Au-TiO2-Coated Spectroscopy-Based Human Teeth Disorder Detection Sensor: Design and Quantitative Analysis
Abstract
1. Introduction
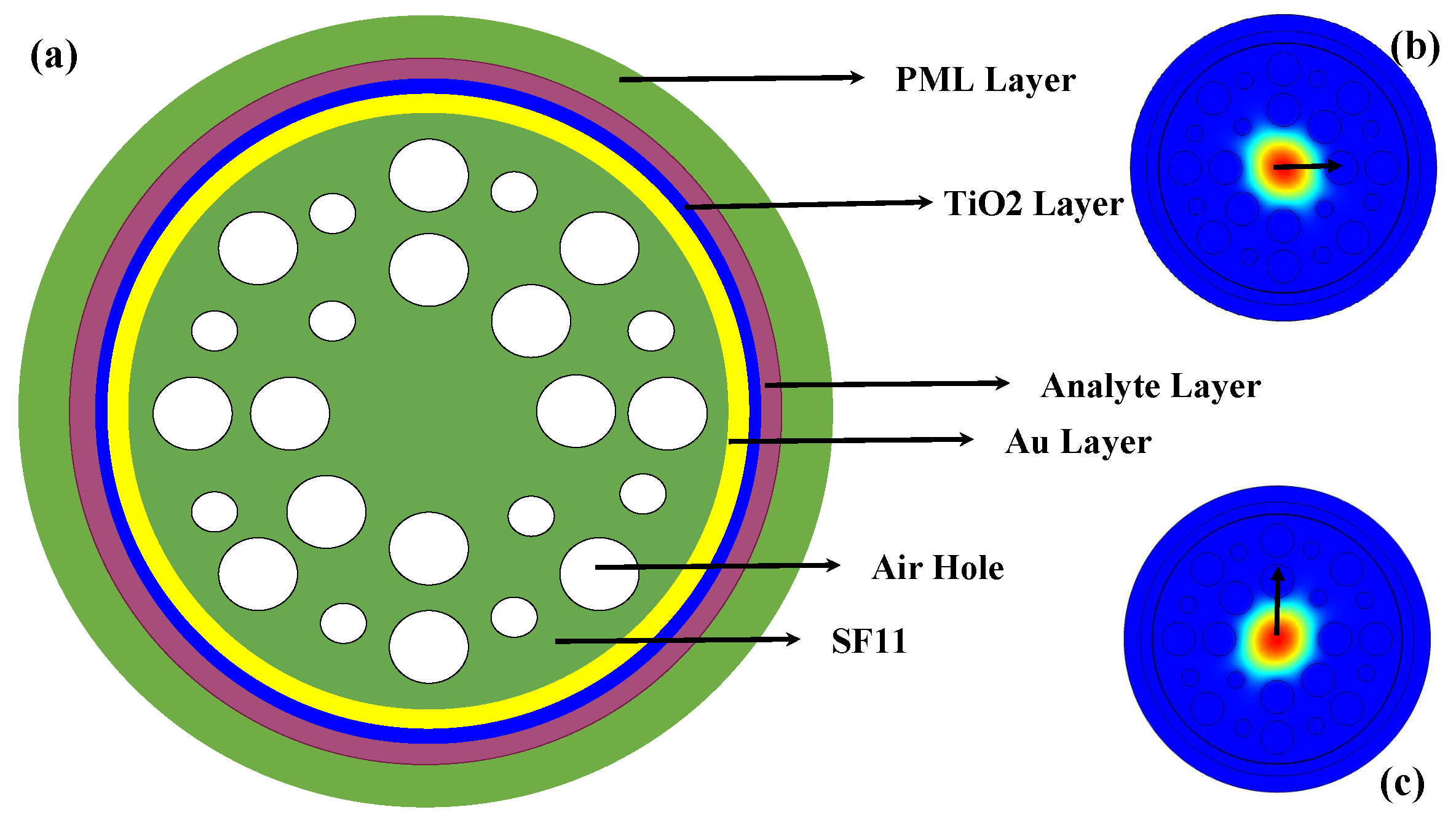
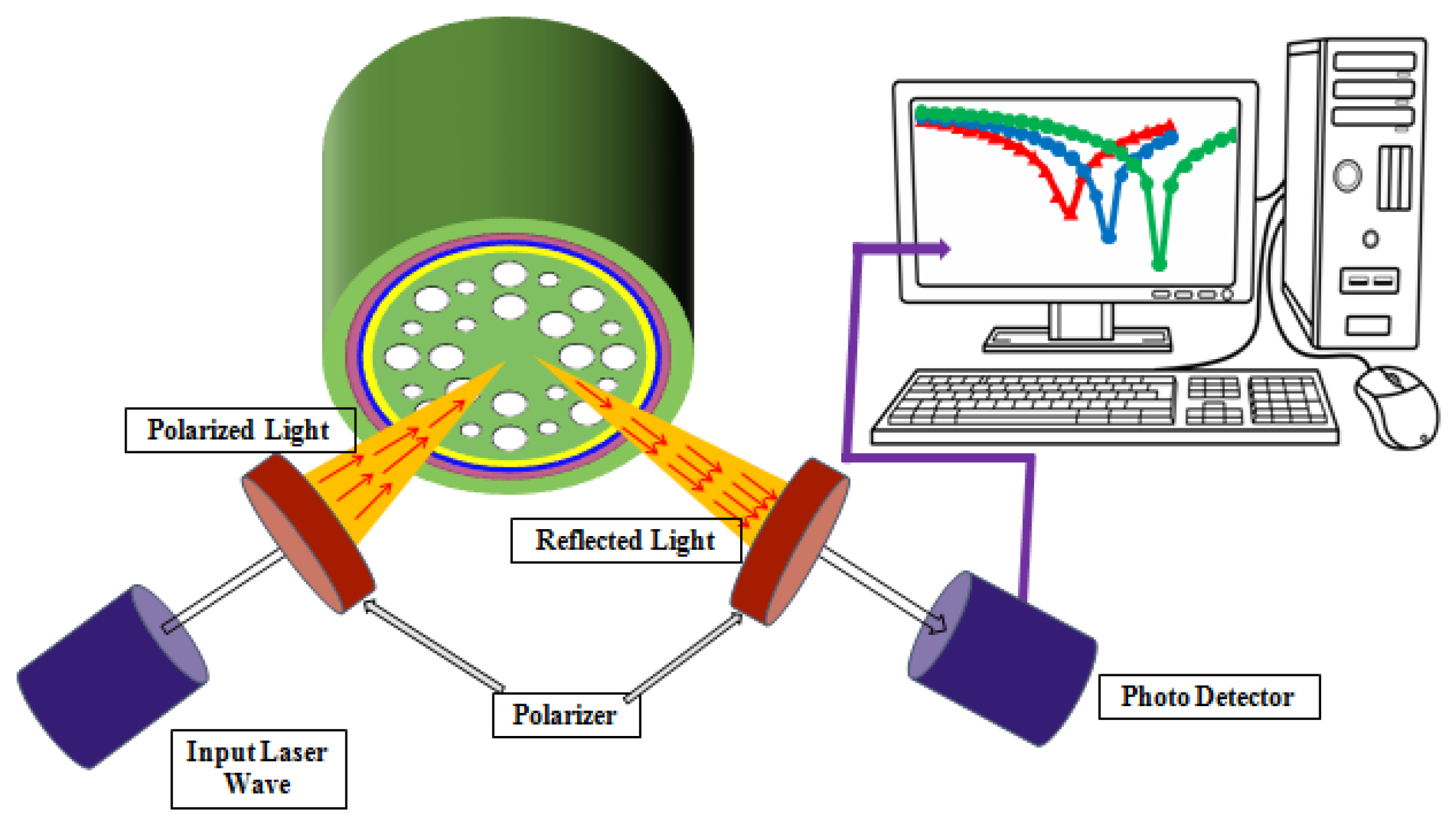
2. Design and Methodology
3. Fabrication
4. Numerical Analysis
4.1. RI of SF11
4.2. RI of Au
4.3. RI of
4.4. Birefringence
4.5. Beat Length (LB)
4.6. Coupling Length (LC)
4.7. Numerical Aperture (NA)
4.8. Power Spectrum
4.9. Transmission Spectrum
4.10. Effective Mode Area (EMA)
4.11. Wavelength Sensitivity
4.12. Confinement Loss (CL)
5. Result Analysis and Discussion
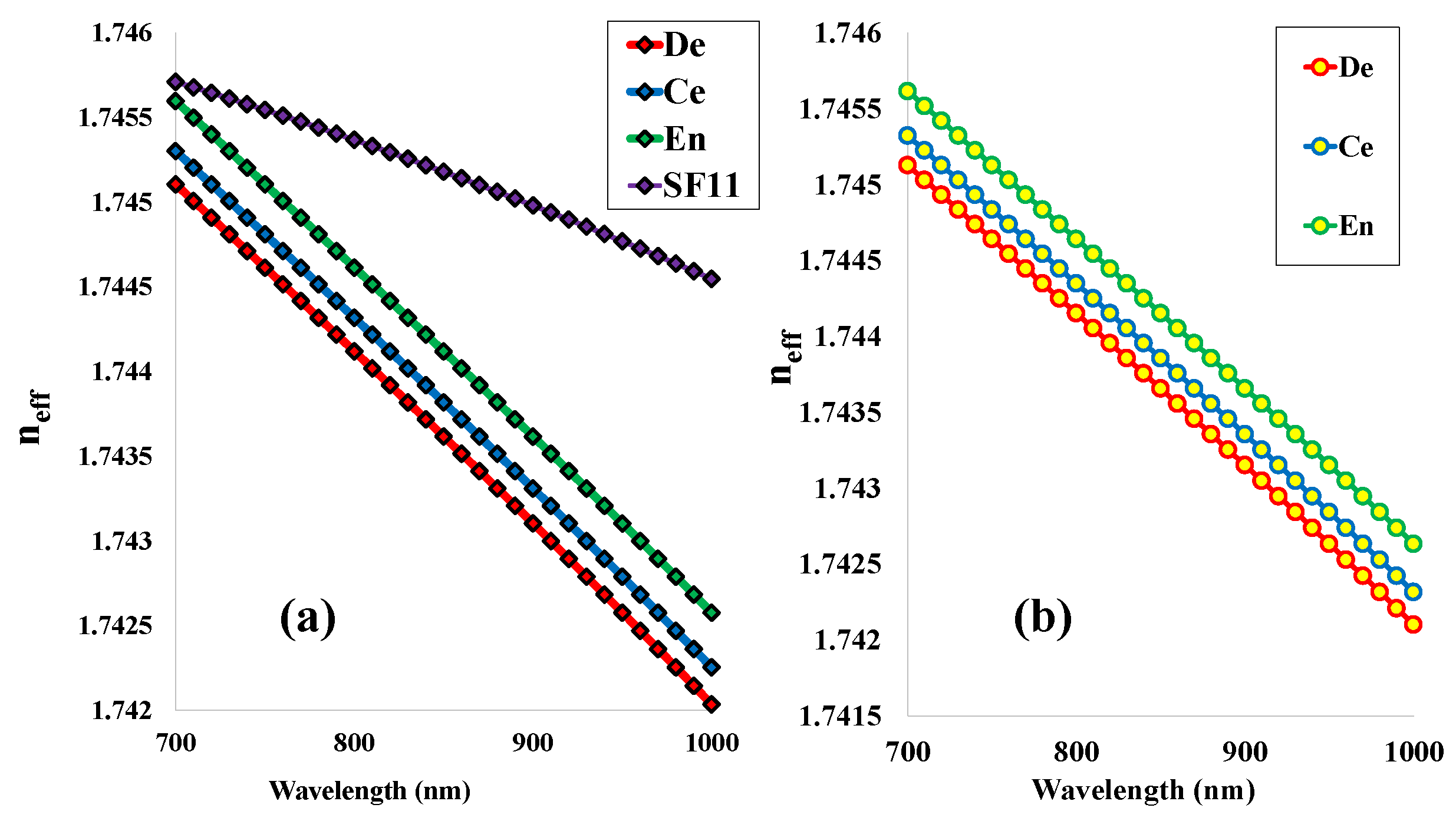
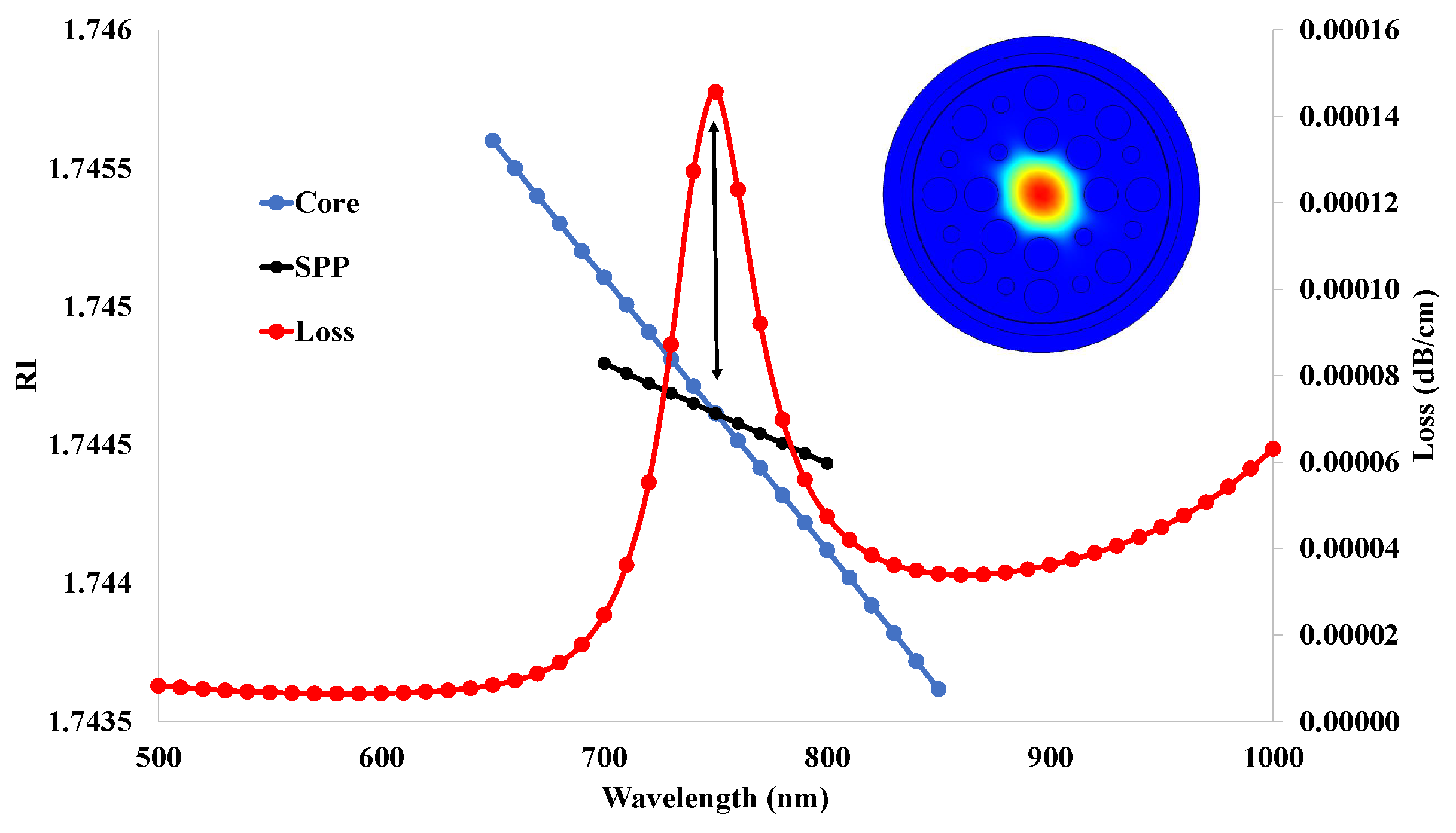
5.1. Effective Refractive Index (RI)
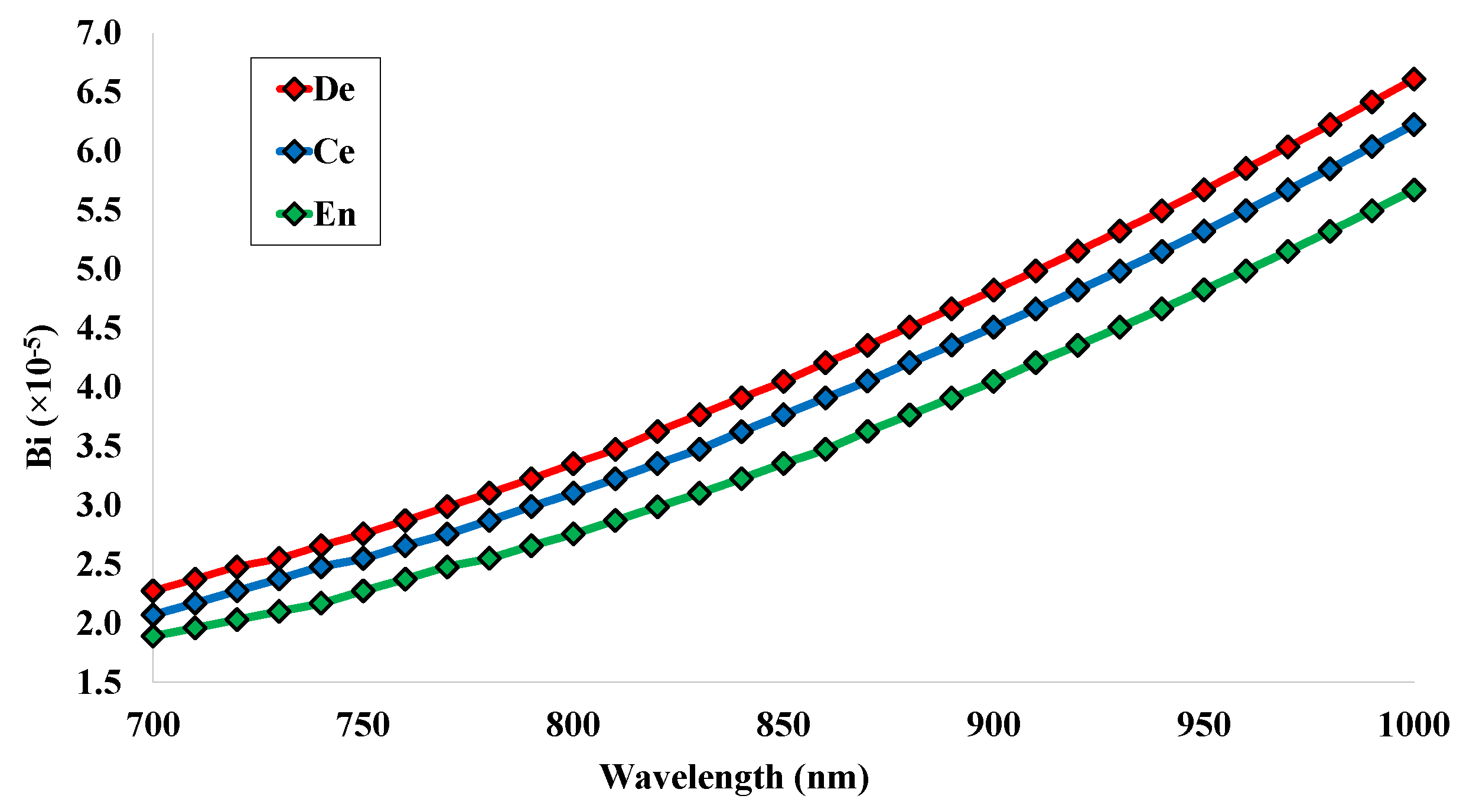
5.2. Birefringence (Bi)
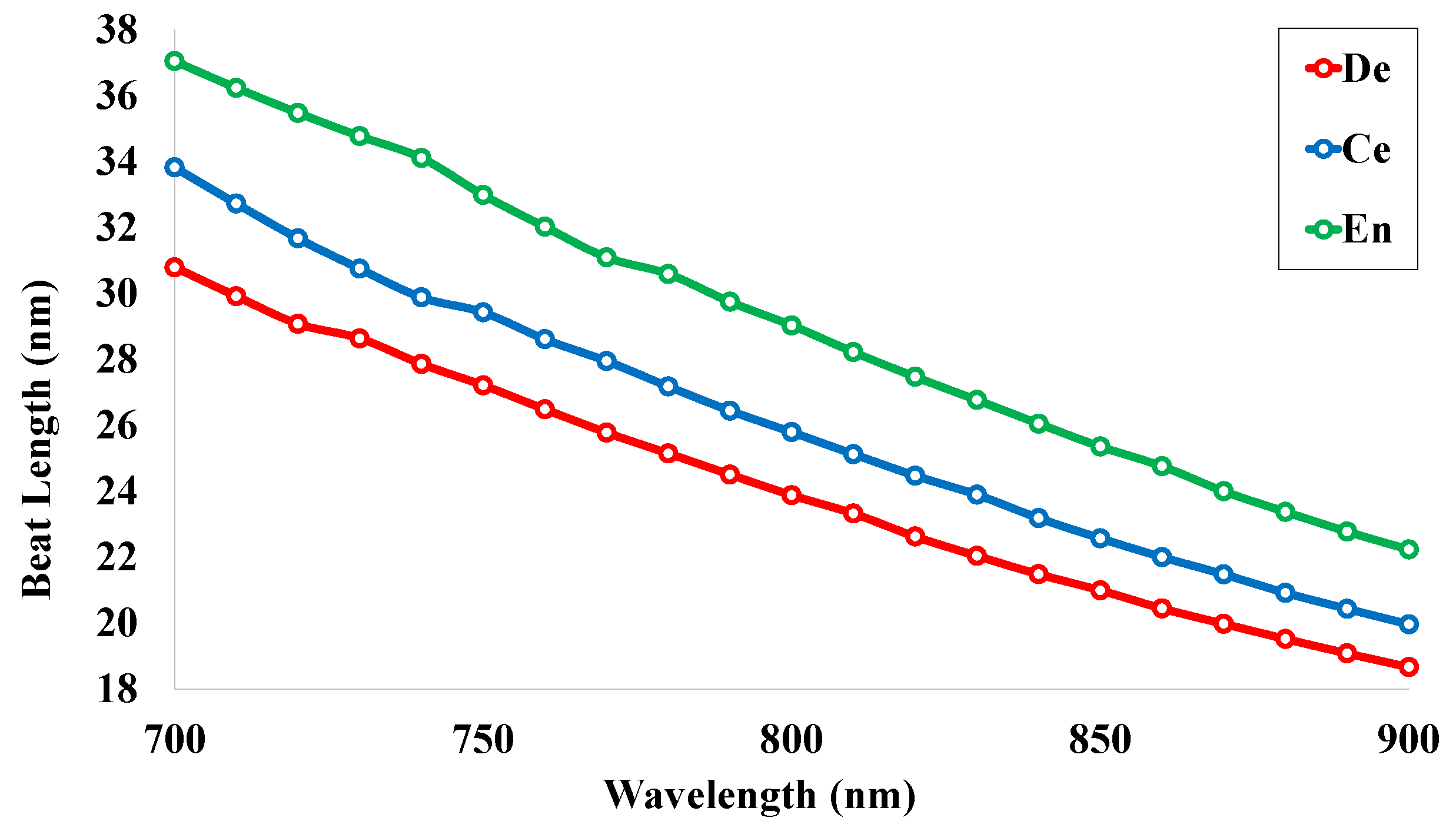
5.3. Beat Length (LB)
5.4. Coupling Length (LC)
5.5. Numerical Aperture (NA)
5.6. Power (P)
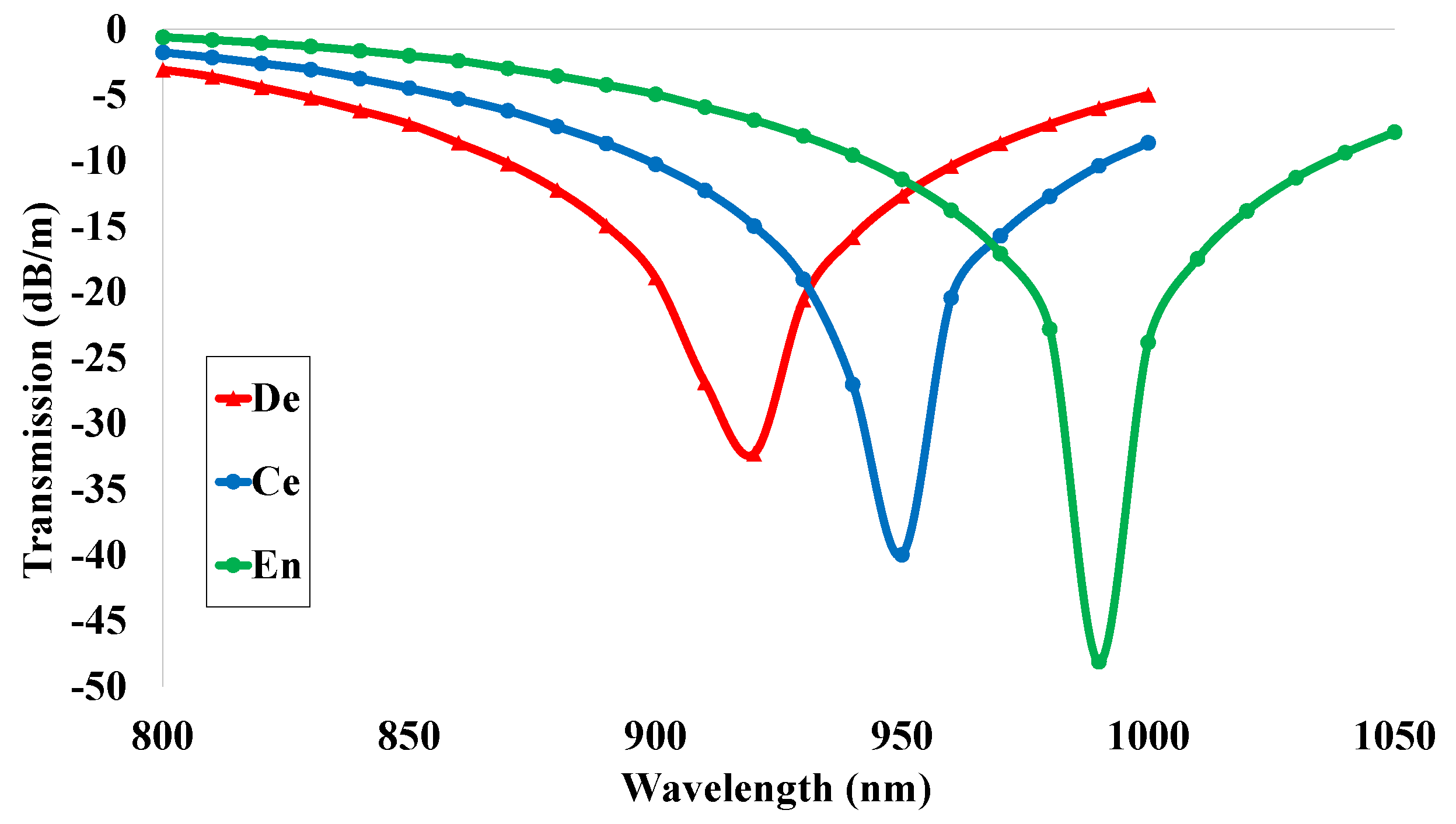
5.7. Transmission (T)
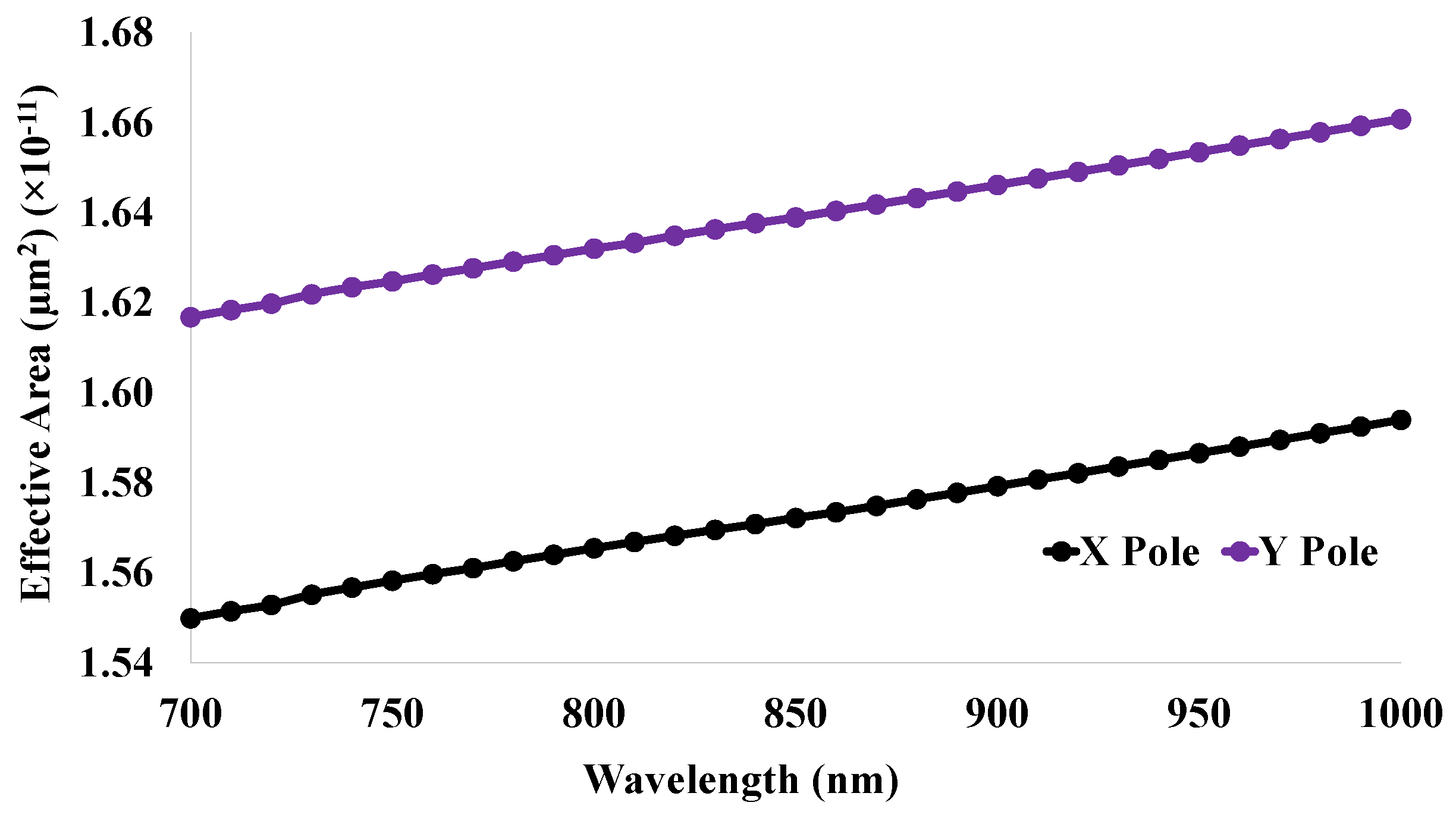
5.8. Effective Mode Area (EMA)
5.9. Wavelength Sensitivity (WS)
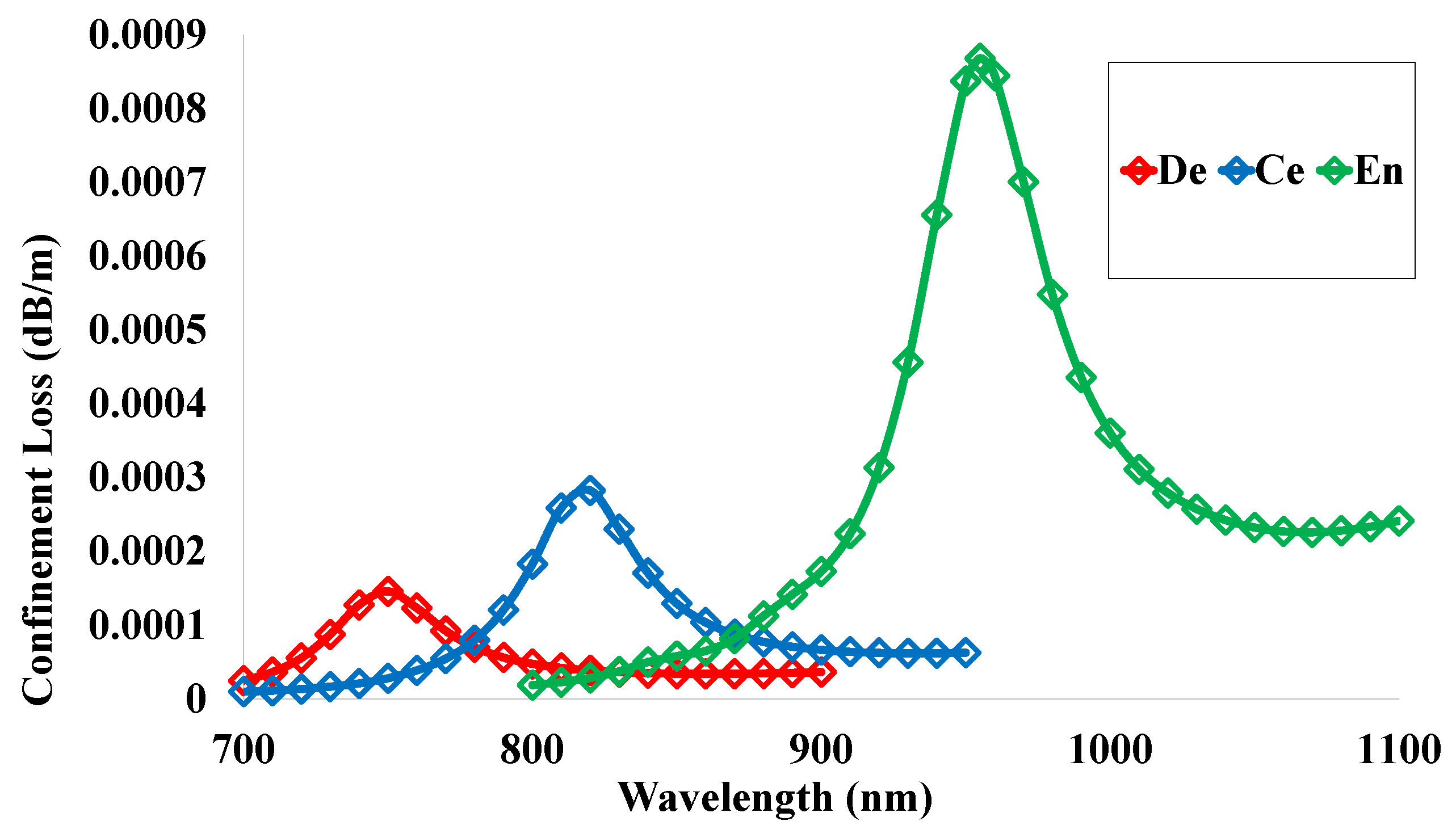
5.10. Confinement Loss (CL)
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goldberg, M.; Kulkarni, A.B.; Young, M.; Boskey, A. Dentin: Structure, Composition and Mineralization: The role of dentin ECM in dentin formation and mineralization. Front. Biosci. Elite Ed. 2011, 3, 711. [Google Scholar] [CrossRef]
- Black, G.V. Descriptive Anatomy of the Human Teeth; SS White Manufacturing Company: Lakewood, NJ, USA, 1897. [Google Scholar]
- Naderi, F.; Mehdiabadi, M.; Kamarehei, F. The therapeutic effects of stem cells from human exfoliated deciduous teeth on clinical diseases: A narrative review study. Am. J. Stem Cells 2022, 11, 28. [Google Scholar]
- Datta, S.; Modak, B.; Chaki, N. A Complete Review on Dental Carious Lesion Detection Methods and its Challenges. Acta Sci. Dent. Sci. 2019, 3, 74–81. [Google Scholar] [CrossRef]
- Esmaeili, S.; Zanjanchi, M.A.; Golmojdeh, H.; Shariati, S. Modification of MCM-410-Based Core-Shell for Construction of a Colorimetric Gas Sensor. IEEE Sens. J. 2021, 21, 17665–17672. [Google Scholar] [CrossRef]
- Song, R.; Hao, X.; Jing, X.; Deng, D.; Li, Y.; Qi, X.; Su, J.; Wu, C. Optical waveguide-type laser interference velocimeter for measurement of ultra-low speeds. IEEE Sens. J. 2022, 22, 12776–12783. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, A.; Qiu, F.; Wang, L.; Yin, X.Y.; Zhao, W.M.; Du, N.N.; Cong, X.W.; Ren, Z.H.; Hua, L.R.; et al. High Sensitivity Coreless Fiber Surface Plasmon Resonance Sensor Based on Au Nano Biconical Particles. IEEE Sens. J. 2021, 22, 256–263. [Google Scholar] [CrossRef]
- Khannanov, M.N.; Vankov, A.B.; Kirpichev, V.E.; Kulik, L.V.; Kukushkin, I.V. Express Analysis of Gas with Surface Enhanced Raman Scattering Waveguide Resonator. IEEE Sens. J. 2021, 21, 24142–24148. [Google Scholar] [CrossRef]
- Pittet, P.; Lu, G.N.; Galvan, J.M.; Ferrigno, R.; Blum, L.J.; Leca-Bouvier, B.D. PCB technology-based electrochemiluminescence microfluidic device for low-cost portable analytical systems. IEEE Sens. J. 2008, 8, 565–571. [Google Scholar] [CrossRef]
- Wu, S.Y.; Zheng, S.S. Detection of partial discharge in GIS and transformer under impulse voltage by fluorescent optical fiber sensor. IEEE Sens. J. 2021, 21, 10675–10684. [Google Scholar] [CrossRef]
- Sen, S.; Abdullah-Al-Shafi, M.; Sikder, A.S.; Hossain, M.S.; Azad, M.M. Zeonex based decagonal photonic crystal fiber (D-PCF) in the terahertz (THz) band for chemical sensing applications. Sens. Bio-Sens. Res. 2021, 31, 100393. [Google Scholar] [CrossRef]
- Islam, M.I.; Ahmed, K.; Sen, S.; Paul, B.K.; Islam, M.S.; Chowdhury, S.; Hasan, M.R.; Uddin, M.S.; Asaduzzaman, S.; Bahar, A.N. Proposed square lattice photonic crystal fiber for extremely high nonlinearity, birefringence, and ultra-high negative dispersion compensation. J. Opt. Commun. 2019, 40, 401–410. [Google Scholar] [CrossRef]
- Leon, M.J.B.M.; Disha, A.S. A simple structure of PCF based sensor for sensing sulfur dioxide gas with high sensitivity and better birefringence. Sens. Int. 2021, 2, 100115. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Verma, R.; Gupta, B.D. Theoretical modeling of a self-referenced dual mode SPR sensor utilizing indium tin oxide film. Opt. Commun. 2016, 369, 131–137. [Google Scholar] [CrossRef]
- Chao, C.T.C.; Kooh, M.R.R.; Chau, Y.F.C.; Thotagamuge, R. November. Susceptible plasmonic photonic crystal fiber sensor with elliptical air holes and external-flat gold-coated surface. Photonics 2022, 9, 916. [Google Scholar] [CrossRef]
- Jilani, A.; Abdel-Wahab, M.S.; Hammad, A.H. Advance deposition techniques for thin film and coating. Mod. Technol. Creat.-Thin-Film. Syst. Coatings 2017, 2, 137–149. [Google Scholar]
- Nielsen, K.; Noordegraaf, D.; Sørensen, T.; Bjarklev, A.; Hansen, T.P. Selective filling of photonic crystal fibres. J. Opt. Pure Appl. Opt. 2005, 7, L13. [Google Scholar] [CrossRef]
- Moznuzzaman, M.; Khan, I.; Islam, M.R. Nano-layered surface plasmon resonance-based highly sensitive biosensor for virus detection: A theoretical approach to detect SARS-CoV-2. AIP Adv. 2021, 11, 065023. [Google Scholar] [CrossRef] [PubMed]
- Uniyal, A.; Chauhan, B.; Pal, A.; Srivastava, V. InP and graphene employed surface plasmon resonance sensor for measurement of sucrose concentration: A numerical approach. Opt. Eng. 2022, 61, 057103. [Google Scholar] [CrossRef]
- Liao, H.; Nehl, C.L.; Hafner, J.H. Biomedical Applications of Plasmon Resonant Metal Nanoparticles. Future Med. 2006, 1, 201–208. [Google Scholar] [CrossRef]
- Chau, Y.F.; Liu, C.Y.; Yeh, H.H.; Tsai, D.P. A comparative study of high birefringence and low confinement loss photonic crystal fiber employing elliptical air holes in fiber cladding with tetragonal lattice. Prog. Electromagn. Res. B 2010, 22, 39–52. [Google Scholar] [CrossRef]
- Kaijage, S.F.; Namihira, Y.; Hai, N.H.; Begum, F.; Razzak, S.A.; Kinjo, T.; Miyagi, K.; Zou, N. Broadband dispersion compensating octagonal photonic crystal fiber for optical communication applications. Jpn. J. Appl. Phys. 2009, 48, 052401. [Google Scholar] [CrossRef]
- Galtarossa, A.; Palmieri, L.; Schiano, M.; Tambosso, T. Measurements of beat length and perturbation length in long single-mode fibers. Opt. Lett. 2000, 25, 384–386. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Sampaolo, A.; Patimisco, P.; Spagnolo, V.; Ma, Y. Ultra-highly sensitive HCl-LITES sensor based on a low-frequency quartz tuning fork and a fiber-coupled multi-pass cell. Photoacoustics 2022, 27, 100381. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, S.B.; Goldberg, B.B.; Ünlü, M.S. Theoretical analysis of numerical aperture increasing lens microscopy. J. Appl. Phys. 2005, 97, 053105. [Google Scholar] [CrossRef]
- Mitu, S.A.; Dey, D.K.; Ahmed, K.; Paul, B.K.; Luo, Y.; Zakaria, R.; Dhasarathan, V. Fe3O4 nanofluid injected photonic crystal fiber for magnetic field sensing applications. J. Magn. Magn. Mater. 2020, 494, 165831. [Google Scholar] [CrossRef]
- Mitu, S.A.; Ahmed, K.; Hossain, M.; Paul, B.K.; Nguyen, T.K.; Dhasarathan, V. Design of magnetic fluid sensor using elliptically hole assisted photonic crystal fiber (PCF). J. Supercond. Nov. Magn. 2020, 33, 2189–2198. [Google Scholar] [CrossRef]
- Agrawal, G.P. Nonlinear Fiber Optics; Academic: San Diego, CA, USA, 2001. [Google Scholar]
- White, T.P.; McPhedran, R.C.; de Sterke, C.M.; Botten, L.C.; Steel, M.J. Confinement losses in microstructured optical fibers. Opt. Lett. 2001, 26, 1660–1662. [Google Scholar] [CrossRef]
- Mortensen, N.A.; Folken, J.R.; Skovgaard, P.M.; Broeng, J. Numerical aperture of single-mode photonic crystal fibers. IEEE Photonics Technol. Lett. 2002, 14, 1094–1096. [Google Scholar] [CrossRef]
- Mitu, S.A.; Ahmed, K.; Al Zahrani, F.A.; Abdullah, H.; Hossain, M.; Paul, B.K. Micro-structure ring fiber–based novel magnetic sensor with high birefringence and high sensitivity response in broad waveband. Plasmonics 2021, 16, 905–913. [Google Scholar] [CrossRef]
- Amin, R.; Abdulrazak, L.F.; Mohammadd, N.; Ahmed, K.; Bui, F.M.; Ibrahim, S.M. GaAs-filled elliptical core-based hexagonal PCF with excellent optical properties for nonlinear optical applications. Ceram. Int. 2022, 48, 5617–5625. [Google Scholar] [CrossRef]
- Ju, J.; Jin, W.; Demokan, M.S. Properties of a highly birefringent photonic crystal fiber. IEEE Photonics Technol. Lett. 2003, 15, 1375–1377. [Google Scholar] [CrossRef]
- Mohammadd, N.; Abdulrazak, L.F.; Tahhan, S.R.; Amin, R.; Ibrahim, S.M.; Ahmed, K.; Bui, F.M. GaP-filled PCF with ultra-high birefringence and nonlinearity for distinctive optical applications. J. Ovonic Res. 2022, 18, 129–140. [Google Scholar] [CrossRef]
- Hossain, M.B.; Bulbul, A.A.M.; Mukit, M.A.; Podder, E. Analysis of optical properties for square, circular and hexagonal photonic crystal fiber. Opt. Photonics J. 2017, 7, 235–243. [Google Scholar] [CrossRef]
- Abdullah, H.; Ahmed, K.; Alam, M.S.; Rashed, A.N.Z.; Mitu, S.A.; Al-Zahrani, F.A.; Kabir, M.A. High sensitivity refractive index sensor based on triple layer MgF2-gold-MgF2 coated nano metal films photonic crystal fiber. Optik 2021, 241, 166950. [Google Scholar] [CrossRef]
- Arman, H.; Olyaee, S. Photonic bandgap fiber-based gas sensor with high sensitivity and high birefringence. J. Comput. Electron. 2022, 21, 1357–1364. [Google Scholar] [CrossRef]
- Leon, M.J.B.M.; Kabir, M.A. Design of a liquid sensing photonic crystal fiber with high sensitivity, bireferingence and low confinement loss. Sens.-Bio-Sens. Res. 2020, 28, 100335. [Google Scholar] [CrossRef]
- Wu, J.; Li, S.; Wang, X.; Shi, M.; Feng, X.; Liu, Y. Ultrahigh sensitivity refractive index sensor of a D-shaped PCF based on surface plasmon resonance. Appl. Opt. 2018, 57, 4002–4007. [Google Scholar] [CrossRef]
- Islam, M.S.; Islam, M.R.; Sultana, J.; Dinovitser, A.; Ng, B.W.H.; Abbott, D. Exposed-core localized surface plasmon resonance biosensor. JOSA B 2019, 36, 2306–2311. [Google Scholar] [CrossRef]




| Constants | Values | Constants | Values |
|---|---|---|---|
| 1.73759695 | 0.013188707 | ||
| 0.313747346 | 0.0623068142 | ||
| 1.89878101 | 155.23629 |
| Sensing | Bi | LB | LC | NA | Pw | Tr | EMA | WS | CL |
|---|---|---|---|---|---|---|---|---|---|
| Analyte | (RIU) | (nm) | (nm) | (RIU) | (dB) | ||||
| De | 2.27 | 30.78 | 15.39 | 0.05 | 0.93 | −32 | 1.55 | 28,948.69 | 0.00015 |
| Ce | 2.07 | 33.82 | 16.91 | 0.04 | 0.95 | −40 | 1.55 | 33,684.99 | 0.00028 |
| En | 1.89 | 37.04 | 18.52 | 0.02 | 0.98 | −48 | 1.55 | 38,396.56 | 0.00087 |
| Structure | Bi (RIU) | WS (nm/RIU) | RS (%) | CL (dB/m) | Ref. |
|---|---|---|---|---|---|
| Triple-layer SPR-based sensor | 3.59 × | 28,046.205 | —— | 3.5 × | [36] |
| High birefringence gas sensor | 8.76 × | —— | 96.7 | 0.11 | [37] |
| Sulfur dioxide gas sensor | 1.0 × | —— | 70 | 0.000136525 | [13] |
| PCF-based liquid sensor | 8 × | —— | 49.13 | 5.583 × | [38] |
| D-shape SPR-based RI sensor | —— | 31,000 | —— | 474.32 | [39] |
| SPR-based biosensor | —— | 34,000 | —— | 0.79 × | [40] |
| D-Shaped germanium-doped PCF sensor | 1.06 × | 5600 | —— | 0.00680 | [18] |
| Human teeth disease detection sensor | 1.89 × | 38,396.56 | 99.99 | 0.00087 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitu, S.A.; Ahmed, K.; Bui, F.M.; Chen, L.; Smirani, L.K.; Patel, S.K.; Sorathiya, V. Au-TiO2-Coated Spectroscopy-Based Human Teeth Disorder Detection Sensor: Design and Quantitative Analysis. Micromachines 2023, 14, 1191. https://doi.org/10.3390/mi14061191
Mitu SA, Ahmed K, Bui FM, Chen L, Smirani LK, Patel SK, Sorathiya V. Au-TiO2-Coated Spectroscopy-Based Human Teeth Disorder Detection Sensor: Design and Quantitative Analysis. Micromachines. 2023; 14(6):1191. https://doi.org/10.3390/mi14061191
Chicago/Turabian StyleMitu, Sumaiya Akhtar, Kawsar Ahmed, Francis M. Bui, Li Chen, Lassaad K. Smirani, Shobhit K. Patel, and Vishal Sorathiya. 2023. "Au-TiO2-Coated Spectroscopy-Based Human Teeth Disorder Detection Sensor: Design and Quantitative Analysis" Micromachines 14, no. 6: 1191. https://doi.org/10.3390/mi14061191
APA StyleMitu, S. A., Ahmed, K., Bui, F. M., Chen, L., Smirani, L. K., Patel, S. K., & Sorathiya, V. (2023). Au-TiO2-Coated Spectroscopy-Based Human Teeth Disorder Detection Sensor: Design and Quantitative Analysis. Micromachines, 14(6), 1191. https://doi.org/10.3390/mi14061191









