Biotechnology and the Chemical Sciences. Anal. Chem. 2014, 86, 3240–3253. [Google Scholar] [CrossRef]
- Baden, T.; Chagas, A.M.; Gage, G.; Marzullo, T.; Prieto-Godino, L.L.; Euler, T. Open Labware: 3-D Printing Your Own Lab Equipment. PLoS Biol. 2015, 13, e1002086. [Google Scholar] [CrossRef]
- Kitson, P.J.; Glatzel, S.; Chen, W.; Lin, C.-G.; Song, Y.-F.; Cronin, L. 3D Printing of Versatile Reactionware for Chemical Synthesis. Nat. Protoc. 2016, 11, 920–936. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Q. The Application of 3D Printing in STEM Education. In Proceedings of the 2018 IEEE International Conference on Applied System Invention (ICASI), Chiba, Japan, 13–17 April 2018; pp. 1115–1118. [Google Scholar] [CrossRef]
- Pinger, C.W.; Geiger, M.K.; Spence, D.M. Applications of 3D-Printing for Improving Chemistry Education. J. Chem. Educ. 2020, 97, 112–117. [Google Scholar] [CrossRef]
- Irwin, J.; Pearce, J.; Anzalone, G.; Oppliger, D. The RepRap 3-D Printer Revolution in STEM Education. In Proceedings of the 2014 ASEE Annual Conference & Exposition Proceedings, ASEE Conferences, Indianapolis, IN, USA, 15–18 June 2014; pp. 24.1242.1–24.1242.13. [Google Scholar]
- Yang, A.; Hsieh, W. Hydrodynamic Focusing Investigation in a Micro-Flow Cytometer. Biomed. Microdevices 2007, 9, 113–122. [Google Scholar] [CrossRef]
- Nichols, B.; Hirt, C.; Hotchkiss, R. Volume of Fluid (VOF) Method for the Dynamics of Free Boundaries. J. Comput. Phys. 1981, 39, 201–225. [Google Scholar]
- Lee, G.B.; Chang, C.C.; Huang, S.B.; Yang, R.J. The hydrodynamic focusing effect inside rectangular microchannels. J. Micromech. Microeng. 2006, 16, 1024. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awate, D.M.; Holton, S.; Meyer, K.; Juárez, J.J. Processes for the 3D Printing of Hydrodynamic Flow-Focusing Devices. Micromachines 2023, 14, 1388. https://doi.org/10.3390/mi14071388
Awate DM, Holton S, Meyer K, Juárez JJ. Processes for the 3D Printing of Hydrodynamic Flow-Focusing Devices. Micromachines. 2023; 14(7):1388. https://doi.org/10.3390/mi14071388
Chicago/Turabian StyleAwate, Diwakar M., Seth Holton, Katherine Meyer, and Jaime J. Juárez. 2023. "Processes for the 3D Printing of Hydrodynamic Flow-Focusing Devices" Micromachines 14, no. 7: 1388. https://doi.org/10.3390/mi14071388
APA StyleAwate, D. M., Holton, S., Meyer, K., & Juárez, J. J. (2023). Processes for the 3D Printing of Hydrodynamic Flow-Focusing Devices. Micromachines, 14(7), 1388. https://doi.org/10.3390/mi14071388







